Abstract
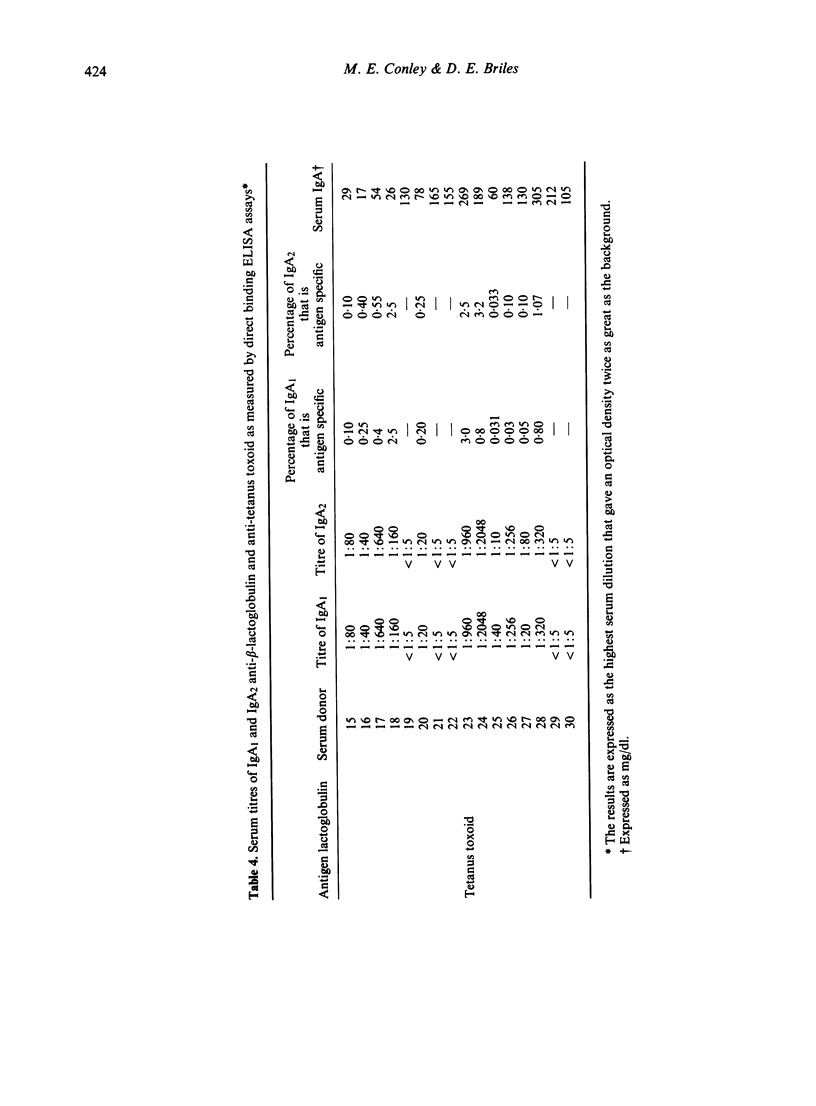
Although there is IgG subclass restriction in the antibody responses to most antigens, our data indicate that the human IgA subclasses, IgA, and IgA2, do not demonstrate a similar antigen specific restriction. We did not find evidence for IgA subclass restriction in the antibody responses to phosphorylcholine (PC), beta lactoglobulin or tetanus toxoid. These antigens were chosen to represent carbohydrate-like versus protein antigens and antigens presented through the mucosal route versus the humoral route. For each of these antigens the proportion of antigen specific IgA that was IgA1 and IgA2 was similar to that of total serum IgA. IgA anti-PC, which is thought to be directed against the phosphorylcholine moieties found on certain bacterial polysaccharides, could be found in the serum of all individuals tested and constituted 0.063-0.088% of the total serum IgA. IgA anti-beta lactoglobulin and anti-tetanus toxoid could be measured only in the serum of selected individuals, usually those with known milk protein sensitivity, or those recently immunized with tetanus toxoid. The lack of marked subclass restriction of IgA responses to these antigens stands in contrast to results obtained by others for IgG antibodies, in which carbohydrates and proteins preferentially stimulate antibodies in different IgG subclasses.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankhurst A. D., Lambert P. H., Miescher P. A. Studies on the thymic dependence of the immunoglobulin classes of the mouse (38570). Proc Soc Exp Biol Med. 1975 Feb;148(2):501–504. doi: 10.3181/00379727-148-38570. [DOI] [PubMed] [Google Scholar]
- Bloemmen J., Eyssen H. Immunoglobulin levels of sera of genetically thymusless (nude) mice. Eur J Immunol. 1973 Feb;3(2):117–118. doi: 10.1002/eji.1830030213. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Conley M. E., Kearney J. F., Lawton A. R., 3rd, Cooper M. D. Differentiation of human B cells expressing the IgA subclasses as demonstrated by monoclonal hybridoma antibodies. J Immunol. 1980 Nov;125(5):2311–2316. [PubMed] [Google Scholar]
- Conley M. E., Koopman W. J. Serum IgA1 and IgA2 in normal adults and patients with systemic lupus erythematosus and hepatic disease. Clin Immunol Immunopathol. 1983 Mar;26(3):390–397. doi: 10.1016/0090-1229(83)90123-x. [DOI] [PubMed] [Google Scholar]
- Gordon J., Murgita R. A. Suppression and augmentation of the primary in vitro immune response by different classes of antibodies. Cell Immunol. 1975 Feb;15(2):392–402. doi: 10.1016/0008-8749(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Gray B. M., Dillon H. C., Jr, Briles D. E. Epidemiological studies of Streptococcus pneumoniae in infants: development of antibody to phosphocholine. J Clin Microbiol. 1983 Nov;18(5):1102–1107. doi: 10.1128/jcm.18.5.1102-1107.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Courtenay B. M., Leuchars E., Davies A. J. Studies on immunological paralysis. VI. Thymic-independence of tolerance and immunity to type 3 pneumococcal polysaccharide. Cell Immunol. 1971 Dec;2(6):614–626. doi: 10.1016/0008-8749(71)90009-8. [DOI] [PubMed] [Google Scholar]
- Huber H., Douglas S. D., Nusbacher J., Kochwa S., Rosenfield R. E. IgG subclass specificity of human monocyte receptor sites. Nature. 1971 Feb 5;229(5284):419–420. doi: 10.1038/229419a0. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L. E., Strober W. Characteristics and regulatory function of murine con A-induced, cloned T cells obtained from Peyer's patches and spleen: mechanisms regulating isotype-specific immunoglobulin production by Peyer's patch B cells. J Immunol. 1982 Aug;129(2):475–483. [PubMed] [Google Scholar]
- Kunkel H. G., Prendergast R. A. Subgroups of gamma-A immune globulins. Proc Soc Exp Biol Med. 1966 Jul;122(3):910–913. doi: 10.3181/00379727-122-31287. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Weigle W. O., Spiegelberg H. L. Immunoglobulins cytophilic for human lymphocytes, monocytes, and neutrophils. J Clin Invest. 1975 Feb;55(2):368–376. doi: 10.1172/JCI107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Mayer L., Fu S. M., Kunkel H. G. Human T cell hybridomas secreting factors for IgA-specific help, polyclonal B cell activation, and B cell proliferation. J Exp Med. 1982 Dec 1;156(6):1860–1865. doi: 10.1084/jem.156.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongini P. K., Paul W. E., Metcalf E. S. T cell regulation of immunoglobulin class expression in the antibody response to trinitrophenyl-ficoll. Evidence for T cell enhancement of the immunoglobulin class switch. J Exp Med. 1982 Mar 1;155(3):884–902. doi: 10.1084/jem.155.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- Potter M. Antigen-binding myeloma proteins in mice. Ann N Y Acad Sci. 1971 Dec 31;190:306–321. doi: 10.1111/j.1749-6632.1971.tb13543.x. [DOI] [PubMed] [Google Scholar]
- Riesen W. F., Skvaril F., Braun D. G. Natural infection of man with group A streptococci. Levels; restriction in class, subclass, and type; and clonal appearance of polysaccharide-group-specific antibodies. Scand J Immunol. 1976;5(4):383–390. doi: 10.1111/j.1365-3083.1976.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yamawaki-Kataoka Y., Nishida Y., Kataoka T., Honjo T. Ordering of mouse immunoglobulin heavy chain genes by molecular cloning. Nature. 1981 Jan 15;289(5794):149–153. doi: 10.1038/289149a0. [DOI] [PubMed] [Google Scholar]
- Slack J., Der-Balian G. P., Nahm M., Davie J. M. Subclass restriction of murine antibodies. II. The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med. 1980 Apr 1;151(4):853–862. doi: 10.1084/jem.151.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19(0):259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Benveniste E., Pineda E. The selective role of membrane IgG in the antigen-induced inhibition of human in vitro antibody synthesis. J Immunol. 1982 Jan;128(1):398–401. [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Immunoregulation in humans: control of antitetanus toxoid antibody production after booster immunization. J Clin Invest. 1978 Dec;62(6):1154–1160. doi: 10.1172/JCI109234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzukida Y., Wang C. C., Putnam F. W. Structure of the A2m(1) allotype of human IgA--a recombinant molecule. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1104–1108. doi: 10.1073/pnas.76.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaerman J. P., Heremans J. F. Subclasses of human immunoglobulin a based on differences in the alpha polypeptide chains. Science. 1966 Aug 5;153(3736):647–649. doi: 10.1126/science.153.3736.647. [DOI] [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]


