Abstract
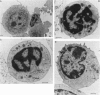
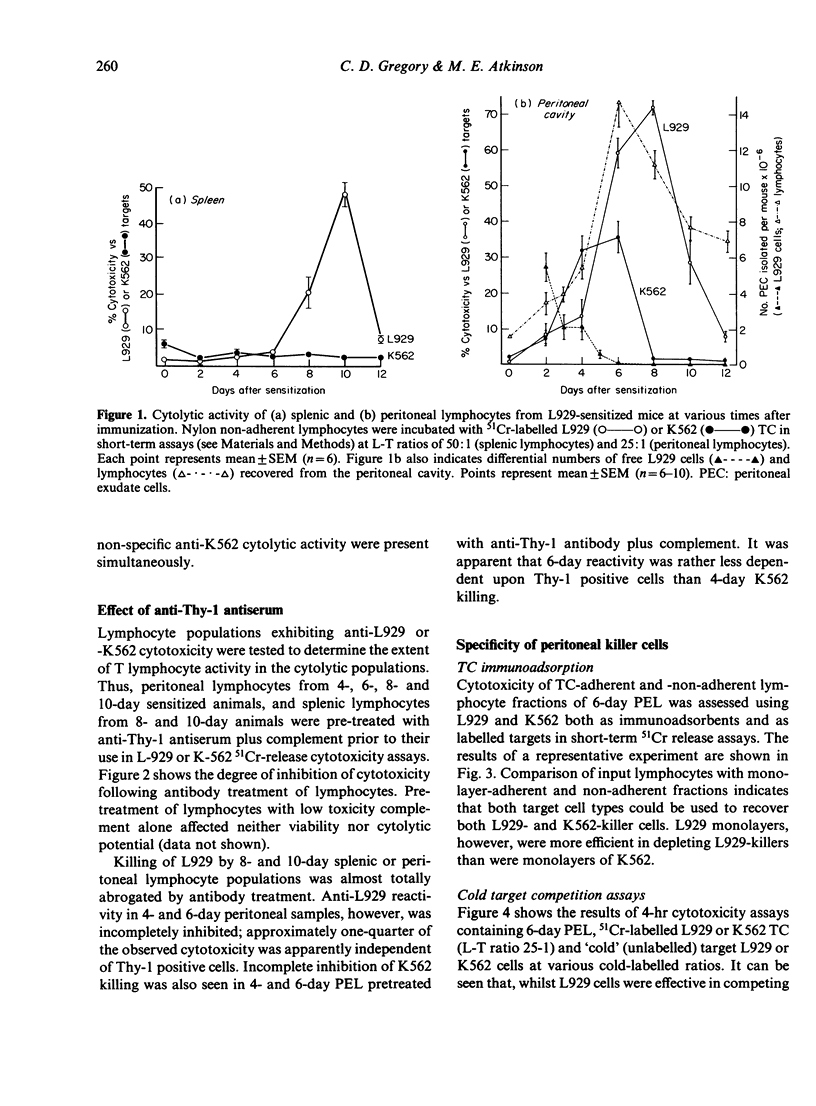
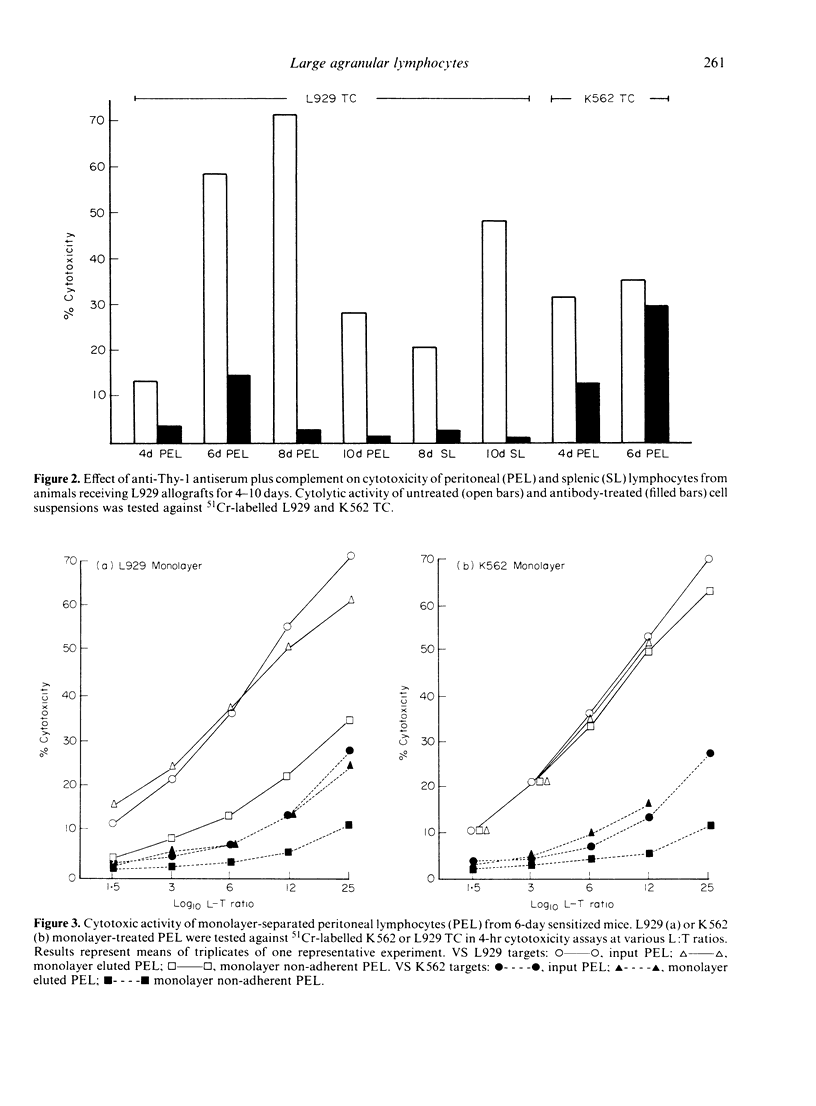
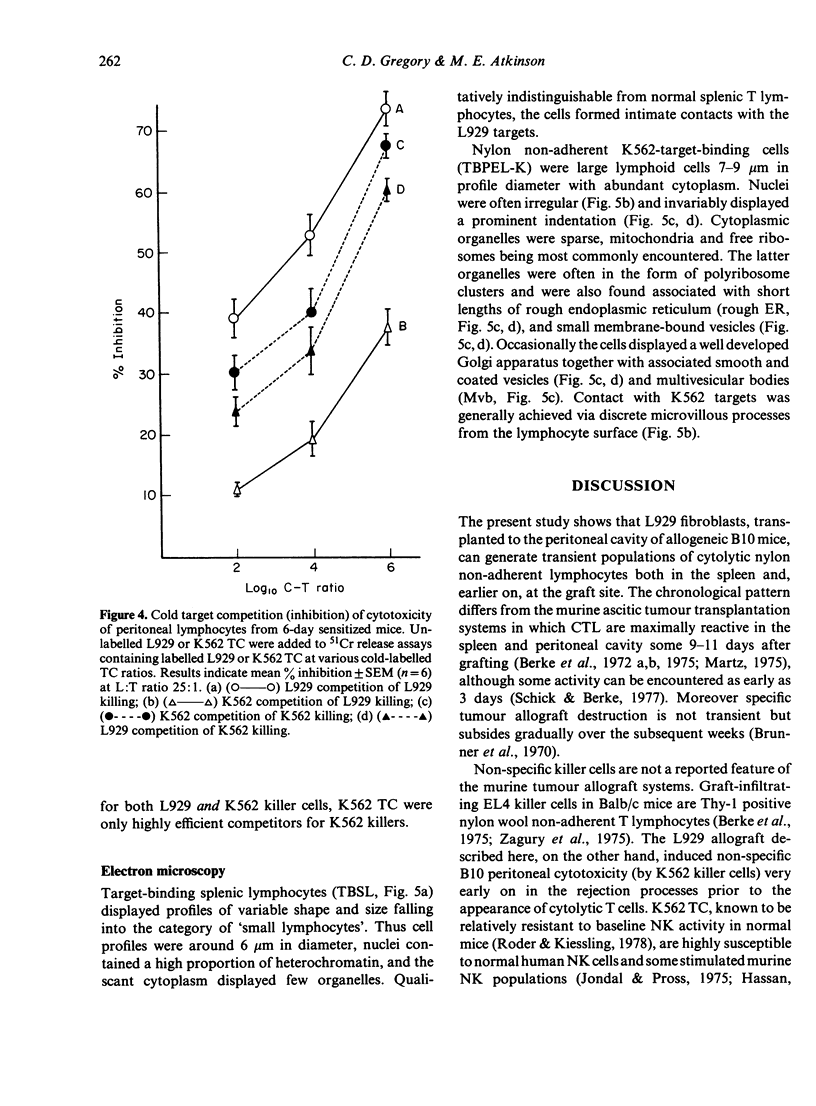
The cytolytic reactivity and ultrastructure of centrally-reactive and allograft-infiltrating lymphocyte populations was investigated in a murine peritoneal allograft system. Animals sensitized with a single intraperitoneal dose of allogeneic L929 fibroblasts generated a population of splenic cytolytic T cells maximally reactive 10 days after immunization. Sensitized splenic lymphocytes, isolated by immunoadsorption on L929 monolayers, were ultrastructurally classified as mature small lymphocytes. At the graft site, cytolytic non-T lymphoid cells displaying the ability to kill K562 target cells, were demonstrable between 4 and 6 days after sensitization. Six-day peritoneal lymphocyte populations were found to contain both cytolytic T cells (L929 killers) and highly reactive K562 killers. Immunoadsorption and cold target competition studies indicated that the K562 killer cells were able to recognize both K562 and L929 targets. K562 target-binding cells appeared to be ultrastructurally immature and were designated 'large agranular lymphocytes'. The role of cytolytic non-T cells in rejecting allografts is discussed.
Full text
PDF


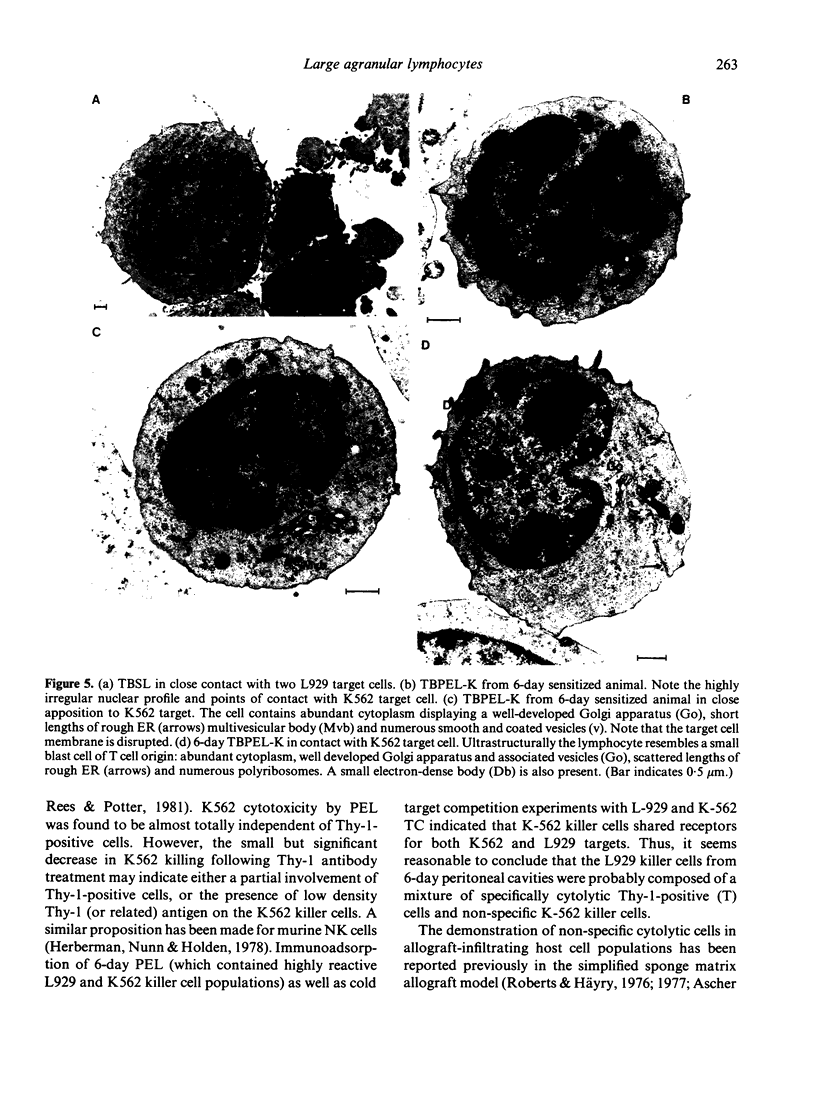


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher N. L., Ferguson R. M., Hoffman R., Simmons R. L. Partial characterization of cytotoxic cells infiltrating sponge matrix allografts. Transplantation. 1979 Apr;27(4):254–259. doi: 10.1097/00007890-197904000-00010. [DOI] [PubMed] [Google Scholar]
- Ascher N. L., Hoffman R., Hanto D. W., Simmons R. L. Cellular events within the rejecting allograft. Transplantation. 1983 Mar;35(3):193–197. [PubMed] [Google Scholar]
- Berke G., Amos D. B. Mechanism of lymphocyte-mediated cytolysis. The LMC cycle and its role in transplantation immunity. Transplant Rev. 1973;17(0):71–107. doi: 10.1111/j.1600-065x.1973.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Berke G., Sullivan K. A., Amos B. Rejection of ascites tumor allografts. I. Isolation, characterization, and in vitro reactivity of peritoneal lymphoid effector cells from BALB-c mice immune to EL4 leukosis. J Exp Med. 1972 Jun 1;135(6):1334–1350. doi: 10.1084/jem.135.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke G., Sullivan K. A., Amos D. B. Tumor immunity in vitro: destruction of a mouse ascites tumor through a cycling pathway. Science. 1972 Aug 4;177(4047):433–434. doi: 10.1126/science.177.4047.433. [DOI] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Rudolf H., Chapuis B. Studies of allograft immunity in mice. I. Induction, development and in vitro assay of cellular immunity. Immunology. 1970 Apr;18(4):501–515. [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Haskill J. S., Häyry P., Radov L. A. Systemic and local immunity in allograft and cancer rejection. Contemp Top Immunobiol. 1978;8:107–170. doi: 10.1007/978-1-4684-0922-2_5. [DOI] [PubMed] [Google Scholar]
- Hassan Z. M., Rees R. C., Potter C. W. Corynebacterium parvum stimulation of adherent and non-adherent cytotoxic cells in mice. Br J Cancer. 1981 Oct;44(4):532–538. doi: 10.1038/bjc.1981.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T. Low density of Thy 1 antigen on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1978 Jul;121(1):304–309. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Staal S., Djeu J. Y. Augmentation of natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic target cells. Int J Cancer. 1977 Apr 15;19(4):555–564. doi: 10.1002/ijc.2910190417. [DOI] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopt U. T., Sullivan W., Simmons R. L. Recruitment of lymphocytes to sponge matrix allografts. Transplant Proc. 1981 Mar;13(1 Pt 2):1086–1090. [PubMed] [Google Scholar]
- Itoh K., Suzuki R., Umezu Y., Hanaumi K., Kumagai K. Studies of murine large granular lymphocytes. II. Tissue, strain, and age distributions of LGL and LAL. J Immunol. 1982 Jul;129(1):395–405. [PubMed] [Google Scholar]
- Jondal M., Pross H. Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975 Apr 15;15(4):596–605. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Suzuki R., Hinuma S., Saitoh F. Studies of murine large granular lymphocytes. I. Identification as effector cells in NK and K cytotoxicities. J Immunol. 1982 Jul;129(1):388–394. [PubMed] [Google Scholar]
- Loveland B. E., Hogarth P. M., Ceredig R., McKenzie I. F. Cells mediating graft rejection in the mouse. I. Lyt-1 cells mediate skin graft rejection. J Exp Med. 1981 May 1;153(5):1044–1057. doi: 10.1084/jem.153.5.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini W., Boraschi D., Alberti S., Aleotti A., Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981 Aug;43(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Martz E. Early steps in specific tumor cell lysis by sensitized mouse T lymphocytes. I. Resolution and characterization. J Immunol. 1975 Jul;115(1):261–267. [PubMed] [Google Scholar]
- Nemlander A., Saksela E., Häyry P. Are "natural killer" cells involved in allograft rejection? Eur J Immunol. 1983 Apr;13(4):348–350. doi: 10.1002/eji.1830130415. [DOI] [PubMed] [Google Scholar]
- Reynolds C. W., Timonen T. T., Holden H. T., Hansen C. T., Herberman R. B. Natural killer cell activity in the rat. Analysis of effector cell morphology and effects of interferon on natural killer cell function in the athymic (nude) rat. Eur J Immunol. 1982 Jul;12(7):577–582. doi: 10.1002/eji.1830120709. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., Häyry P. Effector mechanisms in allograft rejection. II. Density, electrophoresis, and size fractionation of allograft-infiltrating cells demonstrating several classes of killer cells. Cell Immunol. 1977 May;30(2):236–253. doi: 10.1016/0008-8749(77)90068-5. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., Häyry P. Sponge matrix allografts. A model for analysis of killer cells infiltrating mouse allografts. Transplantation. 1976 Jun;21(6):437–445. [PubMed] [Google Scholar]
- Roder J. C., Kiessling R., Biberfeld P., Andersson B. Target-effector interaction in the natural killer (NK) cell system. II. The isolation of NK cells and studies on the mechanism of killing. J Immunol. 1978 Dec;121(6):2509–2517. [PubMed] [Google Scholar]
- Roder J. C., Kiessling R. Target--effector interaction in the natural killer cell system. I. Covariance and genetic control of cytolytic and target-cell-binding subpopulations in the mouse. Scand J Immunol. 1978;8(2):135–144. doi: 10.1111/j.1365-3083.1978.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Schick B., Berke G. Activity of tumor-associated lymphoid cells at short intervals after administration of irradiated syngeneic and allogeneic tumor cells. J Immunol. 1977 Mar;118(3):986–991. [PubMed] [Google Scholar]
- Stulting R. D., Berke G. Nature of lymphocyte-tumor interaction. A general method for cellular immunoabsorption. J Exp Med. 1973 Apr 1;137(4):932–942. doi: 10.1084/jem.137.4.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney N. L., Garovoy M. R., Busch G. J., Strom T. B., Graves M. J., Carpenter C. B. Rejected human renal allografts: recovery and characteristics of infiltrating cells and antibody. Transplantation. 1979 Nov;28(5):421–426. doi: 10.1097/00007890-197911000-00015. [DOI] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Analysis by a single cell cytotoxicity assay of natural killer (NK) cells frequencies among human large granular lymphocytes and of the effects of interferon on their activity. J Immunol. 1982 Jun;128(6):2514–2521. [PubMed] [Google Scholar]
- Timonen T., Saksela E., Ranki A., Häyry P. Fractionation, morphological and functional characterization of effector cells responsible for human natural killer activity against cell-line targets. Cell Immunol. 1979 Nov;48(1):133–148. doi: 10.1016/0008-8749(79)90106-0. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]