Abstract
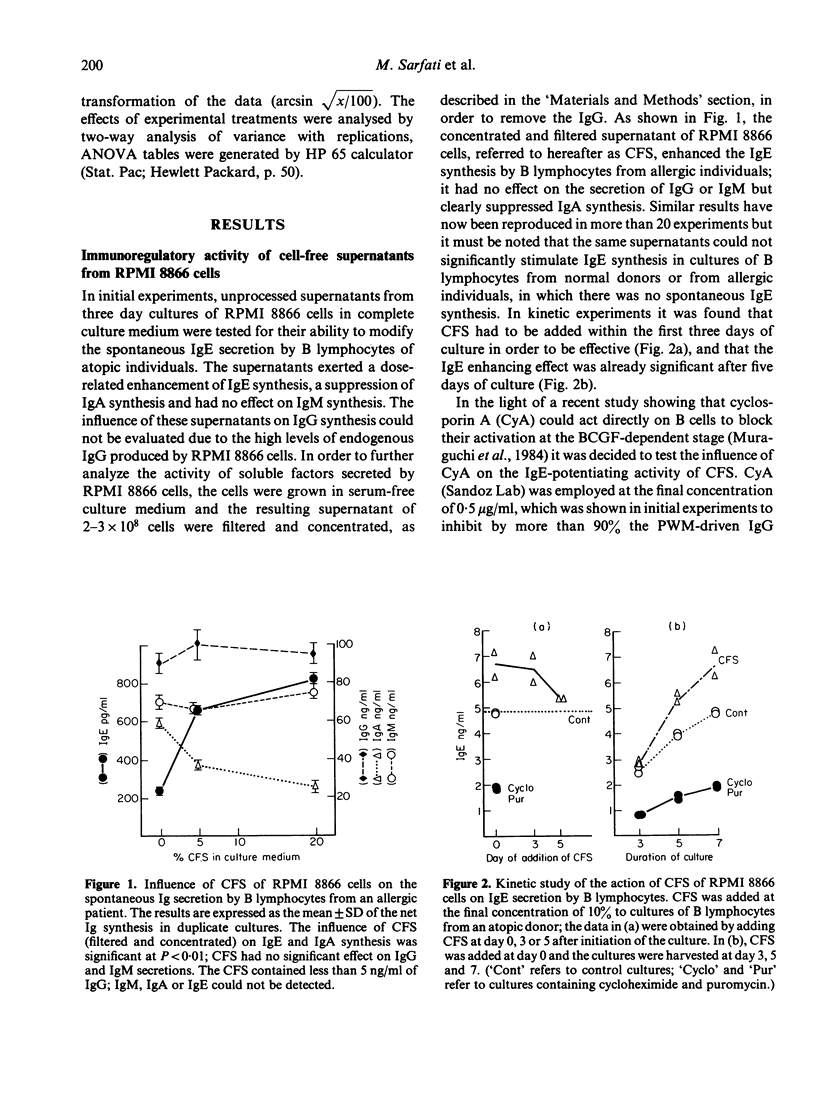
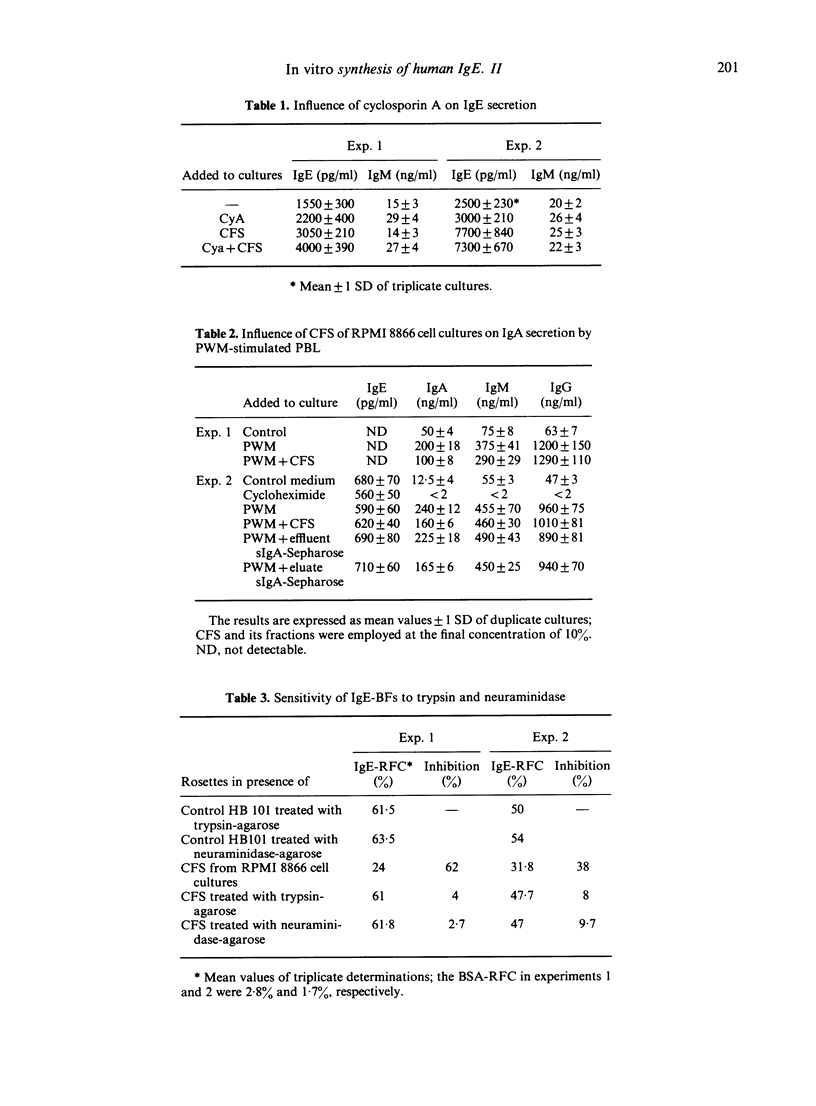
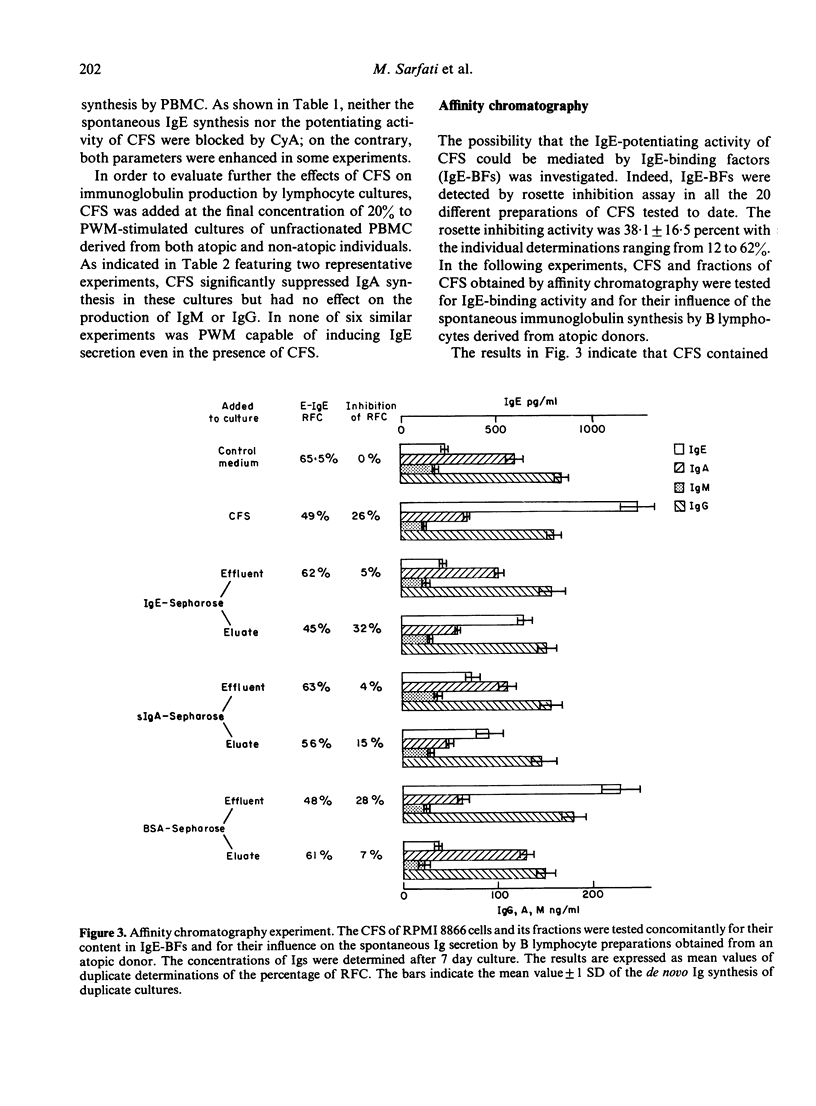
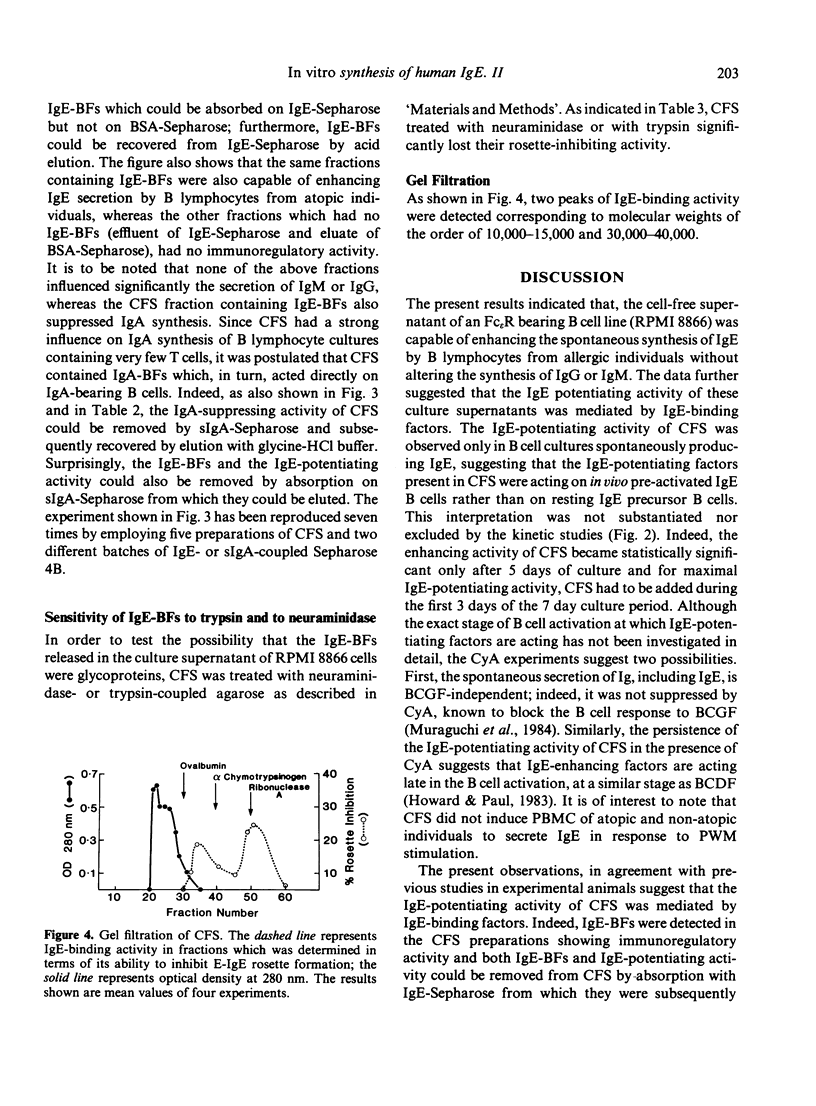
RPMI 8866 lymphoblastoid cells, known to express surface Fc epsilon R, were tested for their ability to regulate the in vitro synthesis of human IgE. Cell-free supernatants (CFS) of RPMI 8866 cells enhanced in a dose-dependent fashion the spontaneous IgE synthesis by B cells of allergic individuals. For maximum activity the CFS had to be added during the first 3 days of culture. CFS did not significantly alter the spontaneous synthesis of IgM or IgG, but they suppressed IgA synthesis both in B cell cultures and in pokeweed mitogen-stimulated peripheral blood mononuclear cells cultures. Cyclosporin A did not suppress either the spontaneous Ig production by B cells nor the IgE-potentiating activity of CFS. The enhancing activity of CFS was related to its content in IgE binding factors (IgE-BFs); these factors were detected by their ability to inhibit the rosetting of RPMI 8866 cells with IgE-coated erythrocytes (E-IgE). Both the IgE-BFs and the IgE-potentiating activity of the supernatants of RPMI 8866 cell cultures could be removed by absorption with IgE-Sepharose, from which they could subsequently be eluted with glycine-HCl buffer. IgE-BFs were identified as glycoproteins on the basis of their sensitivity to trypsin and to neuraminidase. By filtration of the RPMI 8866 cell supernatants through a Sephadex G75 column, IgE-binding activity was found to be associated with two fractions with molecular sizes in the range of 10,000-15,000 and 30,000-40,000. The IgA-suppressing activity of the RPMI 8866 culture filtrates could be absorbed with sIgA-Sepharose from which it was subsequently recovered by elution with glycine-HCl buffer. Most unexpectedly, sIgA-Sepharose also removed IgE-BFs and IgE-potentiating activity from the RPMI 8866 supernatants; both could be recovered by subsequent elution from sIgA-Sepharose with gycline-HCl buffer. These data are provisionally interpreted as indicating that the IgE-BFs secreted by RPMI 8866 cells had affinity for both IgE and sIgA and that they exerted a reciprocal effect on the in vitro synthesis of IgE and IgA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bich-Thuy L. T., Revillard J. P. Selective suppression of human B lymphocyte differentiation into IgG-producing cells by soluble Fc gamma receptors. J Immunol. 1982 Jul;129(1):150–152. [PubMed] [Google Scholar]
- Böyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:7–7. [PubMed] [Google Scholar]
- Conrad D. H., Froese A. Characterization of the target cell receptor for IgE. III. properties of the receptor isolated from rat basophilic leukemia cells by affinity chromatography. J Immunol. 1978 Feb;120(2):429–437. [PubMed] [Google Scholar]
- Deguchi H., Suemura M., Ishizaka A., Ozaki Y., Kishimoto S., Yamamura Y., Kishimoto T. IgE class-specific suppressor T cells and factors in humans. J Immunol. 1983 Dec;131(6):2751–2756. [PubMed] [Google Scholar]
- Gonzalez-Molina A., Spiegelberg H. L. A subpopulation of normal human peripheral B lymphcytes that bind IgE. J Clin Invest. 1977 Apr;59(4):616–624. doi: 10.1172/JCI108679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Molina A., Spiegelberg H. L. Binding of IgE myeloma proteins to human cultured lymphoblastoid cells. J Immunol. 1976 Nov;117(5 PT2):1838–1845. [PubMed] [Google Scholar]
- Hellström U., Spiegelberg H. L. Characterization of human lymphocytes bearing Fc receptors for IgE isolated from blood and lymphoid organs. Scand J Immunol. 1979;9(1):75–86. doi: 10.1111/j.1365-3083.1979.tb02709.x. [DOI] [PubMed] [Google Scholar]
- Hirashima M., Yodoi J., Ishizaka K. Regulatory role of IgE-binding factors from rat T lymphocytes. III. IgE-specific suppressive factor with IgE-binding activity. J Immunol. 1980 Oct;125(4):1442–1448. [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- Huff T. F., Uede T., Ishizaka K. Formation of rat IgE-binding factors by rat-mouse T cell hybridomas. J Immunol. 1982 Aug;129(2):509–514. [PubMed] [Google Scholar]
- Ishizaka K., Sandberg K. Formation of IgE binding factors by human T lymphocytes. J Immunol. 1981 May;126(5):1692–1696. [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Katz D. H. IgE antibody responses in vitro: from rodents to man. Prog Allergy. 1982;32:105–160. [PubMed] [Google Scholar]
- Kishimoto T. IgE class-specific suppressor T cells and regulation of the IgE response. Prog Allergy. 1982;32:265–317. [PubMed] [Google Scholar]
- Kiyono H., McGhee J. R., Mosteller L. M., Eldridge J. H., Koopman W. J., Kearney J. F., Michalek S. M. Murine Peyer's patch T cell clones. Characterization of antigen-specific helper T cells for immunoglobulin A responses. J Exp Med. 1982 Oct 1;156(4):1115–1130. doi: 10.1084/jem.156.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R., Taylor R. B. General methods for the study of cells and serum during the immune response: the response to dinitrophenyl in mice. Clin Exp Immunol. 1969 Apr;4(4):473–487. [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Coppleson L. W. A THREE-CELL INTERACTION REQUIRED FOR THE INDUCTION OF THE PRIMARY IMMUNE RESPONSE in vitro. Proc Natl Acad Sci U S A. 1968 Oct;61(2):542–547. doi: 10.1073/pnas.61.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Butler J. L., Fauci A. S. Sequential requirements for cell cycle progression of resting human B cells after activation by anti-Ig. J Immunol. 1984 Jan;132(1):176–180. [PubMed] [Google Scholar]
- Sarfati M., Rubio-Trujillo M., Wong K., Rector E., Sehon A. H., Delespesse G. In vitro synthesis of IgE by human lymphocytes. I. The spontaneous secretion of IgE by B lymphocytes from allergic individuals: a model to investigate the regulation of human IgE synthesis. Immunology. 1984 Oct;53(2):187–196. [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., O'Connor R. D., Simon R. A., Mathison D. A. Lymphocytes with immunoglobulin E Fc receptors in patients with atopic disorders. J Clin Invest. 1979 Sep;64(3):714–720. doi: 10.1172/JCI109514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodoi J., Hirashima M., Ishizaka K. Regulatory role of IgE-binding factors from rat T lymphocytes. II. Glycoprotein nature and source of IgE-potentiating factor. J Immunol. 1980 Oct;125(4):1436–1441. [PubMed] [Google Scholar]
- Yodoi J., Hirashima M., Ishizaka K. Regulatory role of IgE-binding factors from rat T lymphocytes. V. The carbohydrate moieties in IgE-potentiating factors and IgE-suppressive factors. J Immunol. 1982 Jan;128(1):289–295. [PubMed] [Google Scholar]
- Yodoi J., Ishizaka K. Lymphocytes bearing Fc receptors for IgE. I. Presence of human and rat T lymphocytes with Fc epsilon receptors. J Immunol. 1979 Jun;122(6):2577–2583. [PubMed] [Google Scholar]


