Abstract
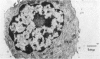
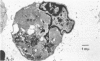
Anti-immunoglobulin (anti-Ig) causes suppression of secretion of immunoglobulin by LPS-activated pig B lymphoblasts. The cellular level at which anti-Ig influences immunoglobulin secretion has been investigated, using soluble anti-Ig which enters B cells, and anti-Ig immobilized onto acrylamide bead or plastic surfaces which can act only at the B-cell surface. Suppression of secretion only occurred with soluble anti-Ig, indicating that the intracellular processing of antibody after its complexing on the cell surface was necessary for suppression to occur. Immobilized anti-Ig acted as effectively as soluble antibody in activation of resting B cells into mitosis, showing that the activating signal of anti-Ig is received at the cell surface. Electron microscopy has shown that the block to secretion after soluble anti-Ig resulted from the accumulation of smooth membrane-bounded intracellular vesicles which, by immunofluorescence, contained immunoglobulin. The formation of vesicles was intimately associated with the intracellular localization of 125I-labelled anti-Ig which was used to follow cellular processing of anti-Ig.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Klaus G. G. Antigen-antibody complexes suppress antibody production by mouse plasmacytoma cells in vitro. Eur J Immunol. 1978 Mar;8(3):217–220. doi: 10.1002/eji.1830080315. [DOI] [PubMed] [Google Scholar]
- Abbas A. K., Klaus G. G. Inhibition of antibody production in plasmacytoma cells by antigen. Eur J Immunol. 1977 Oct;7(10):667–674. doi: 10.1002/eji.1830071003. [DOI] [PubMed] [Google Scholar]
- Andersson J., Bullock W. W., Melchers F. Inhibition of mitogenic stimulation of mouse lymphocytes by anti-mouse immunoglobulin antibodies. I. Mode of action. Eur J Immunol. 1974 Nov;4(11):715–722. doi: 10.1002/eji.1830041103. [DOI] [PubMed] [Google Scholar]
- Antoine J. C., Avrameas S., Gonatas N. K., Stieber A., Gonatas J. O. Plasma membrane and internalized immunoglobulins of lymph node cells studied with conjugates of antibody or its Fab fragments with horseradish peroxidase. J Cell Biol. 1974 Oct;63(1):12–23. doi: 10.1083/jcb.63.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daguillard F., Heiner D. C., Richter M., Rose B. The response of leucocytes of agammaglobulinaemia subjects to phythohaemagglutinin and anti-immunoglobulin antiserum. Clin Exp Immunol. 1969 Feb;4(2):203–211. [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Lipsky P. E. Immunoglobulin secretion by human splenic lymphocytes in vitro: the effects of antibodies to IgM and IgD. J Immunol. 1978 May;120(5):1465–1472. [PubMed] [Google Scholar]
- Inman J. K., Dintzis H. M. The derivatization of cross-linked polyacrylamide beads. Controlled introduction of functional groups for the preparation of special-purpose, biochemical adsorbents. Biochemistry. 1969 Oct;8(10):4074–4082. doi: 10.1021/bi00838a026. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Klein J., Bockman D. E., Cooper M. D., Lawton A. R. B cell differentiation induced by lipopolysaccharide. V. Suppression of plasma cell maturation by anti-mu: mode of action and characteristics of suppressed cells. J Immunol. 1978 Jan;120(1):158–166. [PubMed] [Google Scholar]
- Maino V. C., Hayman M. J., Crumpton M. J. Relationship between enhanced turnover of phosphatidylinositol and lymphocyte activation by mitogens. Biochem J. 1975 Jan;146(1):247–252. doi: 10.1042/bj1460247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J., Corbel C., Leptin M., Lernhardt W., Gerhard W., Zeuthen J. Regulation of B lymphocyte replication and maturation. J Cell Biochem. 1982;19(4):315–332. doi: 10.1002/jcb.240190403. [DOI] [PubMed] [Google Scholar]
- Ottosen P. D., Courtoy P. J., Farquhar M. G. Pathways followed by membrane recovered from the surface of plasma cells and myeloma cells. J Exp Med. 1980 Jul 1;152(1):1–19. doi: 10.1084/jem.152.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. C. Stimulation of mouse lymphocytes by insoluble anti-mouse immunoglobulin. Nature. 1975 Nov 27;258(5533):361–363. doi: 10.1038/258361a0. [DOI] [PubMed] [Google Scholar]
- Sell S., Rowe D. S., Gell P. G. Studies on rabbit lymphocytes in vitro. 3. Proteins, RNA, and DNA synthesis by lymphocyte cultures after stimulation with phytohaemagglutinin, with staphylococcal filtrate, with antiallotype serum, and with heterologous antiserum to rabbit whole serum. J Exp Med. 1965 Oct 1;122(4):823–839. doi: 10.1084/jem.122.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman C. L., Unanue E. R. Control of proliferation and differentiation in B lymphocytes by anti-Ig antibodies and a serum-derived cofactor. Proc Natl Acad Sci U S A. 1978 May;75(5):2401–2405. doi: 10.1073/pnas.75.5.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons D. B., Clarkson C. A. Suppression with anti-Ig of immunoglobulin secretion by pig lymphoblasts. Immunology. 1983 Jul;49(3):491–499. [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Endocytosis by lymphocytes of complexes of anti-Ig with membrane-bound Ig. J Immunol. 1972 Feb;108(2):569–572. [PubMed] [Google Scholar]