Abstract
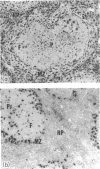
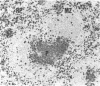
In the present study we investigated the role of mononuclear phagocytes in the pathogenesis of lipopolysaccharide (LPS)-induced lethality and tissue injury. Since hepatic and splenic macrophages are the primary sites of localization of i.v.-injected LPS, we selectively eliminated these macrophages using liposome-encapsulated dichloromethylene diphosphonate (DMDP). After double DMDP-liposome treatment the phagocytic cells in the liver and spleen were completely eliminated, except for the macrophages in the white pulp of the spleen which were affected to a lesser extent by this treatment. An i.v. injection of LPS into DMDP- and saline-pretreated mice showed that the latter animals exhibited febrile-associated symptoms such as lethargy and ruffled fur, but that macrophage elimination abrogated these symptoms. Although after double saline- or DMDP-pretreatment the LD50 appears to be 1 mg and 630 micrograms, respectively, the differences in lethality between both groups of mice were not statistically significant. Therefore, we concluded that hepatic and splenic macrophages are not necessary for LPS-induced lethality. The role of macrophages in LPS-induced local tissue damage was studied by comparing the histopathological changes in hepatic and splenic tissue between DMDP- and saline-pretreated mice. A sublethal dose of LPS induced similar hepatic lesions in macrophage-depleted and saline-pretreated mice, whereas the histopathological changes in the spleen were much more pronounced after DMDP-pretreatment. Particularly in the inner periarteriolar lymphocyte sheath (PALS) of these mice, the number of T cells was considerably reduced and extensive cellular necrosis could be found. These data strongly suggest that the local tissue damage resulting from LPS injection may not be due to its localization in mononuclear phagocytes but rather to interaction with other cell types.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Macrophage activation and nonspecific immunity. Int Rev Exp Pathol. 1978;18:303–346. [PubMed] [Google Scholar]
- Berendt M. J., Newborg M. F., North R. J. Increased toxicity of endotoxin for tumor-bearing mice and mice responding to bacterial pathogens: macrophage activation as a common denominator. Infect Immun. 1980 May;28(2):645–647. doi: 10.1128/iai.28.2.645-647.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- COOPER G. N., STUART A. E. Sensitivity of mice to bacterial lipopolysaccharide following alteration of activity of the reticulo-endothelial system. Nature. 1961 Jul 15;191:294–295. doi: 10.1038/191294a0. [DOI] [PubMed] [Google Scholar]
- Claassen E., Kors N., Van Rooijen N. Influence of carriers on the development and localization of anti-2,4,6-trinitrophenyl (TNP) antibody-forming cells in the murine spleen. II. Suppressed antibody response to TNP-Ficoll after elimination of marginal zone cells. Eur J Immunol. 1986 May;16(5):492–497. doi: 10.1002/eji.1830160505. [DOI] [PubMed] [Google Scholar]
- Claassen E., Van Rooijen N. The effect of elimination of macrophages on the tissue distribution of liposomes containing [3H]methotrexate. Biochim Biophys Acta. 1984 Dec 20;802(3):428–434. doi: 10.1016/0304-4165(84)90360-x. [DOI] [PubMed] [Google Scholar]
- Classen E., Van Rooijen N. Preparation and characteristics of dichloromethylene diphosphonate-containing liposomes. J Microencapsul. 1986 Apr-Jun;3(2):109–114. doi: 10.3109/02652048609031565. [DOI] [PubMed] [Google Scholar]
- Coalson J. J., Benjamin B. A., Archer L. T., Beller B. K., Spaet R. H., Hinshaw L. B. A pathologic study of Escherichia coli shock in the baboon and the response to adrenocorticosteroid treatment. Surg Gynecol Obstet. 1978 Nov;147(5):726–736. [PubMed] [Google Scholar]
- Cook J. A., Dougherty W. J., Holt T. M. Enhanced sensitivity to endotoxin induced by the RE stimulant, glucan. Circ Shock. 1980;7(3):225–238. [PubMed] [Google Scholar]
- Crafton C. G., Di LUZIO N. R. Relationship of reticuloendothelial functional activity to endotoxin lethality. Am J Physiol. 1969 Sep;217(3):736–742. doi: 10.1152/ajplegacy.1969.217.3.736. [DOI] [PubMed] [Google Scholar]
- Filkins J. P. Monokines and the metabolic pathophysiology of septic shock. Fed Proc. 1985 Feb;44(2):300–304. [PubMed] [Google Scholar]
- Freudenberg M. A., Kleine B., Galanos C. The fate of lipopolysaccharide in rats: evidence for chemical alteration in the molecule. Rev Infect Dis. 1984 Jul-Aug;6(4):483–487. doi: 10.1093/clinids/6.4.483. [DOI] [PubMed] [Google Scholar]
- Freudenberg N., Freudenberg M. A., Bandara K., Galanos C. Distribution and localization of endotoxin in the reticulo-endothelial system (RES) and in the main vessels of the rat during shock. Pathol Res Pract. 1985 Mar;179(4-5):517–527. doi: 10.1016/S0344-0338(85)80193-X. [DOI] [PubMed] [Google Scholar]
- Groeneveld P. H., Erich T., Kraal G. In vivo effects of LPS on B lymphocyte subpopulations. Migration of marginal zone-lymphocytes and IgD-blast formation in the mouse spleen. Immunobiology. 1985 Dec;170(5):402–411. doi: 10.1016/S0171-2985(85)80064-4. [DOI] [PubMed] [Google Scholar]
- Groeneveld P. H., Erich T., Kraal G. The differential effects of bacterial lipopolysaccharide (LPS) on splenic non-lymphoid cells demonstrated by monoclonal antibodies. Immunology. 1986 Jun;58(2):285–290. [PMC free article] [PubMed] [Google Scholar]
- Groeneveld P. H., van Rooijen N., Eikelenboom P. In-vivo effects of lipopolysaccharide on lymphoid and non-lymphoid cells in the mouse spleen. Migration of marginal metallophils towards the follicle centres. Cell Tissue Res. 1983;234(1):201–208. doi: 10.1007/BF00217413. [DOI] [PubMed] [Google Scholar]
- Groeneveld P. H., van Rooijen N. In vivo effects of lipopolysaccharide on lymphoid and non-lymphoid cells in the mouse spleen. Reduction of T-lymphocytes and phagocytosis in the inner parts of the periarteriolar lymphocyte sheath. Cell Tissue Res. 1984;236(3):637–642. doi: 10.1007/BF00217233. [DOI] [PubMed] [Google Scholar]
- Groeneveld P. H., van Rooijen N. Localization of intravenously injected lipopolysaccharide (LPS) in the spleen of the mouse. An immunoperoxidase and histochemical study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;48(3):237–245. doi: 10.1007/BF02890132. [DOI] [PubMed] [Google Scholar]
- Hart D. A. Increased sensitivity of Corynebacterium parvum-treated mice to toxic effects of indomethacin and lipopolysaccharide. Infect Immun. 1985 Feb;47(2):408–414. doi: 10.1128/iai.47.2.408-414.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw L. B., Archer L. T., Beller-Todd B. K., Coalson J. J., Flournoy D. J., Passey R., Benjamin B., White G. L. Survival of primates in LD100 septic shock following steroid/antibiotic therapy. J Surg Res. 1980 Feb;28(2):151–170. doi: 10.1016/0022-4804(80)90158-4. [DOI] [PubMed] [Google Scholar]
- Lefer A. M. Eicosanoids as mediators of ischemia and shock. Fed Proc. 1985 Feb;44(2):275–280. [PubMed] [Google Scholar]
- Levy E., Ruebner B. H. Hepatic changes produced by a single dose of endotoxin in the mouse. Light microscopy and histochemistry. Am J Pathol. 1967 Aug;51(2):269–285. [PMC free article] [PubMed] [Google Scholar]
- Loegering D. J. Intravascular hemolysis and RES phagocytic and host defense functions. Circ Shock. 1983;10(4):383–395. [PubMed] [Google Scholar]
- Lotz M. J., Fareed J., Balis J. U. Role of splenic red pulp in endotoxin-induced disseminated intravascular coagulation. Lab Invest. 1981 Nov;45(5):469–476. [PubMed] [Google Scholar]
- Maier R. V., Hahnel G. B. Potential for endotoxin-activated Kupffer's cells to induce microvascular thrombosis. Arch Surg. 1984 Jan;119(1):62–67. doi: 10.1001/archsurg.1984.01390130048009. [DOI] [PubMed] [Google Scholar]
- Maier R. V., Ulevitch R. J. The response of isolated rabbit hepatic macrophages (H-M macrophage) to lipopolysaccharide (LPS). Circ Shock. 1981;8(2):165–181. [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- McCuskey R. S., McCuskey P. A. In vivo and electron microscopic studies of the splenic microvasculature in mice. Experientia. 1985 Feb 15;41(2):179–187. doi: 10.1007/BF02002611. [DOI] [PubMed] [Google Scholar]
- McCuskey R. S., McCuskey P. A., Urbaschek R., Urbaschek B. Species differences in Kupffer cells and endotoxin sensitivity. Infect Immun. 1984 Jul;45(1):278–280. doi: 10.1128/iai.45.1.278-280.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Baughn R. E., Musher D. M., Musher D. M. Effects of BCG infection on the susceptibility of mouse macrophages to endotoxin. Infect Immun. 1979 Apr;24(1):59–64. doi: 10.1128/iai.24.1.59-64.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribi E. E., Cantrell J. L., Von Eschen K. B., Schwartzman S. M. Enhancement of endotoxic shock by N-acetylmuramyl-L-alanyl-(L-seryl)-D-isoglutamine (muramyl dipeptide). Cancer Res. 1979 Nov;39(11):4756–4759. [PubMed] [Google Scholar]
- Schade U., Rietschel E. T. The role of prostaglandins in endotoxic activities. Klin Wochenschr. 1982 Jul 15;60(14):743–745. doi: 10.1007/BF01716568. [DOI] [PubMed] [Google Scholar]
- TANAKA N., NISHIMURA T., YOSHIYUKI T. Histochemical studies on the cellular distribution of endotoxin of Salmonella enteritidis in mouse tissues. Jpn J Microbiol. 1959 Apr;3:191–201. doi: 10.1111/j.1348-0421.1959.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Tobias P. S., Mathison J. C. Regulation of the host response to bacterial lipopolysaccharides. Fed Proc. 1984 Sep;43(12):2755–2759. [PubMed] [Google Scholar]
- Utili R., Abernathy C. O., Zimmerman H. J. Endotoxin effects on the liver. Life Sci. 1977 Feb 15;20(4):553–568. doi: 10.1016/0024-3205(77)90458-1. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Moore R. N., Sipe J. D., Rosenstreich D. L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. I. In vivo studies. J Immunol. 1980 Apr;124(4):2004–2009. [PubMed] [Google Scholar]
- Vogel S. N., Weedon L. L., Wahl L. M., Rosenstreich D. L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. II. T cell modulation of macrophage sensitivity to LPS in vitro. Immunobiology. 1982 Feb;160(5):479–493. doi: 10.1016/S0171-2985(82)80010-7. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res. 1984;238(2):355–358. doi: 10.1007/BF00217308. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R., Kamperdijk E. W. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. Ultrastructural aspects of elimination of marginal zone macrophages. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49(4):375–383. doi: 10.1007/BF02912114. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Use of liposomes as biodegradable and harmless adjuvants. Methods Enzymol. 1983;93:83–95. doi: 10.1016/s0076-6879(83)93036-7. [DOI] [PubMed] [Google Scholar]