Abstract
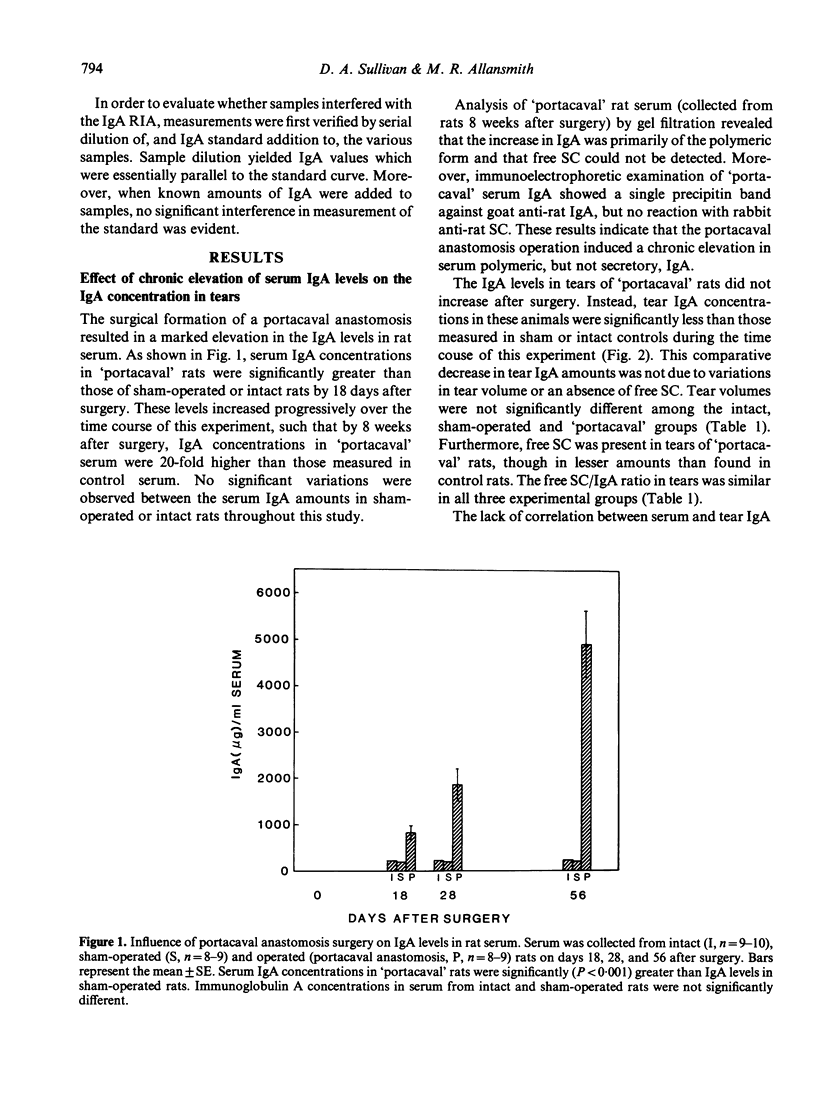
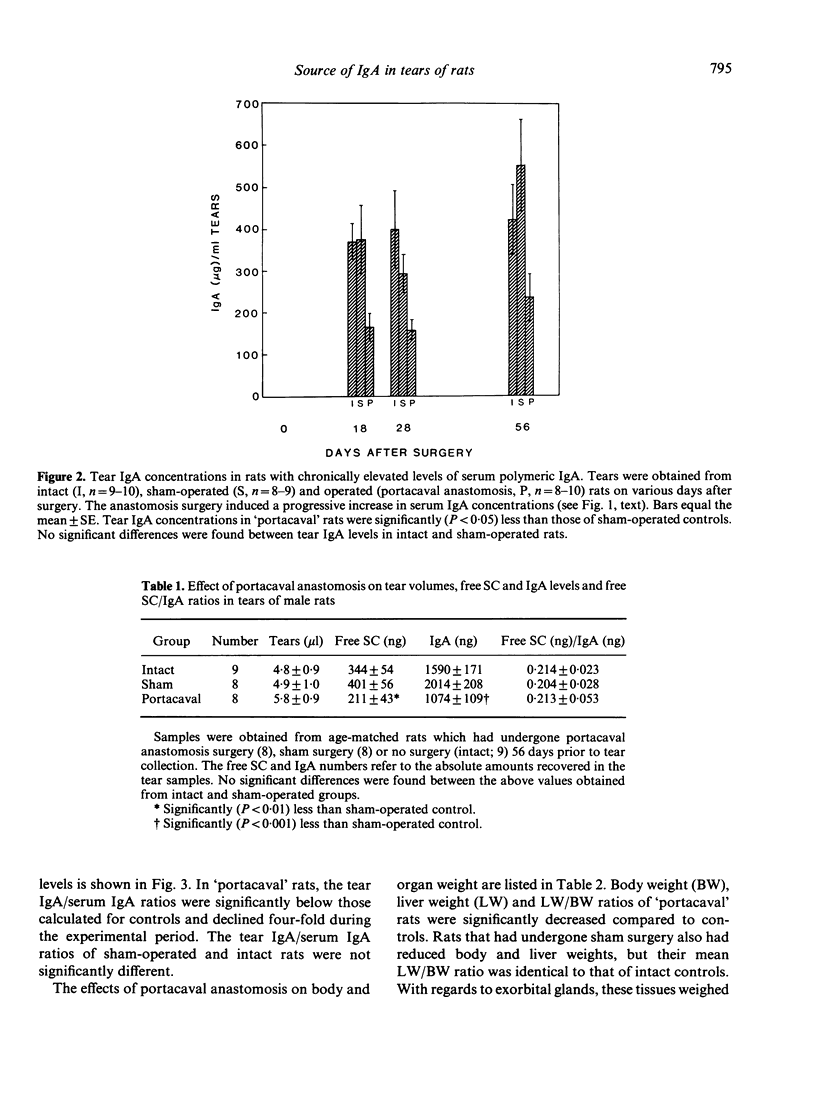
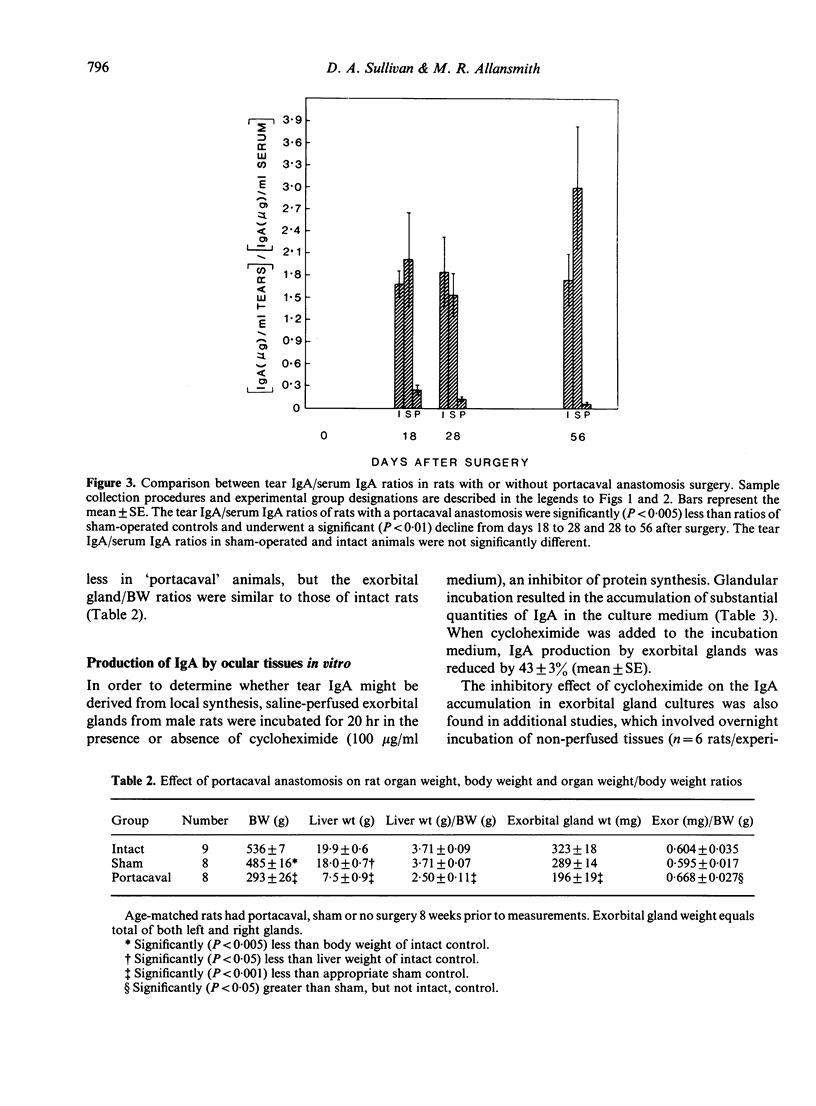
The purpose of the present study was to determine whether IgA in rat tears originates from serum and/or local synthesis. To examine the first possibility, we compared the IgA levels in serum and tears of rats with a portacaval anastomosis. This operation induced a chronic and progressive elevation of polymeric IgA concentrations in serum. By 8 weeks after surgery, serum IgA levels in 'portacaval' rats were 20-fold higher than those of sham-operated or intact controls. In contrast, IgA levels in tears of 'portacaval' rats did not increase after surgery, despite the availability of free secretory component (SC) in tears. The lack of correlation between tear and serum IgA concentrations resulted in a significant decrease in the tear IgA/serum IgA ratio after anastomosis surgery. To assess whether tear IgA might be derived from local synthesis, we cultured various ocular tissues from male rats in the presence or absence of cycloheximide, an inhibitor of protein synthesis. Incubation of exorbital (lacrimal) glands resulted in the accumulation of substantial quantities of IgA in the culture medium. This accumulation was significantly reduced by the presence of cycloheximide. In contrast, cycloheximide had no effect on the amounts of IgA in cultures of 'lids', Harder's glands and globes. These results indicate that IgA in rat tears originates from local synthesis and not from serum transfer. Furthermore, our findings suggest that the rat exorbital gland is responsible for the synthesis and secretion of tear IgA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allansmith M. R., Gillette T. E. Secretory component in human ocular tissues. Am J Ophthalmol. 1980 Mar;89(3):353–361. doi: 10.1016/0002-9394(80)90004-5. [DOI] [PubMed] [Google Scholar]
- Allansmith M. R., Kajiyama G., Abelson M. B., Simon M. A. Plasma cell content of main and accessory lacrimal glands and conjunctiva. Am J Ophthalmol. 1976 Dec;82(6):819–826. doi: 10.1016/0002-9394(76)90056-8. [DOI] [PubMed] [Google Scholar]
- Burns C. A., Ebersole J. L., Allansmith M. R. Immunoglobulin A antibody levels in human tears, saliva, and serum. Infect Immun. 1982 Jun;36(3):1019–1022. doi: 10.1128/iai.36.3.1019-1022.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M. E., Koopman W. J. In vitro regulation of IgA subclass synthesis. I. Discordance between plasma cell production and antibody secretion. J Exp Med. 1982 Dec 1;156(6):1615–1621. doi: 10.1084/jem.156.6.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren U., Ahlstedt S., Hedman L., Wadsworth C., Hanson L. A. Dimeric IgA in the rat is transferred from serum into bile but not into milk. Scand J Immunol. 1981 Jul;14(1):95–98. doi: 10.1111/j.1365-3083.1981.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Fisher M. M., Nagy B., Bazin H., Underdown B. J. Biliary transport of IgA: role of secretory component. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2008–2012. doi: 10.1073/pnas.76.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M., Prendergast R. A., Silverstein A. M. Secretory immune system of rabbit ocular adnexa. Invest Ophthalmol Vis Sci. 1979 Oct;18(10):1093–1096. [PubMed] [Google Scholar]
- Hall J. G., Hopkins J., Orlans E. Studies on the lymphocytes of sheep. III. Destination of lymph-borne immunoblasts in relation to their tissue of origin. Eur J Immunol. 1977 Jan;7(1):30–37. doi: 10.1002/eji.1830070108. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Pribnow J. F. Topical ocular immunization of rabbits. Invest Ophthalmol Vis Sci. 1981 Nov;21(5):753–756. [PubMed] [Google Scholar]
- Halsey J. F., Mitchell C., Meyer R., Cebra J. J. Metabolism of immunoglobulin A in lactating mice: origins of immunoglobulin A in milk. Eur J Immunol. 1982 Feb;12(2):107–112. doi: 10.1002/eji.1830120203. [DOI] [PubMed] [Google Scholar]
- Lamm M. E. Cellular aspects of immunoglobulin A. Adv Immunol. 1976;22:223–290. doi: 10.1016/s0065-2776(08)60550-7. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Sautter V., Preisig R., Bircher J. Hepatic functional deterioration after portacaval shunt in the rat. Effects on sulfobromophthalein transport-maximum, indocyanine green clearance and galactose elimination capacity. Gastroenterology. 1976 Aug;71(2):221–227. [PubMed] [Google Scholar]
- Lemaître-Coelho I., Altamirano G. A., Barranco-Acosta C., Meykens R., Vaerman J. P. In vivo experiments involving secretory component in the rat hepatic transfer of polymeric IgA from blood into bile. Immunology. 1981 Jun;43(2):261–270. [PMC free article] [PubMed] [Google Scholar]
- Lemaître-Coelho I., Jackson G. D., Vaerman J. P. High levels of secretory IgA and free secretory component in the serum of rats with bile duct obstruction. J Exp Med. 1978 Mar 1;147(3):934–939. doi: 10.1084/jem.147.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaître-Coelho I., Yamakido M., Montgomery P. C., Langendries A. E., Vaerman J. P. Selective excretion of IgA in rat bronchial secretions: lack of significant contribution from plasma IgA. Immunol Commun. 1982;11(6):441–453. doi: 10.3109/08820138209050741. [DOI] [PubMed] [Google Scholar]
- Malaty R., Dawson C. R., Wong I., Lyon C., Schachter J. Serum and tear antibodies to Chlamydia after reinfection with guinea pig inclusion conjunctivitis agent. Invest Ophthalmol Vis Sci. 1981 Dec;21(6):833–841. [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R., Arnold R. R., Michalek S. M., Prince S. J., Babb J. L. Selective induction of an immune response in human external secretions by ingestion of bacterial antigen. J Clin Invest. 1978 Mar;61(3):731–737. doi: 10.1172/JCI108986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan S. R., Sarna G. S. Effect of portacaval anastomosis and chronic underfeeding on the hypothalamic-pituitary-gonadal axis in the rat. J Endocrinol. 1981 Jan;88(1):39–47. doi: 10.1677/joe.0.0880039. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Ayyildiz A., Lemaitre-Coelho I. M., Vaerman J. P., Rockey J. H. Induction and expression of antibodies in secretions: the ocular immune system. Ann N Y Acad Sci. 1983 Jun 30;409:428–440. doi: 10.1111/j.1749-6632.1983.tb26887.x. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Khaleel S. A., Goudswaard J., Virella G. Selective transport of an oligomeric IgA into canine saliva. Immunol Commun. 1977;6(6):633–642. doi: 10.3109/08820137709093472. [DOI] [PubMed] [Google Scholar]
- Murray E. S., Charbonnet L. T., MacDonald A. B. Immunity to chlamydial infections of the eye. I. The role of circulatory and secretory antibodies in resistance to reinfection with guinea pig inclusion conjunctivitis. J Immunol. 1973 Jun;110(6):1518–1525. [PubMed] [Google Scholar]
- Pasquali Ronchetti I., Baccarani Contri M., Pittaluga S., Vassanelli P., Zannini P., Maruotti R. A morphometric and ultrastructural study of the long term effect of portacaval anastomosis on the liver of normal male rats. J Submicrosc Cytol. 1983 Jul;15(3):731–749. [PubMed] [Google Scholar]
- Sheldrake R. F., Husband A. J., Watson D. L., Cripps A. W. Selective transport of serum-derived IgA into mucosal secretions. J Immunol. 1984 Jan;132(1):363–368. [PubMed] [Google Scholar]
- Sullivan D. A., Bloch K. J., Allansmith M. R. Hormonal influence on the secretory immune system of the eye: androgen control of secretory component production by the rat exorbital gland. Immunology. 1984 Jun;52(2):239–246. [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Bloch K. J., Allansmith M. R. Hormonal influence on the secretory immune system of the eye: androgen regulation of secretory component levels in rat tears. J Immunol. 1984 Mar;132(3):1130–1135. [PubMed] [Google Scholar]
- Sullivan D. A., Underdown B. J., Wira C. R. Steroid hormone regulation of free secretory component in the rat uterus. Immunology. 1983 Jun;49(2):379–386. [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Wira C. R. Hormonal regulation of immunoglobulins in the rat uterus: uterine response to a single estradiol treatment. Endocrinology. 1983 Jan;112(1):260–268. doi: 10.1210/endo-112-1-260. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Wira C. R. Hormonal regulation of immunoglobulins in the rat uterus: uterine response to multiple estradiol treatments. Endocrinology. 1984 Feb;114(2):650–658. doi: 10.1210/endo-114-2-650. [DOI] [PubMed] [Google Scholar]
- Sullivan D. A., Wira C. R. Variations in free secretory component levels in mucosal secretions of the rat. J Immunol. 1983 Mar;130(3):1330–1335. [PubMed] [Google Scholar]
- Vaerman J. P., André C., Bazin H., Heremans J. F. Mesenteric lymph as a major source of serum IgA in guinea pigs and rats. Eur J Immunol. 1973 Sep;3(9):580–584. doi: 10.1002/eji.1830030911. [DOI] [PubMed] [Google Scholar]
- Vaerman J. P., Lemaître-Coelho I., McSween R. N., Benjamin I. S., Thomas H. C. Increased serum IgA levels in rats after portacaval shunt but not after portacaval transposition. Scand J Immunol. 1981 Aug;14(2):131–136. doi: 10.1111/j.1365-3083.1981.tb00192.x. [DOI] [PubMed] [Google Scholar]


