Abstract
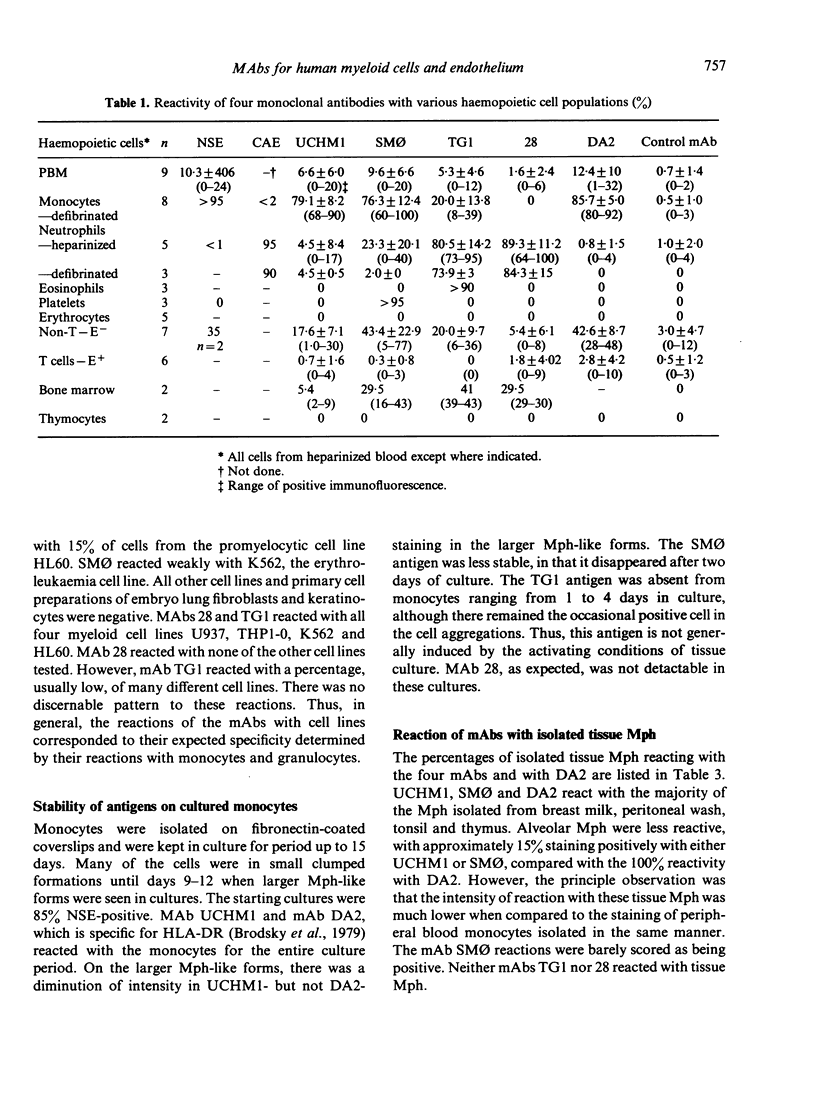
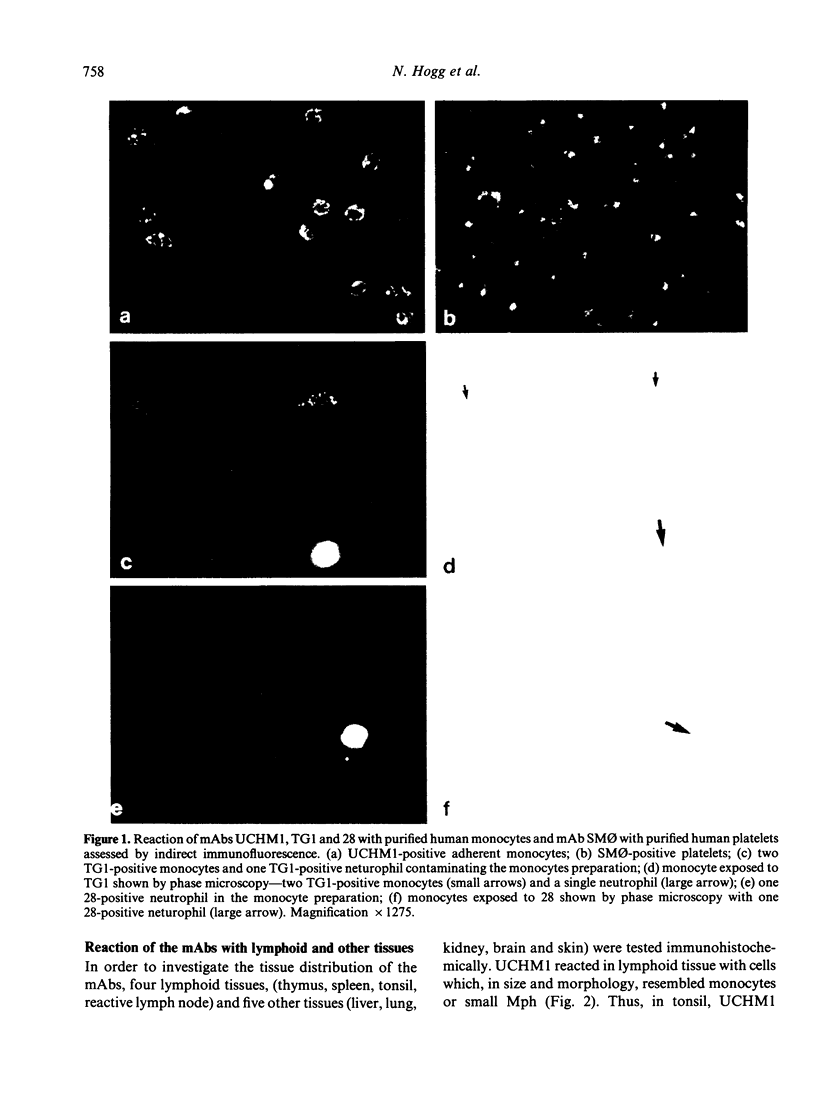
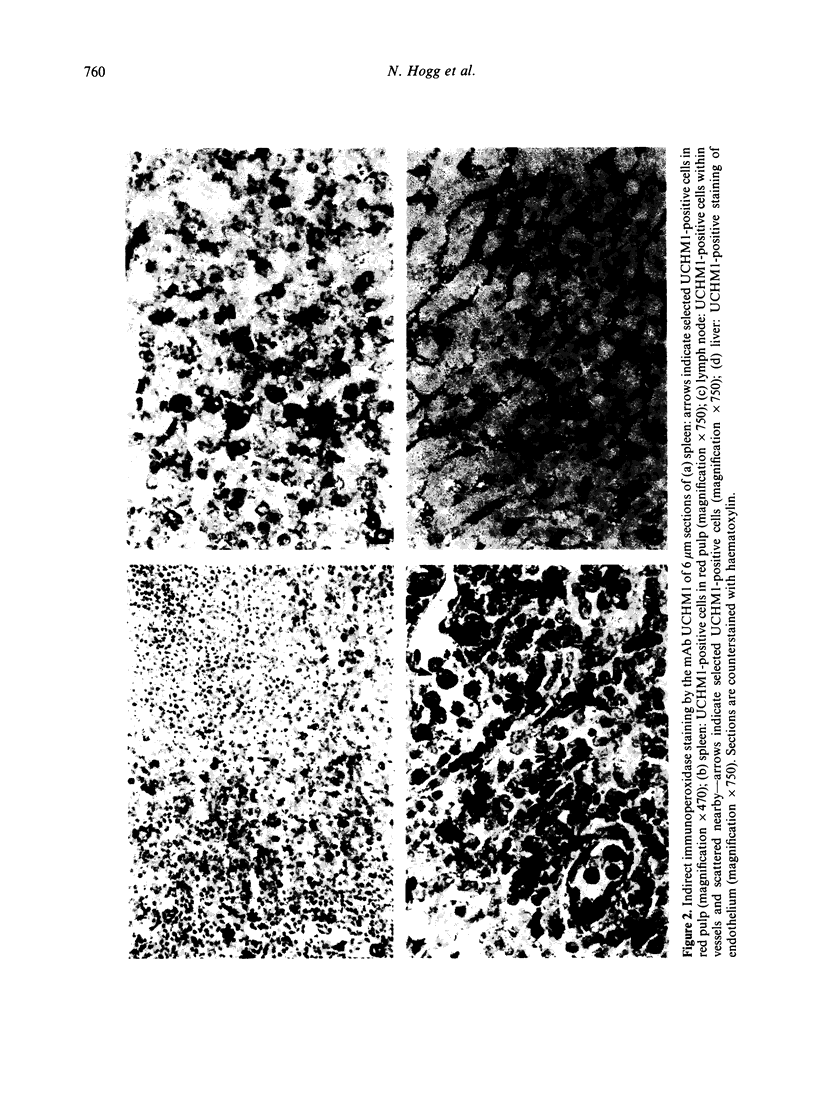
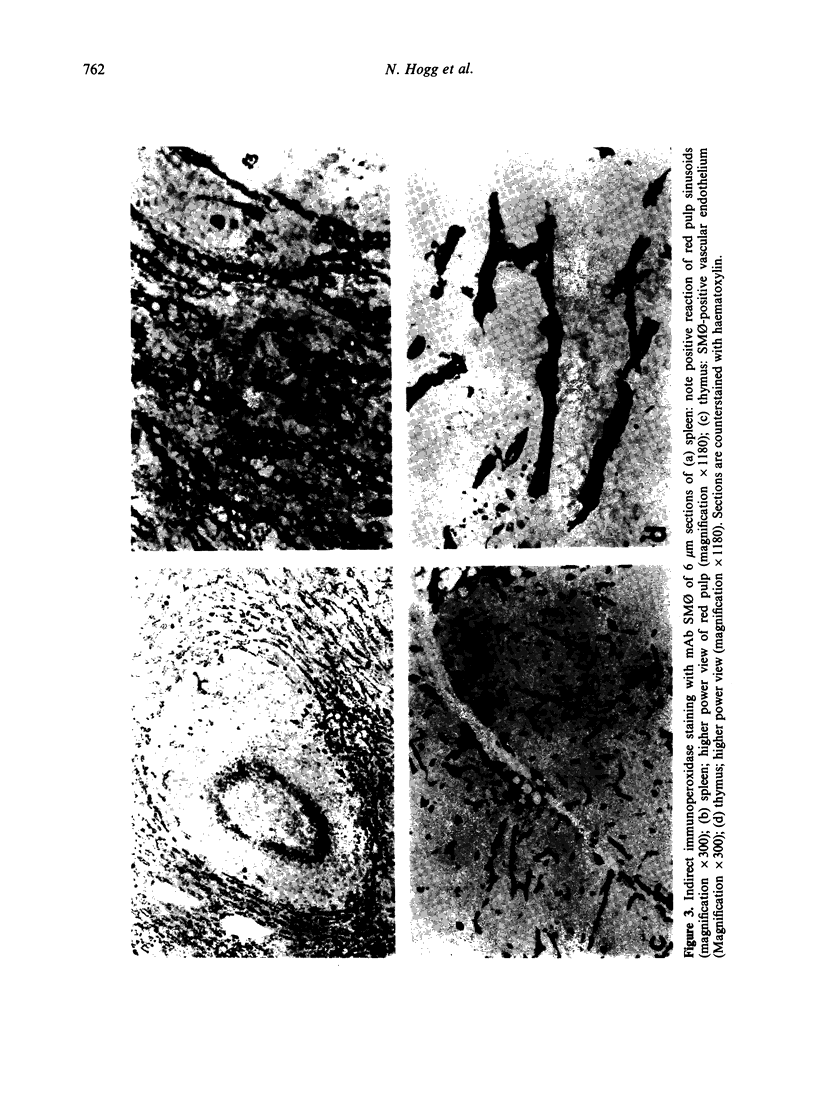
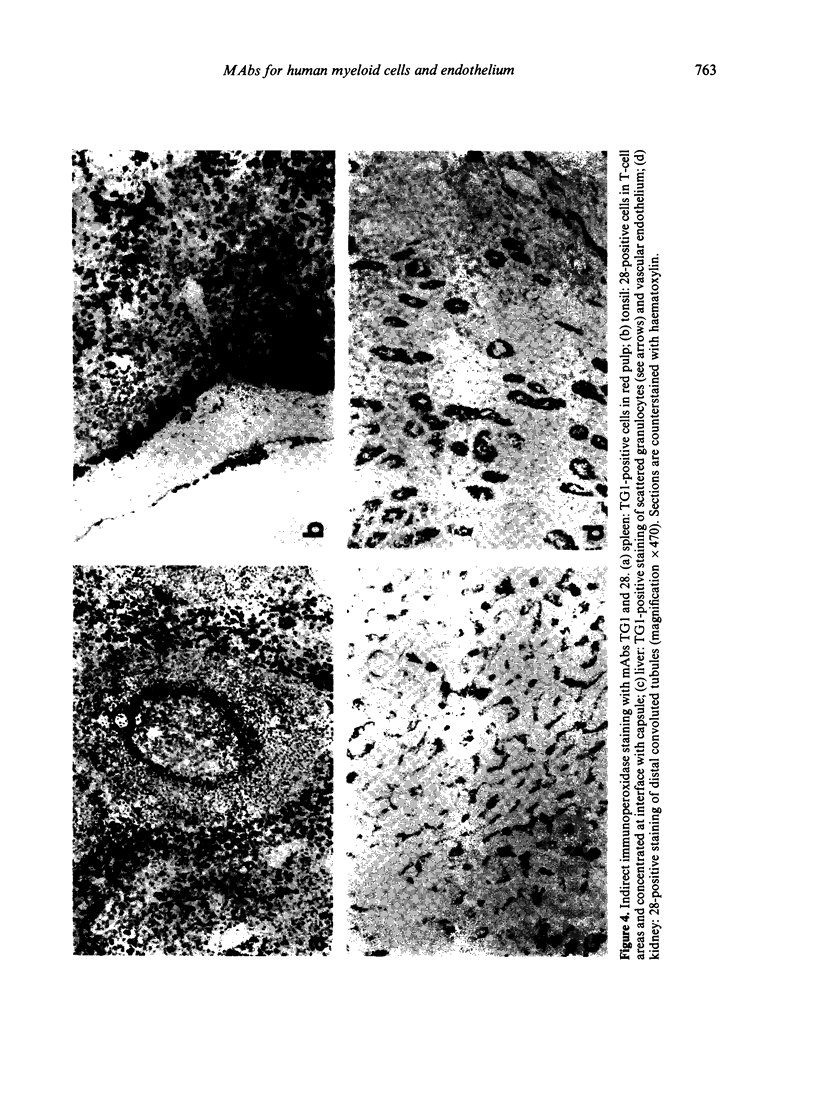
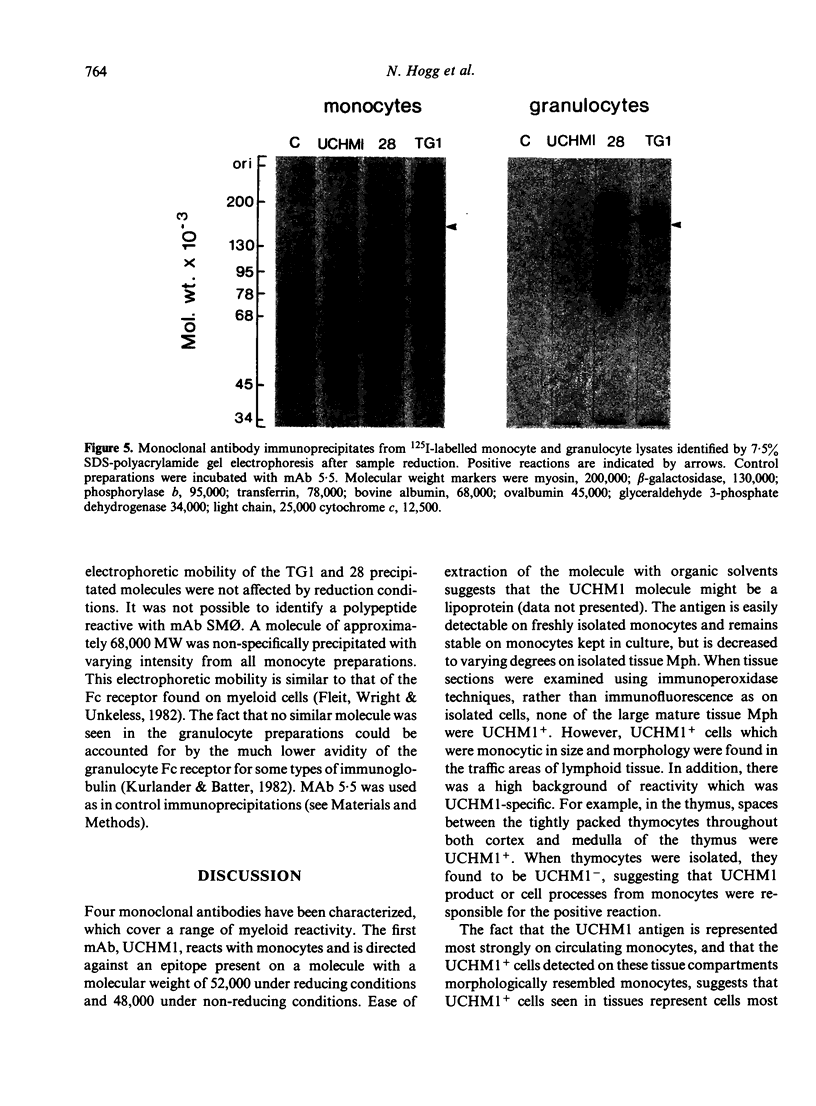
Four monoclonal antibodies against antigens of human myeloid cells have been produced and thoroughly characterized in terms of their reactions with peripheral blood cells, cell lines, nine lymphoid and non-lymphoid tissues and the polypeptides with which they react. UCHM1 and SmO identify antigens present on the majority of blood monocytes and a variable, but lower, proportion of tissue macrophages. From their morphology and location in tissues, these cells appear to be recirculating monocytes. SMO antigen is also present on platelets. In addition, both antibodies stained endothelial cells, SMO in all tissues examined and UCHM1 variably. Biochemical investigation indicated that the UCHM1 antigen is a protein of 52,000 MW while the SMO antigen could not be indentified. The antibodies TG1 and 28 identify antigens mainly present on granulocytes. While mAb 28 reacted with neutrophils, TG1 also stained eosinophils and stained strongly a proportion of monocytes. TG1 also reacted variably with some non-haemopoietic cell lines. Both antibodies reacted predominantly with granulocytes in tissue sections. MAb TG1 precipitated a single polypeptide of 156,000 MW from monocytes and granulocytes, while mAb 28 precipitated non-convalently associated polypeptides of 83,000 and 155,000 MW from granulocytes but only a single molecule from monocytes, corresponding to the lower MW chain of 83,000. The epitope with which mAb 28 reacts appears not to be exposed on the surface of intact monocytes. This suggests that a similar or identical 83,000 MW molecule is made by both neutrophils and monocytes, but that its expression differs according to cell type.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Douglas S. D. Purification of human monocytes on microexudate-coated surfaces. J Immunol. 1978 Apr;120(4):1372–1374. [PubMed] [Google Scholar]
- Alitalo K., Hovi T., Vaheri A. Fibronectin is produced by human macrophages. J Exp Med. 1980 Mar 1;151(3):602–613. doi: 10.1084/jem.151.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Cerilli G. J., Brasile L., Galouzis T., DeFrancis M. E. Clinical significance of antimonocyte antibody in kidney transplant recipients. Transplantation. 1981 Dec;32(6):495–497. doi: 10.1097/00007890-198112000-00009. [DOI] [PubMed] [Google Scholar]
- Dooley D. C., Simpson J. F., Meryman H. T. Isolation of large numbers of fully viable human neutrophils: a preparative technique using percoll density gradient centrifugation. Exp Hematol. 1982 Aug;10(7):591–599. [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Slusarenko M., Cohen J., Reiser J. Monoclonal antibody with specificity for monocytes and neurons. Cell. 1981 Jun;24(3):875–884. doi: 10.1016/0092-8674(81)90113-6. [DOI] [PubMed] [Google Scholar]
- Howard M. A., Montgomery D. C., Hardisty R. M. Factor-VIII-related antigen in platelets. Thromb Res. 1974 May;4(5):617–624. doi: 10.1016/0049-3848(74)90218-7. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W., De los Santos R. P., Hoyer J. R. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest. 1973 Nov;52(11):2737–2744. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Mosher D. F. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978 Jun 1;147(6):1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Kurlander R. J., Batker J. The binding of human immunoglobulin G1 monomer and small, covalently cross-linked polymers of immunoglobulin G1 to human peripheral blood monocytes and polymorphonuclear leukocytes. J Clin Invest. 1982 Jan;69(1):1–8. doi: 10.1172/JCI110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linch D. C., Allen C., Beverley P. C., Bynoe A. G., Scott C. S., Hogg N. Monoclonal antibodies differentiating between monocytic and nonmonocytic variants of AML. Blood. 1984 Mar;63(3):566–573. [PubMed] [Google Scholar]
- Moen T., Moen M., Thorsby E. HLA-D region products are expressed in endothelial cells. Tissue Antigens. 1980 Feb;15(2):112–122. doi: 10.1111/j.1399-0039.1980.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Moraes J. R., Stastny P. A new antigen system expressed in human endothelial cells. J Clin Invest. 1977 Aug;60(2):449–454. doi: 10.1172/JCI108795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng A. K., Zhang Y. H., Russo C., Ferrone S. Immunochemical and functional analysis of Ia-like antigens on human monocyte-macrophages with monoclonal antibodies. Cell Immunol. 1982 Jan 1;66(1):78–87. doi: 10.1016/0008-8749(82)90159-9. [DOI] [PubMed] [Google Scholar]
- Payne C. M. Platelet satellitism: an ultrastructural study. Am J Pathol. 1981 Apr;103(1):116–128. [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Jankiewicz J., Trinchieri G. Binding of platelets to human monocytes: a source of artifacts in the study of the specificity of antileukocyte antibodies. J Immunol Methods. 1982;50(3):269–276. doi: 10.1016/0022-1759(82)90164-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Van Agthoven A., Schlossman S. F., Terhorst C. Structural analysis of differentiation antigens Mo1 and Mo2 on human monocytes. Hybridoma. 1982;1(3):329–337. doi: 10.1089/hyb.1.1982.1.329. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]