Abstract
The molecular mechanism(s) that are responsible for suppressing MyoD’s transcriptional activities in undifferentiated skeletal muscle cells have not yet been determined. We now show that MyoD associates with a histone deacetylase-1 (HDAC1) in these cells and that this interaction is responsible for silencing MyoD-dependent transcription of endogenous p21 as well as muscle-specific genes. Specifically, we present evidence that HDAC1 can bind directly to MyoD and use an acetylated MyoD as a substrate in vitro, whereas a mutant version of HDAC1 (H141A) can not. Further more, this mutant also fails to repress MyoD-mediated transcription in vivo, and unlike wild-type HDAC1 it can not inhibit myogenic conversion, as judged by confocal microscopy. Finally, we show that an endogenous MyoD can be acetylated upon its conversion to a hypophosphorylated state and only when the cells have been induced to differentiate. These results provide for a model which postulates that MyoD may be co-dependent on HDAC1 and P/CAF for temporally controlling its transcriptional activity before and after the differentiation of muscle cells.
Keywords: HDAC1/MyoD/myogenic conversion/skeletal muscle cells
Introduction
Specification of the myogenic lineage and differentiation of skeletal muscle cells are critically dependent on a class of transcription factors (MyoD, Myf-5, myogenin and MRF4) known as the MyoD family of basic helix–loop– helix (bHLH) proteins (Arnold and Winter, 1998). In fact, each has the ability to convert non-muscle cell types into differentiation-competent myogenic cells after ectopic expression in either tissue culture or transgenic mice (reviewed in Lassar et al., 1994). Although the individual roles of these factors are still being elucidated, genetic studies have demonstrated that MyoD and Myf-5 are potentially redundant and act to establish the myogenic lineage, whereas myogenin serves to control the process of terminal differentiation (Arnold and Winter, 1998). This genetic hierarchy is also reflected in in vitro differentiation systems. For example, myogenin RNA and the RNA of muscle structural genes are produced in abundance only after muscle cells constitutively expressing either MyoD or Myf-5 are induced to differentiate (Hollenberg et al., 1993; Lassar et al., 1994).
MyoD engages the cell cycle machinery to effect cell cycle withdrawal prior to the differentiation process (reviewed in Lassar et al., 1994). Insights into how this might occur have recently come from several studies, and the current view is that the cyclin-dependent kinase (cdk) inhibitors p21 and p57, which negatively regulate cell cycle progression (Harper and Elledge, 1996), play important roles in this process. Indeed, both of these proteins have been found to be highly expressed in vivo during embryonic muscle differentiation and in myogenic cells that have been stimulated to differentiate in culture (Maione and Amati, 1997; Reynaud et al., 1999; P.Zhang et al., 1999). Moreover, it is now clear that MyoD is responsible in part for upregulating p21 in muscle cells that have been induced to differentiate in vitro (Halevy et al., 1995; Otten et al., 1997).
Members of the MyoD family function in association with ubiquitously expressed bHLH factors known as E proteins (e.g. E12 and E47) (Lassar et al., 1994). As such, these heterodimers promote muscle-specific transcription by binding to a simple consensus sequence of CANNTG, termed an E-box, present in the regulatory regions of many genes expressed in skeletal muscle (Blackwell and Weintraub, 1990; Weintraub, 1993). Evidence indicates that MyoD–E12 heterodimers can also collaborate with members of the myocytes enhancer factor 2 (MEF2) family to activate muscle-specific genes and myogenesis (Naya and Olson, 1999). Essential to this cooperation are the myogenic residues alanine and threonine, which reside in the basic domain of MyoD (Lassar et al., 1994). It has been postulated that these amino acids may be necessary for maintaining MyoD in an ideal conformation while it activates transcription (Ma et al., 1994), and for that matter they may also be important for its acetylation (Sartorelli et al., 1999).
Although MyoD is constitutively expressed in proliferating myoblasts, it is nevertheless unable to function as a transcriptional activator and thereby execute the myogenic program (Lassar et al., 1994). The precise mechanism by which the transcriptional activity of MyoD is suppressed in these cells is still unclear, although several models have been proposed. One in particular invokes the Id factor, which can heterodimerize with E proteins and, to a lesser extent, with MyoD, thereby attenuating MyoD’s ability to interact with regulatory elements of muscle-specific genes (Benezra et al., 1990). The notion that Id inhibits MyoD function in its entirety, however, has been elegantly disputed with the use of MyoD–E protein chimeras (Neuhold and Wold, 1993). Other factors reported to inhibit MyoD functions are the murine twist protein and Mist1, both of which can disrupt the DNA binding and transactivating functions of MyoD (Spicer et al., 1996; Lemercier et al., 1998). Cyclin-dependent kinase 4 (cdk4) also appears to have this effect by binding directly to MyoD without kinase activity (J.-M.Zhang et al., 1999a). However, inactive cdk4 is also highly expressed in differentiated cells (Wang and Walsh, 1996), and exactly what effect this might have on MyoD activity is still unclear. Another model credits phosphorylation for negatively affecting MyoD’s activities, since ectopic expression of cyclin D1, the regulator of cdk4, inhibits MyoD’s ability to transactivate E-box-containing reporter genes (Rao et al., 1994; Skapek et al., 1995). However, although MyoD is one of several highly phosphorylated forms in proliferating myoblasts (Tapscott et al., 1989), evidence suggests that this type of modification may be more in keeping, at least in part, with its stability (Song et al., 1998; Kitzmann et al., 1999; Tintignac et al., 2000).
Although the preceding models may help to explain the suppression of MyoD’s transcriptional activities in proliferating myoblasts, the possibility of yet more major pathways contributing to this phenomenon needs to be considered in view of recent findings. For example, the ability of the lymphoid lineage-determining factors Ikaros and Aiolos to repress rather than activate transcription in proliferating lymphocytes appears to correspond, in part, with their association with mSin3-HDAC (Kim et al., 1999; Koipally et al., 1999), a complex that includes the histone deacetylases HDAC1 and HDAC2 (Ng and Bird, 2000). These enzymes have recently been implicated in transcriptional repression and are believed to be responsible for silencing mammalian genes by deacetylating core histones (Ng and Bird, 2000). Besides HDAC1 and HDAC2, at least four additional histone deacetylases (HDAC3–6) are known (Ng and Bird, 2000), and recent work on HDAC4 indicates a link to MEF2 in that an MEF2-activated reporter is repressed by this enzyme (Miska et al., 1999).
Given that MyoD displays regulatory events similar to that of MEF2 and the mouse proteins Ikaros and Aiolos, all of which appear to attract different HDAC complexes, we asked whether a histone deacetylase activity might also be extending to MyoD and, thereby, affecting its transcriptional activity in skeletal muscle cells. The resulting data provide strong support for the model that this enzyme modulates MyoD activity and that MyoD deacetylation is indeed a critical determinant in the myogenic program.
Results
MyoD is acetylated only in differentiated muscle cells
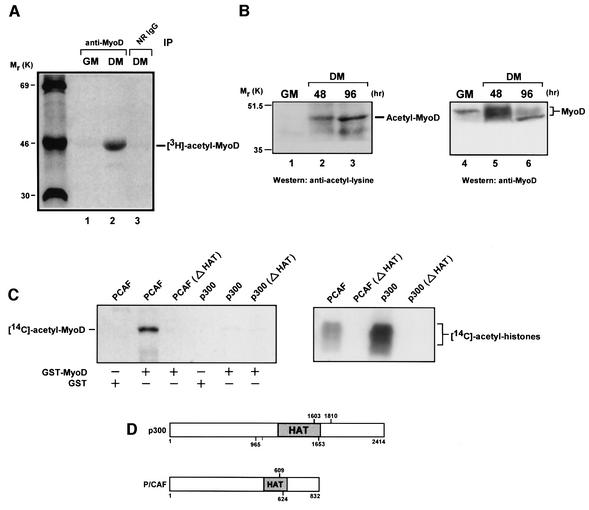
Prior to investigating the possibility that histone deacetylases might be controlling the silencing of MyoD’s transcriptional activities, we thought it important to examine initially the circumstances under which an endogenous MyoD might be acetylated in muscle cells. Although others have recently shown that an ectopically expressed MyoD can in fact be acetylated in these cells (Sartorelli et al., 1999), this study did not reveal the circumstances under which this modification might occur. To address this issue, we immunoprecipitated MyoD from extracts of undifferentiated or differentiated C2C12 (C2) muscle cells that had been pulse labeled with sodium [3H]acetate for 1.5 h. C2 cells are a well characterized murine skeletal muscle cell line that can be induced by serum deprivation to differentiate in vitro (Yaffe and Saxel, 1977). As seen in the autoradiogram of Figure 1A, endogenous MyoD can indeed be acetylated, but interestingly, the modification occurred only after these cells were exposed to differentiation medium (DM) as opposed to growth medium (GM).
Fig. 1. Acetylation of MyoD occurs only in differentiated cells and solely by P/CAF in vitro. (A) Proliferating C2 muscle cells (GM) or C2 cells switched to DM for 24 h were pulse labeled with [3H]sodium acetate for 1.5 h. Extracts were prepared and immunoprecipitated in parallel with anti-MyoD polyclonal antibody or normal rabbit IgG (NR IgG). Immune complexes were analyzed by 10% SDS–PAGE and processed for fluorography. (B) Western analysis of proteins in nuclear extracts of proliferating C2 cells (GM) and C2 cells cultured in DM for the indicated times. Acetylated proteins were identified by anti-acetyl-lysine antibodies (lanes 1–3). Re-probing of the same blot with anti-MyoD confirmed the acetylated species to be MyoD (lanes 4–6). Note that because MyoD and the immunoglobulin heavy chain migrate extremely close to one another on SDS–polyacrylamide gels due to the similarity in molecular weight, the probing of immune complexes of MyoD from C2 cells cultured in GM or DM with an anti-acetyl-lysine antibody was problematic; and only occasionally were the results identical to those in lanes 1–3 (data not shown). It should also be noted that in these, as well as in the rest of the experiments described below, the protein content of extracts from differentiated or undifferentiated C2 cells was normalized as described in Materials and methods. (C) GST (2 µg), GST–MyoD (1 µg) or histones (5 µg) were incubated with [14C]acetyl-CoA and either wild-type P/CAF, P/CAF (Δ609–624), p300 (965–1810) or p300 (Δ1603–1653). Reaction products were separated by SDS–PAGE and visualized by fluorography. (D) Diagram of recombinant p300 and P/CAF showing the endpoints of mutant deletions in the HAT domain.
The observation that MyoD is highly phosphorylated in proliferating myoblasts, but undergoes substantial dephosphorylation once these cells are induced to differentiate (Kitzmann et al., 1999), raises the prospect that only a hypophosphorylated form of MyoD may be capable of acetylation. To address this possibility, we performed western blot analysis on extracts from differentiated or undifferentiated cells, using as probes an anti-acetyl-lysine antibody and an antibody specific for MyoD on the same membrane. Even though an anti-MyoD antibody was clearly able to recognize a hyperphosphorylated form of MyoD (Figure 1B, lane 4), the anti-acetyl-lysine antibody was unable to detect an acetylated form of MyoD in cells maintained in GM (Figure 1B, lane 1), consistent with the preceding results. In contrast, an acetylated form of MyoD was clearly visible in cells cultured in DM (Figure 1B, lanes 2 and 3) and this coincided with a MyoD showing a minimal amount of phosphorylation throughout the course of differentiation (Figure 1B, lanes 5 and 6). In this respect, the fact that a purified glutathione S-transferase (GST)–MyoD is a much poorer substrate for acetylation after phosphorylation by cdks is consistent with these results (data not shown). Taken together, these data directly demonstrate that an endogenous MyoD can in fact be acetylated in muscle cells, but only after they have been induced to differentiate in culture. Moreover, our results also suggest that hyperphosphorylated forms of MyoD may lack the physical structure to become modified by an acetyltransferase, indicating therefore that phosphorylation events may be critical to the scheduling of MyoD’s acetylation.
MyoD is acetylated by P/CAF, but not by p300 in vitro
P/CAF and p300/CBP are transcriptional co-activators that possess intrinsic histone acetyltransferase (HAT) activity (Ogryzko et al., 1996; Yang et al., 1996), and other than core histones, these factors can also acetylate sequence-specific transcription factors such as p53, E2F1 and GATA-1 (Gu and Roeder, 1997; Boyes et al., 1998; Martinez-Balbas et al., 2000). Previous work has documented a role for both P/CAF and p300/CBP in mediating MyoD-dependent transcription (Puri et al., 1997a), and recently it has been shown that an exogenously expressed P/CAF can acetylate a transfected MyoD in non-muscle cells (Sartorelli et al., 1999). In light of the fact that MyoD has been found in a multimeric complex consisting of p300/CBP and P/CAF in differentiated muscle cells (Puri et al., 1997b), we decided to determine whether either or both of these acetylases could acetylate MyoD in vitro. The results in Figure 1C show that P/CAF is extremely efficient in acetylating a GST–MyoD fusion protein, as judged by the use of [14C]acetyl-CoA. The integrity of this acetylation is verified by the fact that no signal was detected in reactions containing GST and P/CAF or GST–MyoD and a P/CAF mutant lacking part of the HAT domain (Figure 1C and D). Although p300 failed to acetylate GST–MyoD, it was able to incorporate 14C into core histones under the same conditions (Figure 1C). Finally, the fact that P/CAF, but not p300, was able to increase MyoD’s DNA binding affinity after acetylation in vitro (data not shown) is consistent with the findings of others (Sartorelli et al., 1999).
MyoD can associate with P/CAF, but only in differentiated muscle cells
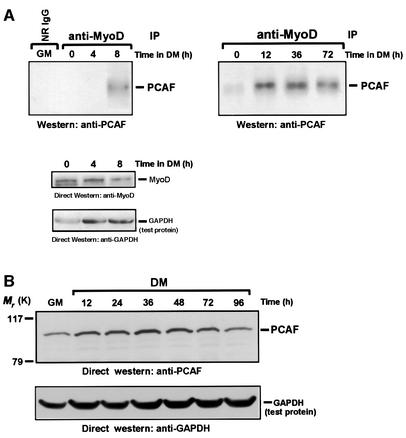
The in vitro results described in the preceding experiments strongly indicate that p300/CBP may lack the potential to acetylate MyoD in vivo. If true, the acetylation of MyoD, which is noticeably restricted to differentiated muscle cells (Figure 1A and B), is most likely then a direct consequence of P/CAF's activities. Therefore, to investigate whether MyoD might be interacting with P/CAF as muscle cells begin to differentiate, we prepared whole-cell extracts from C2 cells cultured in GM or DM for the indicated times. After normalizing for protein content (see Materials and methods), the extracts were immunoprecipitated, in parallel, with a control antibody or an antibody directed against MyoD. Immunoprecipitates were then examined for the presence of P/CAF by western blot analysis, using antibodies specific for P/CAF. As seen in Figure 2A, there was hardly any association between MyoD and P/CAF in proliferating myoblasts (GM), whereas by 8 h and up until the end of this experiment (72 h) an interaction between these two proteins was clearly observable once the cells were placed into DM. What is highly relevant to this result is that the steady-state levels of both P/CAF and MyoD protein remained nearly constant under these conditions (Figure 2 and data not shown). Immune complexes of MyoD recovered from these same extracts were also examined for the presence of p300/CBP, and similarly to what has been observed in other experiments (Eckner et al., 1996), we could find no evidence for the appearance of this protein, as judged by western blotting (data not shown). Perhaps the simplest explanation for this result is that the epitopes that would otherwise be recognized by the MyoD antibody have become inaccessible because of the binding of p300/CBP. MyoD–p300/CBP complexes are presumed to exist in muscle cells only because others have shown that an in vitro translated p300 can bind directly to a purified GST–MyoD fusion protein (Eckner et al., 1996; Yuan et al., 1996).
Fig. 2. The association between P/CAF and MyoD occurs only in differentiated cells. (A) Western analysis of immunoprecipitates of MyoD obtained from extracts of proliferating C2 cells and C2 cells cultured in DM for the indicated times. The probe was anti-P/CAF. The lower panel represents the level of MyoD in extracts of C2 cells cultured in DM for the indicated times, as judged by western analysis with the use of anti-MyoD. An anti-GAPDH antibody was also used to ensure equal loading. (B) The level of P/CAF in extracts of proliferating cells (GM) or cells cultured in DM for the indicated times was analyzed by western blotting using anti-P/CAF antibody as probe. The membrane was also probed with anti-GAPDH to monitor equal loading of the extracts.
From these experiments, we conclude that although MyoD can associate with P/CAF in muscle cells, this interaction can only occur after the cells have been induced to differentiate.
MyoD can associate with HDAC1 in undifferentiated cells and bind directly to HDAC1 in vitro
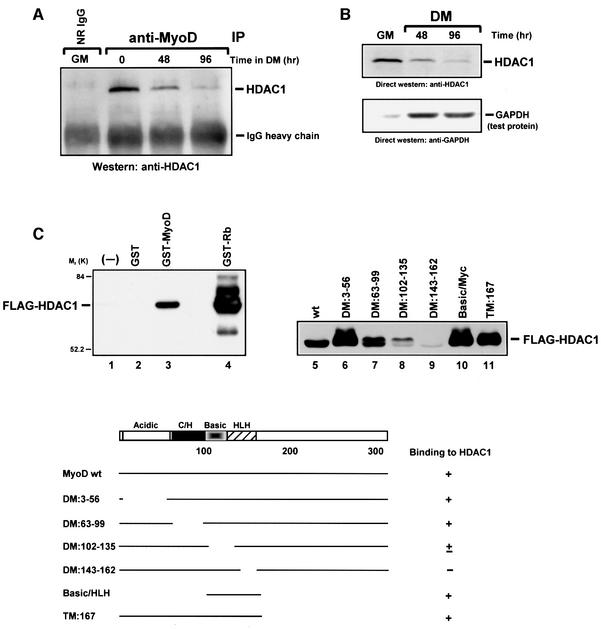
The absence of an acetylated form of MyoD in undifferentiated muscle cells (Figure 1) suggested to us that MyoD might be a target of deacetylase activity, and as such, the enzymes controlling this event would most likely be the HDAC histone deacetylases. Although HDACs actively engage in deacetylating core histones to mediate transcriptional repression (Ng and Bird, 2000), a role for these enzymes in deacetylating other proteins has recently been acknowledged because of the number of transcription factors that can apparently be deacetylated by HDACs in vivo (Brown et al., 2000). To investigate whether MyoD might be interacting with histone deacetylases, we examined immune complexes of MyoD for the presence of the histone deacetylase HDAC1. As demonstrated by western analysis (Figure 3A), anti-MyoD immunoprecipitates from myoblasts cultured in GM contained a significant amount of HDAC1. However, after these cells were switched to DM, the amount of HDAC1 in the precipitates began to diminish over time. The regression in the MyoD–HDAC1 interaction observed during the course of differentiation is most likely because of the decline in the levels of HDAC1 protein that begins at around 48 h, and nearly disappears by 96 h (Figure 3B). The underlying basis for the change in the levels of HDAC1 is still unclear. Finally, that MyoD could be immunoprecipitated with HDAC1 after being co-expressed in 10T1/2 cells (data not shown) provides further evidence that these proteins can indeed interact in vivo.
Fig. 3. HDAC1 associates with MyoD in undifferentiated cells and binds directly to MyoD in vitro. (A) Immunoprecipitates of MyoD from extracts of proliferating (GM) or differentiated (DM) C2 cells were analyzed by western blotting for the presence of HDAC1, using anti-HDAC1 as probe. (B) The level of HDAC1 in extracts of proliferating C2 cells (GM) or in C2 cells switched to DM for the indicated times was analyzed by western blotting using anti-HDAC1 as probe. As a control for equal loading, the membrane was also probed with anti-GAPDH. (C) Equal molar quantities of purified GST (lane 2), GST–MyoD (lanes 3 and 5), GST–Rb pocket protein (lane 4) or mutant GST–MyoD proteins (lanes 6–11) were pre-bound to glutathione beads and then incubated with BSA for 2 h. Beads with or without attached protein (lane 1) were incubated in parallel with an equivalent amount of baculovirus-expressed, FLAG-epitope-tagged HDAC1, and after extensive washing the bound material was subject to analysis by western blotting using anti-FLAG as probe. A summary of these results is shown in the bottom panel. DM, deletion mutant; TM, truncation mutant. The basic/HLH region includes amino acids 102–166.
We next performed in vitro binding assays to determine whether MyoD could interact directly with HDAC1. After incubating a purified FLAG-epitope-tagged HDAC1 protein (Hassig et al., 1998) with a GST–MyoD fusion protein, we were able to establish that HDAC1 could bind specifically to a full-length MyoD, but not to GST (Figure 3C). To rule out further the possibility of a non-specific interaction, we also incubated HDAC1 with a GST–Rb (amino acids 373–928) fusion protein (Ewen et al., 1993), and consistent with previous results (Brehm et al., 1998; Magnaghi-Jaulin et al., 1998), we saw that the HDAC1 protein could also bind to retinoblastoma protein (Rb). We then repeated this experiment with a series of GST–MyoD deletion and truncation mutants (Lassar et al., 1989) in order to determine the site on MyoD with which HDAC1 interacts. As shown in Figure 3C (lanes 10 and 11, respectively), HDAC1 strongly bound the N-terminal fragment (amino acids 1–167) as well as a fragment spanning the highly basic and HLH regions (amino acids 102–166) of MyoD. In contrast, removing amino acids 143–162 from MyoD greatly affected its ability to interact with HDAC1 (lane 9), as did amino acids 102–135, albeit to a lesser extent (lane 8). Taken together, these results clearly show that HDAC1 can interact directly with MyoD, and the site on MyoD that appears to be responsible for this interaction is that which includes the basic and HLH motifs.
MyoD associates with deacetylase activity in undifferentiated cells, and HDAC1 can deacetylate MyoD in vitro
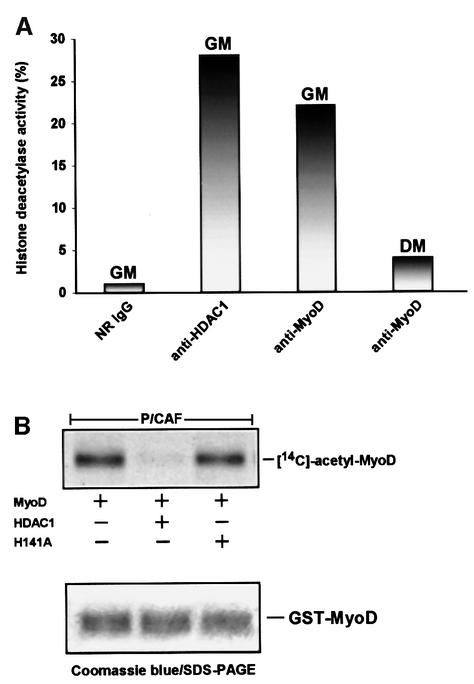
The results presented thus far indicate that MyoD can associate with HDAC1 in vivo and that the interaction between these two proteins might be direct. In considering this, we were prompted to examine whether MyoD might be associated with a histone deacetylase activity in C2 cells. For this purpose, we immunoprecipitated extracts of cells, cultured either in GM or in DM for 96 h, with either a control antibody or antibodies specific for MyoD or HDAC1. The derived precipitates were then separately incubated with [14C]acetate-labeled histones (acetylated by p300 in vitro), and afterwards each of the reaction mixtures was resolved on SDS–polyacrylamide gels followed by fluorography. Figure 4A shows that immune complexes of HDAC1 or MyoD recovered from cells cultured in GM were almost comparable in their efficiency to deacetylate the labeled histones, whereas immune complexes of MyoD obtained from extracts of cells cultured in DM showed noticeably minimal activity. Incidentally, the HDAC activity that was found in association with these immune complexes was specific because of its sensitivity to trichostatin A (TSA), an inhibitor of deacetylase activity (data not shown).
Fig. 4. Histone deacetylase activity associates with MyoD in undifferentiated cells, and HDAC1 alone can deacetylate MyoD in vitro. (A) Immunoprecipitates of MyoD or HDAC1 were recovered from extracts of C2 cells cultured in either GM or DM for 96 h using the indicated antibodies. The precipitates were then separately added to [14C]acetate-labeled histones, which were previously acetylated in vitro by FLAG-p300 in the presence of [14C]acetyl-CoA. The deacetylase assays were performed as described in Materials and methods, and the reaction mixtures were then separated by 18% SDS–PAGE and visualized by fluorography. The intensity of the bands on the gel was quantified by using NIH Image software, and the values were then normalized with respect to the control antibody (NR IgG). Results are expressed as a relative percentage. (B) GST–MyoD was incubated with purified FLAG-P/CAF in the presence of [14C]acetyl-CoA, captured by glutathione–agarose beads, and then separately incubated with the following: FLAG-HDAC1, FLAG-H141A or without either. Deacetylase reactions were separated by 10% SDS–PAGE, stained with Coomassie Blue (bottom panel) and then treated accordingly (see Materials and methods) for viewing by fluorography.
Having established that endogenous MyoD is associated with deacetylase activity in undifferentiated cells, we wanted to determine whether an acetylated form of MyoD could serve as a substrate for HDAC1 in vitro. For this analysis, we expressed and immunopurified from Jurkat T cells a FLAG-epitope-tagged HDAC1 protein and, in addition, a mutant version of HDAC1 (H141A). H141A has been shown previously to carry a mutation that completely abolishes deacetylase activity (Hassig et al., 1998). Equivalent concentrations of each of these proteins, as judged by an HDAC1 western blot (data not shown), were then tested for their ability to deacetylate a GST– MyoD protein, which had been acetylated by P/CAF in vitro. As shown in Figure 4B, the HDAC1 enzyme was fully active in deacetylating a [14C]acetate-labeled MyoD, whereas the enzyme (H141A) bearing the mutation was not. In the same figure, an SDS–polyacrylamide gel stained with Coomassie Brilliant Blue verifies that an equal amount of MyoD protein was used in each of the reactions. It must be noted that although the recombinant versions of HDAC1 and HDAC1 H141A retain the ability to interact with HDAC components (Hassig et al., 1998), it is unlikely that they would be associated with HDAC2 upon their recovery from Jurkat T cells, since these cells express very little, if any, of this deacetylase (Hassig et al., 1998). Therefore, we can not exclude the possibility that HDAC2 might be equally proficient in using an acetylated MyoD as a substrate as well.
Based on the results so far, we conclude that an HDAC1 deacetylase activity associates with MyoD in proliferating myoblasts, but that this situation is essentially reversed in differentiated cells. Moreover, since HDAC1 can specifically deacetylate MyoD in vitro, we strongly believe that the interaction between these two proteins may sustain MyoD in a deacetylated form and therefore may help to orchestrate, at least in part, the silencing of MyoD’s transcriptional activities in undifferentiated cells.
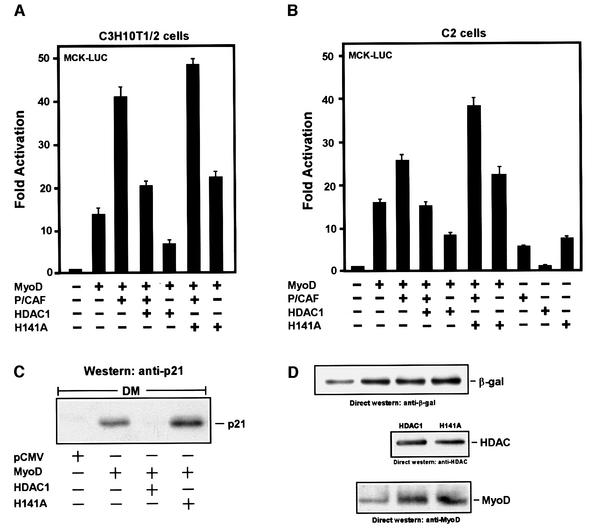
HDAC1 inhibits P/CAF’s ability to augment MyoD-dependent transcription in vivo
In light of MyoD’s association with HDAC1 in undifferentiated muscle cells, we used the mouse embryonic cell line 10T1/2 to determine whether this interaction might be relevant to P/CAF’s ability to potentiate MyoD-dependent transcription. Parenthetically, these cells do not express endogenous MyoD, but have the ability to undergo phenotypic/myogenic conversion after transfection with MyoD (Weintraub, 1993). Therefore, 10T1/2 cells were co-transfected with vectors expressing MyoD, P/CAF, HDAC1 or the HDAC1 H141A mutant along with a naturally occurring muscle creatine kinase (MCK) enhancer element fused to a luciferase reporter gene. To normalize for transfection efficiency and to control for non-specific effects, a β-gal expression plasmid under the control of the CMV promoter was included in each of the transfections. Consistent with previous reports (Puri et al., 1997b), we found that P/CAF could augment MyoD-dependent transcription from the MCK enhancer in DM-cultured cells by at least 3-fold (Figure 5A). In addition, this stimulation was HAT dependent since a P/CAF mutant without its catalytic domain failed to enhance this activity (data not shown). More importantly, however, co-transfection with HDAC1 inhibited P/CAF’s ability to activate MyoD-dependent transcription, and this repression was equally pronounced in the absence of P/CAF. The HDAC1 H141A mutant, on the other hand, not only failed to inhibit MyoD-directed transcription but also stimulated it, regardless of whether P/CAF was ectopically expressed or not. As yet, we can not explain the underlying basis for this stimulation; however, the events associated with this mutant suggest that HDAC1 normally plays a role in silencing the transcriptional activities of MyoD. Finally, HDAC1’s repressive effect on MyoD-dependent transcription was severely compromised when the cells were treated with the histone deacetylase inhibitor TSA, and when transfected alone, the expressions of P/CAF or HDAC1 had no significant effect on the basal transcriptional activity of the enhancer in the absence of MyoD (data not shown).
Fig. 5. HDAC1 inhibits P/CAF’s ability to enhance MyoD-dependent transcription. (A and B) Proliferating 10T1/2 or C2 cells were co-transfected with the luciferase reporter vector and the indicated expression plasmids: pCMV-MyoD, pCMV-P/CAF, pCMV-HDAC1, pCMV-H141A and pCMV-β-Gal. Twenty-four hours post-transfection, the cells were switched to DM for 36 h, harvested, and assayed for reporter gene activity. Values from luciferase assays are expressed as fold activation and represent the average of three independent experiments (error bars, SD) that were normalized to β-galactosidase activity (internal standard). (C) Proliferating 10T1/2 cells were co-transfected with pCMV-β-Gal along with the indicated plasmids. After 24 h, the transfected cells were switched to differentiation medium (DM) and 48 h later the cells were harvested. Extracts from each of the transfected cells were adjusted for β-Gal activity and equivalent levels were then separated by 10% SDS–PAGE. Endogenous p21 was detected by using western blot analysis with an antibody specific for this protein. (D) The same amount of extracts as described in (C) were analyzed by western blotting for equivalent expression of β-Gal, FLAG-epitope-tagged HDAC1, H141A and MyoD, using antibodies specific for each of these proteins.
For relevancy, we thought it important to perform the above experiments in C2 muscle cells, and as detailed in Figure 5B the results turned out to be strikingly similar. Thus, the fact that HDAC1 and P/CAF can also affect the transcriptional behavior of an endogenously expressed MyoD argues strongly that their functions are authentic, at least in these assays. Taken together, these results strongly support the notion that both P/CAF and HDAC1 play important roles in controlling the transcriptional behavior of MyoD in muscle cells.
HDAC1 can affect MyoD’s ability to stimulate the endogenous promoter of p21
The experiments described above indicate that HDAC1 can compromise MyoD’s ability to promote transcription from a transfected plasmid containing the MCK enhancer. However, since plasmid DNA does not completely recapitulate the native chromatin structure (Jeong and Stein, 1994), it was important to determine whether HDAC1 could also inhibit MyoD’s capacity to activate transcription from an endogenous gene, and most appropriately, p21. Indeed, this non-muscle gene has previously been shown to be upregulated by MyoD in response to muscle cell differentiation (Halevy et al., 1995). Further more, although the p21 promoter contains multiple E-box elements to affect the binding of MyoD (Novitch et al., 1999), it importantly does not have MEF2 binding sites (data not shown). Therefore, a control plasmid or a plasmid encoding MyoD was transfected into 10T1/2 cells, alone or in combination with a vector expressing either HDAC1 or H141A, and afterwards the cells were switched to DM. As an internal control, the pCMV-β-gal expression vector was also included in each transfection, and after the cells were harvested the levels of β-gal activity in each of the samples were determined, accordingly. These values were then used as a basis for adjusting each of the extracts in order to reflect equivalent expression of the transfected genes of interest (Figure 5D). Afterwards, the adjusted extracts were analyzed in parallel on an SDS–polyacrylamide gel. As can be seen in Figure 5C, MyoD was quite capable of activating transcription from the endogenous p21 gene in the adjusted extract, as judged by western analysis. Co-expression with HDAC1, however, overcame MyoD’s imposed upregulation of p21, and not surprisingly this effect did not occur when using the HDAC1 H141A mutant. These observations reinforce the previous conclusions that HDAC1 can inhibit MyoD in activating transcription; however, most importantly, they suggest that this activity can occur in the context of chromatin architecture.
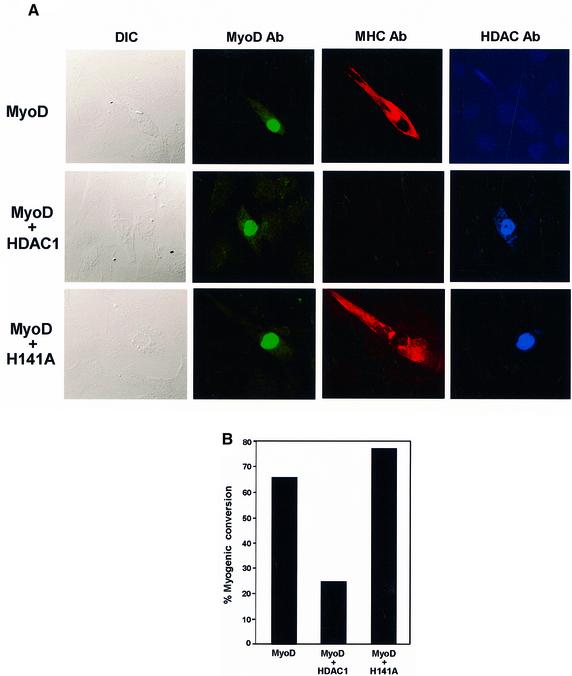
HDAC1 can inhibit myogenic conversion but the H141A mutant can not
The myosin heavy chain (MHC) is a muscle-specific protein, and because it is expressed only in differentiated cells it is often used as a phenotypic marker for myogenic conversion (Olson, 1992). We used MHC in conjunction with confocal microscopy to help us investigate whether HDAC1 could specifically affect MyoD’s function in initiating myogenesis in 10T1/2 cells. Cells were transfected with a MyoD expression vector either alone or in combination with vectors encoding HDAC1 or the mutant version, HDAC1 H141A. After 48 h in differentiation medium, the cells were fixed and immunostained with antibodies specific for MyoD (green), MHC (red) and HDAC (blue). As shown in Figure 6A, cells that were efficiently transfected with MyoD alone stained positive for both MyoD and MHC, and perhaps more importantly they displayed the beginnings (i.e. elongation) of morphological differentiation, which would eventually culminate in the formation of multinucleated myotubes. In striking contrast, although the cells that were co-transfected with MyoD and HDAC1 stained positive for both of these proteins, there was no evidence of staining for MHC, nor was there any change in the morphology of the cells. The integrity of this effect was confirmed by the fact that cells co-transfected with HDAC1 H141A and MyoD also stained positive for MHC and showed signs of differentiation. These results, together with those presented in a histogram (Figure 6B), provide strong support for the idea that HDACs are indeed functionally relevant in retaining MyoD in a deacetylated form, at least in the context of its ability to promote the myogenic program in non-muscle cells.
Fig. 6. HDAC1 inhibits myogenic conversion of fibroblasts to muscle cells. (A) 10T1/2 cells grown on glass coverslips were transfected in parallel with the indicated plasmids. Twenty-four hours later, the transfected cells were switched to DM, and after 48 h the cultures were fixed and immunostained with a rabbit polyclonal antibody specific for MyoD (green), a mouse monoclonal antibody specific for MHC (red) and a goat polyclonal antibody specific for HDAC1 and mutant version H141A (blue). Confocal fluorescence microscopy of random fields of cells was performed accordingly (see Materials and methods), and the left-most panels represent corresponding fields obtained by differential interference contrast (DIC) microscopy. (B) The histogram depicts the percentage of cells undergoing myogenic conversion as described in Materials and methods. The experiments were performed three separate times with comparable results.
Discussion
The fact that MyoD is expressed in proliferating myoblasts prior to terminal differentiation leads one to think that there must be mechanisms in these cells that restrain MyoD from activating muscle-specific genes. The experiments reported here provide the first direct evidence that HDAC complexes may be directly involved in controlling such an activity. Together, the functional and biochemical data obtained are consistent with the proposal that HDACs may act to sustain MyoD in a deacetylated and transcriptionally repressed form until muscle cells are induced to differentiate. Once this occurs, MyoD is converted to an acetylated and transcriptionally active form, a process most likely mediated by P/CAF. In this respect, therefore, it appears that acetylation and deacetylation may be functionally linked in controlling the transcriptional activities of MyoD, with consequences of having either a negative or a positive effect on genes that are specific to the myogenic process.
Previous experiments have suggested a role for mammalian mSin3-HDAC or NuRD complexes in mediating transcriptional repression, primarily by modulating the structure of chromatin. As such, these complexes contain the enzymes HDAC1 and HDAC2, which principally catalyze the deacetylation of histones in order to impede the assembly or recruitment of transcriptional activators to their respective promoters (Ng and Bird, 2000). However, evidence now indicates that deacetylases can also affect the behavior of several transcription factors. For example, the activity of Sp1 in mediating transcriptional repression rather than activation appears to be regulated by HDAC1 (Doetzlhofer et al., 1999). The p53 protein uses HDAC complexes to repress transcription in vivo by physically associating with mSin3 at the promoters of specific genes (Murphy et al., 1999). Intriguingly, although p53 can be acetylated in vivo (Gu and Roeder, 1997; Sakaguchi et al., 1998; Liu et al., 1999; Martinez-Balbas et al., 2000), there is as yet no evidence that p53 itself is a direct target for deacetylases. Finally, the E2F1 transcription factor appears to be negatively regulated by deacetylases as well; in fact, HDAC1 in association with Rb can deacetylate E2F1 in vitro (Martinez-Balbas et al., 2000).
In light of the observations mentioned above, a key point apparent from the data presented here is a role for HDAC complexes that may go beyond the deacetylation of histones and involve the direct deacetylation of MyoD, at least in undifferentiated muscle cells. This hypothesis is compatible with our demonstration that MyoD can bind directly to HDAC1 and act as a substrate for this enzyme following its acetylation in vitro (Figures 3 and 4). Interestingly, the site on MyoD with which HDAC1 appears to interact is the bHLH domain, a region necessary for converting 10T1/2 fibroblasts into muscle cells (Lassar et al., 1994) and which includes lysines that can be acetylated (Sartorelli et al., 1999; Polesskaya et al., 2000). Equally important is our observation that MyoD can be found in association with histone deacetylase activity in undifferentiated rather than in differentiated muscle cells, and this finding, together with the aforementioned results, is in excellent accord with MyoD’s inability to activate transcription in proliferating myoblasts. It is worth noting that the histone deacetylase activity that was found in association with MyoD turned out to be sensitive to treatment with the TSA inhibitor, indicating therefore that the enzymatic activity was being contributed by HDAC, and not by any other factor. The likelihood of HDAC1 specifically mediating this activity is supported by the fact that this protein can stably interact with MyoD in undifferentiated cells; nonetheless, we still can not exclude the possibility of HDAC2 contributing to this activity as well. Although the exact composition of the MyoD– HDAC complex has yet to be defined, we can tentatively rule out the inclusion of Rb, only because its presence could not be detected in immune complexes of MyoD recovered from extracts of undifferentiated muscle cells (A.Mal, unpublished data), a result consistent with that of previously published work (J.-M.Zhang et al., 1999b). Although we have no direct proof, this leads us to believe that Rb is most likely uninvolved in recruiting HDAC1 to MyoD-containing promoters, as in the case of E2F1 (Brehm et al., 1998; Luo et al., 1998; Magnaghi-Jaulin et al., 1998).
A direct relationship between MyoD and HDAC complexes is also underlined by the fact that HDAC1 can effectively inhibit MyoD-mediated transcriptional activation in differentiated model systems. Accordingly, when 10T1/2 or C2 cells were co-transfected with MyoD or MyoD together with P/CAF, HDAC1 was highly efficient in repressing transcription from a reporter gene with the MCK enhancer, whereas an enzymatically compromised mutant version of HDAC1 (Hassig et al., 1998) was not. Moreover, very similar findings were observed when a reporter plasmid containing synthetic E-boxes (4RE-Luc) was used instead, and in this case, HDAC1’s ability to inhibit MyoD’s activity was at least 6-fold (data not shown). Importantly, this result demonstrates that a MyoD–HDAC1 complex can function uniquely in repressing transcription from a regulatory element that does not contain an MEF2 binding site, unlike MCK. Finally, we do not believe that any of these results are peculiar to the chromatin packaging of transfected DNA, since HDAC1 was found to act similarly in the context of native chromatin. Specifically, the MyoD-mediated activation of the endogenous p21 gene was dramatically silenced in 10T1/2 cells after co-transfection with HDAC1, but again not with the H141A mutant. The fact that exogenous MyoD can increase the levels of endogenous p21 in 10T1/2 cells is consistent with previous studies (Guo et al., 1995; Halevy et al., 1995; Parker et al., 1995).
Ectopic expression of MyoD in 10T1/2 cells also leads to the induction of myogenin and skeletal muscle-specific genes (Davis et al., 1987), and this work strongly suggests that a transfected HDAC1 can repress MyoD-dependent transcription of these other genes as well. Indeed, results obtained by confocal microscopy demonstrate quite convincingly that HDAC1, in contrast to the H141A mutant, can prevent MyoD from converting these cells to a myogenic phenotype, as judged by the lack of MHC expression and the formation of myotubes. As such, we believe that these findings, together with those reported above, are particularly important for the following reasons. Foremost, this is the first evidence that HDAC1 may in fact be regulating the acetylation levels of MyoD, and presumably that of any histone protein that may be functionally linked to MyoD-dependent promoters. Such a regulatory mechanism, under the proper conditions, would undoubtedly shift the balance between MyoD’s ability to activate or repress transcription, and thereby be functionally relevant to processes of muscle cell differentiation, at least in an in vitro model system. However, during the preparation of this manuscript, others described a situation whereby it appeared that only the deacetylases HDAC4 and HDAC5 could affect MyoD’s ability to initiate myogenesis, specifically by repressing MEF2 activity (Lu et al., 2000). We suspect that this discrepancy originates in part from the way in which the two studies were conducted systematically. Nonetheless, the MEF2 factor has been shown to bind HDAC4 (Miska et al., 1999), and since MEF2-regulated genes are activated in a temporal fashion during differentiation (Naya and Olson, 1999), it is reasonable to assume that MEF2 could indeed be a target for HDAC4- or HDAC5-mediated repression throughout this process. However, we would argue that the repression of MEF2-regulated genes by HDAC4 and HDAC5 does not necessary preclude the possibility of MyoD’s transcriptional activities being controlled in part by HDAC1, as demonstrated here, especially while muscle cells remain in a proliferating state.
A role for acetylation in regulating MyoD’s transcriptional activities with respect to myogenic conversion has been demonstrated recently (Sartorelli et al., 1999), and our data, alongside those from previous experiments (Puri et al., 1997b), strongly indicate that the histone acetyltransferase P/CAF may be responsible for this modification. For instance, although P/CAF is relatively abundant in proliferating myoblasts, it chooses not to interact with MyoD until the cells have been induced to differentiate, and this association continues throughout the myogenic process (Figure 2). Perhaps more importantly, the kinetics of the MyoD–P/CAF interaction coincides with the time when MyoD begins to show evidence of acetylation (data not shown), providing further proof of P/CAF’s role in modifying this protein during myogenesis. That an exogenously expressed MyoD can become highly acetylated in differentiated cells has also been described recently (Sartorelli et al., 1999). To some extent, this also occurs when MyoD is forcibly expressed to a high level in proliferating myoblasts (Sartorelli et al., 1999). The shifting of the equilibria within the deacetylase/acetylase pool because of the forced expression of MyoD offers a possible explanation for the latter effect. Arguably, however, our findings strongly indicate that endogenous MyoD is not acetylated in proliferating myoblasts, in contrast to what was recently described by others (Polesskaya et al., 2000). Finally, although acetylation may be regulating MyoD’s ability to activate transcription, it is conceivable that other forms of modifications may also be important to this process. Indeed, our studies suggest that a hypophosphorylated rather than a hyperphosphorylated form of MyoD may be more subject to acetylation as cells undergo differentiation. In this respect, it is interesting that during the early phases of myogenic differentiation, the cdk inhibitor p57Kip2 increases markedly and that this in turn results in the accumulation of a hypophosphorylated MyoD (Reynaud et al., 1999). As such, this could act as a signal for the reshuffling of deacetylases and acetylases relative to MyoD’s modification and subsequent activity. Thus, the possibility of phosphorylation and acetylation being used in combination to alter the transcriptional function of MyoD is a notion that is currently being investigated.
Apart from P/CAF, a role for the histone acetyltransferase p300/CBP in helping MyoD to promote myogenic differentiation has also been suggested. This assumption is based primarily on p300/CBP’s ability to augment MyoD-dependent transcription from E-box-containing reporter genes and to bind directly to MyoD in vitro (Eckner et al., 1996; Yuan et al., 1996; Puri et al., 1997a; Sartorelli et al., 1997). Definitive proof, however, of a direct interaction between these two proteins in myogenic cells has yet to be established. We note that the use of an antibody specific for p300 has enabled others to establish in differentiated cells complexes of p300/CBP containing either MyoD or P/CAF (Yuan et al., 1996; Puri et al., 1997b). A contrasting situation is seen, however, when immune complexes of MyoD from differentiated cells are analyzed, in that the contents appear to be restricted to P/CAF (Figure 2; Puri et al., 1997b) since p300/CBP can not be detected, at least under these conditions (Eckner et al., 1996; A.Mal, unpublished data). In considering these results, together with the fact that P/CAF can interact directly with p300/CBP in vivo (Yang et al., 1996; Korzus et al., 1998), it is not unreasonable to suggest that P/CAF may be functioning at times as a bridging molecule between MyoD and p300/CBP, at least in vivo. Indeed, this possibility would be consistent with experiments indicating that the HAT activity of P/CAF, and not p300, is required for MyoD-dependent transcription in non-muscle cells (Puri et al., 1997b).
That MyoD can associate with two acetylases in vivo, yet be a substrate for only one in vitro (Figure 1; Sartorelli et al., 1999), is not unprecedented, since very similar findings have been seen with the erythroid Krüppel-like factor (EKLF), a transcriptional activator that interacts with P/CAF and p300/CBP in vivo but is acetylated only by p300 in vitro (Zhang and Bieker, 1998). Although our in vitro experiments demonstrate an ability of p300 to specifically acetylate histones, it does not have the capability to acetylate MyoD efficiently, whereas P/CAF can perform this activity effectively (Figure 1). We do recognize, however, that others have provided evidence of p300 being capable of acetylating MyoD in vitro (Polesskaya et al., 2000). In view of this, it is perhaps important to point out that we have on occasions observed this as well, but only after viewing an autoradiogram that had been exposed for an extreme length of time; in fact, 14 times longer than for P/CAF. Nevertheless, the significance of these apparently contradictory findings awaits further study, and whether there will be circumstances in which p300/CBP plays a uniquely important role in acetylating MyoD in vivo is yet to be seen.
Finally, it must be noted that previous studies have shown that sodium butyrate, an inhibitor of histone deacetylases, can inhibit muscle cell differentiation by interfering with the functions of MyoD and myogenin (Johnston et al., 1992), but, conversely, inhibit cell proliferation and stimulate the differentiation of colonic epithelial cells. This inconsistency might relate to the fact that butyrate is rather unspecific and can affect some other enzymes (e.g. alkaline phosphatase and dipeptidyl peptidase IV) as well (Kruh, 1982; Siavoshian et al., 2000). Equally important is the fact that butyrate can also stimulate—independently of its effect on HDACs—the expression of cyclin D (Siavoshian et al., 1997, 2000), a cell cycle protein that when over expressed can in fact inhibit myogenesis (Skapek et al., 1995, 1996).
In conclusion, our results establish a novel mechanism for explaining, in part, the temporal control of MyoD-mediated transcription in skeletal muscle cells. Apart from this, our findings also provide the first example of a non-histone transcriptional regulator that can perhaps serve as a substrate for both HAT and HDAC activities in vivo, and thereby strengthen the notion that these enzymes are not limited to modifying histones to effect gene activation.
Materials and methods
Tissue culture and transfection
C3H10T1/2 mouse fibroblasts and simian virus 40 (SV40) large T-antigen (T-Ag)-transformed Jurkat T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with antibiotics and 10% fetal bovine serum (FBS). C2C12 (C2) skeletal muscle cells (kindly provided by N.Rosenthal, Massachusetts General Hospital) were also maintained in DMEM supplemented with antibiotics, but with 20% FBS (GM). To induce differentiation, C2 myoblasts were switched to DM consisting of DMEM containing 2% horse serum and 10 µg/ml insulin (Halevy et al., 1995; Parker et al., 1995). Cytosine arabinoside (10 µM) was added 12 h later for a period of 36 h to eliminate proliferating, non-differentiating myoblasts (Guo et al., 1995). When cultured under these conditions, the C2 cells begin to form morphologically and biochemically differentiated myotubes within 24 h, as previously reported (Neville et al., 1997). A monoclonal antibody (MF20), which specifically stains sarcomeric skeletal MHCs, was used in immunofluorescence to quantitate the extent of differentiation in these cells (Andres and Walsh, 1996). After incubation in DM for 96 h, the percentage of MHC-positive cells was usually >95%. Cultures of 10T1/2 cells were transfected by the Lipofectamine (Gibco-BRL) or calcium phosphate co-precipitation method (Promega), and SV40 T-Ag Jurkat T cells by the Superfect procedure (Qiagen). All transfections were carried out according to the manufacturers’ instructions. Transfection of C2 myoblasts was performed according to the calcium phosphate co-precipitation protocol as described previously (Mal et al., 2000). Briefly, after 16 h in medium containing the precipitated DNA, the cells were washed and incubated in fresh DMEM containing either 10 or 20% FBS for an additional 24 h. Afterwards, the cells were switched to DM for 36–48 h to induce differentiation.
Expression vectors and purification of protein
The following plasmids (carrying an SV40 origin of replication) have been described previously: pBJ5/HDAC1-F and pBJ5/H141A-F encode a FLAG-epitope-tagged wild-type and a mutant version H141A of HDAC1, respectively (Hassig et al., 1998); pCSA-MyoD expresses full-length MyoD from the CMV promoter (Skapek et al., 1996); (–650)MCK-Luc contains the MCK promoter and enhancer (Guo and Walsh, 1997); pCI-P/CAF encodes FLAG-epitope-tagged P/CAF (Yang et al., 1996) from the CMV promoter; and pGEX-MyoD (constructed and kindly provided by A.Lassar) and pGEX-Rb (Ewen et al., 1993) encode GST–MyoD and GST–Rb fusion proteins, respectively. pGEX expression vectors encoding mutant GST–MyoD fusion proteins lacking the acidic N-terminus (DM:3–56), the cysteine-histidine-rich region (DM:63–99), the highly basic region (DM:102–135), the HLH or the myc homology region (DM:143–162), the C-terminal half of the protein (TM:167–318), or a fusion protein containing only the basic and HLH region of MyoD (amino acids 102–166) have also been described previously (Lassar et al., 1989). Recombinant baculoviruses expressing FLAG-epitope-tagged p300 (965–1810) with a HAT domain, p300 (Δ1603–1653) with a mutagenized HAT domain, wild-type P/CAF and P/CAF (Δ609–624) with a mutagenized HAT domain have been described previously (Ogryzko et al., 1996; Yang et al., 1996). pcM/HDAC1-F and pcM/H141A-F, encoding FLAG-epitope-tagged HDAC1 and H141A from the CMV promoter, respectively, were constructed by subcloning the NotI–EcoRI fragment of pBJ5/HDAC1-F or pBJ5/H141A-F into the expression vector pcDNA3.1(–) obtained from Invitrogen. A vector (CMV-β) expressing β-galactosidase was from Clontech.
FLAG-epitope-tagged HDAC1 (kindly provided by Dr S.L.Schreiber) and FLAG-epitope-tagged p300 or P/CAF, as well as their derivatives, were purified from High-Five insect cells (Invitrogen), as described previously (Ogryzko et al., 1996). Briefly, 48 h post-infection, the cells were harvested and lysed in buffer A [20 mM Tris–HCl pH 8.0, 10% glycerol, 5 mM MgCl2, 0.1% Tween-20, 1 mM phenylmethylsulfonyl fluoride (PMSF)] containing 0.5 M KCl. After centrifugation, the clear supernatant was incubated with anti-FLAG M2 antibody–agarose beads for 1–2 h and subsequently washed in buffer A containing 150 mM KCl. Bound proteins were eluted from the beads in the same buffer containing 0.1 mg/ml FLAG-peptide, resolved by SDS–PAGE, and examined by Coomassie Blue staining or western blotting for purity. Eluted proteins were stored at –70°C following dialysis in buffer A containing 0.15 M KCl. The recovery of FLAG-epitope-tagged HDAC1 or H141A from extracts of SV40 T-Ag Jurkat T cells was also conducted in a similar manner. Specifically, cells were transfected with either pBJ5/HDAC1-F or pBJ5/H141A-F, and 3.5 h post-transfection the medium was removed and replaced with fresh medium containing 50 µg/ml 12-O-tetra decanoyl-phorbol-13-acetate (PMA) and 10 µg/ml phytohemagglutinin (PHA). After 48 h, cells were harvested, lysed in buffer A, and subjected to immunoaffinity procedures, as described above. Purification of GST–MyoD and GST–Rb was as described previously (Mal et al., 1996). After purification, each protein was dialyzed against buffer [20 mM Tris–HCl pH 8.0, 10% glycerol, 5 mM MgCl2, 1 mM PMSF, 0.5 mM dithiothreitol (DTT), 0.5 mM EDTA] containing 150 mM KCl, prior to storage at –70°C.
Extracts, immunoprecipitations and western blotting
Anti-MyoD (C20 or M-318), anti-HDAC1 (H51), anti-p300 (N15), anti-p21 (C19) and normal rabbit IgG (NR-IgG) were purchased from Santa Cruz Biotechnology. Polyclonal anti-HDAC1 (Bartl et al., 1997) was kindly provided by C.Seiser. Anti-GAPDH and anti-acetyl-lysine antibody were obtained from BioDesign and New England BioLabs, respectively. Antibody to P/CAF (Yang et al., 1996) was generously provided by Y.Nakatani. Anti-β-Gal and the monoclonal anti-FLAG antibody M2 were purchased from Promega and Sigma, respectively. In most cases, whole-cell extracts were prepared by resuspending cells in lysis buffer with freshly added inhibitors (50 mM HEPES–KOH pH 7.0, 150 mM NaCl, 10% glycerol, 1 mM DTT, 1 mM EDTA, 0.1 mM sodium orthovanadate, 5 mM sodium pyrophosphate, 5 mM NaF, 1 mM PMSF, 5 µg/ml aprotinin, 5 µg/ml leupeptin, 2.5 µg/ml pepstatin) containing 0.1% NP-40. After 1 h at 4°C, the cells were sonicated three times for 5 s with 15 s intervals. The lysate was then centrifuged twice at 15 000 g for 30 min at 4°C. Protein concentrations of the supernatants were determined by the Bio-Rad protein assay system, and the supernatants were stored at –70°C. Lysates of C2 cells cultured in GM or DM were prepared as follows: cells were collected by centrifugation and the packed cell volume (pcv) was then measured. Afterwards, the cells were resuspended in 5 pcv of hypotonic buffer (10 mM HEPES–KOH pH 7.9, 10 mM NaCl, 1.5 mM MgCl2, 0.5 mM DTT, 0.5 mM PMSF) and kept on ice for 10 min. Following centrifugation, the swollen pellets were again suspended in hypotonic buffer (2 pcv), and then dounced in a homogenizer until >95% of the cells were lysed with intact nuclei. Nuclei were counted by a hemocytometer, and afterwards the lysate was adjusted to a final concentration of 50 mM HEPES–KOH pH 7.9, 150 mM NaCl, 0.1% NP-40, 2 mM EDTA, 0.5 mM DTT, 5 µg/ml protease inhibitors, 1 mM sodium orthovanadate, 5 mM sodium pyrophosphate and 5 mM NaF. Afterwards, the lysate was sonicated, clarified by centrifugation, and assayed for protein concentration by the Bio-Rad procedure. Protein concentration was expressed as mg/1 × 106 nuclei, and all lysates were subsequently used for immunoprecipitations and western blot analysis. Nuclear extracts of C2 cells cultured in GM or DM were prepared according to the method of Andrews and Faller (1991). Briefly, pelleted cells in microfuge tubes were resuspended in buffer (10 mM HEPES–KOH pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.2 mM PMSF) and allowed to swell on ice for 10 min. After centrifugation, the supernatant was discarded and the pellet resuspended in cold buffer (20 mM HEPES–KOH pH 7.9, 25% glycerol, 1.5 mM MgCl2, 420 mM NaCl, 0.5 mM DTT, 0.2 mM EDTA, 0.2 mM PMSF) for high salt extraction. Cell debris was removed by centrifugation, and the supernatant was stored at –70°C. Immunoprecipitations were carried out as previously described (Mal et al., 2000). For western blot analysis, extracts or immunoprecipitated products were separated by SDS–PAGE followed by liquid transfer to PVDF. Immunodetection was performed by probing the membrane with the primary antibody and then the secondary antibody followed by the ECL method (Amersham). In some cases, the membranes were also probed with anti-GAPDH to check for the loading of equal amounts of protein. It has been shown previously that the levels of GAPDH do not change in C2 cells, whether they are differentiated or not (Hu and Olson, 1990).
In vivo acetylation assays
To detect acetylated MyoD in vivo, C2 cells were cultured in either GM or DM for 24 h and then transferred to the same medium containing 1 mCi/ml [3H]sodium acetate (2–5 Ci/mmol) (NEN Life Science). After 1.5 h, the cells were lysed accordingly (see above), and the resulting extracts immunoprecipitated with either anti-MyoD or NR-IgG. The precipitated products were then resolved on 10% SDS–PAGE gels. Gels containing the [3H]acetate-labeled MyoD were fixed and enhanced by immersing the gels in a commercial fluorography enhancing solution (EN3HANCE, NEN Life Science) (Gu and Roeder, 1997), and after drying the gels were subject to autoradiography at –70°C.
Protein acetylase and deacetylase assays
Protein acetyltransferase assays were performed as previously described (Gu and Roeder, 1997). Reaction mixtures (30 µl) consisting of buffer (50 mM Tris–HCl pH 8.0, 10% glycerol, 1 mM DTT, 0.1 mM EDTA, 1 mM PMSF, 10 mM sodium butyrate) containing 1 µl of [14C]acetyl-CoA (55 mCi/mmol; Amersham) and 1 or 2 µg of protein substrate with either 150 ng of p300 (965–1810), p300 (Δ1603–1653), wild-type P/CAF or P/CAF (Δ609–624) were incubated for 20 min at 30°C. Each reaction was then run on either a 10 or an 18% SDS–polyacrylamide gel. Prior to autoradiography at –70°C, the gels were fixed and enhanced, as described above. Calf thymus histones were purchased from Boehringer Mannheim.
The deacetylase assay was carried out as described previously (Taunton et al., 1996). After heating for 5 min at 95°C to inactivate P/CAF acetylase activity (Liu et al., 1999), a [14C]acetate-incorporated MyoD was captured by glutathione–agarose beads. Afterwards, the beads were washed three times in buffer A containing 150 mM KCl, and then twice in deacetylase buffer B (50 mM Tris–HCl pH 8.0, 150 mM NaCl, 10% glycerol). After this, the beads were resuspended in 50 µl of buffer B containing either 500 ng of FLAG-epitope-tagged HDAC1 or mutant version H141A (recovered from SV40 T-Ag Jurkat T cells), and the mixtures were then incubated for 1 h at 37°C. To inhibit deacetylase activity, 400 nM TSA was added to buffer B containing FLAG-epitope-tagged HDAC1 for 10 min prior to the addition of beads with bound [14C]acetyl-MyoD. Each reaction was run on a 10% SDS–polyacrylamide gel, and the gels were then stained with Coomassie Blue, enhanced, and subject to autoradiography at –70°C.
Assay for deacetylase-associated activity
To determine the deacetylase activity in immunoprecipitates of MyoD or HDAC1, equal amounts of cell extracts (1.0 mg) prepared from C2 cells cultured in either GM or DM for 96 h were immunoprecipitated with control antibody (NR-IgG) or antibodies specific for MyoD or HDAC1. After four washes with lysis buffer and three washes with deacetylase buffer B, the precipitates were resuspended in 45 µl of buffer B. A solution (5 µl) containing [14C]acetyl histones, prepared as described above, was then added to each of the mixtures, and the reactions were allowed to proceed for 1 h at 37°C. For inhibition, the reaction mixtures were pre-incubated with TSA (400 nM) for 10 min prior to the addition of [14C]acetyl histones. Each of the samples were then solubilized with SDS–PAGE buffer and resolved on an 18% SDS–polyacrylamide gel. The gels were then fixed, enhanced, dried and subjected to autoradiography at –70°C. The signals of labeled proteins were quantitated by NIH image software after scanning with a MicrotecK apparatus (Model Scan Maker III).
Gene reporter assays and deacetylase inhibition assays
C2 or 10T1/2 cells at 30–40% confluency were co-transfected with a total of 10 µg of DNA by the calcium phosphate co-precipitation method (Promega). After 24 h post-transfection, the cells were switched to DM for 36 h and then lysed in a reporter lysis buffer (Promega). Luciferase activity (Promega Luciferase Assay System) was determined and normalized by the level of β-galactosidase (Promega β-galactosidase Enzyme Assay System), as described previously (Puri et al., 1997b). The plasmids used in the assay were pCSA-MyoD (1.2 µg), pCI-P/CAF (3.0 µg), pcM/HDAC1-F (2.5 µg), pcM/H141A-F (2.5 µg), pCMV-β (0.5 µg) and the LUC reporter gene (0.4 µg).
For determining HDAC1’s effect on MyoD in up-regulating endogenous p21, 10T1/2 cells were transfected with 12 µg of DNA using the lipofectamine reagent (Gibco-BRL). After 24 h post-transfection, the cells were transferred to DM and cultured for 48 h. Afterwards, the cells were lysed in lysis buffer and centrifuged for the collection of clear supernatant. The plasmids used in this experiment were pCSA-MyoD (2.0 µg), pcM/HDAC1-F (3.0 µg), pcM/H141A-F (3.0 µg) and pCMV-β (1.0 µg). The level of β-galactosidase activity in each co-transfection was measured accordingly, and the values were then normalized for equal loading of extracts onto SDS–PAGE gels.
GST pull-down assays
Beads coated with equal molar quantities of GST or GST fusion proteins were incubated in 1 ml of binding buffer (25 mM HEPES–KOH pH 7.4, 250 mM NaCl, 0.5 mM DTT, 0.5 mM EDTA, 0.1% NP-40, 0.5 mM PMSF) containing bovine serum albumin (BSA) (5 mg/ml) for 2 h at 4°C. Afterwards, the beads were washed three times with binding buffer without BSA, and then resuspended in 50 µl of binding buffer with equal molar amounts of baculovirus-expressed FLAG-epitope-tagged HDAC1. After 30 min on ice, the mixture was supplemented with 950 µl of binding buffer and then rocked for 2 h at 4°C. Afterwards, the beads were washed four times with binding buffer, centrifuged, and resuspended in SDS–PAGE sample buffer. The denatured proteins were then resolved by a 10% SDS–polyacrylamide gel for western blot analysis.
Confocal immunofluorescence
10T1/2 cells cultured on glass coverslips were transfected in parallel with pCSA-MyoD (1.0 µg) or pCSA-MyoD (1.0 µg) together with pcM/HDAC1-F (2.5 µg) or pcM/H141A-F (2.5 µg) and an arbitrary amount of control plasmid equal to 5 µg of DNA per transfection. At 24 h post-transfection, the transfected cells were switched to DM for 48 h and then washed extensively with phosphate-buffered saline (PBS) containing 2.5 mM MgCl2. The cells were then fixed in cold methanol (20%), rehydrated with PBS, blocked with PBS containing 3% BSA, washed with PBS containing 0.1% NP-40, and then incubated with a mixture of primary antibodies: anti-MyoD, anti-HDAC1 and anti-MHC at a dilution of 1:25, 1:25 and 1:50, respectively. After 2 h of incubation, the coverslips were washed extensively with 0.1% NP-40 in PBS and then subjected to secondary antibodies raised in donkey and purchased from Jackson ImmunoResearch. These included fluorescein isothiocyanate- conjugated anti-rabbit IgG (1:1000), AMCA-conjugated anti-goat IgG (1:500) and Texas Red-conjugated anti-mouse IgG (1:1500). After 1 h of incubation, the coverslips were washed and then mounted on glass slides. Confocal images were collected using a Leica TCS-SP spectral laser scanning confocal microscope equipped with two argon lon lasers (364 and 488 nm), as well as a krypton/argon lon laser (568 nm). The lens was a PlanApo 63×, 1.32 N.A. oil immersion. Images were exported into Adobe Photoshop for processing and printing. In a field of ∼200 cells transfected with pCMV-MyoD, pCMV-MyoD + HDAC1 or pCMV-MyoD + H141A, the percentage of myogenic conversion was calculated based on MHC expression, as judged by antibody staining.
Acknowledgments
Acknowledgements
We thank A.Lassar for the plasmid constructs pGEX-MyoD and pCSA-MyoD, K.Walsh for (–650)MCK-luc, and S.L.Schreiber for pBJ5/HDAC1-F, pBJ5/H141A-F and baculovirus expressing HDAC1-F. We also thank Dr Judy Drazda for assistance with the confocal microscope. This work was supported by grants from the NIH (GM54014) and American Heart Association.
References
- Andres V. and Walsh,K. (1996) Myogenin expression, cell cycle withdrawal and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J. Cell Biol., 132, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N.C. and Faller,D.V. (1991) A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res., 19, 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold H.-H. and Winter,B. (1998) Muscle differentiation: more complexity to the network of myogenic regulators. Curr. Opin. Genet. Dev., 8, 539–544. [DOI] [PubMed] [Google Scholar]
- Bartl S., Taplick,J., Lagger,G., Khier,H., Kuchler,K. and Seiser,C. (1997) Identification of mouse histone deacetylase 1 as a gowth factor-inducible gene. Mol. Cell. Biol., 17, 5033–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R., Davis,R.L., Lockshon,D., Turner,D.L. and Weintraub,H. (1990) The protein Id: a negative regulator of helix–loop–helix DNA binding proteins. Cell, 61, 49–59. [DOI] [PubMed] [Google Scholar]
- Blackwell T.K. and Weintraub,H. (1990) Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science, 250, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Boyes J., Byfield,P., Nakatani,Y. and Ogryzko,V. (1998) Regulation of activity of the transcription factor GATA-1 by acetylation. Nature, 396, 594–598. [DOI] [PubMed] [Google Scholar]
- Brehm A., Miska,E.A., McCance,D.J., Reid,J.L., Bannister,A.J. and Kouzarides,T. (1998) Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature, 391, 597–601. [DOI] [PubMed] [Google Scholar]
- Brown C.E., Lechner,T., Howe,L. and Workman,J.L. (2000) The many HATs of transcription coactivators. Trends Biochem. Sci., 25, 15–19. [DOI] [PubMed] [Google Scholar]
- Davis R.L., Weintraub,H. and Lassar,A.B. (1987) Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell, 51, 987–1000. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A., Rotheneder,H., Laggar,G., Koranda,M., Kurtev,V., Brosch,G., Wintersberger,E. and Seiser,C. (1999) Histone deacetylase 1 can repress transcription by binding to Sp1. Mol. Cell. Biol., 19, 5504–5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner R., Yao,T.-P., Oldread,E. and Livingston,D.M. (1996) Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev., 10, 2478–2490. [DOI] [PubMed] [Google Scholar]
- Ewen M.E., Sluss,H.K., Sherr,C.J., Matsushime,H., Kato,J.-Y. and Livingston,D.M. (1993) Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell, 73, 487–497. [DOI] [PubMed] [Google Scholar]
- Gu W. and Roeder,R.G. (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell, 90, 595–606. [DOI] [PubMed] [Google Scholar]
- Guo K. and Walsh,K. (1997) Inhibition of myogenesis by multiple cyclin–cdk complexes. J. Biol. Chem., 272, 791–797. [DOI] [PubMed] [Google Scholar]
- Guo K., Wang,J., Andres,V., Smith,R.C. and Walsh,K. (1995) MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol. Cell. Biol., 15, 3823–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Novitch,B.G., Spicer,D.B., Skapek,S.X., Rhee,J., Hannon,G.J., Beach,D. and Lassar,A.B. (1995) Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science, 267, 1018–1021. [DOI] [PubMed] [Google Scholar]
- Harper J.W. and Elledge,S. (1996) Cdk inhibitors in development and cancer. Curr. Opin. Genet. Dev., 6, 56–64. [DOI] [PubMed] [Google Scholar]
- Hassig C.A., Tong,J.K., Fleischer,T.C., Owa,T., Grable,P.G., Ayer,D.E. and Schreiber,S.L. (1998) A role for histone deacetylase activity in HDAC1-mediated transcriptional repression. Proc. Natl Acad. Sci. USA, 95, 3519–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg S.M., Cheng,P.F. and Weintraub,H. (1993) Use of a conditional MyoD transcription factor in studies of MyoD trans-activation and muscle determination. Proc. Natl Acad. Sci. USA, 90, 8028–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.-S. and Olson,E.N. (1990) Functional receptors for transforming growth factor-β are retained by biochemically differentiated C2 myocytes in growth factor-deficient medium containing EGTA but down-regulated during terminal differentiation. J. Biol. Chem., 265, 7914–7919. [PubMed] [Google Scholar]
- Jeong S. and Stein,A. (1994) Micrococcal nuclease digestion of nuclei reveals extended nucleosome ladders having anomalous DNA lengths for chromatin assembled on non-replicating plasmids in transfected cells. Nucleic Acids Res., 22, 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L.A., Tapscott,S.J. and Eisen,H. (1992) Sodium butyrate inhibits myogenesis by interfering with the transcriptional activation function of MyoD and myogenin. Mol. Cell. Biol., 12, 5123–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. et al. (1999) Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity, 10, 345–355. [DOI] [PubMed] [Google Scholar]
- Kitzmann M., Vandromme,M., Schaeffer,V., Carnac,G., Labbe,J.-C., Lamb,N. and Fernandez,A. (1999) cdk1- and cdk2-mediated phosphorylation of MyoD Ser200 in growing C2 myoblasts: role in modulating MyoD half-life and myogenic activity. Mol. Cell. Biol., 19, 3167–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koipally J., Renold,A., Kim,J. and Georgopoulos,K. (1999) Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J., 18, 3090–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E., Torchia,J., Rose,D., Xu,L., Kurokawa,R., Mclnerney,E., Mullen,T., Glass,C. and Rosenfeld,M. (1998) Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science, 279, 703–707. [DOI] [PubMed] [Google Scholar]
- Kruh J. (1982) Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol. Cell. Biochem., 42, 65–82. [DOI] [PubMed] [Google Scholar]
- Lassar A.B., Buskin,J.N., Lockshon,D., Davis,R.L., Apone,S., Hauschka,S.D. and Weintraub,H. (1989) MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell, 58, 823–831. [DOI] [PubMed] [Google Scholar]
- Lassar A.B., Skapek,S.X. and Novitch,B. (1994) Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr. Opin. Cell Biol., 6, 788–794. [DOI] [PubMed] [Google Scholar]
- Lemercier C., To,R.Q., Carrasco,R.A. and Konieczny,S.F. (1998) The basic helix–loop–helix transcription factor Mist1 functions as a transcriptional repressor of myoD. EMBO J., 17, 1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Scolnick,D.M., Trievel,R.C., Zhang,H.B., Marmorstein,R., Halazonetis,T.D. and Berger,S.L. (1999) p53 sites are acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol. Cell. Biol., 19, 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., McKinsey,T.A., Zhang,C.-L. and Olson,E.N. (2000) Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol. Cell, 6, 233–244. [DOI] [PubMed] [Google Scholar]
- Luo R.X., Postigo,A.A. and Dean,D.C. (1998) Rb interacts with histone deacetylase to repress transcription. Cell, 92, 463–473. [DOI] [PubMed] [Google Scholar]
- Ma P., Rould,M.A., Weintraub,H. and Pabo,C.O. (1994) Crystal structure of MyoD bHLH domain–DNA complex: perspectives on DNA recognition and implications for transcriptional activation. Cell, 77, 451–459. [DOI] [PubMed] [Google Scholar]
- Magnaghi-Jaulin L., Groisman,R., Naguibneva,I., Robin,P., Lorain,S., LeVillain,J.P., Troalen,F., Trouche,D. and Harel-Bellan,A. (1998) Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature, 391, 601–604. [DOI] [PubMed] [Google Scholar]
- Maione R. and Amati,P. (1997) Interdependence between muscle differentiation and cell-cycle control. Biochim. Biophys. Acta, 1332, M19–M30. [DOI] [PubMed] [Google Scholar]
- Mal A., Poon,R.Y.C., Howe,P.H., Toyoshima,H., Hunter,T. and Harter,M.L. (1996) The E1A oncoprotein disables the CDK inhibitor p27Kip1 in TGF-β treated cells. Nature, 380, 262–265. [DOI] [PubMed] [Google Scholar]
- Mal A., Chattopadhyay,D., Ghosh,M.K., Poon,R.Y.C., Hunter,T. and Harter,M.L. (2000) p21 and retinoblastoma protein control the absence of DNA replication in terminally differentiated muscle cells. J. Cell Biol., 149, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Balbas M., Bauer,U.-M., NielsenS.J., Brehm,A. and Kouzarides,T. (2000) Regulation of E2F1 activity by acetylation. EMBO J., 19, 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska E.A., Karlsson,C., Langley,E., Nielsen,S.J., Pines,J. and Kouzarides,T. (1999) HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J., 18, 5099–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M., Ahn,J., Walker,K.K., Hoffman,W.H., Evans,R.M., Levine,A.J. and George,D.L. (1999) Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev., 13, 2490–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya F.J. and Olson,E. (1999) MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr. Opin. Cell Biol., 11, 683–688. [DOI] [PubMed] [Google Scholar]
- Neuhold L.A. and Wold,B. (1993) HLH forced dimers: Tethering MyoD to E47 generates a dominant positive myogenic factor insulated from negative regulation by Id. Cell, 74, 1033–1042. [DOI] [PubMed] [Google Scholar]
- Neville C., Rosenthal,N., McGrew,M., Bogdanova,N. and Hauschka,S. (1997) Skeletal muscle cultures. In Emerson,C.P.,Jr and Sweeney,H.L. (eds), Methods in Muscle Biology. Academic Press, New York, NY, pp. 85–114. [PubMed]
- Ng H.H. and Bird,A. (2000) Histone deacetylases: silencers for hire. Trends Biochem. Sci., 25, 121–126. [DOI] [PubMed] [Google Scholar]
- Novitch B.G., Spicer,D.B., Kim,P.S., Cheung,W.L. and Lassar,A.B. (1999) pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr. Biol., 9, 449–459. [DOI] [PubMed] [Google Scholar]
- Ogryzko V.V., Schiltz,R.L., Russanova,V., Howard,B.H. and Nakatani,Y. (1996) The transcriptional coactivators p300 and CBP are histone acetytransferases. Cell, 87, 953–959. [DOI] [PubMed] [Google Scholar]
- Olson E.N. (1992) Interplay between proliferation and differentiation within the myogenic lineage. Dev. Biol., 154, 261–272. [DOI] [PubMed] [Google Scholar]
- Otten A.D., Firpo,E.J., Gerber,A.N., Brody,L.L., Roberts,J.M. and Tapscott,S.J. (1997) Inactivation of MyoD-mediated expression of p21 in tumor cell lines. Cell Growth Differ., 8, 1151–1160. [PubMed] [Google Scholar]
- Parker S.B., Eichele,G., Zhang,P., Rawls,A., Sands,A.T., Bradley,A., Olson,E.N., Harper,J.W. and Elledge,S.J. (1995) p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science, 267, 1024–1027. [DOI] [PubMed] [Google Scholar]
- Polesskaya A., Duquet,A., Naguibneva,I., Weise,C., Vervisch,A., Bengal,E., Hucho,F., Robin,P. and Harel-Bellan,A. (2000) CBP/p300 activates MyoD by acetylation. J. Biol. Chem., 275, 34359–34364. [DOI] [PubMed] [Google Scholar]
- Puri P.L., Avantaggiati,M.L., Balsano,C., Sang,N., Graessmann,A., Giordano,A. and Levero,M. (1997a) p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J., 16, 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P.L. et al. (1997b) Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol. Cell, 1, 35–45. [DOI] [PubMed] [Google Scholar]
- Rao S.S., Chu,C. and Kohtz,D.S. (1994) Ectopic expression of cyclin D1 prevents activation of gene transcription by myogenic basic helix–loop–helix regulators. Mol. Cell. Biol., 14, 5259–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud E.G., Pelpel,K., Guillier,M., Leibovitch,M.P. and Leibovitch,S.A. (1999) p57Kip2 stabilizes the MyoD protein by inhibiting cyclin E–cdk2 kinase activity in growing myoblasts. Mol. Cell. Biol., 19, 7621–7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi K., Herrera,J.E., Saito,S., Miki,T., Bustin,M., Vassilev,A., Anderson,C.W. and Appella,E. (1998) DNA damage activates p53 through a phosphorylation–acetylation cascade. Genes Dev., 12, 2831–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V., Huang,J., Hamamori,Y. and Kedes,L. (1997) Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol. Cell. Biol., 17, 1010–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V., Puri,P.L., Hamamori,Y., Ogryzko,V., Chung,G., Nakatani,Y., Wang,J.Y.J. and Kedes,L. (1999) Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell, 4, 725–734. [DOI] [PubMed] [Google Scholar]
- Siavoshian S., Blottiere,H.M., Cherbut,C. and Galmiche,J.P. (1997) Butyrate stimulates cyclin D and p21 and inhibits cyclin-dependent kinase 2 expression in HT-29 colonic epithelial cells. Biochem. Biophys. Res. Commun., 232, 169–172. [DOI] [PubMed] [Google Scholar]
- Siavoshian S., Segain,J.P., Kornprobst,M., Connet,C., Cherbut,C., Galmiche,J.P. and Blottiere,H.M. (2000) Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut, 46, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skapek S.X., Rhee,J., Spicer,D.B. and Lassar,A.B. (1995) Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science, 267, 1022–1024. [DOI] [PubMed] [Google Scholar]
- Skapek S.X., Rhee,J., Kim,P.S., Novitch,B.G. and Lassar,A.B. (1996) Cyclin-mediated inhibition of muscle gene expression via a mechanism that is independent of pRB hyperphosphorylation. Mol. Cell. Biol., 16, 7043–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A., Wang,Q., Goebl,M.G. and Harrington,M.A. (1998) Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol. Cell. Biol., 18, 4994–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer D.B., Rhee,J., Cheung,W.L. and Lassar,A.B. (1996) Inhibition of myogenic bHLH and MEF2 transcription factors by the bHLH protein twist. Science, 272, 1476–1480. [DOI] [PubMed] [Google Scholar]
- Tapscott S.J., Lassar,A.B., Davis,R.L. and Weintraub,H. (1989) 5-bromo-2′-deoxyuridine blocks myogenesis by extinguishing expression of MyoD1. Science, 245, 532–536. [DOI] [PubMed] [Google Scholar]
- Taunton J., Hassig,C.A. and Schreiber,S.L. (1996) A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science, 272, 408–411. [DOI] [PubMed] [Google Scholar]
- Tintignac L.A., Leibovitch,M.P., Kitzmann,M., Fernandez,A., Ducommun,B., Meijer,L. and Leibovitch,S.A. (2000) Cyclin E–cdk2 phosphorylation promotes late G1-phase degradation of MyoD in muscle cells. Exp. Cell Res., 259, 300–307. [DOI] [PubMed] [Google Scholar]
- Wang J. and Walsh,K. (1996) Inhibition of retinoblastoma protein phosphorylation by myogenesis-induced changes in the subunit composition of the cyclin-dependent kinase 4 complex. Cell Growth Differ., 7, 1471–1478. [PubMed] [Google Scholar]
- Weintraub H. (1993) The MyoD family and myogenesis: redundancy, networks and thresholds. Cell, 75, 1241–1244. [DOI] [PubMed] [Google Scholar]
- Yaffe D. and Saxel,O. (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature, 270, 725–727. [DOI] [PubMed] [Google Scholar]
- Yang X.-J., Ogryzko,V.V., Nishikawa,J., Howard,B.H. and Nakatani,Y. (1996) A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature, 382, 319–324. [DOI] [PubMed] [Google Scholar]
- Yuan W., Condorelli,G., Caruso,M., Felsani,A. and Giordano,A. (1996) Human p300 protein is a coactivator for the transcription factor MyoD. J. Biol. Chem., 271, 9009–9013. [DOI] [PubMed] [Google Scholar]
- Zhang J.-M., Wei,Q., Zhao,X. and Paterson,B.M. (1999a) Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with cdk4. EMBO J., 18, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-M., Zhao,X., Wei,Q. and Paterson,B.M. (1999b) Direct inhibition of G1 cdk kinase activity by MyoD promotes myoblast cell cycle withdrawal and terminal differentiation. EMBO J., 18, 6983–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Wong,C., Liu,D., Finegold,M., Harper,J.W. and Elledge,S.J. (1999) p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes Dev., 13, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. and Bieker,J.J. (1998) Acetylation and modulation of erythroid Krüppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl Acad. Sci. USA, 95, 9855–9860. [DOI] [PMC free article] [PubMed] [Google Scholar]