Abstract
The Escherichia coli dinI gene is one of the LexA-regulated genes, which are induced upon DNA damage. Its overexpression conferred severe UV sensitivity on wild-type cells and resulted in the inhibition of LexA and UmuD processing, reactions that are normally dependent on activated RecA in a complex with single-stranded (ss)DNA. Here, we study the mechanism by which DinI inhibits the activities of RecA. While DinI neither binds to ssDNA nor prevents the formation of RecA nucleoprotein filament, it binds to active RecA filament, thereby inhibiting its coprotease activity but not the ATPase activity. Furthermore, even under in vitro conditions where UmuD cleavage dependent on RecA–ssDNA–adeno sine-5′-(3-thiotriphosphate) is blocked in the presence of DinI, LexA is cleaved normally. This result, taken together with electron microscopy observations and linear dichroism measurements, indicates that the ternary complex remains intact in the presence of DinI, and that the affinity to the RecA filament decreases in the order LexA, DinI and UmuD. DinI is thus suited to modulating UmuD processing so as to limit SOS mutagenesis.
Keywords: coprotease/LexA/linear dichroism/recombinase/UmuD
Introduction
The Escherichia coli RecA protein has pivotal roles in DNA recombination and repair, and it has been extensively studied with respect to its structure and function (Kowalczykowski et al., 1994; Friedberg et al., 1995; Roca and Cox, 1997; Kuzminov, 1999). RecA promotes strand pairing and exchange reactions between homologous DNA molecules, and as such is often called a recombinase. In addition, RecA promotes the cleavage of LexA, UmuD and prophage repressors such as λcI in DNA-damaged cells. Since LexA and other proteins are self-cleaved under certain conditions in vitro, even in the absence of RecA, the term coprotease was coined for the activity of RecA in promoting the cleavage of LexA and other proteins (Little, 1984).
Binding of RecA to single-stranded (ss)DNA is a prerequisite for both the recombinase and coprotease activities, leading to the formation of a nucleoprotein filament or so-called activated RecA. In vitro studies suggest that ATP (or dATP) is required for filament formation. LexA is the repressor of many DNA damage-inducible genes, including lexA itself and recA, and it undergoes a self-cleavage reaction upon interaction with a RecA nucleoprotein filament, thereby resulting in induction of many LexA-regulated genes. This reaction to DNA damage is called the SOS response. Thus, RecA functions as a sensor of DNA damage by virtue of its ability to bind ssDNA generated as a result of interrupted DNA replication at sites of DNA damage. Such binding results in RecA activation, and consequent amplification of the inducing signal by promoting the processing of LexA and other proteins. Most of the induced gene products function in repairing DNA damage, but the product of the umuDC operon, which is most tightly regulated at the transcriptional level by the LexA repressor, functions in DNA damage-induced mutagenesis. The nascent UmuD protein is inactive for mutagenesis and needs to be converted to an active form (UmuD′) by an intermolecular self-cleavage reaction, which is promoted by interaction with a RecA nucleoprotein filament (McDonald et al., 1998). Two UmuD′ molecules interact with one UmuC molecule to form a UmuD′2C complex (Woodgate et al., 1989), which functions as DNA polymerase V to bypass DNA lesions in conjunction with RecA, at the risk of causing mutations (Reuven et al., 1999; Tang et al., 1999, 2000). Such DNA damage-inducible mutagenesis is called SOS mutagenesis.
Interactions between a RecA nucleoprotein filament and homologous DNA, or proteins such as LexA and UmuD, are mutually competitive. For example, excess amounts of either ssDNA or double-stranded (ds)DNA inhibited RecA coprotease activity in vitro (Craig and Roberts, 1980, 1981; Takahashi and Schnarr, 1989; Rehrauer et al., 1996). Conversely, in vivo overproduction of both UmuD′ and UmuC resulted in the inhibition of RecA-dependent homologous DNA recombination (Sommer et al., 1993). An uncleavable form of LexA protein (LexA-S119A) also inhibited DNA strand-exchange activity of RecA in vitro (Harmon et al., 1996). Furthermore, UmuD′2C inhibited in vitro LexA cleavage mediated by a RecA filament (Rehrauer et al., 1998). It is believed that such inhibition is achieved by LexA and UmuD′2C binding to the deep helical groove of the RecA nucleoprotein filament (Yu and Egelman, 1993; Frank et al., 2000), to which homologous DNA also binds (Story et al., 1992).
Recently, we reported that the LexA-regulated dinI gene encodes a small protein (81 amino acids) that is involved in regulating RecA functions. We first identified the dinI gene as a multicopy suppressor of the cold-sensitive phenotype caused by the dinD68 mutation (Yasuda et al., 1996). While dinI null mutants showed no difference in UV sensitivity from the parental strain, dinI overexpression conferred severe UV sensitivity on wild-type cells (Yasuda et al., 1998). In dinI-overexpressed cells, processing of LexA and UmuD after DNA damage was blocked, and homologous DNA recombination, as measured by P1 phage transduction, was also suppressed. Conversely, processing of UmuD occurred more rapidly and extensively in dinI null mutants than in the wild type, thus conferring dinI mutants with a mutator phenotype. However, no discernible change in the regulation of LexA after DNA-damaging treatment was observed between isogenic dinI and wild-type strains (our unpublished result). Furthermore, in an in vitro system with purified DinI, RecA and UmuD proteins, DinI inhibited UmuD processing even after a stable RecA–ssDNA–adenosine-5′-(3-thiotriphosphate) (ATPγS) complex was formed. In comparison, DinI did not inhibit the cleavage of LexA under the same conditions. These results suggested that DinI might directly interact with the RecA nucleoprotein filament, thereby inhibiting the UmuD processing. How ever, the question as to why DinI differentially affected LexA and UmuD processing remained unanswered.
In this study, we investigated the interaction between DinI and RecA nucleoprotein filaments using various biochemical and physicochemical methods. Moreover, we compared DinI, LexA-S119A and UmuD-K97A for their ability to inhibit RecA coprotease activity, which enabled us to clarify why DinI inhibits UmuD processing more efficiently than LexA cleavage both in vivo and in vitro.
Results
DinI does not inhibit the formation of an active RecA–ssDNA complex
In vivo overexpression of dinI resulted in the inhibition of both coprotease and recombinase activities of RecA (Yasuda et al., 1998). There are at least three different explanations for the inhibitory mechanism: (i) DinI may interact with either ssDNA or free RecA not bound to ssDNA, so as to prevent the formation of an active RecA–ssDNA complex; (ii) DinI may interact with the active RecA nucleoprotein filament to prevent it from interacting with LexA, UmuD or homologous DNA; (iii) DinI may interact with the RecA nucleoprotein filament in such a way as to dissociate the ternary complex necessary for activation. We have examined all of these possibilities.
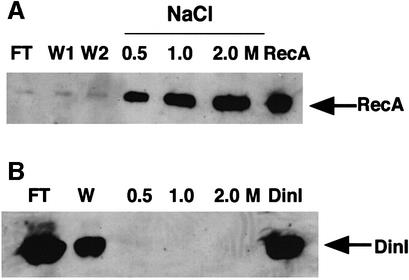
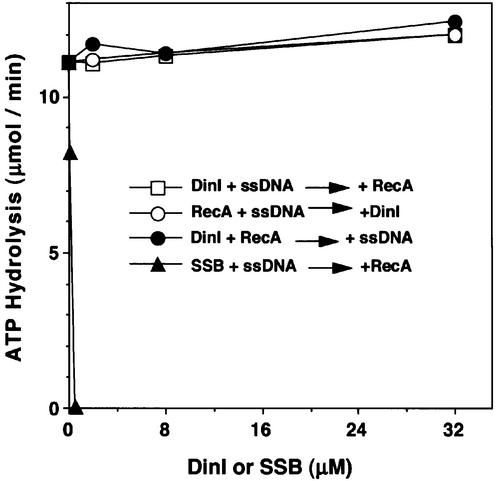
We first tested binding of DinI to an ssDNA–cellulose column. As expected, RecA bound to the column and was eluted with higher concentrations of NaCl (Figure 1A). In contrast, DinI was recovered in the flow-through and wash fractions (Figure 1B), indicating that DinI does not bind to the ssDNA–cellulose. We then examined the effect of DinI on the ssDNA-dependent ATPase activity of RecA. DinI itself showed no ATPase activity in the absence or presence of ssDNA (data not shown). As shown in Figure 2, DinI did not affect the ATPase activity of RecA, even in a 30-fold excess over RecA, when it was mixed with poly(dT) either before or after the addition of RecA to the reaction mixture, while the E.coli ssDNA-binding protein (SSB) very effectively inhibited the ATPase activity when premixed with poly(dT). No inhibition of the ATPase activity was observed when DinI and RecA were premixed and ssDNA then added to the mixture. These results, therefore, exclude the possibility that DinI inhibits the interaction between RecA and ssDNA or the access of ATP to the RecA–ssDNA complex.
Fig. 1. DinI protein does not bind to ssDNA. ssDNA binding assays for RecA (A) and DinI (B) were performed as described in Materials and methods. FT and W represent flow-throw and wash fraction, respectively. In the case of RecA, the wash was performed twice (W1 and W2). In lane RecA or DinI, purified RecA or DinI protein, respectively, was applied to the gel.
Fig. 2. DinI does not inhibit the ssDNA-dependent ATP hydrolysis promoted by RecA protein. DinI, SSB, RecA (all at 1 µM) and poly(dT) (3 µM) were added in different orders. DinI was added to the reaction mixture after RecA and poly(dT) were pre-incubated at 37°C for 5 min (open circles). RecA was added after DinI (open squares) or SSB (closed triangles) was pre-incubated with poly(dT). Poly(dT) was added to the reaction mixture after RecA and DinI were pre-incubated (closed circles). After adding the components, the mixtures were incubated at 37°C for a further 5 min, and ATP hydrolysis reactions were started with the addition of ATP (1 mM).
Interaction between DinI and activated RecA in vivo
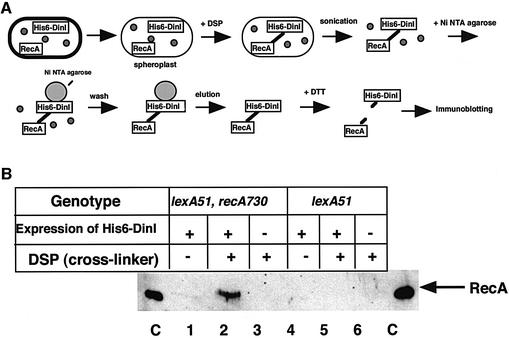
Next, we examined whether DinI could interact with free RecA monomer or activated RecA nucleoprotein filament in vivo using a cross-linker, dithiobis(succinimidylpropionate) (DSP). The reagent has a disulfide bond in the center, which can be cleaved by the addition of dithiothreitol (DTT) (see Figure 3A for the experimental scheme). We used two E.coli strains, DE192 (lexA51) and DE667 (lexA51 recA730), which were transformed with a plasmid overproducing a recombinant N-terminal His-tagged DinI. The His6-DinI protein behaved exactly as the normal DinI protein in vivo and in vitro: overproducing the His6-DinI protein in the cells blocked SOS induction after UV irradiation, and the purified His6-DinI protein inhibited RecA-dependent UmuD processing in vitro (data not shown). The above two E.coli strains carry the lexA51(Def) mutation, which inactivates LexA repressor activity. One strain, DE192, overproduces the wild-type RecA protein, while the other, DE667, overproduces the coprotease-constitutive RecA730 protein. The RecA730 protein is believed to be constitutively activated by binding to ssDNA regions that are transiently generated during DNA replication in the absence of DNA damage because of a higher DNA-binding affinity (Lavery and Kowalczykowski, 1992).
Fig. 3. Cross-linking between DinI and activated RecA. (A) A schematic presentation of the procedure used. (B) DinI binds to activated RecA. The experiments were carried out with DE667 (lexA51 recA730) or DE192 (lexA51), both carrying pHR255 (lacIq). In lanes 1, 2, 4 and 5, the cells carried pYP92 (His6-DinI). In lanes 3 and 6, the cells carried pQE9 (vector). In lanes 2, 3, 5 and 6, samples were treated with DSP. In lanes C, purified RecA protein was loaded.
After overproduction of the His6-DinI protein, the cells from both strains were converted to spheroplasts, to which DSP was added to cross-link any interacting proteins. The cell extracts were prepared and mixed with Ni-NTA–agarose, which binds His6-DinI together with any protein(s) cross-linked to it. The His6-DinI protein was eluted from the Ni-NTA–agarose. After treatment with DTT to cleave the disulfide bond present in DSP, the eluted samples were subjected to SDS–PAGE followed by western blot analysis with anti-RecA antibodies as the probe. As seen in Figure 3B, the RecA band was observed in the sample from DE667 with the coprotease-constitutive RecA730, but not from DE192 with the wild-type RecA. Because we detected no or little difference in the total amounts of RecA and His6-DinI proteins between DE667 and DE192 (data not shown), the above result should imply that DinI interacts in vivo with activated RecA but not with free RecA monomers.
Interaction between DinI and a RecA nucleoprotein filament
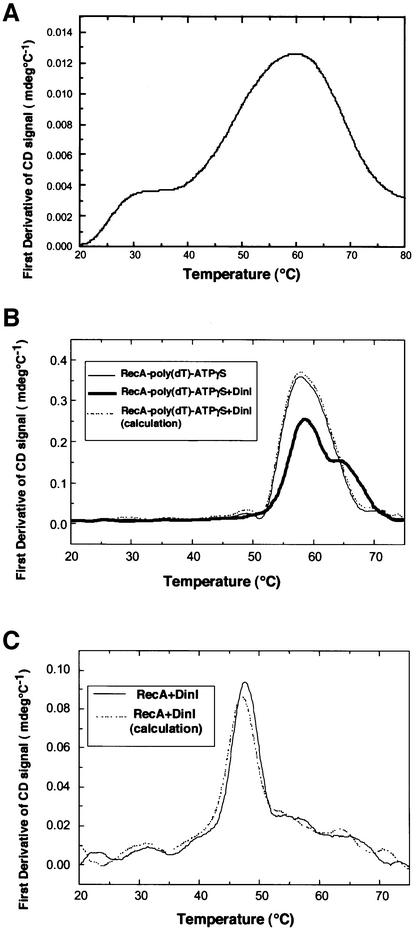
We verified an interaction between DinI and RecA nucleoprotein filament by spectroscopic measurements. First, we used circular dichroism (CD), which relates to the secondary structure of proteins. The CD spectrum of DinI (not shown) indicated that the protein contains a large proportion (70%) of α-helix, in good agreement with the prediction from its amino acid sequence. DinI was heat denatured at ∼58°C (Figure 4A), and RecA was also unfolded at ∼58°C when it formed a complex with poly(dT) and ATPγS (Figure 4B). As shown in Figure 4B, when DinI was added to the RecA–poly(dT)– ATPγS ternary complex at a molar ratio to RecA monomer of 1:1, two different melting temperatures of 58 and 65°C were observed. In contrast, the addition of DinI to RecA in the absence of poly(dT) generated only a slight increase in the melting temperature of RecA (Figure 4C). This slight difference (∼0.5°C) is of limited significance, although it could indicate a very weak interaction between DinI and free RecA under the in vitro conditions used. Our CD measurements, therefore, clearly indicate that DinI interacts with the RecA–poly(dT)–ATPγS complex, stabilizing it against thermal unfolding.
Fig. 4. Effect of DinI on the thermal stability of RecA. Thermal unfolding of DinI and RecA proteins was detected by CD change upon temperature elevation, and presented by its first derivative, d(CD)/dT. (A) DinI alone (8.3 µM). (B) RecA–poly(dT)–ATPγS complex (thin line); mixture of DinI and RecA–poly(dT)–ATPγS complex (thick line); expected theoretical curve of the mixture when there is no interaction between DinI and RecA–poly(dT)–ATPγS complex (broken line). RecA, 8.3 µM; DinI, 8.3 µM; poly(dT), 24.9 µM. (C) RecA with ATPγS (solid line) in the absence of ssDNA; expected theoretical curve of the mixture when there is no interaction between DinI and RecA with ATPγS (broken line). RecA, 4 µM; DinI, 4 µM.
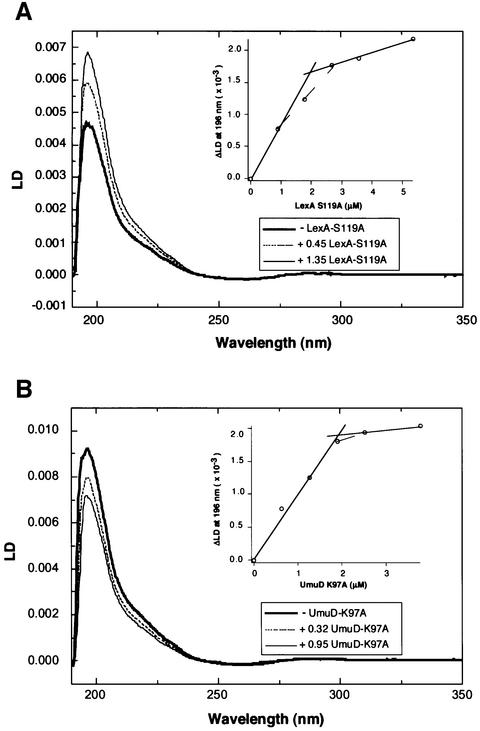
We then studied the interaction of DinI with a RecA nucleoprotein filament by flow linear dichroism (LD) spectroscopy. RecA nucleoprotein filaments can easily be flow-oriented and provide large LD signals (Norden et al., 1990, 1992). By contrast, small proteins, like DinI, do not provide any LD signal because they can not be oriented by flow. However, if a protein binds to a RecA nucleoprotein filament and is flow-oriented together with the filament, the addition of such a protein to the RecA filament should generate a change in the LD signal. To examine the validity of this argument, we used lysozyme as a negative control and LexA-S119A (an uncleavable mutant form of LexA) as a positive control. When RecA (4 µM) and poly(dT) (12 µM in nucleotides) were mixed in the presence of 50 µM ATPγS and placed for >3 h at room temperature, an LD signal was observed. The addition of lysozyme to the RecA–poly(dT)–ATPγS complex did not generate any change in the LD signal (not shown), but the addition of LexA-S119A to the ternary complex caused an increase in intensity of the positive LD signal at ∼196 nm, without significant change in the signal at ∼260 nm (Figure 5A), indicating an additional positive signal from LexA. We applied this method to study the interaction of UmuD-K97A, a non-cleavable mutant form of UmuD, with the RecA nucleoprotein filament. In contrast to LexA-S119A, the addition of UmuD-K97A to the RecA–poly(dT)–ATPγS complex caused a decrease in the LD signal at ∼196 nm (Figure 5B), implying that UmuD-K97A bound to the filament and generated a negative LD signal. In the inset of Figure 5A and B, the changes in the LD value at 196 nm were plotted against the amount of LexA-S119A or UmuD-K97A added to the reaction mixture. The results suggest that LexA-S119A and UmuD-K97A bind to the RecA–poly(dT)–ATPγS complex at the molar ratio to RecA monomer of 1:2, although more experiments are necessary to determine the stoichiometry definitely by LD measurements. LD can thus detect the interaction between a RecA nucleoprotein filament and proteins that bind to it.
Fig. 5. Effect of LexA-S119A and UmuD-K97A on the LD signal of RecA–poly(dT)–ATPγS complex. RecA (4 µM) and poly(dT) (12 µM in nucleotides) were used. LD was measured with a shear force of 20/s at 25°C. (A) LexA-S119A was added at a molar ratio to RecA of 0.23, 0.45, 067, 0.89 or 1.35. Only the spectra for RecA alone or with LexA-S119A added at 0.45 or 1.35 molar ratio are shown. (B) UmuD-K97A was added at a molar ratio to RecA of 0.16, 0.32, 0.47, 0.63 or 0.95. Only the spectra for RecA alone or with UmuD-K97A added at 0.32 or 0.95 molar ratio are shown. In the insets of (A) and (B), the change in the LD value at 196 nm was plotted against the molar ratio of LexA-S119A or UmuD-K97A to RecA.
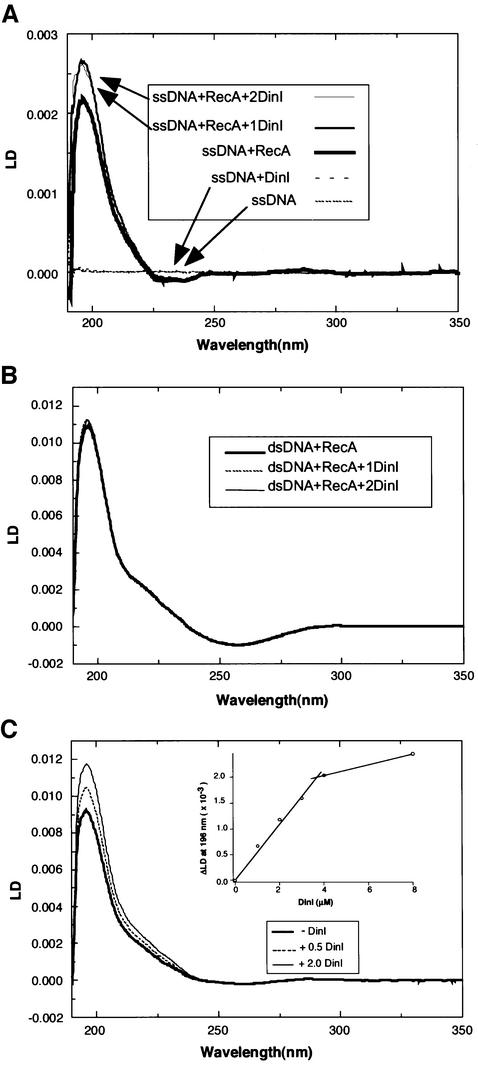
To investigate the interaction between DinI and RecA nucleoprotein filament by the LD method, we used either poly(dεA) or poly(dT) as ssDNA, and native calf thymus DNA as dsDNA. The LD signal of free poly(dεA) was too small to distinguish from the baseline. When RecA (4 µM) and poly(dεA) (12 µM) were mixed in the presence of 50 µM ATPγS, a significant LD signal was observed. No LD signal appeared upon the addition of DinI alone to poly(dεA) (Figure 6A), supporting the previous result that DinI does not bind directly to ssDNA. However, the addition of DinI (4 µM) to RecA–poly(dεA)–ATPγS and RecA–poly(dT)–ATPγS complexes increased the LD signals (Figure 6A and C). In the inset of Figure 6C, the changes in the LD value at 196 nm were plotted against the amount of DinI added to the reaction mixture. The result suggests that DinI binds to the RecA nucleoprotein filament at a ratio to RecA monomer of 1:1, while the results shown in the inset of Figure 5A and B suggest that LexA and UmuD bind at a ratio of 1:2. In contrast to such results with RecA–ssDNA–ATPγS complexes, the addition of DinI caused little or no effect on the LD signal of the RecA–dsDNA–ATPγS complex (Figure 6B). These results indicate that DinI interacts with RecA bound to ssDNA, but not with that bound to dsDNA.
Fig. 6. Effect of DinI on the LD signal of RecA–DNA–ATPγS complexes. LD was measured with a shear force of 60/s (A and C) or 20/s (B) at 20°C. (A) poly(dεA) (12 µM in nucleotides) was mixed with 4 µM RecA alone or plus DinI (4 or 8 µM). The curves of ssDNA alone or with DinI are almost at baseline. (B) RecA (4 µM) and calf thymus dsDNA (12 µM) were mixed with or without DinI (8 µM). All the curves are almost superimposed. (C) RecA (4 µM) and poly(dT) (12 µM) were mixed with 1, 2, 3, 4 or 8 µM DinI. Only the spectra for RecA alone or with DinI added at 0.5 or 2.0 molar ratio are shown. In the inset of (C), the LD value at 196 nm was plotted against the molar ratio of DinI added to RecA.
DinI does not dissociate the RecA–ssDNA–ATPγS complex
From the results described above, it is unlikely that DinI dissociates the RecA–ssDNA–ATPγS ternary complex. This was confirmed directly as described below.
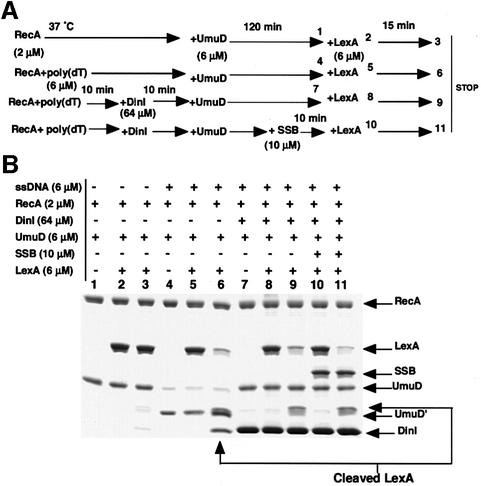
In our previous in vitro experiments with the purified proteins, activated RecA-dependent UmuD processing was effectively inhibited in the presence of DinI, but LexA processing was not (Yasuda et al., 1998). If DinI inhibited UmuD processing by dissociating the Rec–ssDNA–ATPγS complex, then the cleavage of LexA added later to the reaction mixture containing the ternary complex, DinI and UmuD should also be inhibited. However, it may be argued that even if RecA–ssDNA–ATPγS complex is disassembled by the addition of DinI, reassociation of RecA monomers with ssDNA might occur and thereby promote preferential LexA cleavage. Such reassociation can, however, be blocked in the presence of SSB, a potent competitor for binding to ssDNA (Figure 2). In fact, SSB completely prevented LexA processing when it was mixed with RecA, ssDNA and ATPγS prior to the addition of LexA (data not shown). As shown in Figure 7B (the experimental scheme is given in Figure 7A), UmuD and LexA were cleaved in the presence of RecA–poly(dT)– ATPγS (lanes 4–6) but not in its absence (lanes 1–3). In the presence of DinI, UmuD processing was inhibited (lanes 7–11), but LexA subsequently added to the same reaction mixture was cleaved normally in the presence or absence of SSB (lanes 9 and 11). This result indicates that the RecA–poly(dT)–ATPγS complex remains active for LexA cleavage in the presence of DinI, while being inactive for UmuD processing. We believe, therefore, that these findings eliminate the possibility that DinI dissociates the RecA–ssDNA–ATPγS ternary complex.
Fig. 7. DinI does not dissociate a RecA–ssDNA–ATPγS complex. (A) The experimental scheme. (B) SDS–PAGE analysis of the reaction products. The in vitro reactions were performed as described in Materials and methods. Proteins and ssDNA were added to the reaction mixture as indicated in (A). The lane number corresponds to the sampling time taken from the reaction mixture, as indicated by the number in (A).
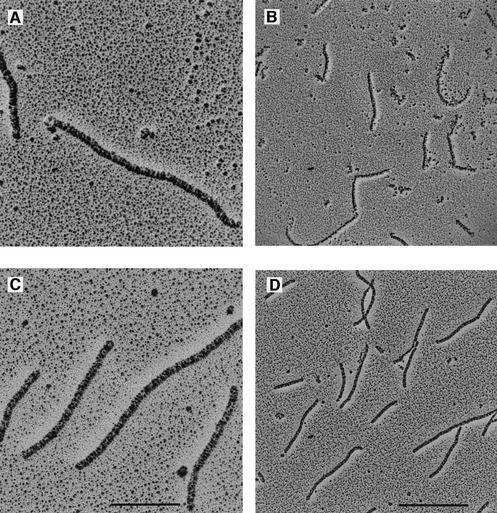
We visualized the RecA–ssDNA–ATPγS complex structure with or without DinI by electron microscopy (Figure 8). When RecA was mixed with poly(dT) (average length 400 nucleotides) in the presence of ATPγS and placed at 37°C, the intermolecular end-joining of RecA nucleoprotein filaments was observed (Figure 8A and B), as previously reported (Register and Griffith, 1986). We observed very similar structures when DinI was added after the RecA–poly(dT)–ATPγS complex was placed at 37°C for 1 h (Figure 8C and D), indicating that the ternary complex was maintained in the presence of DinI. Since DinI is so small, we did not expect any difference in the structure of the RecA filament in the presence or absence of DinI. We did, however, notice that the striated pattern of the RecA nucleoprotein filament seemed to be more condensed in the presence of DinI than in the absence of it (compare Figure 8C with A).
Fig. 8. Electron micrographs of RecA–poly(dT)–ATPγS complex without DinI (A and B) and with DinI (C and D). The bars indicate 200 nm (A and C) and 500 nm (B and D).
Competitive binding of DinI, LexA and UmuD to a RecA nucleoprotein filament
The results described above support the notion that DinI interacts with a RecA nucleoprotein filament so as to prevent it from interacting with UmuD. Why, then, does DinI inhibit UmuD processing but not LexA cleavage? We reasoned that such distinct effects could be caused by differences in the binding affinity of each protein to a RecA nucleoprotein filament. To address this question, we assayed the inhibitory effects of DinI, LexA-S119A and UmuD-K97A on RecA coprotease activities with either LexA or UmuD as the substrate.
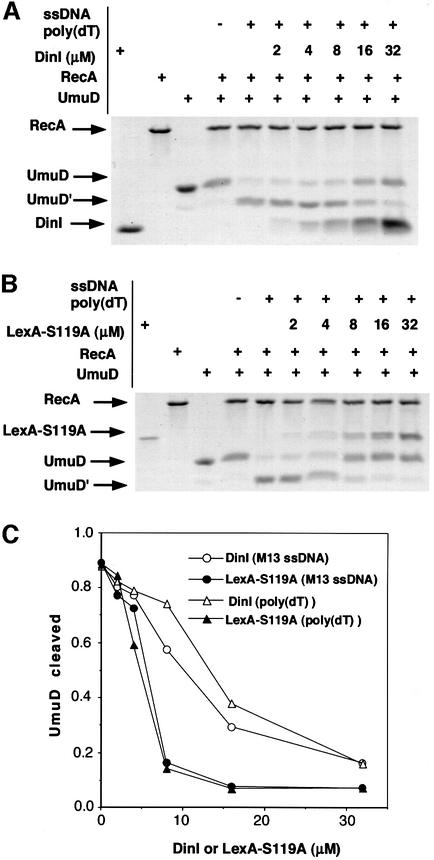
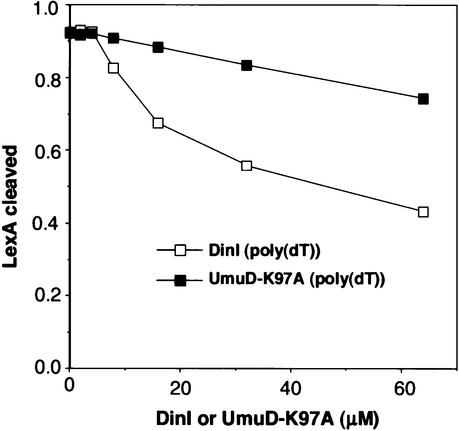
We first compared the effect of LexA-S119A and DinI on the inhibition of UmuD processing, using either poly(dT) or M13 DNA as ssDNA (Figure 9). In both cases, LexA-S119A inhibited UmuD processing more efficiently than did DinI, as shown in Figure 9C where the extent of inhibition is plotted against the amount of LexA-S119 or DinI added to the reaction. Next, we compared the effects of DinI and UmuD-K97A on inhibiting LexA cleavage. LexA cleavage occurred much faster than UmuD processing: LexA was completely cleaved within 15 min at 37°C, while complete UmuD processing took ∼3 h under the same conditions. When DinI or UmuD-K97A was added to the RecA–poly(dT)–ATPγS complex and then LexA added to the mixture, LexA was completely cleaved after a further 15 min incubation at 37°C (data not shown). We subsequently performed the reaction at 20°C instead of 37°C, in order to slow down the LexA cleavage reaction. Indeed, complete LexA cleavage took ∼30 min at 20°C (data not shown). Under these conditions, we could detect inhibitory effects of DinI and UmuD-K97A on LexA cleavage, although the extent of inhibition was much weaker than that of LexA-S119A or DinI on UmuD processing. The result summarized in Figure 10 demonstrates that DinI inhibited LexA cleavage more efficiently than UmuD-K97A. The above experiments to measure the RecA coprotease activity were carried out in the presence of ATPγS, which stabilizes the RecA nucleoprotein filament. However, when LexA cleavage was measured at 37°C in the presence of ATP instead of ATPγS, no inhibition was observed upon the addition of DinI (data not shown). This eliminates the possibility that the failure of DinI to inhibit LexA cleavage in vitro is an artifact caused by ATPγS.
Fig. 9. Inhibition of UmuD processing by DinI and LexA-S119A. (A and B) The in vitro reactions were performed as described in Materials and methods. Different amounts of DinI (A) or LexA-S119A (B) were added to the reaction mixtures to measure the inhibition of processing of UmuD (6 µM). The proteins were analyzed by 16.5% SDS–PAGE with a Tricine buffer system. (C) The densities of the UmuD and UmuD′ bands in the presence of poly(dT) (A and B) or M13 ssDNA (data not shown) were measured, and the relative amounts of UmuD′ against UmuD + UmuD′ were plotted against the amount of DinI or LexA-S119A added to the reaction.
Fig. 10. Inhibition of LexA processing by DinI and UmuD-K97A. The in vitro reactions in the presence of poly(dT) were performed as described in Materials and methods at 20°C. Different amounts of DinI or UmuD-K97A were added to the reaction mixtures to measure the inhibition of cleavage of LexA (6 µM). The densities of the LexA bands were measured, and the relative amounts of the cleaved LexA were plotted against the amount of DinI or UmuD-K97A added to the reaction.
Discussion
Direct binding of DinI to the RecA filament for the inhibition of RecA functions
In this study, we have examined in detail how DinI inhibits RecA coprotease activities, by a variety of in vivo and in vitro experiments. All of our results are consistent with the notion that DinI interacts with RecA nucleoprotein filament in a competitive manner with LexA and UmuD. As visualized by electron microscopy observations, the RecA filament structure is maintained in the presence of DinI, thus eliminating the possibility that DinI dissociates the RecA nucleoprotein complex.
Three-dimensional electron micrograph reconstruction studies suggested that LexA binds to the activated RecA filament within its deep helical groove (Yu and Egelman, 1993). This site is also believed to be the secondary DNA-binding site essential for DNA strand exchange (Story et al., 1992). Cross-linking studies and mutational analyses of RecA protein suggest that this site is also the UmuD binding site (Lee and Walker, 1996; Nastri et al., 1997; Konola et al., 1998). Furthermore, UmuD′2C was recently shown by cryo-electron microscopy studies to bind uniformly along the filament’s deep helical groove under saturating conditions (Frank et al., 2000). Our results shown in Figures 9 and 10 suggest that DinI also binds to the same site, or an overlapping one, in the RecA nucleoprotein filaments. At the present time, however, we can not completely exclude the possibility that binding of DinI to the RecA filament generates an allosteric effect on the binding of other proteins to the filament. In any case, despite its relatively small size, RecA can interact with multiple proteins and DNA, which compete with each other. This provides a mechanism whereby it can regulate different pathways of DNA repair, especially switching between recombination repair and error-prone translesion synthesis.
Differential binding affinities of LexA, UmuD and DinI to the RecA filament
There are two possible (non-mutually exclusive) explanations for the stronger inhibitory effect of DinI on UmuD processing than on LexA cleavage: (i) there is a difference in the binding affinity of these proteins to the RecA filament; (ii) there is a difference in the rate of cleavage. We can not discard the second possibility because, even in the RecA-independent cleavage reaction, LexA cleavage occurs faster than UmuD processing (Burckhardt et al., 1988). However, since our previous analysis indicated that the limiting step in RecA-dependent cleavage of LexA is the binding between LexA and RecA filament (Takahashi et al., 1986), the most probable explanation is that there is a difference in binding affinity, with the order being LexA > DinI > UmuD. Indeed, support for this hypothesis is found in Figure 9 where LexA-S119A inhibited UmuD processing more efficiently than did DinI. In contrast, the inhibitory effects of DinI and UmuD-K97A on LexA cleavage were weaker and only detected when LexA cleavage was assayed at 20°C. Under these conditions, DinI inhibited LexA cleavage more efficiently than UmuD-K97A, implying that DinI has a higher affinity for the RecA nucleoprotein filament than does UmuD.
In this regard, Frank et al. (1993) reported that UmuD′ binds to a RecA nucleoprotein filament less tightly than UmuD. In comparison, the UmuD′2C complex may bind to the RecA filament with a higher affinity, as it inhibits RecA-mediated homologous recombination both in vivo and in vitro, whereas UmuD and UmuD′ do not. Furthermore, UmuD′2C was also shown to inhibit LexA cleavage effectively even at 37°C (Rehrauer et al., 1998). We observed that when dinI was overexpressed in lexA51 (Def) Δ umuDC mutant cells carrying a plasmid that can produce UmuD′C, UV-induced mutagenesis was not inhibited (our unpublished result). It implied that overexpression of DinI did not inhibit the interaction of UmuD′2C with the RecA filament in vivo, while effectively inhibiting the cleavage of LexA and UmuD (Yasuda et al., 1998). We therefore infer that UmuD′2C has a higher affinity for a RecA nucleoprotein filament than does DinI, UmuD or UmuD′. Thus, once formed, the UmuD′2C complex is preferentially guided to the tip of a RecA nucleoprotein filament (Sommer et al., 1998; Frank et al., 2000) to exert its function as DNA polymerase V and bypass lesions in DNA. The multiple and elaborate steps to regulate the formation of the error-prone DNA polymerase V might have evolved as the last resort to restrict its action, thus avoiding any ‘gratuitous’ mutagenesis (Woodgate and Levine, 1996). It is believed that soon after translesion synthesis is completed the error-prone enzyme DNA polymerase V is again replaced by the replicative enzyme DNA polymerase III. For the elongation by DNA polymerase III to occur, it is presumed that RecA should be dissociated from the DNA template, because the RecA filament was shown to inhibit DNA polymerase III action (Shwartz and Livneh, 1989). How such switching from one DNA polymerase to another occurs at or around a DNA lesion blocking normal replication is an interesting question to be clarified by further experiments.
DinI inhibited the LexA cleavage when it was overproduced, but no discernible difference (in terms of recovery) in the amount of intact LexA in the late phase of the SOS response was found between the wild-type and dinI mutant cells (our unpublished result). In contrast, the inhibitory effect of DinI on UmuD processing was observed when it was expressed at the normal single-copy level of the gene, although much less drastically than when overexpressed (Yasuda et al., 1998). These in vivo observations can be explained by the differences in the affinity to the RecA filament among LexA, DinI and UmuD described here. Thus, the intermediate affinity of DinI is more suited to a fine-tuning role in UmuDC-dependent mutagenesis, rather than as a general regulator of the global SOS response.
Detection of protein–protein interaction with LD
The LD method has been used to investigate the detailed structure of RecA–DNA complexes and chromatin (Kubista et al., 1985; Takahashi et al., 1987; Norden et al., 1990, 1992; Hagmar et al., 1992). In this study, we demonstrated that the interaction between RecA nucleoprotein filament and other proteins could also be studied by this method. If a protein binds to RecA filaments and is flow-oriented together with them, some change in the LD signal intrinsic to the RecA filaments is expected. Consistent with this expectation, the addition of LexA-S119A or UmuD-K97A to a RecA–ssDNA–ATPγS complex caused an increase or decrease in the LD signal in the far-UV region, where the signals from aromatic amino acids and peptide bonds of proteins are dominant over the signal from DNA bases. No significant change was observed in the signal at ∼260 nm where the signals from DNA are dominant. These proteins bind to the RecA filament without modifying the filament organization. The absence of any change upon the addition of lysozyme shows that the presence of small non-orientable molecules does not disturb the observation of LD signal even in the far-UV region. The signal change in the far-UV region is probably due to preferential orientation of some aromatic amino acid residues or peptide bonds of the interacting protein.
The addition of DinI, LexA-S119A or UmuD-K97A largely changed the LD signal at ∼196 nm. Based on such changes in the LD signal in relation to the amount of protein added to the RecA filament, we could estimate the stoichiometry of the interaction of DinI, LexA or UmuD with RecA. LexA and UmuD were considered to bind in 1:2 stoichiometry with RecA. On the basis of electron micrograph reconstruction studies, Yu and Egelman (1993) have shown that LexA binds in 1:2 stoichiometry with RecA. Furthermore, UmuD′2C also binds in 1:2 stoichiometry with RecA (Rehrauer et al., 1998; Frank et al., 2000). On the other hand, DinI appears to bind in 1:1 stoichiometry with RecA. Every RecA subunit has two different LexA binding sites. Yu and Egelman (1993) described that the presence of one LexA binding at each site on two adjacent RecA protomers may physically clash with the ability of an additional LexA molecule to bind to the next unoccupied sites. DinI protein is small (9 kDa) compared with LexA (22 kDa), UmuD (15 kDa) and UmuD′2C (72 kDa), so that binding of DinI to one RecA protomer may not affect its binding to the adjacent ones. Further investigations are required for our full understanding of the interactions between RecA nucleoprotein filaments and other proteins.
Materials and methods
Strains, plasmids, chemicals and media
Strain DE192 (provided by R.Woodgate, NIH, USA) is F– lexA51(Def) sulA211 rpsL31 (lac-pro)XIII (Ennis et al., 1985). Strain DE667 (provided by R.Woodgate via T.Nohmi) is F– lexA51(Def) recA730 sulA211 thi-1 Δ(lac-gpt)5 Δ(uvrB-chlA) mtl-1 rpsL31. The plasmid pHR255 (provided by H.Hara, National Institute of Genetics, Japan) carries a 1.2 kb PstI–KpnI lacIq fragment of pMJR1560 (Stark, 1987) in a derivative pACYC184, which was used to regulate expression of the gene under the control of the tac promoter. pYP92 was constructed by inserting the dinI-coding region between the BamHI and BglII sites of the expression vector pQE9. pSNS123, to overproduce LexA-119A (Slilaty and Little, 1987), was provided by S.C.Kowalczykowski (University of California, Davis, CA). pEC69 overproducing UmuD-K97A and an E.coli strain RW382 [a Δ(umuDC)595::cat derivative of BL21(DE3)] were provided by R.Woodgate (McDonald et al., 1998). ATP, dATP and dTTP were purchased from Amersham-Pharmacia Biotech. ATPγS was purchased from Boehringer Mannheim Biotech. DSP was purchased from Pierce.
Proteins and antibodies
DinI was purified as described (Yasuda et al., 1998). Antibodies against the purified DinI protein were raised in rabbits. RecA protein was purified as described (Morimatsu et al., 1995). Anti-RecA antibodies were a gift from A.Shinohara (Osaka University, Japan). Wild-type and LexA-S119A mutant LexA proteins were purified from strain AK101 carrying pTH227 (Horii et al., 1981) or strain GW2730 carrying pSNS123 (Slilaty and Little, 1987), as previously described. UmuD-K97A protein was purified as described (McDonald et al., 1998). SSB was purchased from Amersham-Pharmacia Biotech.
DNA substrates
Poly(dT) and poly(dA) were prepared by enzymatic extension of synthesized oligo(dT) (15mer) and oligo(dA) (15mer), respectively, with terminal deoxynucleotidyl transferase (Boehringer Mannheim Biotech). Poly(dεA) was prepared by chemical modification of poly(dA) (Cazenave et al., 1983). The average length of poly(dT) and poly(dεA) was ∼400mer.
ssDNA binding assay
Heat-denatured calf thymus DNA–cellulose (Amersham-Pharmacia Biotech) was used. This cellulose was packed into a column and equilibrated with buffer A (20 mM Tris–HCl pH 7.5, 4 mM MgCl2, 1 mM DTT, 0.1 mM ATPγS) containing 50 mM NaCl. Then, 50 µg of DinI or RecA were loaded onto the column. After washing the column with the buffer, the proteins bound to the column were eluted with buffer A containing 0.5, 1.0 or 2.0 M NaCl, and then separated by either 12.5 or 19.6% SDS–PAGE (Ito et al., 1980). RecA or DinI was detected by immunoblotting analysis.
ATP hydrolysis assay
ATP hydrolysis was measured by an enzyme-coupled spectroscopic assay as described (Mikawa et al., 1998). The assay was performed in 40 mM Tris–HCl pH 7.5, 50 mM NaCl, 2 mM MgCl2, 2 mM DTT, 2 mM phosphoenolpyruvate, 0.32 mM NADH and 25 U/ml each of pyruvate kinase and lactate dehydrogenase at 37°C. The absorbance was measured with a Hitachi spectrophotometer, model U-3000.
CD measurements
CD was measured on a Jasco J-715 spectropolarimeter using a 0.1 cm pathlength quartz cuvette. The temperature of the cuvette was controlled by a programmable Peltier thermoelectric system. Thermal unfolding of proteins was examined by monitoring the change in CD signal at 220 nm (bandwidth 5 nm) upon temperature elevation from 20 to 80°C at a rate of 1°C/min. The experiments were performed in 40 mM Tris–HCl pH 7.5, 30 mM NaCl, 4 mM MgCl2, with or without 50 µM ATPγS.
LD measurements
LD was measured on a Jasco J-715 spectropolarimeter with LD mode. The samples were flow-oriented by circulating the solution through a circulating 0.05 cm pathlength quartz cell (Hellema, Germany) with a chemically inert HPLC pump (Jasco, Japan). A flow rate of 2 or 5 ml/min was used. LD spectra were measured with a bandwidth of 2 nm and a scan rate of 50 nm/min, and averaged over four scans to increase the signal/noise ratio. The samples were prepared in 40 mM Tris–HCl pH 7.5, 30 mM NaCl, 4 mM MgCl2 and 50 µM ATPγS, and incubated for >3 h at room temperature. To measure an interaction between RecA–DNA– ATPγS complex and DinI, LexA-S119A, UmuD-K97A or lysozyme, LD was measured 20 min after the addition of each of the proteins to RecA–DNA–ATPγS.
Cross-linking
The DE667 or DE192 cells carrying pHR225 and either pYP92 or pQE9 were grown to log phase in L-broth containing chloramphenicol (100 µg/ml), ampicillin (50 µg/ml) and isopropyl-β-d-thiogalacto pyranoside (IPTG) (0.1 mM) at 37°C. After the addition of sodium azide at a final concentration of 0.03%, the cultures (1 ml each) were placed on ice for 5 min and the cells were harvested. The cells, resuspended in 100 µl of 50 mM HEPES–KOH pH 8.0, 20% sucrose, were mixed with 1/10 volume of 1 mg/ml lysozyme suspension in 0.1 M EDTA pH 8.0 and kept on ice for 30 min. The lysozyme-treated cells were harvested again and resuspended in 100 µl of the same buffer without lysozyme. The samples were then treated with DSP (1/25 volume of 30 mg/ml solution in dimethylsulfoxide to give a final concentration of 1.2 mg/ml) at 4°C for 15 h. Mock treatment samples were supplemented with dimethylsulfoxide. After the addition of 400 µl of buffer A (20 mM phosphate pH 7.4, 10 mM imidazole, 8 M urea), the DSP-treated cells were disrupted by sonication. The lysed cells were mixed with 50 µl of Ni-NTA–agarose for 2 h at 4°C. After washing with buffer A three times, the His6-DinI proteins bound to the Ni–NTA agarose were eluted with buffer B (20 mM phosphate pH 7.4, 0.5 M imidazole). Then, in order to cleave the disulfide bond in the center of DSP, the samples were mixed with the SDS–PAGE sample buffer containing DTT (100 mM). After incubation at 37°C for 30 min, the samples were subjected to 10% SDS–PAGE.
Immunoblotting analysis
After SDS–PAGE, separated proteins were electrotransferred to an Immobilon P membrane (Millipore) and subsequently probed with rabbit antibodies raised against DinI or RecA. The target proteins were visualized using the ECL western blotting analysis system (Amersham-Pharmacia Biotech).
Electron microscopy
For electron microscopy, RecA (6.5 µM) was mixed with poly(dT) (19.5 µM in nucleotides) in 40 mM Tris–HCl pH 7.5, 50 mM NaCl, 4 mM MgCl2, 2 mM DTT, 1 mM ATPγS, 40% glycerol, at 37°C for 20 min. After the RecA–poly(dT)–ATPγS complex was formed, DinI (13 µM) was added and further incubated at 37°C for 40 min. Specimens were prepared for low-angle rotary shadowing as described previously (Hirako et al., 1998), and observed using a Hitachi H-7100 electron microscope.
LexA and UmuD cleavage assay
Unless indicated otherwise, the cleavage reactions were carried out in the standard buffer containing 40 mM Tris–HCl pH 7.5, 50 mM NaCl, 4 mM MgCl2, 2 mM DTT, 1 mM ATPγS. RecA (2 µM) and ssDNA (6 µM in nucleotides) were pre-incubated at 37°C for 10 min after mixing in the above buffer. To measure the effect of DinI or LexA-S119A on UmuD processing, the mixtures were supplemented with different amounts of DinI or LexA-S119A, and further incubated at 37°C for an additional 10 min. After the addition of UmuD (6 µM), the reaction mixtures were incubated at 37°C for a further 3 h. To measure the effect of DinI or UmuD-K97A on LexA cleavage, the mixtures were supplemented with different amounts of DinI or UmuD-K97A and further incubated at 37°C for an additional 10 min. After the addition of LexA (6 µM), the mixtures were incubated at 20°C for a further 30 min. The reaction products were analyzed by 12.5% SDS–PAGE with the standard buffer system or by 16.5% SDS–PAGE with the Tricine buffer system for the other assays, and stained with Coomassie Blue R-250. The densities of the bands were quantified using Sigma Gel software (Jandel Corporation).
Cleavage of the LexA protein added to the mixture containing RecA–ssDNA–ATPγS, DinI and UmuD was examined as follows. After pre-incubation as above, the reaction mixture containing RecA (2 µM) and poly(dT) (6 µM) was mixed with DinI (64 µM). After a 10 min incubation, UmuD (6 µM) was added to the reaction mixture and incubated for a further 2 h. Then, the mixture was supplemented with LexA (6 µM) and incubated for a further 15 min. When necessary, SSB (10 µM) was added 2 h after the addition of UmuD, and the mixtures were incubated for 10 min before the addition of LexA. The reactions were terminated by addition of the SDS–PAGE sample buffer and the products were analyzed as described above.
Acknowledgments
Acknowledgements
We thank many people who enabled us to complete this work: S.C.Kowalczykowski for the plasmid to purify LexA-S119A protein; A.Kihara for information about in vivo cross-linking; K.Shigesada for advice in determining stoichiometries; and the engineers of Jasco Co. Ltd (Japan) for proposing the use of an HPLC pump for the observation of LD. We are very grateful to H.Iwasaki for purified UmuD protein, T.Nohmi for bacterial strains, A.Shinohara for anti-RecA antibodies, and R.Masui for purified RecA protein. We are deeply indebted to R.Woodgate for providing us with purified UmuD-K97A protein and the UmuD-K97A-overproducing plasmid/strain, and also for improving the manuscript. This work was supported in part by a research grant-in-aid from the Ministry of Education, Science and Culture, Japan to H.O., Inoue Research Award for Young Scientists to T.Y., and a research grant from Association pour la Recherche sur le Cancer (no. 9364) to M.T.
References
- Burckhardt S.E., Woodgate,R., Scheuermann,R.H. and Echols,H. (1988) UmuD mutagenesis protein of Escherichia coli: overproduction, purification and cleavage by RecA. Proc. Natl Acad. Sci. USA, 85, 1811–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Toulme,J.J. and Helene,C. (1983) Binding of RecA protein to single-stranded nucleic acids: spectroscopic studies using fluorescent polynucleotides. EMBO J., 2, 2247–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N.L. and Roberts,J.W. (1980) E.coli recA protein-directed cleavage of phage λ repressor requires polynucleotide. Nature, 283, 26–30. [DOI] [PubMed] [Google Scholar]
- Craig N.L. and Roberts,J.W. (1981) Function of nucleoside triphosphate and polynucleotide in Escherichia coli recA protein-directed cleavage of phage λ repressor. J. Biol. Chem., 256, 8039–8044. [PubMed] [Google Scholar]
- Ennis D.G., Fisher,B., Edmiston,S. and Mount,D.W. (1985) Dual role for Escherichia coli RecA protein in SOS mutagenesis. Proc. Natl Acad. Sci. USA, 82, 3325–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E.G., Hauser,J., Levine,A.S. and Woodgate,R. (1993) Targeting of the UmuD, UmuD′ and MucA′ mutagenesis proteins to DNA by RecA protein. Proc. Natl Acad. Sci. USA, 90, 8169–8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E.G., Cheng,N., Do,C.C., Cerritelli,M.E., Bruck,I., Goodman,M.F., Egelman,E.H., Woodgate,R. and Steven,A.C. (2000) Visualization of two binding sites for Escherichia coli UmuD′2C complex (DNA pol V) on RecA–ssDNA filaments. J. Mol. Biol., 297, 585–597. [DOI] [PubMed] [Google Scholar]
- Friedberg E.G., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis. ASM Press, Washington, DC.
- Hagmar P., Norden,B., Baty,D., Chartier,M. and Takahashi,M. (1992) Structure of DNA–RecA complexes studied by residue differential linear dichroism and fluorescence spectroscopy for a genetically engineered RecA protein. J. Mol. Biol., 226, 1193–1205. [DOI] [PubMed] [Google Scholar]
- Harmon F.G., Rehrauer,W.M. and Kowalczykowski,S.C. (1996) Interaction of Escherichia coli RecA protein with LexA repressor. II. Inhibition of DNA strand exchange by the uncleavable LexAS119A repressor argues that recombination and SOS induction are competitive processes. J. Biol. Chem., 271, 23874–23883. [PubMed] [Google Scholar]
- Hirako Y., Usukura,J., Uematsu,J., Hashimoto,T., Kitajima,Y. and Owaribe,K. (1998) Cleavage of BP180, a 180-kDa bullous pemphigoid antigen, yields a 120-kDa collagenous extracellular polypeptide. J. Biol. Chem., 273, 9711–9717. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa,T., Nakatani,T., Hase,T., Matsubara,H. and Ogawa,H. (1981) Regulation of SOS functions: purification of E.coli LexA protein and determination of its specific site cleaved by the RecA protein. Cell, 27, 515–522. [DOI] [PubMed] [Google Scholar]
- Ito K., Date,T. and Wickner,W. (1980) Synthesis, assembly into the cytoplasmic membrane and proteolytic processing of the precursor of coliphage M13 coat protein. J. Biol. Chem., 255, 2123–2130. [PubMed] [Google Scholar]
- Konola J.T., Guzzo,A., Gow,J.B., Walker,G.C. and Knight,K.L. (1998) Differential cleavage of LexA and UmuD mediated by recA Pro67 mutants: implications for common LexA and UmuD binding sites on RecA. J. Mol. Biol., 276, 405–415. [DOI] [PubMed] [Google Scholar]
- Kowalczykowski S.C., Dixon,D.A., Eggleston,A.K., Lauder,S.D. and Rehrauer,W.M. (1994) Biochemistry of homologous recombination in Escherichia coli. Microbiol. Rev., 58, 401–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubista M., Hard,T., Nielsen,P.E. and Norden,B. (1985) Structural transitions of chromatin at low salt concentrations: a flow linear dichroism study. Biochemistry, 24, 6336–6342. [DOI] [PubMed] [Google Scholar]
- Kuzminov A. (1999) Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol. Mol. Biol. Rev., 63, 751–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery P.E. and Kowalczykowski,S.C. (1992) Biochemical basis of the constitutive repressor cleavage activity of recA730 protein. A comparison to recA441 and recA803 proteins. J. Biol. Chem., 267, 20648–20658. [PubMed] [Google Scholar]
- Lee M.H. and Walker,G.C. (1996) Interactions of Escherichia coli UmuD with activated RecA analyzed by cross-linking UmuD monocysteine derivatives. J. Bacteriol., 178, 7285–7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J.W. (1984) Autodigestion of lexA and phage λ repressors. Proc. Natl Acad. Sci. USA, 81, 1375–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J.P., Frank,E.G., Levine,A.S. and Woodgate,R. (1998) Intermolecular cleavage by UmuD-like mutagenesis proteins. Proc. Natl Acad. Sci. USA, 95, 1478–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T., Masui,R. and Kuramitsu,S. (1998) RecA protein has extremely high cooperativity for substrate in its ATPase activity. J. Biochem., 123, 450–457. [DOI] [PubMed] [Google Scholar]
- Morimatsu K., Horii,T. and Takahashi,M. (1995) Interaction of Tyr103 and Tyr264 of the RecA protein with DNA and nucleotide cofactors: fluorescence study of engineered proteins. Eur. J. Biochem., 228, 779–785. [DOI] [PubMed] [Google Scholar]
- Nastri H.G., Guzzo,A., Lange,C.S., Walker,G.C. and Knight,K.L. (1997) Mutational analysis of the RecA protein L1 region identifies this area as a probable part of the co-protease substrate binding site. Mol. Microbiol., 25, 967–978. [DOI] [PubMed] [Google Scholar]
- Norden B., Elvingson,C., Eriksson,T., Kubista,M., Sjoberg,B., Takahashi,M. and Mortensen,K. (1990) Structure of a RecA–DNA complex from linear dichroism and small-angle neutron-scattering in flow-oriented solution. J. Mol. Biol., 216, 223–228. [DOI] [PubMed] [Google Scholar]
- Norden B., Elvingson,C., Kubista,M., Sjoberg,B., Ryberg,H., Ryberg,M., Mortensen,K. and Takahashi,M. (1992) Structure of RecA–DNA complexes studied by combination of linear dichroism and small-angle neutron scattering measurements on flow-oriented samples. J. Mol. Biol., 226, 1175–1191. [DOI] [PubMed] [Google Scholar]
- Register J.C. III and Griffith,J. (1986) RecA protein filaments can juxtapose DNA ends: an activity that may reflect a function in DNA repair. Proc. Natl Acad. Sci. USA, 83, 624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehrauer W.M., Lavery,P.E., Palmer,E.L., Singh,R.N. and Kowalczykowski,S.C. (1996) Interaction of Escherichia coli RecA protein with LexA repressor. I. LexA repressor cleavage is competitive with binding of a secondary DNA molecule. J. Biol. Chem., 271, 23865–23873. [PubMed] [Google Scholar]
- Rehrauer W.M., Bruck,I., Woodgate,R., Goodman,M.F. and Kowalczykowski,S.C. (1998) Modulation of RecA nucleoprotein function by the mutagenic UmuD′C protein complex. J. Biol. Chem., 273, 32384–32387. [DOI] [PubMed] [Google Scholar]
- Reuven N.B., Arad,G., Maor-Shoshani,A. and Livneh,Z. (1999) The mutagenesis protein UmuC is a DNA polymerase activated by UmuD′, RecA and SSB and is specialized for translesion replication. J. Biol. Chem., 274, 31763–31766. [DOI] [PubMed] [Google Scholar]
- Roca A.I. and Cox,M.M. (1997) RecA protein: structure, function and role in recombinational DNA repair. Prog. Nucleic Acid Res. Mol. Biol., 56, 129–223. [DOI] [PubMed] [Google Scholar]
- Shwartz H. and Livneh,Z. (1989) RecA protein inhibits in vitro replication of single-stranded DNA with DNA polymerase III holoenzyme of Escherichia coli. Mutat. Res., 213, 165–173. [DOI] [PubMed] [Google Scholar]
- Slilaty S.N. and Little,J.W. (1987) Lysine-156 and serine-119 are required for LexA repressor cleavage: a possible mechanism. Proc. Natl Acad. Sci. USA, 84, 3987–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S., Bailone,A. and Devoret,R. (1993) The appearance of the UmuD′C protein complex in Escherichia coli switches repair from homologous recombination to SOS mutagenesis. Mol. Microbiol., 10, 963–971. [DOI] [PubMed] [Google Scholar]
- Sommer S., Boudsocq,F., Devoret,R. and Bailone,A. (1998) Specific RecA amino acid changes affect RecA–UmuD′C interaction. Mol. Microbiol., 28, 281–291. [DOI] [PubMed] [Google Scholar]
- Stark M.J. (1987) Multicopy expression vectors carrying the lac repressor gene for regulated high-level expression of genes in Escherichia coli. Gene, 51, 255–267. [DOI] [PubMed] [Google Scholar]
- Story R.M., Weber,I.T. and Steitz,T.A. (1992) The structure of the E.coli recA protein monomer and polymer. Nature, 355, 318–325. [DOI] [PubMed] [Google Scholar]
- Takahashi M. and Schnarr,M. (1989) Investigation of RecA– polynucleotide interactions from the measurement of LexA repressor cleavage kinetics. Presence of different types of complex. Eur. J. Biochem., 183, 617–622. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Daune,M. and Schnarr,M. (1986) Fluorescence study of the RecA dependent proteolysis of LexA, the repressor of the SOS system in Escherichia coli. FEBS Lett., 196, 215–218. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Kubista,M. and Norden,B. (1987) Linear dichroism study of RecA–DNA complexes. Structural evidence and binding stoichiometries. J. Biol. Chem., 262, 8109–8111. [PubMed] [Google Scholar]
- Tang M., Shen,X., Frank,E.G., O’Donnell,M., Woodgate,R. and Goodman,M.F. (1999) UmuD′2C is an error-prone DNA polymerase, Escherichia coli pol V. Proc. Natl Acad. Sci. USA, 96, 8919–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Pham,P., Shen,X., Taylor,J.S., O’Donnell,M., Woodgate,R. and Goodman,M.F. (2000) Roles of E.coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature, 404, 1014–1018. [DOI] [PubMed] [Google Scholar]
- Woodgate R. and Levine,A.S. (1996) Damage inducible mutagenesis: recent insights into the activities of the Umu family of mutagenesis proteins. In Lindahl,T. (ed.), Cancer Surveys: Genetic Instability in Cancer. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 117–140. [PubMed]
- Woodgate R., Rajagopolan,M., Lu,C. and Echols,H. (1989) UmuC mutagenesis protein of Escherichia coli: purification and interaction with UmuD and UmuD′. Proc. Natl Acad. Sci. USA, 86, 7301–7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Nagata,T. and Ohmori,H. (1996) Multicopy suppressors of the cold-sensitive phenotype of the pcsA68 (dinD68) mutation in Escherichia coli. J. Bacteriol., 178, 3854–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Morimatsu,K., Horii,T., Nagata,T. and Ohmori,H. (1998) Inhibition of Escherichia coli RecA coprotease activities by DinI. EMBO J., 17, 3207–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. and Egelman,E.H. (1993) The LexA repressor binds within the deep helical groove of the activated RecA filament. J. Mol. Biol., 231, 29–40. [DOI] [PubMed] [Google Scholar]