Abstract
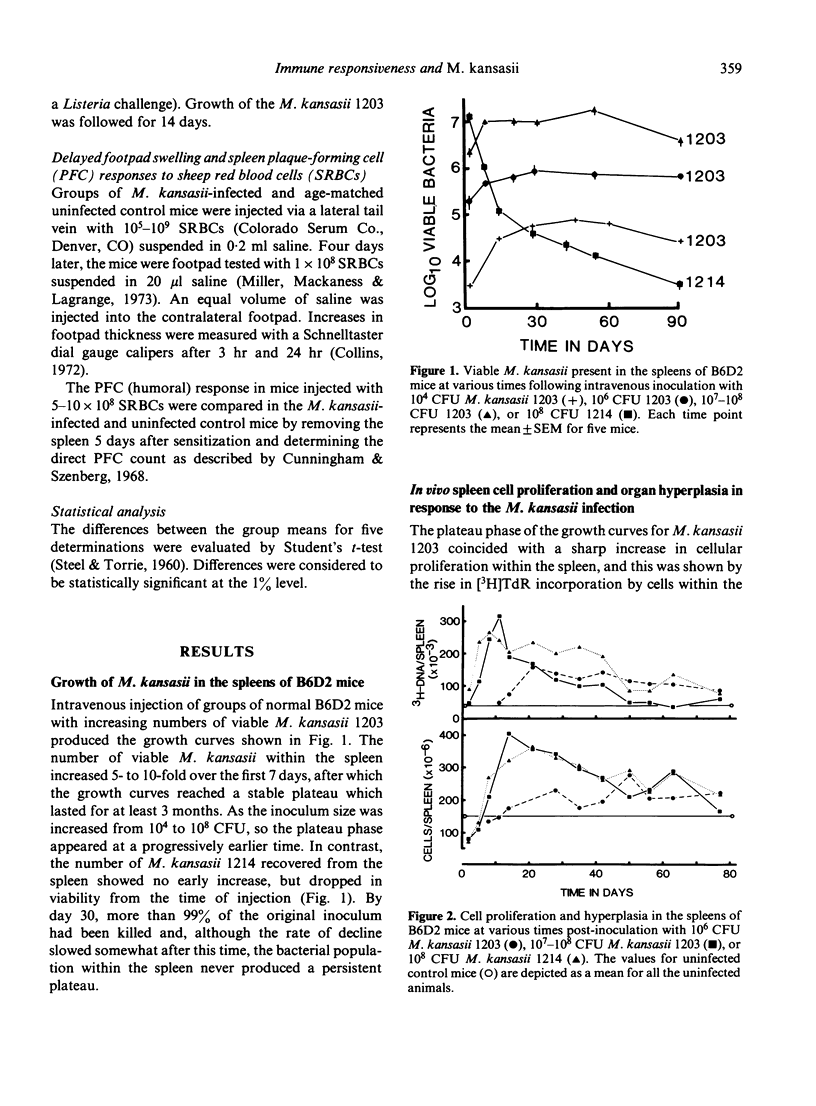
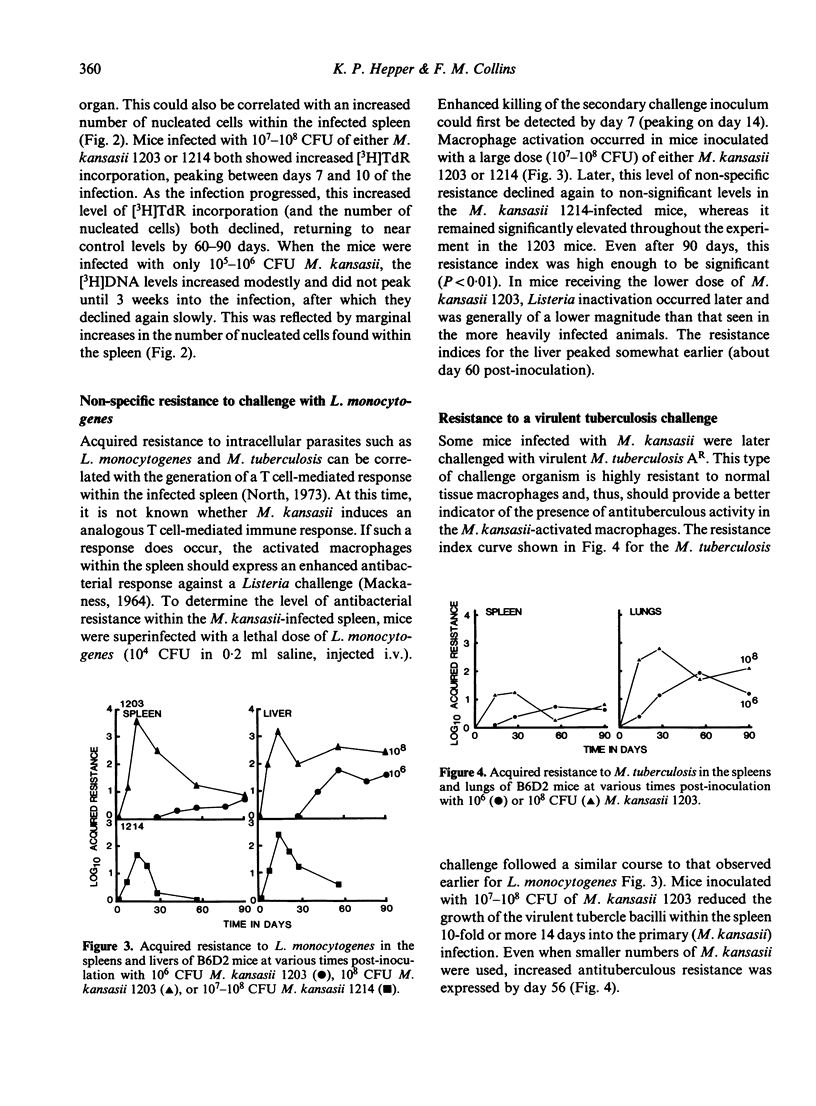
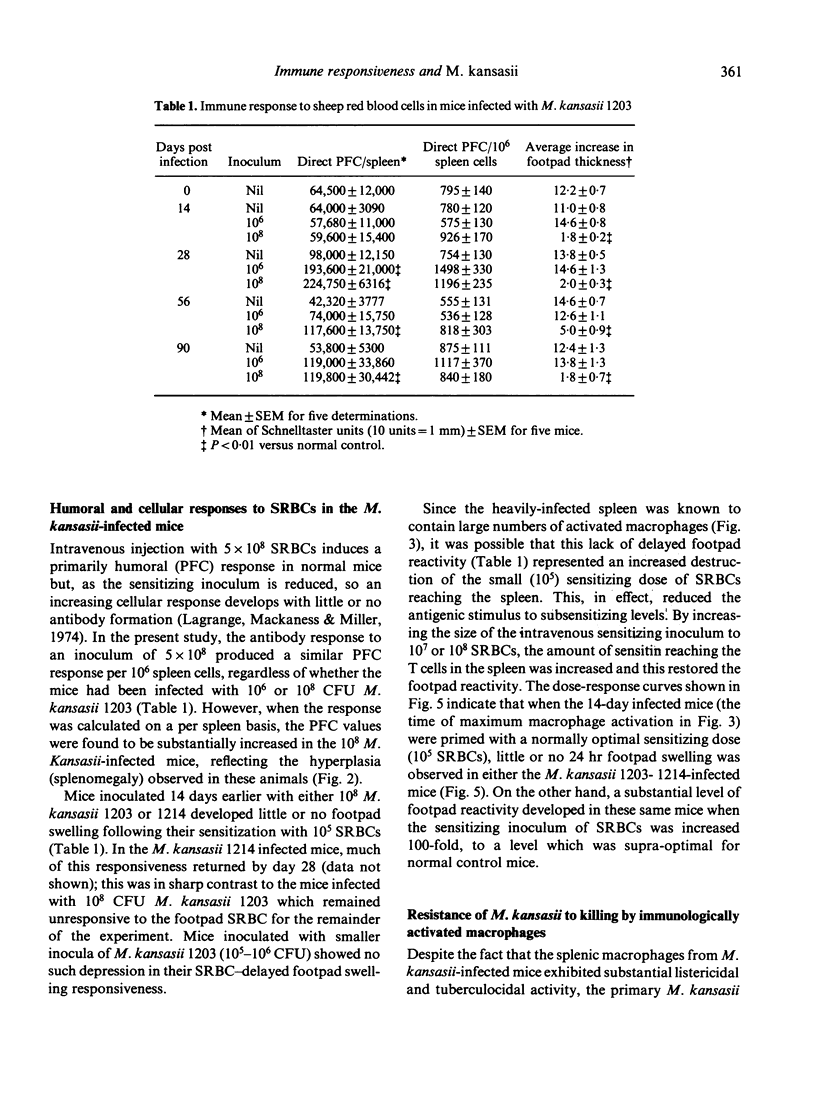
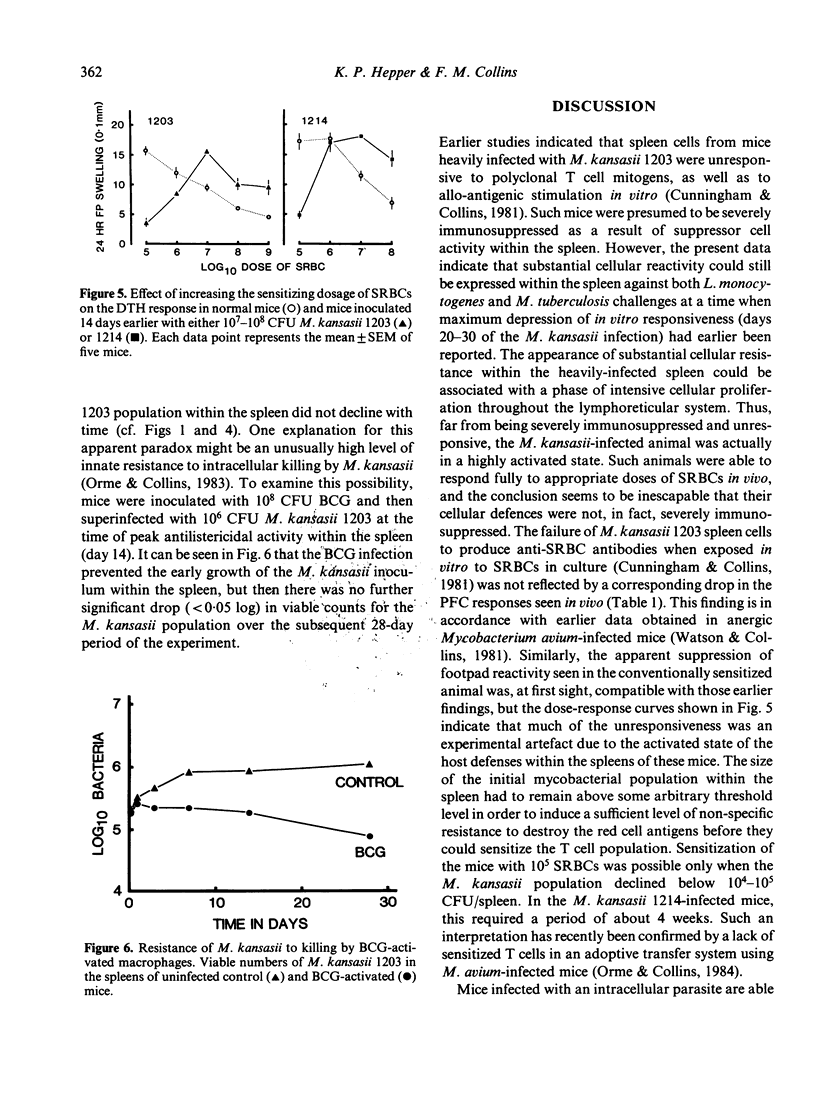
Growth of Mycobacterium kansasii TMC 1203 in B6D2 F1 hybrid mice was associated with increased splenic cellular proliferation, hyperplasia and the generation of non-specific antibacterial resistance. Both responses were dose dependent; the larger the inoculum, the more rapid and extensive the cellular response. However, such mice were still unable to reduce the mycobacterial load within the tissues, apparently because of their inherent resistance to inactivation by immunologically activated macrophages. On the other hand, mice infected with the non-persistent strain of M. kansasii 1214 exhibited only a transient increase in non-specific (anti-listeria) resistance which rapidly declined as the number of viable mycobacteria within the spleen fell below an arbitrary threshold level. Mice infected with either M. kansasii 1203 or 1214 could be immunized with sheep red blood cells (SRBCs), an unrelated T cell-dependent antigen. The humoral (PFC) response was not affected by the mycobacterial load within the spleen. However, the delayed footpad swelling reaction was severely depressed. The latter could be restored merely by increasing the size of the intravenous sensitizing inoculum 100-fold. The present study indicates that mice chronically infected with M. kansasii are not severely immunosuppressed (as had been inferred from earlier in vitro lymphoproliferation studies) but are fully capable of responding to appropriate in vivo stimuli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins F. M., Cunningham D. S. Systemic Mycobacterium kansasii infection and regulation of the alloantigenic response. Infect Immun. 1981 May;32(2):614–624. doi: 10.1128/iai.32.2.614-624.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Immunogenicity of various mycobacteria and the corresponding levels of cross-protection developed between species. Infect Immun. 1971 Dec;4(6):688–696. doi: 10.1128/iai.4.6.688-696.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Morrison N. E., Montalbine V. Immune response to persistent mycobacterial infection in mice. Infect Immun. 1978 May;20(2):430–438. doi: 10.1128/iai.20.2.430-438.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Wayne L. G., v Montalbine The effect of cultural conditions on the distribution of Mycobacterium tuberculosis in the spleens and lungs of specific pathogen-free mice. Am Rev Respir Dis. 1974 Aug;110(2):147–156. doi: 10.1164/arrd.1974.110.2.147. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. The effect of inoculum size on the immune response to BCG infection in mice. Immunology. 1971 Aug;21(2):369–381. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. E., Mackaness G. B., Lagrange P. H. Immunopotentiation with BCG. II. Modulation of the response to sheep red blood cells. J Natl Cancer Inst. 1973 Nov;51(5):1669–1676. doi: 10.1093/jnci/51.5.1669. [DOI] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Immune response to atypical mycobacteria: immunocompetence of heavily infected mice measured in vivo fails to substantiate immunosuppression data obtained in vitro. Infect Immun. 1984 Jan;43(1):32–37. doi: 10.1128/iai.43.1.32-37.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Resistance of various strains of mycobacteria to killing by activated macrophages in vivo. J Immunol. 1983 Sep;131(3):1452–1454. [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. The specificity of suppressor T cells induced by chronic Mycobacterium avium infection in mice. Clin Exp Immunol. 1981 Jan;43(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]


