Abstract
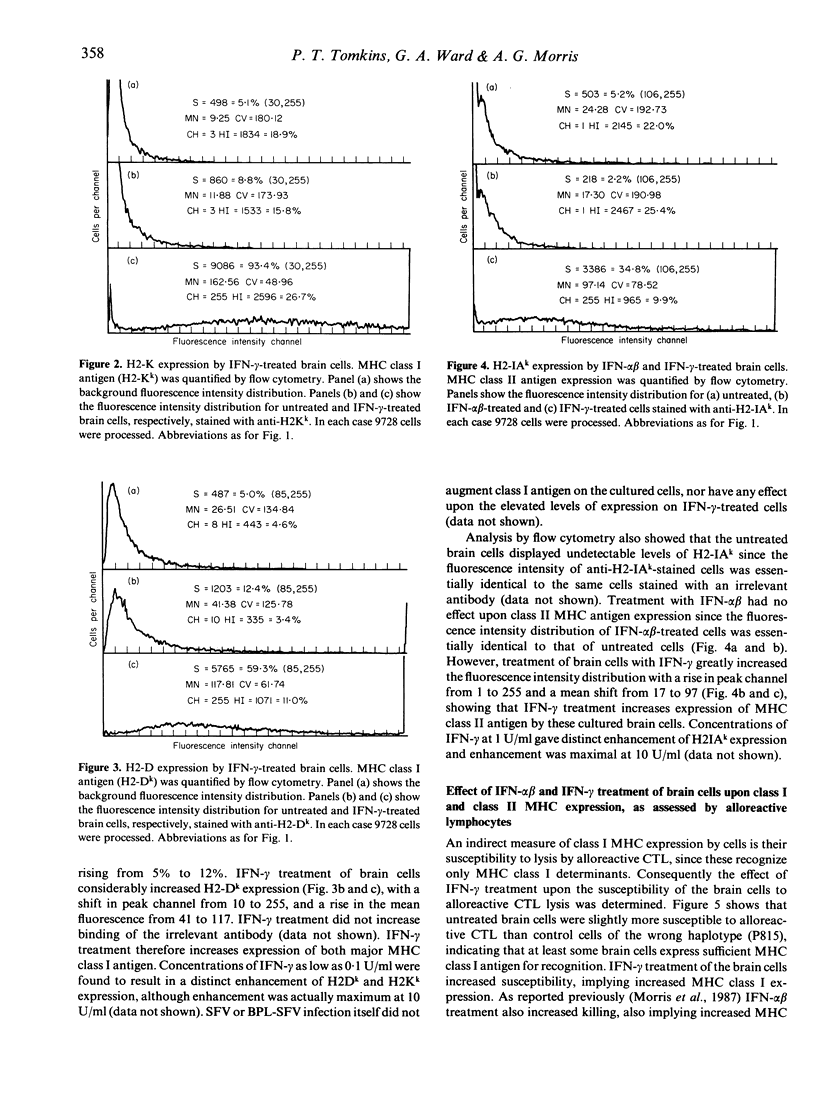
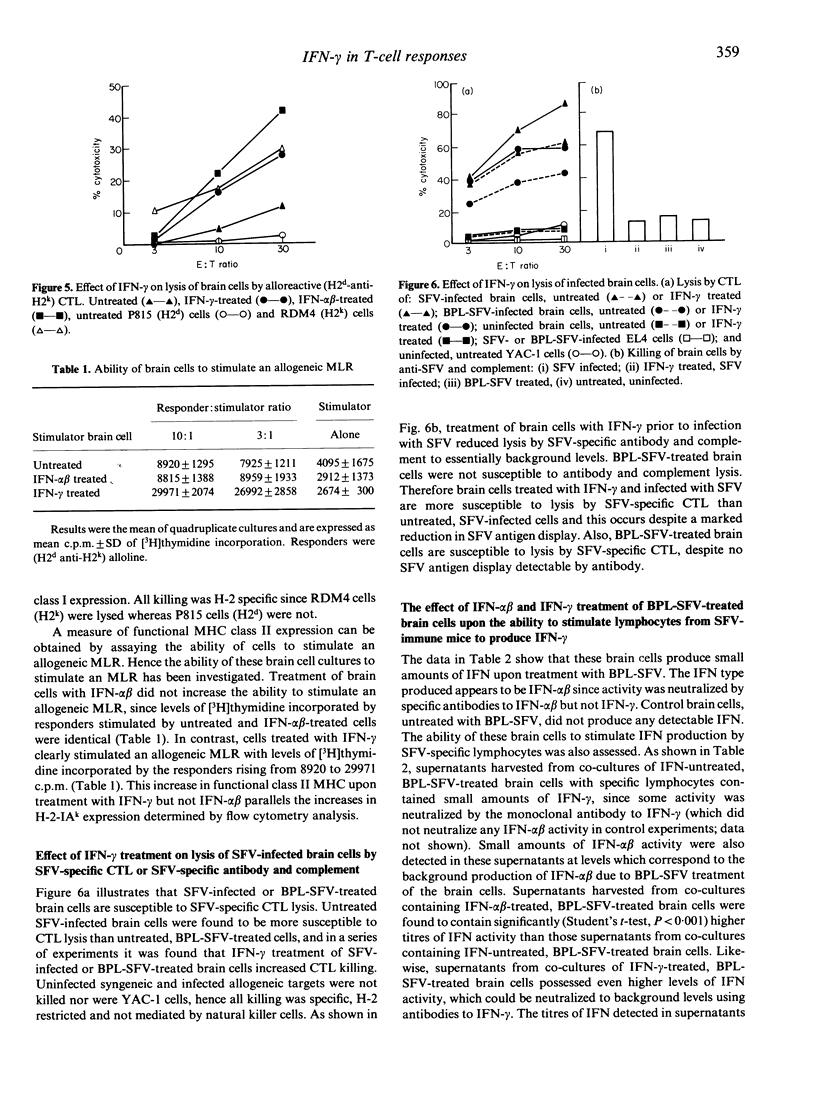
Primary brain cell cultures prepared from newborn C3H mice were infected with Semliki Forest virus (SFV) or treated with a beta-propiolactone-inactivated preparation of SFV (BPL-SFV). The effects of recombinant interferon-gamma (IFN-gamma) treatment on SFV replication, SFV antigen display, major histocompatibility complex (MHC) class I and class II antigen expression, susceptibility to lysis by SFV-specific cytotoxic T lymphocytes (CTL) and the ability to stimulate SFV-specific T lymphocytes to release IFN-gamma were determined. The IFN-gamma treatment prevented replication of SFV, as determined by incorporation of [3H]uridine into SFV-RNA, and reduced expression of SFV antigens on the cell surface, as determined by lysis with antibody and complement or indirect immunofluorescence. BPL-SFV-treated brain cells expressed no SFV antigen detectable by lysis with antibody and complement or indirect immunofluorescence. IFN-gamma increased expression of MHC class I and class II antigens, measured by indirect immunofluorescence, susceptibility to killing by alloreactive T-cell lines and ability to stimulate an allogeneic mixed lymphocyte reaction (MLR). Brain cells infected with SFV or treated with BPL-SFV were susceptible to killing by the CTL. The killing was MHC restricted and neither uninfected nor untreated cells were killed. IFN-gamma treatment prior to SFV infection or BPL-SFV treatment resulted in an augmentation of lysis by the CTL, indicating that even where SFV antigen expression is reduced or present at very low levels, in the context of enhanced MHC class I expression cells remain susceptible to CTL killing. Brain cells treated with BPL-SFV stimulated SFV-specific T cells to release IFN-gamma. Pretreatment of brain cells with IFN-alpha beta or IFN-gamma prior to BPL-SFV treatment markedly increased the ability of the cells to stimulate the SFV-specific T cells to release IFN-gamma. Release of IFN-gamma was MHC restricted and brain cells untreated with BPL-SFV did not stimulate IFN-gamma release. IFN-gamma released by T cells stimulated with BPL-SFV-treated brain cells increased class II MHC expression by brain cells as assessed by indirect immunofluorescence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. J., Johnston M. D., Westmacott L. M., Burke D. C. Department of Biological Sciences, University of Warwick, Coventry, CV47AL, England. J Gen Virol. 1974 Dec;25(3):381–390. doi: 10.1099/0022-1317-25-3-381. [DOI] [PubMed] [Google Scholar]
- Blackman M. J., Morris A. G. Gamma interferon production and cytotoxicity of spleen cells from mice infected with Semliki Forest virus. J Gen Virol. 1984 May;65(Pt 5):955–961. doi: 10.1099/0022-1317-65-5-955. [DOI] [PubMed] [Google Scholar]
- Blackman M. J., Morris A. G. The effect of interferon treatment of targets on susceptibility to cytotoxic T-lymphocyte killing: augmentation of allogeneic killing and virus-specific killing relative to viral antigen expression. Immunology. 1985 Nov;56(3):451–457. [PMC free article] [PubMed] [Google Scholar]
- Bradish C. J., Allner K., Maber H. B. The virulence of original and derived strains of Semliki forest virus for mice, guinea-pigs and rabbits. J Gen Virol. 1971 Aug;12(2):141–160. doi: 10.1099/0022-1317-12-2-141. [DOI] [PubMed] [Google Scholar]
- Cunningham A. L., Nelson P. A., Fathman C. G., Merigan T. C. Interferon gamma production by herpes simplex virus antigen-specific T cell clones from patients with recurrent herpes labialis. J Gen Virol. 1985 Feb;66(Pt 2):249–258. doi: 10.1099/0022-1317-66-2-249. [DOI] [PubMed] [Google Scholar]
- Fierz W., Endler B., Reske K., Wekerle H., Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985 Jun;134(6):3785–3793. [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Klein J., Juretic A., Baxevanis C. N., Nagy Z. A. The traditional and a new version of the mouse H-2 complex. Nature. 1981 Jun 11;291(5815):455–460. doi: 10.1038/291455a0. [DOI] [PubMed] [Google Scholar]
- Meager A., Burke D. C. Production of interferon by ultraviolet radiation inactivated Newcastle disease virus. Nature. 1972 Feb 4;235(5336):280–282. doi: 10.1038/235280a0. [DOI] [PubMed] [Google Scholar]
- Momburg F., Koch N., Möller P., Moldenhauer G., Hämmerling G. J. In vivo induction of H-2K/D antigens by recombinant interferon-gamma. Eur J Immunol. 1986 May;16(5):551–557. doi: 10.1002/eji.1830160516. [DOI] [PubMed] [Google Scholar]
- Morris A. G., Lin Y. L., Askonas B. A. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982 Jan 14;295(5845):150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- Morris A., Tomkins P. T., Maudsley D. J., Blackman M. Infection of cultured murine brain cells by Semliki Forest virus: effects of interferon-alpha beta on viral replication, viral antigen display, major histocompatibility complex antigen display and lysis by cytotoxic T lymphocytes. J Gen Virol. 1987 Jan;68(Pt 1):99–106. doi: 10.1099/0022-1317-68-1-99. [DOI] [PubMed] [Google Scholar]
- Panitch H. S., Hirsch R. L., Haley A. S., Johnson K. P. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987 Apr 18;1(8538):893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- Tedeschi B., Barrett J. N., Keane R. W. Astrocytes produce interferon that enhances the expression of H-2 antigens on a subpopulation of brain cells. J Cell Biol. 1986 Jun;102(6):2244–2253. doi: 10.1083/jcb.102.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Capra J. D. The protein products of the murine 17th chromosome: genetics and structure. Adv Immunol. 1978;26:147–193. doi: 10.1016/s0065-2776(08)60230-8. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- Zamvil S., Nelson P., Trotter J., Mitchell D., Knobler R., Fritz R., Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. 1985 Sep 26-Oct 2Nature. 317(6035):355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]



