Abstract
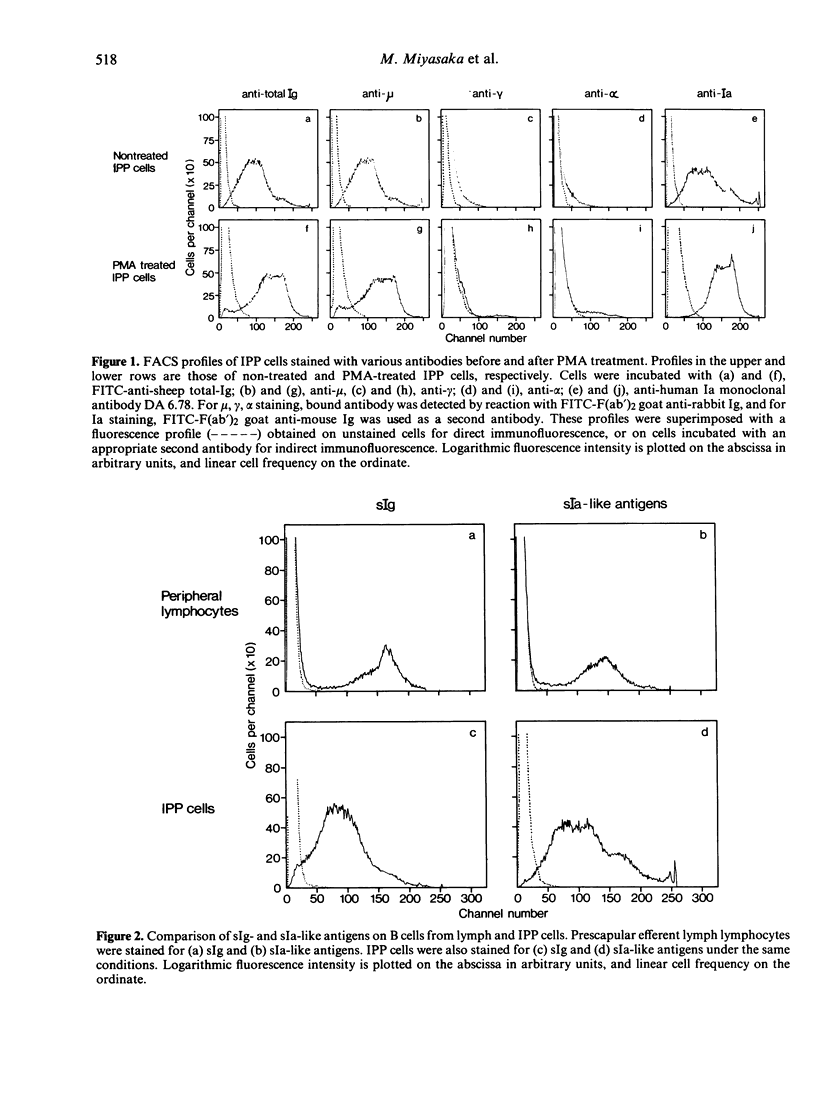
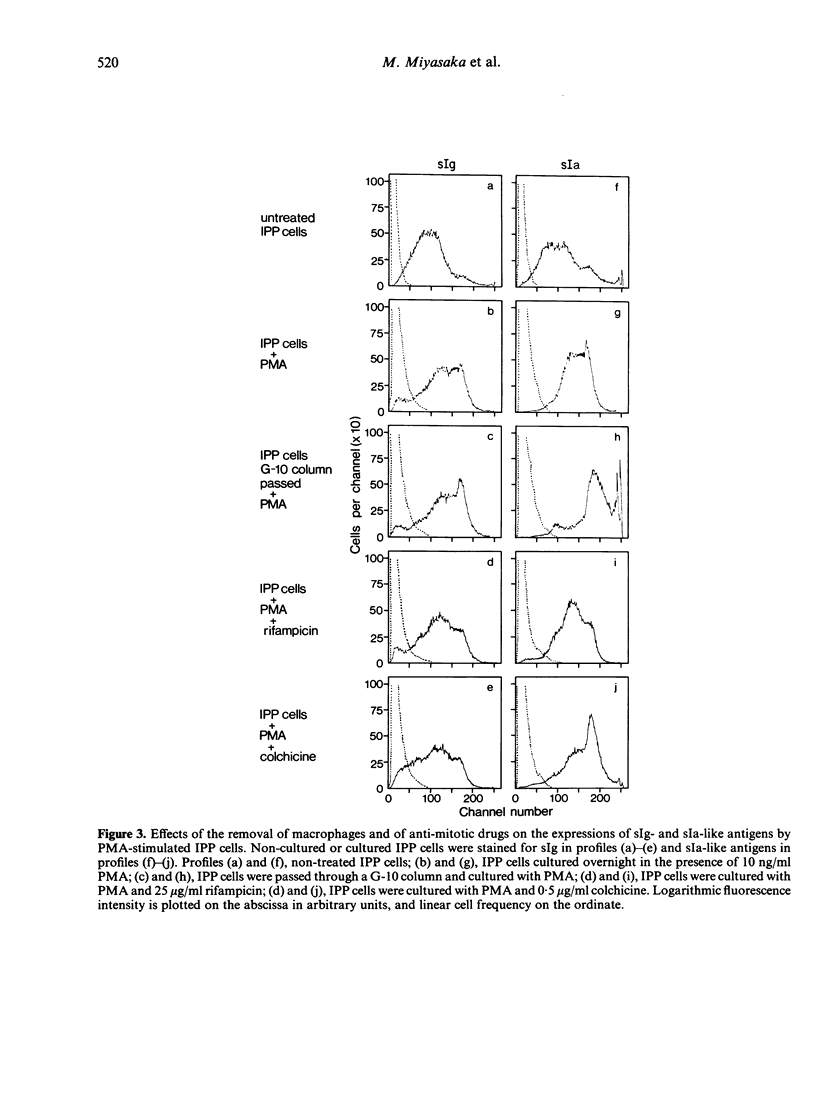
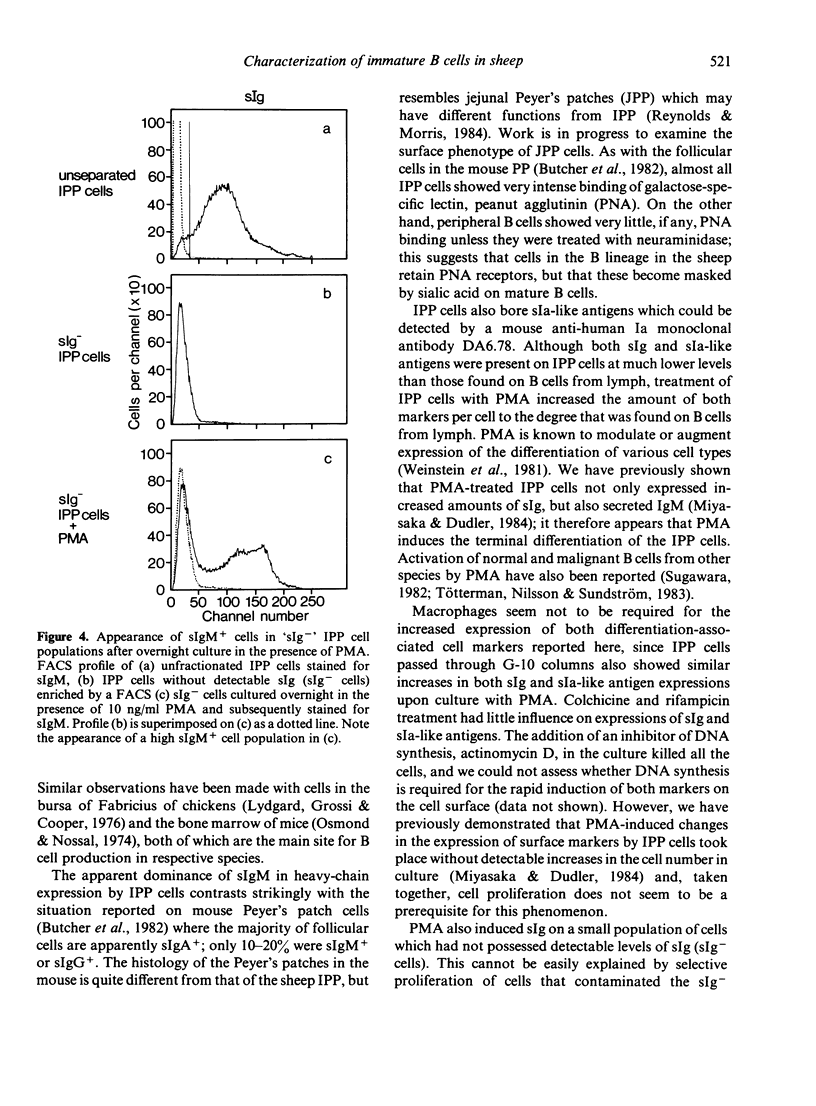
The ileal Peyer's patches (IPP) of sheep may be a primary lymphoid organ for b cells since they have a number of important features in common with the bursa of Fabricius of chickens. We have examined the surface phenotype of IPP cells. Approximately 90% to 95% of IPP cells are 'low sIgM+'; that is, they have surface IgM, but in much smaller amounts than peripheral B cells, which are 'high sIgM+'. IPP cells with sIgG or sIgA are very rare. Upon exposure to a tumour promotor, phorbol myristate acetate (PMA), in vitro, low sIgM+ cells differentiated into high sIgM+ cells. The amount of Ia-like antigens on the surface also increased after PMA treatment. Approximately 5% of IPP cells bore no identifiable markers. However, these cells could also be induced into high sIgM+ cells upon exposure to PMA; this may indicate the presence of precursors of sIgM+ cells within the IPP. While PNA (peanut agglutinin) binds strongly to the vast majority of IPP cells, it binds very little, if at all, to B cells obtained from the periphery, unless they have been treated with neuraminidase; this suggests that cells in the B lineage retain their PNA receptors, but that these become masked by sialic acid on mature B cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher E. C., Rouse R. V., Coffman R. L., Nottenburg C. N., Hardy R. R., Weissman I. L. Surface phenotype of Peyer's patch germinal center cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982 Dec;129(6):2698–2707. [PubMed] [Google Scholar]
- Gathings W. E., Lawton A. R., Cooper M. D. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977 Nov;7(11):804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- Landreth K. S., Rosse C., Clagett J. Myelogenous production and maturation of B lymphocytes in the mouse. J Immunol. 1981 Nov;127(5):2027–2034. [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Lydyard P. M., Grossi C. E., Cooper M. D. Ontogeny of B cells in the chicken. I. Sequential development of clonal diversity in the bursa. J Exp Med. 1976 Jul 1;144(1):79–97. doi: 10.1084/jem.144.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy P. J., Willcox N., Catty D. Early precursors of B lymphocytes. I. Rabbit/mouse species differences in the physical properties and surface phenotype of pre-B cells, and in the maturation sequence of early B cells. Eur J Immunol. 1981 Feb;11(2):76–85. doi: 10.1002/eji.1830110203. [DOI] [PubMed] [Google Scholar]
- McPhee D., Pye J., Shortman K. The differentiation of T lymphocytes. V. Evidence for intrathymic death of most thymocytes. Thymus. 1979 Nov;1(3):151–162. [PubMed] [Google Scholar]
- Miyasaka M., Dudler L. Differentiation of ovine immature B cells upon exposure to phorbol myristate acetate. Int Arch Allergy Appl Immunol. 1984;74(3):281–283. doi: 10.1159/000233559. [DOI] [PubMed] [Google Scholar]
- Miyasaka M., Heron I., Dudler L., Cahill R. N., Forni L., Knaak T., Trnka Z. Studies on the differentiation of T lymphocytes in sheep. I. Recognition of a sheep T-lymphocyte differentiation antigen by a monoclonal antibody T-80. Immunology. 1983 Jul;49(3):545–553. [PMC free article] [PubMed] [Google Scholar]
- Osmond D. G., Nossal G. J. Differentiation of lymphocytes in mouse bone marrow. I. Quantitative radioautographic studies of antiglobulin binding by lymphocytes in bone marrow and lymphoid tissues. Cell Immunol. 1974 Jul;13(1):117–131. doi: 10.1016/0008-8749(74)90232-9. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Megson M., Owen J. J., Cooper M. D. Early production of intracellular IgM by B-lymphocyte precursors in mouse. Nature. 1976 Jan 22;259(5540):224–226. doi: 10.1038/259224a0. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D., Morris B. The effect of antigen on the development of Peyer's patches in sheep. Eur J Immunol. 1984 Jan;14(1):1–6. doi: 10.1002/eji.1830140102. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D., Morris B. The evolution and involution of Peyer's patches in fetal and postnatal sheep. Eur J Immunol. 1983 Aug;13(8):627–635. doi: 10.1002/eji.1830130805. [DOI] [PubMed] [Google Scholar]
- Reynolds J. D., Pabst R. The emigration of lymphocytes from Peyer's patches in sheep. Eur J Immunol. 1984 Jan;14(1):7–13. doi: 10.1002/eji.1830140103. [DOI] [PubMed] [Google Scholar]
- Ryser J. E., Vassalli P. Mouse bone marrow lymphocytes and their differentiation. J Immunol. 1974 Sep;113(3):719–728. [PubMed] [Google Scholar]
- Sugawara I. The immunoglobulin production of human peripheral B lymphocytes induced by phorbol myristate acetate. Cell Immunol. 1982 Sep 1;72(1):88–96. doi: 10.1016/0008-8749(82)90285-4. [DOI] [PubMed] [Google Scholar]
- Tötterman T. H., Nilsson K., Sundström C. Phorbol ester-induced differentiation of chronic lymphocytic leukaemia cells. Nature. 1980 Nov 13;288(5787):176–178. doi: 10.1038/288176a0. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


