Abstract
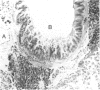
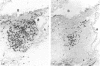
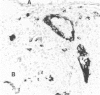
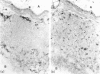
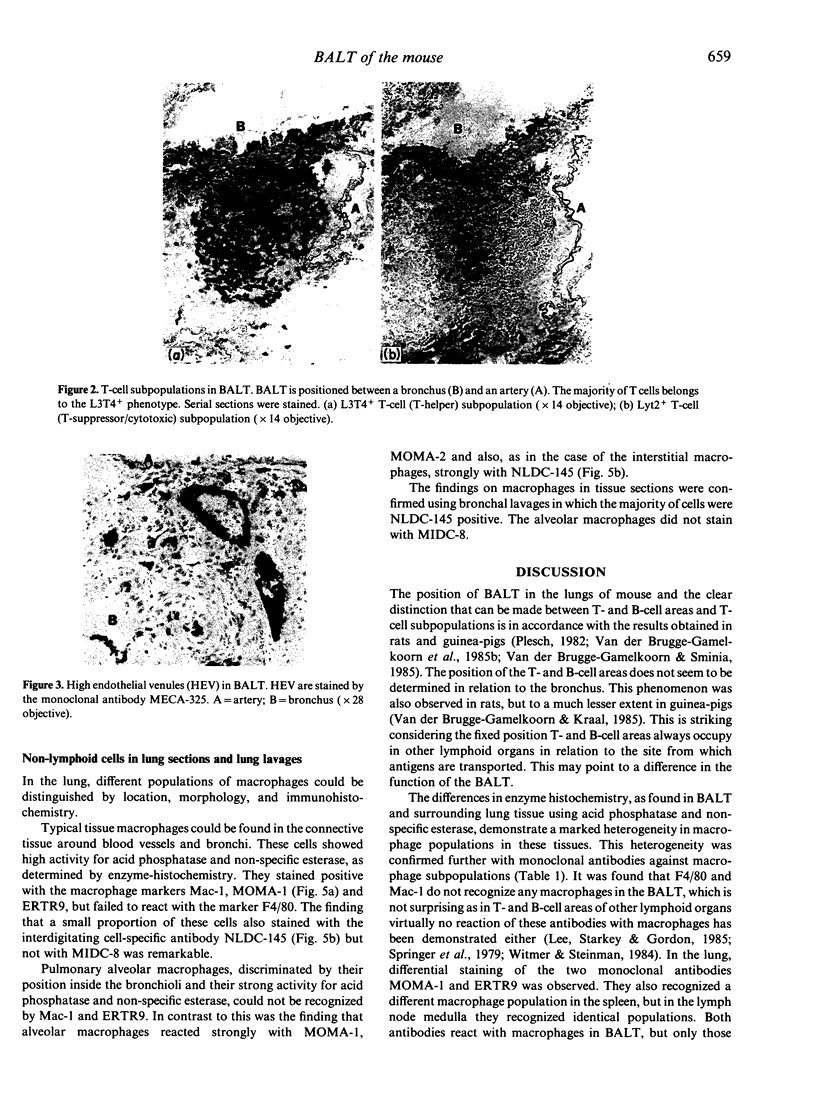
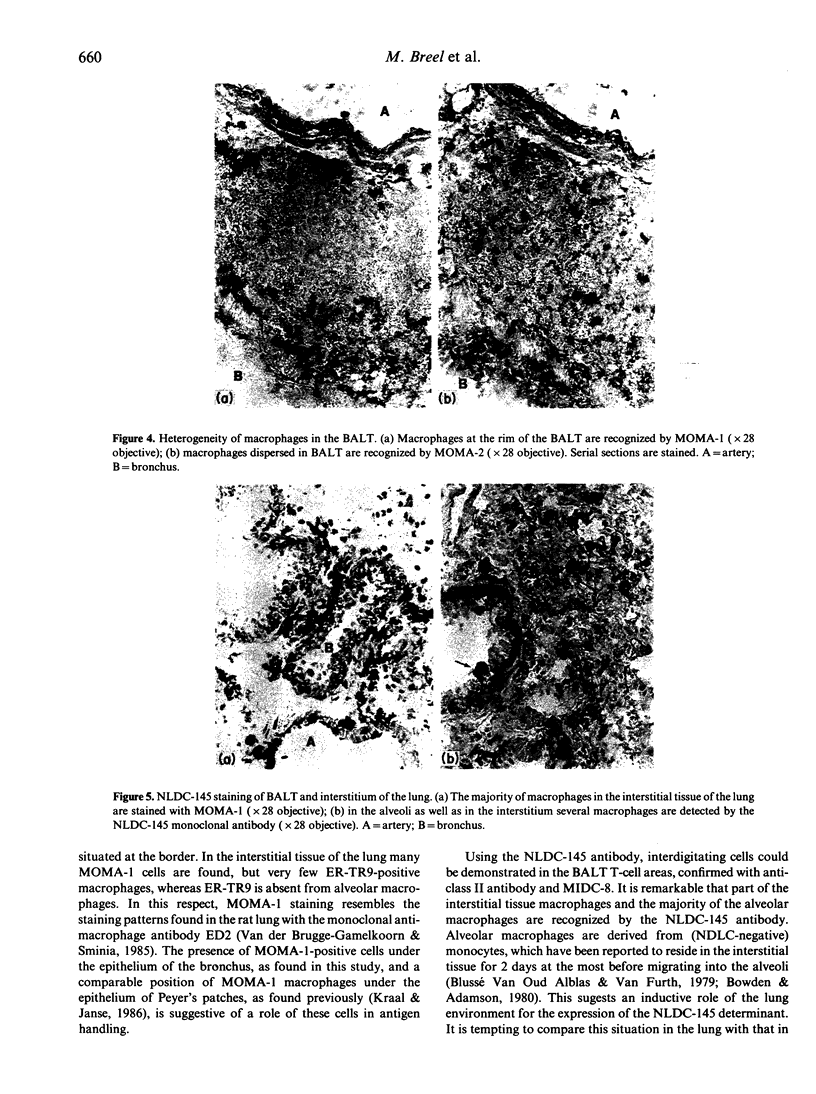
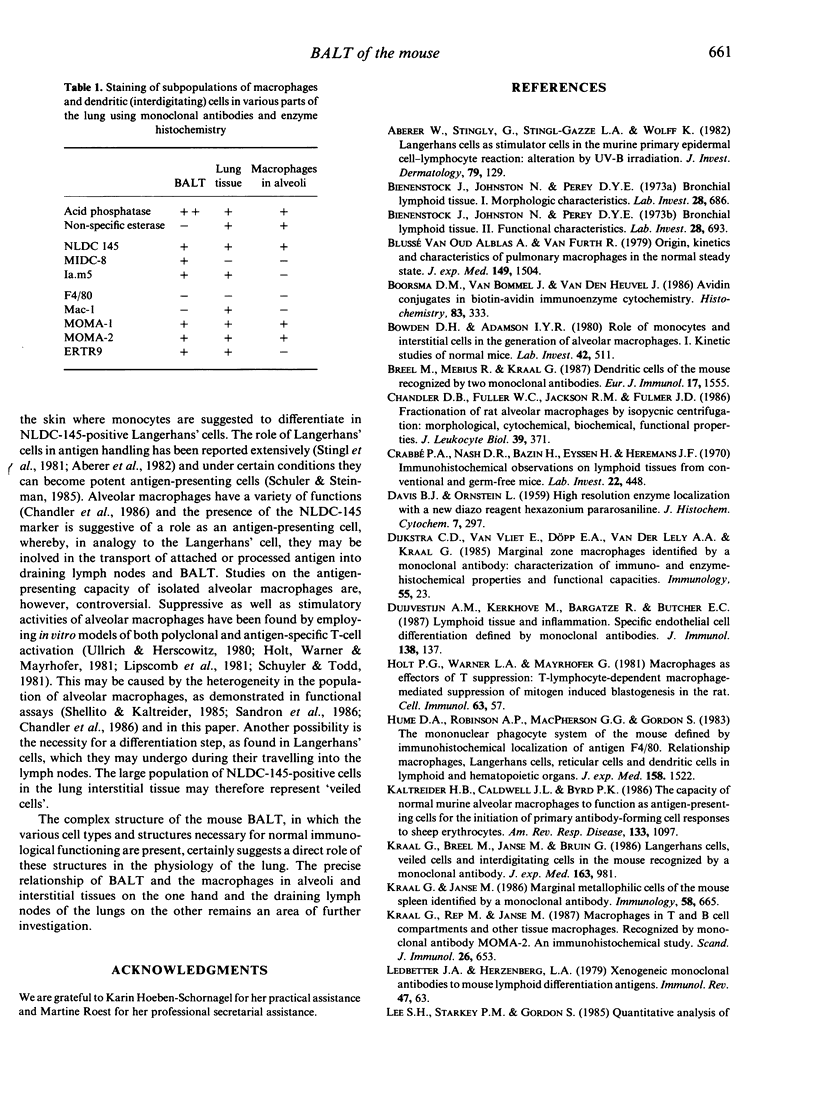
Lymphoid and non-lymphoid subpopulations were investigated in the lung of the mouse with immunocyto-, immunohisto- and enzyme-histochemical methods. Special attention was paid to the cell populations in bronchus-associated lymphoid tissue (BALT), which is positioned between a bronchus and an artery. In BALT, discrete T- and B-cell areas can be found. The majority of the T cells belong to the L3T4+ (T-helper) subpopulation. In the T-cell area interdigitating cells can be recognized by anti-class II antibodies as well as by specific monoclonal antibodies, NLDC-145 and MIDC-8. Macrophage subpopulations can be discriminated by location, enzyme reactivity and various macrophage-specific monoclonal antibody markers. On the outer rim of BALT macrophages are recognized by the MOMA-1 and ERTR9 antibody. Macrophages dispersed in BALT can only be discriminated with the MOMA-2 antibody. The macrophage markers F4/80 and Mac-1 show no reactivity in BALT. In lung, tissue macrophages around bronchi and blood vessels are predominantly recognized by the MOMA-1 and MOMA-2 antibody, and a minor population by the ERTR9 antibody. Alveolar macrophages show heterogeneity with the MOMA-1, MOMA-2 and NLDC-145 antibody. The relationship between alveolar macrophages and antigen-presenting cells is discussed here.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberer W., Stingl G., Stingl-Gazze L. A., Wolff K. Langerhans cells as stimulator cells in the murine primary epidermal cell-lymphocyte reaction: alteration by UV-B irradiation. J Invest Dermatol. 1982 Aug;79(2):129–135. doi: 10.1111/1523-1747.ep12500040. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973 Jun;28(6):686–692. [PubMed] [Google Scholar]
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. II. Functional characterisitics. Lab Invest. 1973 Jun;28(6):693–698. [PubMed] [Google Scholar]
- Boorsma D. M., Van Bommel J., Vanden Heuvel J. Avidin-HRP conjugates in biotin-avidin immunoenzyme cytochemistry. Histochemistry. 1986;84(4-6):333–337. doi: 10.1007/BF00482959. [DOI] [PubMed] [Google Scholar]
- Breel M., Mebius R. E., Kraal G. Dendritic cells of the mouse recognized by two monoclonal antibodies. Eur J Immunol. 1987 Nov;17(11):1555–1559. doi: 10.1002/eji.1830171105. [DOI] [PubMed] [Google Scholar]
- Chandler D. B., Fuller W. C., Jackson R. M., Fulmer J. D. Fractionation of rat alveolar macrophages by isopycnic centrifugation: morphological, cytochemical, biochemical, and functional properties. J Leukoc Biol. 1986 Apr;39(4):371–383. doi: 10.1002/jlb.39.4.371. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Nash D. R., Bazin H., Eyssen H., Heremans J. F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Invest. 1970 May;22(5):448–457. [PubMed] [Google Scholar]
- Holt P. G., Warner L. A., Mayrhofer G. Macrophages as effectors of T suppression: T-lymphocyte-dependent macrophage-mediated suppression of mitogen-induced blastogenesis in the rat. Cell Immunol. 1981 Sep 1;63(1):57–70. doi: 10.1016/0008-8749(81)90028-9. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltreider H. B., Caldwell J. L., Byrd P. K. The capacity of normal murine alveolar macrophages to function as antigen-presenting cells for the initiation of primary antibody-forming cell responses to sheep erythrocytes in vitro. Am Rev Respir Dis. 1986 Jun;133(6):1097–1104. doi: 10.1164/arrd.1986.133.6.1097. [DOI] [PubMed] [Google Scholar]
- Kraal G., Breel M., Janse M., Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986 Apr 1;163(4):981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986 Aug;58(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Rep M., Janse M. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. An immunohistochemical study. Scand J Immunol. 1987 Dec;26(6):653–661. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Starkey P. M., Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985 Mar 1;161(3):475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Lipscomb M. F., Toews G. B., Lyons C. R., Uhr J. W. Antigen presentation by guinea pig alveolar macrophages. J Immunol. 1981 Jan;126(1):286–291. [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Plesch B. E. Histology and immunohistochemistry of bronchus associated lymphoid tissue (BALT) in the rat. Adv Exp Med Biol. 1982;149:491–497. doi: 10.1007/978-1-4684-9066-4_69. [DOI] [PubMed] [Google Scholar]
- Sandron D., Reynolds H. Y., Venet A., Laval A. M., Israel-Biet D., Chretien J. Human alveolar macrophage subpopulations isolated on discontinuous albumin gradients: functional data in normals and sarcoid patients. Eur J Respir Dis. 1986 Oct;69(4):226–234. [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler M. R., Todd L. S. Accessory cell function of rabbit alveolar macrophages. Am Rev Respir Dis. 1981 Jan;123(1):53–57. doi: 10.1164/arrd.1981.123.1.53. [DOI] [PubMed] [Google Scholar]
- Shellito J., Kaltreider H. B. Heterogeneity of immunologic function among subfractions of normal rat alveolar macrophages. II. Activation as a determinant of functional activity. Am Rev Respir Dis. 1985 May;131(5):678–683. doi: 10.1164/arrd.1985.131.5.678. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stingl G., Gazze-Stingl L. A., Aberer W., Wolff K. Antigen presentation by murine epidermal langerhans cells and its alteration by ultraviolet B light. J Immunol. 1981 Oct;127(4):1707–1713. [PubMed] [Google Scholar]
- van der Brugge-Gamelkoorn G. J., Dijkstra C. D., Sminia T. Characterization of pulmonary macrophages and bronchus-associated lymphoid tissue (BALT) macrophages in the rat. An enzyme-cytochemical and immunocytochemical study. Immunobiology. 1985 Jul;169(5):553–562. doi: 10.1016/S0171-2985(85)80009-7. [DOI] [PubMed] [Google Scholar]
- van der Brugge-Gamelkoorn G. J., Kraal G. The specificity of the high endothelial venule in bronchus-associated lymphoid tissue (BALT). J Immunol. 1985 Jun;134(6):3746–3750. [PubMed] [Google Scholar]
- van der Brugge-Gamelkoorn G. J., Sminia T. T-cells and T-cells subsets in rat bronchus associated lymphoid tissue (BALT) in situ and in suspension. Adv Exp Med Biol. 1985;186:323–329. doi: 10.1007/978-1-4613-2463-8_39. [DOI] [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]