Abstract
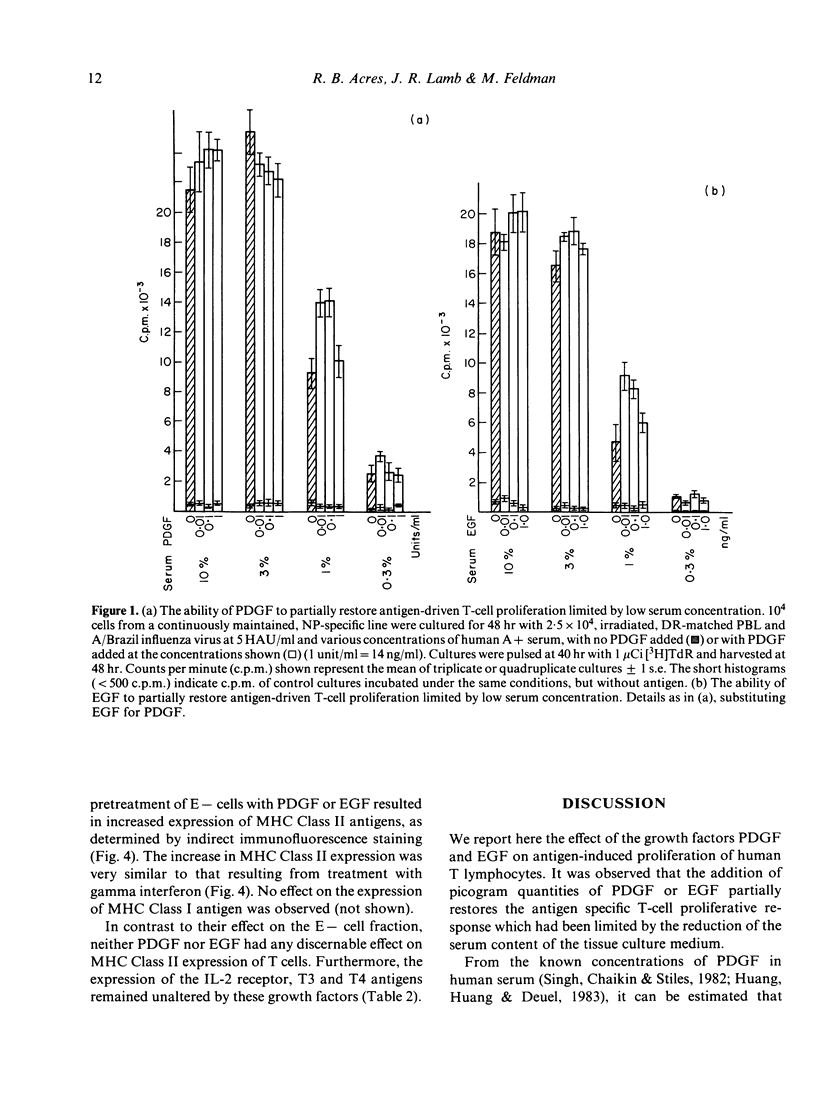
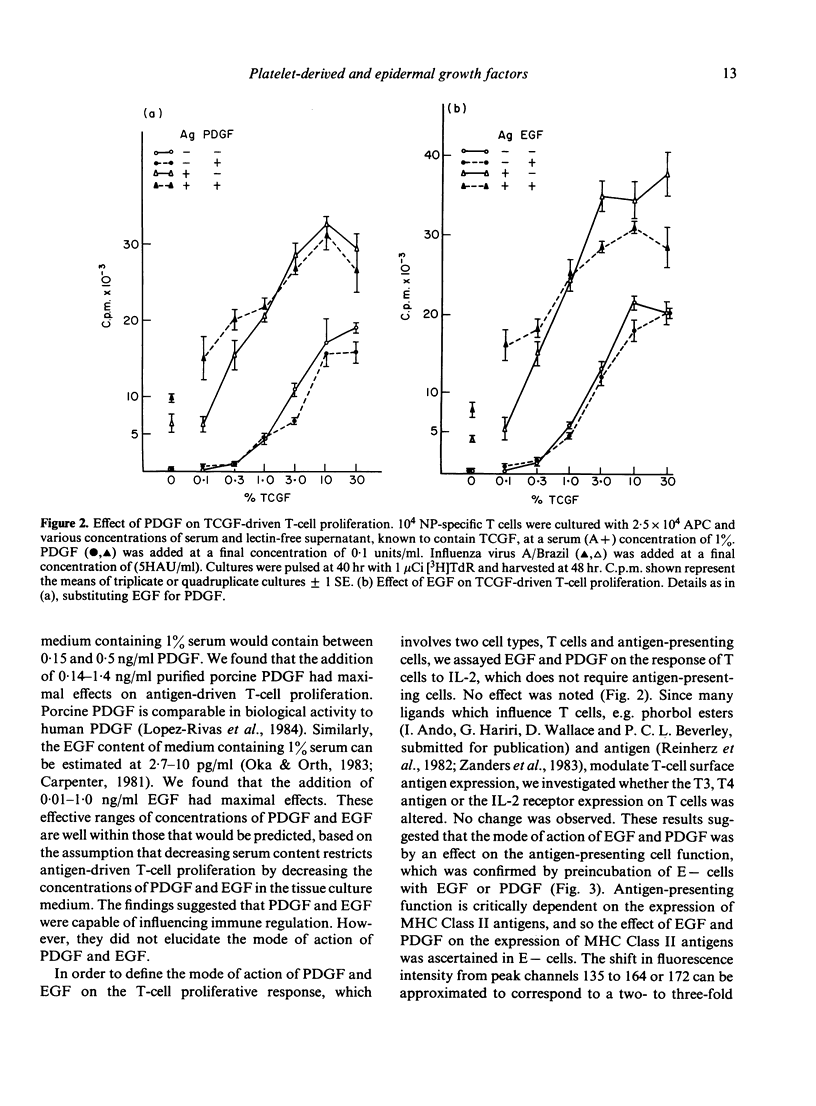
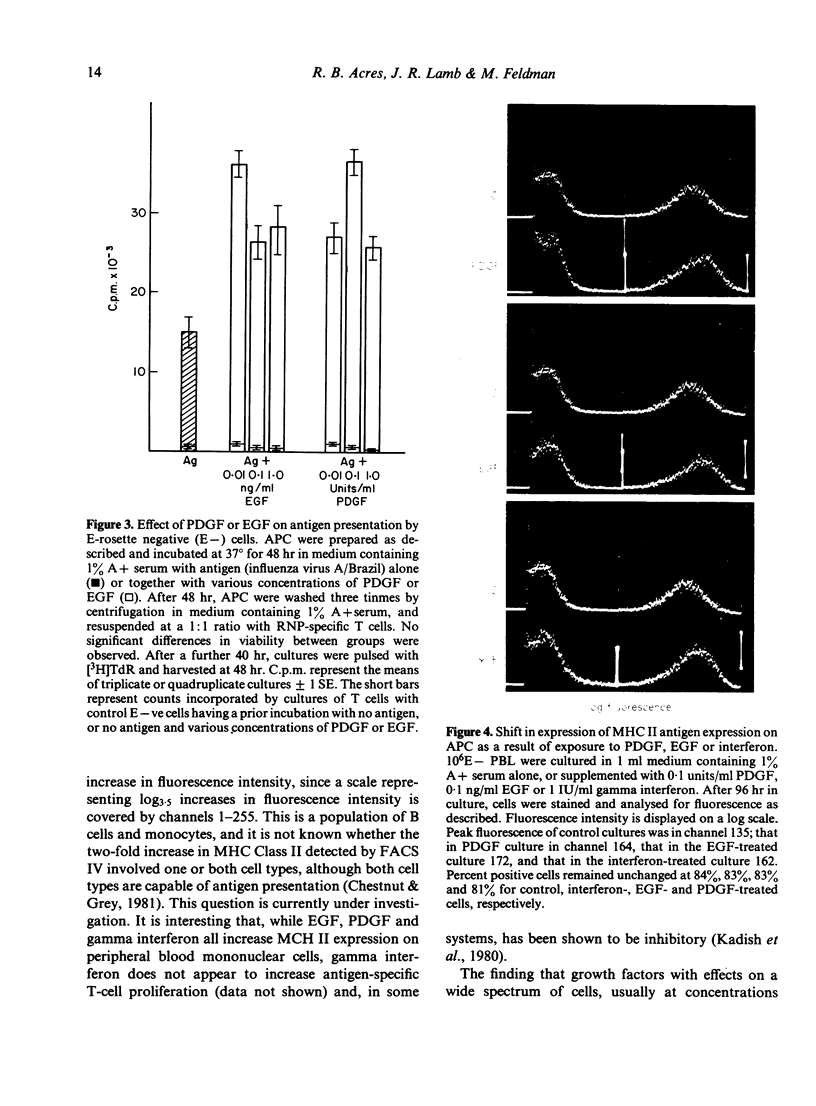
When the serum content of tissue culture medium is reduced from 10% to 1%, the capacity of T cells to proliferate in response to antigen within that medium is dramatically reduced. Physiological concentrations of platelet-derived growth factor (PDGF) or epidermal growth factor (EGF) are able to partially replace the requirement for serum, in that they are able to increase antigen-driven T-cell proliferation at a serum concentration of 1%. Neither growth factor is mitogenic for T cells in the absence of antigen, and neither is able to act synergistically with T-cell growth factor (TCGF) or IL-2) in the absence of antigen. Antigen-presenting cells (APC) pulsed with antigen in the presence of PDGF or EGF are able to stimulate antigen-specific T-cell proliferation to a greater extent than antigen-presenting cells pulsed in the absence of exogenous PDGF or EGF. Both growth factors increase the expression of MHC Class II antigens on antigen-presenting cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chesnut R. W., Grey H. M. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981 Mar;126(3):1075–1079. [PubMed] [Google Scholar]
- Dainiak N., Davies G., Kalmanti M., Lawler J., Kulkarni V. Platelet-derived growth factor promotes proliferation of erythropoietic progenitor cells in vitro. J Clin Invest. 1983 May;71(5):1206–1214. doi: 10.1172/JCI110869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Human platelet-derived growth factor: radioimmunoassay and discovery of a specific plasma-binding protein. J Cell Biol. 1983 Aug;97(2):383–388. doi: 10.1083/jcb.97.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadish A. S., Tansey F. A., Yu G. S., Doyle A. T., Bloom B. R. Interferon as a mediator of human lymphocyte suppression. J Exp Med. 1980 Mar 1;151(3):637–650. doi: 10.1084/jem.151.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Woody J. N., Hartzman R. J., Eckels D. D. In vitro influenza virus-specific antibody production in man: antigen-specific and HLA-restricted induction of helper activity mediated by cloned human T lymphocytes. J Immunol. 1982 Oct;129(4):1465–1470. [PubMed] [Google Scholar]
- Lamb J. R., Zanders E. D., Feldmann M., Lake P., Eckels D. D., Woody J. N., Beverley P. C. The dissociation of interleukin-2 production and antigen-specific helper activity by clonal analysis. Immunology. 1983 Nov;50(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Orth D. N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983 Jul;72(1):249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Stroobant P., Waterfield M. D., Deuel T. F., Keehan M. Platelet-derived growth factor elicits cyclic AMP accumulation in Swiss 3T3 cells: role of prostaglandin production. Cell. 1983 Aug;34(1):265–272. doi: 10.1016/0092-8674(83)90157-5. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Singh J. P., Chaikin M. A., Stiles C. D. Phylogenetic analysis of platelet-derived growth factor by radio-receptor assay. J Cell Biol. 1982 Nov;95(2 Pt 1):667–671. doi: 10.1083/jcb.95.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno Y., Arai S., Takishima T. Induction of immunoglobulin-producing human peripheral blood lymphocytes in serum-free medium. J Immunol Methods. 1982 Jul 30;52(2):255–265. doi: 10.1016/0022-1759(82)90052-7. [DOI] [PubMed] [Google Scholar]
- Tees R., Schreier M. H. Selective reconstitution of nude mice with long-term cultured and cloned specific helper T cells. Nature. 1980 Feb 21;283(5749):780–781. doi: 10.1038/283780a0. [DOI] [PubMed] [Google Scholar]
- Zanders E. D., Lamb J. R., Feldmann M., Green N., Beverley P. C. Tolerance of T-cell clones is associated with membrane antigen changes. Nature. 1983 Jun 16;303(5918):625–627. doi: 10.1038/303625a0. [DOI] [PubMed] [Google Scholar]