Abstract
Dictyostelium discoideum DdRacGap1 (DRG) contains both Rho-GEF and Rho-GAP domains, a feature it shares with mammalian Bcr and Abr. To elucidate the physiological role of this multifunctional protein, we characterized the enzymatic activity of recombinant DRG fragments in vitro, created DRG-null cells, and studied the function of the protein in vivo by analysing the phenotypic changes displayed by DRG-depleted cells and DRG-null cells complemented with DRG or DRG fragments. Our results show that DRG-GEF modulates F-actin dynamics and cAMP-induced F-actin formation via Rac1-dependent signalling pathways. DRG’s RacE-GAP activity is required for proper cytokinesis to occur. Additionally, we provide evidence that the specificity of DRG is not limited to members of the Rho family of small GTPases. A recombinant DRG-GAP accelerates the GTP hydrolysis of RabD 30-fold in vitro and our complementation studies show that DRG-GAP activity is required for the RabD-dependent regulation of the contractile vacuole system in Dictyostelium.
Keywords: actin/contractile vacuole/Dictyostelium/RabD/Rac
Introduction
Human Bcr (breakpoint cluster region), Abr (active Bcr related) and the Drosophila 23E12 protein (Heisterkamp et al., 1989, 1993; Tan et al., 1993; Chuang et al., 1995) are members of a group of proteins that share the following functional domains: a Rho-GEF (G-nucleotide exchange factor) or Dbl-homology domain (DH), which accelerates the exchange of GDP for GTP and thereby activates the GTPase (Bourne et al., 1990; Boguski and McCormick, 1993), a Rho-GAP domain (GTPase activating protein), which is essential to elevate the slow intrinsic GTPase activity (Wittinghofer et al., 1997), and a pleckstrin homology (PH) domain, which is involved in protein– protein interactions or binding of acidic phospholipids (Rebecchi and Scarlata, 1998; Katan and Allen, 1999). Bcr has an additional N-terminal protein kinase domain, which is not present in any other member of this protein family. The human bcr gene is situated on chromosome 22 in a region involved in the Philadelphia translocation, a chromosome abnormality present in the leukaemic cells of patients with chronic myeloid leukaemia or acute lymphoblastic leukaemia. This translocation results in fusion of part of bcr with abl, the gene encoding the c-Abl protein tyrosine kinase, producing Bcr/Abl fusion proteins (Daley et al., 1990; Heisterkamp et al., 1990; Voncken et al., 1998). The normal biological functions of intact Bcr include the Rac1-dependent regulation of NADPH-oxidase in neutrophils and an involvement in mediating cellular effects of glucocorticoids (Voncken et al., 1995, 1998). Biological functions of Abr and 23E12 remain largely unknown, although there is biochemical evidence that Abr can catalyse both G-nucleotide exchange and GTPase activity of CDC42 and Rac proteins (Chuang et al., 1995). A Dictyostelium discoideum protein that is related to this family of multifunctional regulators of small GTPases has been described as DdRacGap (Ludbrook et al., 1997) and DdRacGap1 (Chung et al., 2000), and will be referred to as DRG for conciseness for the remainder of this communication. DRG differs from other Bcr-related proteins in that the organization of these three domains is inverted. In DRG a Src homology 3 (SH3) domain follows the N-terminal GAP domain and a PH domain is sandwiched by two DH domains in the C-terminal part of the protein (Figure 1).
Fig. 1. Diagrammatic representation of Dictyostelium DRG and DRG expression constructs. DRG has a calculated molecular weight of 148.5 kDa and an isoelectric point of 7.2. The predicted amino acid sequence of DRG shows a clear modular composition of the protein. The N-terminus contains a GAP-SH3 module with the GAP domain showing high homology to Rac/Rho-specific GTPase activating proteins (up to 47% homology). Then follows a stretch of 450–500 amino acids of which almost 50% are proline, serine and threonine residues (PST domain). Two Dbl-homology domains (DH1 and DH2) sandwich the PH domain at the C-terminus of the DRG protein. Full-length DRG (FL), a construct containing both Dbl homology domains with the central PH domain (2D) and a construct containing the GTPase activating region with the Src homology 3 domain (GAP-SH3) were expressed in Dictyostelium DRG-null cells. The 2D and GAP constructs were expressed in and purified from bacteria. The 2D construct contains amino acids 779–1335, the GAP-SH3 construct amino acids 1–320, and the GAP construct amino acids 28–184.
The Rho family of Ras-related GTP-binding proteins, which includes the Rho, Rac and CDC42 subfamilies, regulates diverse cellular processes ranging from rearrangements of the actin-based cytoskeleton (Vojtek and Cooper, 1995; Ridley, 1997; Hall, 1998; Aspenstrom, 1999) to the activation of mitogen-activated protein kinases, cell motility, transcription and DNA synthesis (Coso et al., 1995; Minden et al., 1995; Olson et al., 1995). Upon stimulation, members of the Rho subfamily control the formation of stress fibres and focal adhesion complexes. In contrast, Rac GTPases regulate growth factor-induced membrane ruffling and have been shown to act downstream of Ras in growth control. CDC42 is involved in the formation of filopodia in mammalian cells and cell polarization in yeast (Ridley and Hall, 1992; Tapon and Hall, 1997; Jones et al., 1998; Rebecchi and Scarlata, 1998). Dictyostelium discoideum contains at least 14 different rac genes, rac1A-C and racA-F1/2, G-J (Bush et al., 1993; Rivero et al., 1999). Overexpression of one of the rac1 genes leads to dramatic changes in F-actin organization directly affecting cell motility, endocytosis, development and cytokinesis (Chung et al., 2000; Dumontier et al., 2000). It has been proposed that the members of the Rac1 subfamily control the actin-based cytoskeleton via Dgap1, which interacts specifically with Rac1-GTP but does not act as a GAP for these Racs. Deletion of Dgap1 led to a defect in cytokinesis and aberrant regulation of actin (Faix et al., 1998; Dumontier et al., 2000). Overexpression of racC resulted in the formation of unusual F-actin-rich structures at the plasma membrane and an increase in phagocytic activity. Inhibition of PI3-kinase partly reverts the effects of racC overexpression. This suggests that a PI3-kinase acts downstream of RacC (Seastone et al., 1998). RacE is involved in the regulation of cortical tension, actin organization at the cortex, and cleavage furrow formation during cytokinesis (Larochelle et al., 1996; Gerald et al., 1998).
To investigate the physiological functions of DRG, we used a multifaceted approach combining biochemical, molecular genetic and cell biological analysis. Our results show that Rac1A is not the preferred interaction partner of DRG. Instead, DRG-GAP activity regulates diverse cellular processes such as organization of the contractile vacuole system and cytokinesis by linking to RabD- and RacE-dependent signalling pathways, while DRG-GEF activity regulates localization of F-actin and cAMP-induced F-actin formation via Rac1-dependent signalling pathways.
Results
Domain organization of DRG
DRG consists of 1335 amino acids and shows a clear domain organization. The N-terminal part of the protein has a high degree of similarity to Rho-GAPs followed by an SH3 domain. Analysis with the Web-based tools ProfileScan and SMART (Bucher et al., 1996; Schultz et al., 1998; Hofmann et al., 1999; Ponting et al., 1999) revealed that the C-terminal part of DRG contains a second DH domain that has not been reported previously. The two DRG-DH domains display ∼35% identity to each other and to other DH domains. Both contain the three structurally conserved regions (scr1–3) that are typical for DH domains (Boguski and McCormick, 1993; Soisson et al., 1998). Sandwiched between DH1 and DH2 lies the PH domain. The polypeptide sequence between the SH3 domain and DH1 is characterized by the frequent occurrence of repeats of up to six proline, glycine, serine or threonine residues. Twelve asparagine residues form the longest repeat. Asparagine repeats are frequently observed in Dictyostelium proteins and it has been speculated that they play a role in mediating protein–protein interactions (Wienke et al., 1999).
Characterization of DRG-null cells
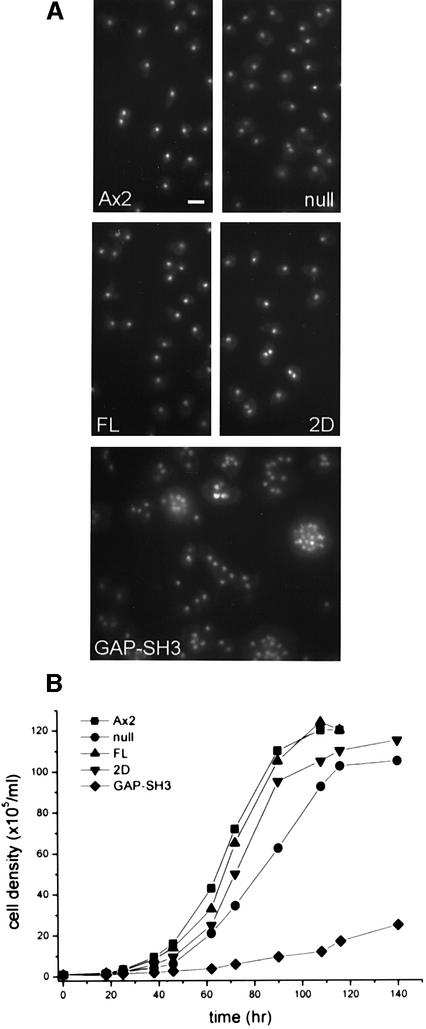
In order to study the in vivo function of DRG, we generated DRG-null cells. Clones were tested for the deletion of the gene by Southern blotting and PCR analysis. The absence of DRG protein was further confirmed by immunoblot analysis using antibody MK-101, generated against a peptide from the SH3 domain of DRG. Due to the deletion of the gene, 36 of the 102 transformants analysed failed to produce DRG. All null cell lines displayed normal growth rates in suspen sion cultures and on bacterial lawns (Figure 2A). They displayed many forms of cell movement, extending and retracting filopodia and lamellopodia, and were able to ingest bacteria by phagocytosis.
Fig. 2. (A) Growth characteristics of DRG-null and DRG transformant cells. Cells were methanol fixed and stained with 4′-6-diamidine-2-phenylindole (DAPI) to visualize the cell nucleus. Most cells contain one and sometimes two nuclei. Null cells expressing DRG-GAP-SH3 are larger and contain generally more than one nucleus up to fifteen nuclei per cell. The scale bar represents 10 µm and is valid for all five pictures. (B) The graph shows growth of cells in suspension culture. Cells were diluted to 1 × 105 ml–1 and cell density was determined. Doubling times of 10 h for wild-type Ax2, FL and 2D cells, 12 h for DRG-null and 22 h for GAP-SH3 cells were determined.
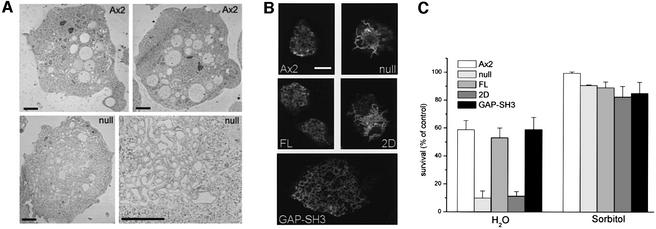
To study the morphological consequences of the drg knockout in detail, cells from clone HDM100 were fixed and their ultrastructure observed in an electron microscope (Figure 3A). A large tubular–vesicular network was observed in DRG-null cells, which was not present in the wild type. The localization of specific organelle markers was investigated by immunofluorescence to identify the nature of this compartment. Mitochondria, Golgi apparatus and endosomal compartments appeared normal in size and organization (data not shown). However, a dramatic difference in the morphology of the contractile vacuole system was observed with an antibody directed against the 100 kDa subunit of the V-H+-ATPase (Figure 3B). This monoclonal antibody predominantly stains the contractile vacuole. In wild-type cells the contractile vacuole system is found in the periphery of the cell and consists of long tubular structures and several vacuoles. In DRG-null cells the contractile vacuole system has a condensed appearance and a non-peripheral localization. The morphological alterations observed by laser scanning confocal microscopy correlate well with the electron micrographs shown in Figure 3A. Next we examined the osmosensitivity of DRG-null cells to detect the functional competence of their contractile vacuole system. Cells were incubated either in water or 350 mM sorbitol, which are hypotonic and hypertonic conditions, respectively. The survival rate of cells after incubation in hypertonic medium was unaltered but under hypotonic conditions a 10-fold decrease was observed (Figure 3C).
Fig. 3. DRG is involved in regulation of the contractile vacuole and osmosensitivity. (A) Electron micrographs of wild-type Ax2 and DRG-null cells. In contrast to the wild-type Ax2 (top), the DRG-null cells show a marked increase in a vesicular tubular structure in the centre of the cell (bottom). A close-up of these structures in the DRG-null cells is shown in the bottom-right panel. The scale bars are 2 µm. (B) Confocal immunofluorescence images demonstrating the contractile vacuole system in Ax2 cells, DRG-null cells and DRG-null cells expressing full-length DRG (FL), DRG-2D (2D) or DRG-GAP-SH3 (GAP-SH3). A monoclonal antibody against the α-subunit of the vacuolar ATPase was used and detected by a Cy3-conjugated secondary antibody. The scale bar represents 10 µm. DRG-null cells and the 2D cells contain a condensed contractile vacuole system while all the other cells are comparable to wild type. The scale bar is 10 µm. (C) Osmosensitivity of DRG-null and DRG transformant cells. Cells were incubated in hypotonic conditions (H2O) and hypertonic conditions (350 mM sorbitol), and survival was determined by plating cells on Klebsiella lawns. Cell lines containing a distorted contractile vacuole system (see B) also display increased osmosensitivity under hypotonic conditions. The data shown are the mean of three independent experiments ± SD.
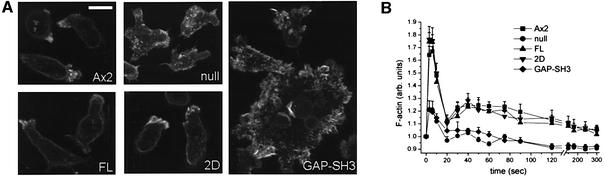
DRG is developmentally regulated with the highest levels of expression observed between 6 and 12 h of development. Expression is 8-fold lower in vegetative cells and decreases gradually during the late stages of multicellular development (data not shown). Changes in F-actin distribution during development were visualized by staining fixed cells with TRITC-labelled phalloidin. In growing null cells no significant difference in the abundance and localization of F-actin was observed (data not shown). In aggregation-competent cells, after 8 h of development, there was a marked difference in the distribution of F-actin between DRG-null and wild type (Figure 4A). Null cells displayed cytosolic aggregates of F-actin and lacked the smooth cortical actin staining observed with wild-type cells. Cortical F-actin in DRG-null cells appeared to be discontinuous, increased in thickness and less polarized (Figure 4A).
Fig. 4. F-actin distribution in aggregation-competent cells. (A) Cells were allowed to settle on glass coverslips, fixed in paraformaldehyde and stained for F-actin as described in Materials and methods. Wild-type Ax2, FL and 2D cells show a polarized F-actin organization and a smooth cortical F-actin staining. DRG-null cells display a more disorganized F-actin distribution with aggregates in the cell and a thicker and discontinuous cortical staining. The larger GAP-SH3 cells show a large number of micro spikes and also a dramatically changed cortical actin staining. The scale bar represents 10 µm and is valid for all five pictures. (B) cAMP-induced actin polymerization response. Aggregation-competent cells at 2 × 107 cells/ml were stimulated with 1 µM cAMP and samples were taken at the indicated times. F-actin content was determined as described in Materials and methods. DRG-null cells and GAP-SH3 cells have a strongly decreased response. The mean of three independent experiments ± SEM are presented.
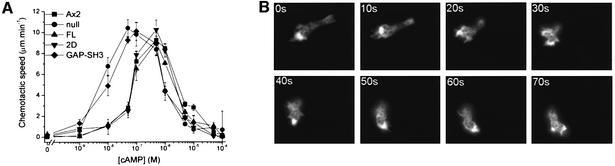
During the Dictyostelium developmental cycle cells aggregate by actively moving up a cAMP gradient. It has been shown that extracellular cAMP evokes fast and highly transient changes in the F-actin cortex (Hall et al., 1988). Approximately 5 s after stimulation of cells with cAMP an increase in the amount F-actin is seen, which decreases rapidly, reaching almost basal level after 20 s. A second lower and slower peak is observed after 30–60 s. DRG-null cells show a strong decrease in the first F-actin peak following cAMP treatment (Figure 4B) and a complete lack of the second peak. The cells motile behaviour in a spatial gradient of cAMP was measured using a micropipette-based assay and the radial bioassay method described by Browning et al. (1995). The radial bioassay was used as it facilitates dose–response experiments for cAMP-induced chemotaxis. Micropipette-based assay and radial bioassay gave similar results for the maximal chemotactic speed. For wild-type and DRG-null cells, movement at ∼10 µm/min was observed (Figure 5A). The cAMP concentration at which DRG-null cells started to move directionally was at least 5-fold lower than for wild-type cells (Figure 5A).
Fig. 5. (A) Chemotaxis of cells was determined using the radial bioassay described in Materials and methods. The mean of three independent experiments ± SEM are presented. (B) Fluorescence microscopy of YFP–DRG expressing cells. YFP–DRG cells were allowed to settle on chambered cover glasses, washed and incubated for at least 6 h in Mes-starvation buffer. Time-lapse photography was performed with 10-s intervals.
Analysis of chemoattractant-induced F-actin formation, following stimulation with increasing concentrations of cAMP, revealed a similar increase in the sensitivity of DRG-null cells for cAMP as observed for chemotaxis (data not shown). The dose–response curve was shifted to the left, showing that the maximal response in DRG-null cells could be induced with ∼10-fold lower cAMP concentrations in comparison with wild type. The shift in chemoattractant-induced F-actin, a process that is independent of phosphodiesterase activity, indicates that the observed differences in the chemotaxis assay were not caused by alterations in phosphodiesterase activity between the different cell lines. Differences in sensitivity to the chemoattractant cAMP are expected to have consequences for the aggregation behaviour of the null cells. Cells were incubated either submerged in Mes-starvation buffer on plastic dishes or on non-nutrient agar and left to aggregate. The DRG-null cells did not show the normal thick, branched aggregation streams but had a tendency to migrate solitarily towards the aggregation centre (data not shown). The difference in aggregation behaviour resulted in an ∼2-fold reduced size of the tight aggregates. The resulting fruiting bodies were smaller but showed normal morphology and carried viable spores.
Expression of full-length DRG in null cells led to a complete reversion of the null phenotype. The rescued cells had a functional and morphologically intact contractile vacuole system. They displayed normal F-actin organization and wild-type-like chemotaxis.
Cellular localization of DRG
A yellow fluorescent protein (YFP)–DRG fusion construct was generated and transformed into wild-type and DRG-null cells to study the cellular localization of DRG in vivo. Expression of the construct was found to be ∼1.5- to 2-fold higher than the endogenous level of DRG. Cell fractionation experiments were performed with wild-type cells and DRG was detected on immunoblots using polyclonal antibody MK101 directed against the protein. These experiments showed that in vegetative cells DRG mainly resides in the cytosol. In aggregation-competent cells, up to 50% of DRG was found in a crude membrane preparation (data not shown) and YFP–DRG was clearly enriched at the leading edge of aggregation-competent cells. Changes in direction of movement were generally preceded by a redistribution of YFP–DRG towards the site of pseudopod extension (Figure 5B).
Functional dissection of the DRG domains
Separate constructs for the production of the regions involved in the GAP and GEF activities were generated and transformed into null cells to dissect the functional importance of each of these activities and to identify the interacting GTPases. Transformants producing the 2D construct (Figure 1) consisting of the PH domain sandwiched by the Dbl domains showed the same alterations of the contractile vacuole system as null cells (Figure 3B). Consequently they also displayed increased osmosensitivity under hypo-osmotic conditions (Figure 3C). In contrast, the actin-dependent consequences of DRG depletion were partially rescued by the 2D construct (Figure 4A). These cells showed smooth cortical F-actin staining and enrichment of F-actin at the leading edge of the cell. Chemotaxis and cAMP-induced F-actin formation appeared to be normal.
Expression of the GAP-SH3 construct in the null cells resulted in complete recovery of the contractile vacuole system (Figure 3B). The transformant’s resistance to osmotic shock was similar to wild-type cells, suggesting that its contractile vacuole system is functional (Figure 3C). The organization of F-actin in aggregation-competent cells was more severely disturbed by expression of GAP-SH3 in DRG-null cells (Figure 4A). In addition to the aberrant localization of F-actin in the DRG-null cell line, the GAP-SH3 transformed cells displayed a large number of short actin-rich extensions on the cell surface. The dramatically reduced cAMP-induced F-actin formation as well as the increased sensitivity for cAMP during chemotaxis were not affected by expression of GAP-SH3 in the null cells (Figures 4B and 5A).
Cytokinesis was severely affected by expression of the GAP-SH3 construct in the null cells. The transformed cells displayed an increase in cell size and number of nuclei (Figure 2A). They also showed a decreased growth rate in suspension culture (Figure 2B) as well as in stationary adhesion cultures (data not shown). Wild-type Ax2 cells have a doubling time of 9 h whereas DRG-null cells double every 11 h. Null cells expressing full-length DRG or DRG-GEF display wild-type growth characteristics but overexpression of GAP-SH3 resulted in a doubling time of >25 h.
DRG displays both GAP and GEF activity
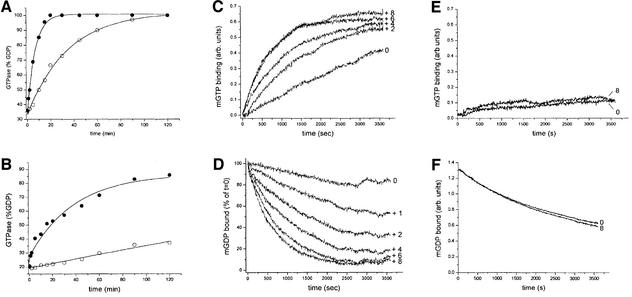
Using the program SMART, we predicted the DRG to contain both GAP and G-nucleotide exchange activity (Schultz et al., 1998). Using bacterially expressed GAP and Rac1A we could confirm that DRG-GAP stimulates Rac1A GTPase activity. The fact that human RhoA was found to be a better substrate than Dictyostelium Rac1A indicated that Rac1A might not be the preferred in vivo substrate (Ludbrook et al., 1997). Additionally two phenotypes have been linked to the DRG-GAP activity. Overexpression of GAP-SH3 in null cells resulted in aberrant cytokinesis and reversion of the contractile vacuole system defect. Both regulation of cytokinesis and the contractile vacuole system have been reported to depend on small GTPases. RacE is essential for proper cytokinesis. A member of the Rab subfamily of small GTPases, the Rab4-like RabD, is required for normal function and morphology of the contractile vacuole system. DRG-GAP stimulates GTPase activity of both RacE and RabD (Figure 6A and B). An equimolar amount of GAP increases the GTPase activity of RacE 5.3-fold (from 0.28 × 10–1 to 1.49 × 10–1 min–1). For RabD, a 30-fold increase was observed (from 0.086 × 10–2 to 2.58 × 10–2 min–1). The use of native or post-translationally modified proteins might result in higher rates of activation than observed here for the bacterially expressed GTPases and DRG fragments.
Fig. 6. GTPase-activating and G-nucleotide exchange activity of Dictyostelium DRG. (A) 5 µM RacE or (B) 5 µM RabD was loaded with radioactive [α-32P]GTP and incubated with (solid circles) or without (open circles) 5 µM purified DRG-GAP. Samples were taken at the timepoints indicated and the percentage of GDP was determined with a phosphoimager. GAP clearly enhances the GTPase activity of RacE and RabD. Rac1A nucleotide exchange was determined by two independent methods. (C) Mant-GTP binding to Rac1A results in an increase in fluorescence. Increasing concentrations of 2D were added (2–8 µM). (D) Mant-GDP dissociation from Rac1A in the presence of an increasing concentration (1–8 µM) of the 2D construct and 200 µM GTP. Mant-GDP release was measured as a decrease of mant fluorescence (365/445 nm) as described in Materials and methods. The rates determined from mant-GDP dissociation and mant-GTP association are very similar. (E) Mant-GTP binding to RacC or (F) mant-GDP dissociation from RacE in the presence or absence of 8 µM 2D. No acceleration of exchange activity by 2D was observed for these two Rac proteins.
To demonstrate that DRG also possesses G-nucleotide exchange activity, we bacterially produced a fragment containing both DH domains and the PH domain, purified it to homogeneity and measured GDP–GTP exchange on Dictyostelium small GTPases (Figure 6C and D). Mant-GDP displacement or mant-GTP association measurements showed that the 2D construct was capable of catalysing G-nucleotide exchange on Dictyostelium Rac1A. Both types of measurements with 2D produced similar rates of stimulation of G-nucleotide exchange activity. For the highest concentration of 2D (8 µM) a 13-fold increase in the initial rate of GDP release was measured. In contrast, no exchange activity was detected for RacC, RacE, RasG and RabD. The data for RacC and RacE are shown in Figure 6E and F. The 13-fold increase in exchange activity for Rac1A by DRG-2D is very similar to the acceleration observed for Rac1 by Bcr- and Abr-GEF or for RhoA by Lfc or Lsc. However, the maximal rate of acceleration was reached at a molar ratio of 1:1 compared with 1:3 observed for DRG-GEF (Chuang et al., 1995; Glaven et al., 1996).
Discussion
DRG was originally isolated in a PCR-based screen for members of the Rho-GAP family in Dictyostelium. The protein was shown to have GAP activity for human RhoA and Dictyostelium Rac1A, and sequence comparison revealed that the protein contains, in addition to the N-terminal GAP-domain, an SH3 domain and a DH-PH module (Ludbrook et al., 1997). Our results show the presence of a further DH domain at the extreme C-terminus of the protein and that a bacterially expressed construct containing both DH domains and the PH domain displays GEF activity for Rac1A in vitro.
DRG as effector of Rac1 GTPases
Rac1A together with Rac1B and Rac1C forms the Dictyostelium Rac1 subfamily. The overall sequence identity of the three GTPases is >90% and their effector domains are identical to each other, but not to other Rac-like GTPases, indicating that they have a unique set of effector proteins in common (Bush et al., 1993; Rivero et al., 1999). This conclusion is supported by experimental findings showing that the members of the Rac1 subfamily play an identical role in regulating a wide range of actin-based events in Dictyostelium, including the formation of cell-surface extensions, cell polarity during chemotaxis, endocytosis and cytokinesis. The fact that both dominant-negative and constitutively active mutant forms of Rac1 display defective F-actin regulation suggests that cycling between the active GTP- and the inactive GDP-bound forms is essential for proper function of Rac1 (Chung et al., 2000; Dumontier et al., 2000).
The role of DRG as effector of Rac1 GTPases was supported by a recent study which showed that disruption of drg and overexpression of constitutively active Rac1BQ61L lead to similar changes in the basal level of F-actin assembly, dynamic reorganization of F-actin in response to the chemo-attractant cAMP, and cell polarity during chemotaxis. These phenotypic changes associated with depletion of DRG were attributed to the lack of Rac1-GAP activity in these cells (Chung et al., 2000). Our analysis of DRG-null cells supports a role of DRG in Rac1-dependent signalling but suggests a different mode of action and additional roles for the protein. The Rac1A-GEF activity displayed by the 2D construct in vitro and the in vivo rescue of the actin-dependent consequences of DRG depletion by this construct reveal the functional importance of DRG’s Rac1-GEF activity (Figure 4A). Our results also indicate that DRG’s Rac1-GEF activity, but not its Rac1-GAP activity, is required to keep Rac1 proteins cycling between the active GTP- and the inactive GDP-bound forms (Figure 7). The increased number of short actin-rich extensions and the severe cytokinesis defect that is associated with the overexpression of the GAP-SH3 construct can be explained by a build-up of the inactive forms of Rac1 in these cells. However, of all Dictyostelium GTPases tested, DRG-GAP showed the lowest activity with Rac1A, making DRG an unlikely candidate for a genuine Rac1-GAP.
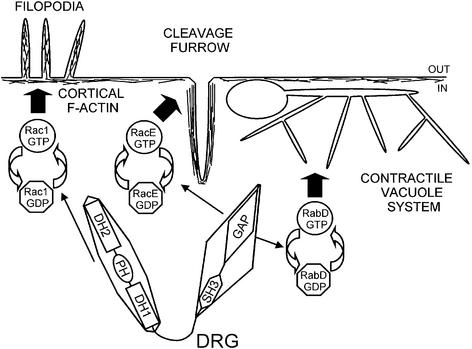
Fig. 7. Scheme demonstrating the role of DRG in cytokinesis, contractile vacuole function and F-actin organization during chemotaxis. DRG-GAP activity plays a major role in cytokinesis by regulating the nucleotide state of RacE, which is of vital importance for the cortical tension during cell division. DRG-GAP also plays a critical role in organization and function of the contractile vacuole in Dictyostelium by an as yet unknown mechanism involving the Rab4 homologue RabD. The DRG G-nucleotide exchange activity is vital for Rac1-dependent F-actin organization and filopodia formation during chemotaxis of the cells towards a cAMP source. See text for further details.
DRG affects RacE-dependent signalling
Similarly to Rac1, RacE is involved in regulating a range of actin-based events including organization of F-actin during cytokinesis. However, the cytokinesis defects displayed by RacE-null cells and Rac1A mutant cells show clear differences. RacE plays an essential role in cleavage furrow formation by regulating the cortical tension of mitotic cells. Depletion of RacE induces a conditional, dominant-negative phenotype. RacE-null cells die when grown in suspension culture, although cells grown in stationary culture on Petri dishes look normal and grow at almost normal rates (Larochelle et al., 1996). Overexpression of constitutively active RacE but not of dominant-negative RacE reverted this cytokinesis defect, showing that RacE in the GTP-bound form, and not cycling between the GDP- and GTP-bound forms, is necessary for proper functioning of the protein (Larochelle et al., 1997). In contrast, both dominant-negative and constitutively active Rac1 mutants displayed a more severe cytokinesis defect in stationary culture, resulting in the formation of large multinuclear cells and an increased frequency of amitotic cell divisions, but displayed almost normal doubling rates in suspension cultures (Dumontier et al., 2000).
DRG-depleted cells showed no growth deficiencies both in shaking culture and on bacterial lawns. However, overexpression of GAP-SH3 in DRG-null cells led to a cytokinesis defect similar to that observed in RacE-null cells. Biochemical assays confirmed that DRG-GAP efficiently stimulates the GTPase activity of RacE. In contrast, DRG-GEF did not enhance GDP–GTP exchange on RacE. This suggests that DRG-GAP activity regulates the amount of RacE-GTP in vivo and that this activity must be tightly regulated in order to allow for proper cytokinesis (Figure 7).
DRG affects RabD-dependent signalling
The small GTPase RabD, a Dictyostelium Rab4 homologue, facilitates communication between the contractile vacuole system and the endosomal pathway, and plays a critical role in the regulation of the structure and function of the contractile vacuole system. The contractile vacuole pumps water actively out of Dictyostelium cells when they are exposed to hypotonic conditions. Overexpression of RabDN121I leads to mis-sorting of both lysosomal markers and contractile vacuole membrane proteins (Bush et al., 1996). The contractile vacuole system of DRG-null cells is clearly impaired in its function. The dramatically changed morphology of the contractile vacuole system in DRG-null cells is similar to that observed in cells overexpressing RabDN121I (Figure 7). The fact that overexpression of the GAP-SH3 construct rescues the osmosensitive phenotype and restores the morphology of the contractile vacuole led us to extend the biochemical characterization of DRG’s GAP and GEF activities to the Rab family. The basal GTPase activity of RabD was increased 30-fold by DRG-GAP, which was the highest activation by DRG-GAP observed for the Dictyostelium small GTPases tested. No effect of DRG-GEF was observed on the rate of GDP–GTP exchange for RabD. This result demonstrates that DRG-GAP regulates the GTPase activity of members of the Rab and the Rho/Rac family.
Our finding that DRG-GAP interacts with members of the Rab family was unexpected. The DRG-GAP domain was predicted to be specific for members of the Rho family of small GTPases by all sequence analysis programs used (Altschul et al., 1990, 1997; Schultz et al., 1998; Ponting et al., 1999). The highest degree of sequence similarity was found with the GAP domains of Bcr and Abr, which have been shown to catalyse the GTPase activity of Rac and CDC42 in vitro (Chuang et al., 1995; Wittinghofer et al., 1997).
Similarly to other GAPs, DRG-GAP is predicted to be predominantly α-helical in structure. It contains a conserved arginine residue at position 51 that is thought to act as a catalytic ‘arginine finger’. Furthermore, our results show that DRG can interact with GTPases that display high diversity in the effector loop region and that it displays highly variable degrees of interaction with GTPases that have similar effector loop regions. This suggests that the effector loop region is not contributing to the specificity of the interaction. DRG-GAP may interact in a similar way with the C-terminal region of GTPases, as was described for the interaction of the yeast Rab homologue YPT and its GAP Gypt1 (Rak et al., 2000).
The fact that DRG-GAP affects the contractile vacuole system by regulating the Dictyostelium Rab4 homologue RabD is intriguing. Schmidt et al. (1997) identified a compartment in neuroendocrine cells with similar morphology to the contractile vacuole system in Dictyostelium. This sub-plasmalemmal tubulocisternal system is characterized by narrow channels connected to the cell surface, giving rise to synaptic-like microvesicles, the counterpart of neuronal synaptic vesicles. It was proposed that the Dictyostelium contractile vacuole system is a specialized version of this eukaryotic cell compartment (Gabriel et al., 1999). The Abr and Bcr proteins are both produced at high levels in brain tissue. The role of Abr and Bcr in the brain is still not understood but our results point in a new direction for which the biochemical activation spectrum of these proteins should be re-evaluated.
Materials and methods
Reagents
Standard chemicals were purchased from Sigma; restriction enzymes, polymerases and DNA-modifying enzymes were from Roche Molecular Biochemicals; blasticidin-S was from ICN. TRITC-labelled phalloidin was a gift from Dr Faulstich. Antibodies against mitoporin, vacuolin, comitin and the 100 kDa subunit of vacuolar ATPase were generously supplied by Dr M.Maniak, Dr A.Noegel and Dr G.Gerisch. Polyclonal peptide antibodies against the DRG-SH3 (MK-101) domain were produced by Biogenes (Berlin, Germany).
Cell growth and transformation
Dictyostelium cells were grown in HL-5C medium (Watts and Ashworth, 1970). Cells were transformed by electroporation (de Hostos et al., 1991). Blasticidin and G418 were used as selectable markers at 5 and 10 µg/µl, respectively. In order to obtain aggregation-competent cells, cells were washed three times in Mes-starvation buffer (20 mM MES, 2 mM MgCl2, 0.2 mM CaCl2), plated on Mes-starvation agar plates, incubated for 16 h at 6°C, harvested and shaken for 1 h at 1 × 107 cells/ml at 22°C. In order to observe aggregation or full development cells were plated on Mes-starvation agar plates or in chambered cover glasses (Nunc) at ∼5 × 105 cells/cm2 and were allowed to develop further at 22°C.
Generation of DRG-null cells and cells expressing DRG domains
The BglII fragment (bp 558–3099) from the genomic clone (DDBJ/EMBL/GenBank accession No. Y10159) was replaced with a blasticidin resistance cassette and the linearized disruption construct was transformed in Ax2 cells. Transformants were selected using 5 µg/µl blasticidin-S, recloned on Klebsiella aerogenes lawns, and checked for successful knock-outs by western blotting using polyclonal DRG-SH3 antibody MK-101. PCR and genomic DNA blotting were then used to analyse positive clones. Several knockout clones were used for this study and no difference between all analysed clones could be detected. The DNA coding for full-length DRG, DRG-2D (amino acids 779–1335) and DRG-GAP-SH3 (amino acids 1–320) was generated by PCR and cloned into pDXA that allows for expression of these constructs under control of the constitutive actin15 promoter (Manstein et al., 1995). These plasmids were transformed in DRG-null cells. Cells were selected in medium containing 10 µg/µl G418 and checked for expression of the proper polypeptide by western blotting. For YFP–DRG a genomic DNA encoding the full-length DRG protein was cloned into the pDXA-YFP vector using BamHI and XbaI. The resulting construct encodes a polypeptide with YFP at the N-terminus followed by a linker (SGSGSG) and DRG.
Protein purification
Small GTPases Rac1A, RacC, RasG and RacE were purified as glutathione S-transferase fusion proteins in Escherichia coli. Cells were induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and grown overnight at room temperature before lysis by French press. The high-speed supernatant was loaded on a glutathione–Sepharose column. The column was washed and protein was eluted by cleavage with thrombin on the column. Thrombin at 10 U/ml was run through the column at slow flow rates (80 µl/min). The eluate was passed through a downstream-attached p-amino-benzamidine column to remove thrombin. The DNA constructs encoding both DH domains (2D) containing amino acids 779–1335, the GAP domain containing amino acids 28–184 and RabD (gift of J.Cardelli) were cloned in the pQE30 vector (Qiagen, Germany), which allows expression of His-tagged proteins in E.coli. Cells expressing 2D and RabD were induced with 0.5 mM IPTG and grown at room temperature for 5 and 16 h, respectively. Protein was purified by lysing the bacteria with a French press and loading a high-speed supernatant on a Ni-NTA column (Qiagen, Germany). The protein was eluted with 300 mM imidazole.
GDP–GTP exchange assay
Loading of small GTPases with GTP or mant-GTP was performed by incubation on ice for 2 min in Tris–EDTA buffer [50 mM Tris pH 7.5, 50 mM NaCl, 40 mM EDTA, 5 mM dithiothreitol (DTT)] and subsequently adding 100 mM MgCl2 (final concentration) in the presence of a 10-fold excess of nucleotide. The mant-GTP was allowed to be hydrolysed by the GTPase. Remaining non-bound nucleotide was removed by running the incubation mixture over a NAP-10 column (Pharmacia Amersham), eluting with 50 mM Tris pH 7.5, 2 mm MgCl2, 5 mM NaCl, 1 mM DTT. Mant-GDP (2 µM) loaded GTPase was incubated with 200 µM GTP and various concentrations of the 2D polypeptide, and fluorescence (365/445 nm) change was measured using an SLM-8000 fluorimeter.
GTPase activity
Loading of small GTPases with [α-32P]GTP was performed as described above. Radioactively labelled GTPases (5 µM final concentration) were incubated at 21°C with or without DRG-GAP, and samples were quenched by addition of trichloroacetic acid (0.5%) at the desired times. After neutralization, samples were loaded on PEI-cellulose thin layer chromatography plates with 1.8 M LiCl as running solution. GDP and GTP were quantified with a Fuji phosphoimager using Imagequant software.
Electron microscopy
Transmission electron microscopy for Figure 3 was carried out as previously described (Parsons et al., 1995; Steyer et al., 1997). Shortly, monolayers of cells on thin glass coverslips were rapidly frozen in liquid ethane. Following freeze substitution, specimens were embedded in Lowicryl HM-20 at low temperature and ultra-thin sections were observed.
Fluorescence microscopy
Cells at a density of 3 × 105 cells/ml were allowed to settle on 12-mm diameter coverslips for 15 min. The fixation method used for studying intracellular morphology was immersion in methanol at –85°C followed by a temperature gradient from –85 to –35°C for 30 min (Neuhaus et al., 1998). The fixed cells were rehydrated in phosphate-buffered saline (PBS) and blocked in PBS containing 3% bovine serum albumin (BSA) followed by incubation in primary antibody for 16 h at 4°C. For staining mitochondria, Golgi complex, late endosomes and the contractile vacuole system, the monoclonal antibodies 70-100-1, 190-340-8, 221-1-1 and an antibody recognizing the 100 kDa subunit of the vacuolar ATPase were used. All primary antibodies were diluted 1:5. After extensive washing cells were labelled with Cy3-conjugated secondary antibody (Amersham Pharmacia) at 1:500 dilution. DAPI was used at a concentration of 0.1 µg/ml to stain cell nuclei. For staining of F-actin, cells were fixed with 1% paraformaldehyde, 0.1% glutaraldehyde, 0.1% Triton X-100 in PHEM (60 mM PIPES pH 6.4, 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2). Cells were then treated twice with 2 mg/ml NaBH4 in PHEM to reduce background fluorescence. After rinsing, cells were blocked in Gel/BSA buffer (20 mM Tris pH 8.0, 0.9% NaCl, 0.1% BSA, 0.02% gelatin) and incubated with 0.2 µM TRITC-phalloidin in the same buffer for 16 h at 4°C. Images were recorded using a Zeiss Axiovert 135 inverted microscope equipped with a Quantix camera (Photometrics, Tucson, AZ) that was controlled by IP-Lab software or on a Leica TCS-NT confocal microscope system.
Osmosensitivity measurement
Cells were incubated at 2 × 106 cells/ml in either water (hypotonic), 350 mM sorbitol (hypertonic) or HL-5C medium (control) in shaking culture at room temperature for 45 min. EDTA (2.5 mM) was added to prevent the cells from forming aggregates. After incubation, 100 cells were plated on bacterial lawns and after 3–4 days survival rates were determined by counting colonies appearing on the bacterial lawns. At least three plates were counted per incubation condition.
Actin polymerization assay
Chemoattractant-induced F-actin formation was measured as described (Hall et al., 1988; Peracino et al., 1998). Briefly, aggregation-competent cells were resuspended at 2 × 107 cells/ml. Cells were stimulated with 1 µM cAMP and 50 µl samples were taken at various timepoints. The reaction was terminated by addition of 450 µl of 3.7% formaldehyde, 0.1% Triton X-100, 0.25 µM TRITC-phalloidin in 20 mM potassium phosphate, 10 mM PIPES, 5 mM EGTA, 2 mM MgCl2 pH 6.8. After staining for 1 h, samples were centrifuged for 5 min at 15 000 g. Pellets were extracted with 700 µl of methanol for 16 h and fluorescence (540/565 nm) was read in an SLM-8000 fluorimeter.
Chemotaxis
Chemotaxis was determined as described by Browning et al. (1995). Aggregation-competent cells were spotted at high density on small agar plates (20 mM Tris pH 7.0) with various concentrations of cAMP in the agar. The radius of the area covered by cells was determined before and after an incubation period of 3 h at 21°C. The increase in radius is a direct measure for net chemotaxis. For the micro-needle assay we filled an Eppendorf femtotip with 100 µM cAMP and using an Eppendorf Injectman placed the needle in a field of aggregation-competent cells attached to a glass surface. We applied a continuous flow with an Eppendorf Femtojet and observed the cells with a Zeiss Axiovert 135 microscope equipped with a Princeton Instruments CCD camera.
Acknowledgments
Acknowledgements
We thank Ursula Rühl for excellent technical assistance, and F.Jon Kull and Roger S.Goody for critically reading the manuscript. We are especially grateful to G.Gerisch, A.Noegel and M.Maniak for providing monoclonal antibodies, J.Cardelli for the RabD clone, and F.Rivero and A.De Lozanne for several Rac constructs. We thank Kenneth C.Holmes for support and encouragement. The Max-Planck-Society (Germany) supported this work. M.L.W.K. was supported by a long-term fellowship from the European Molecular Biology Organization.
References
- Altschul S.F., Gish,W., Miller,W., Myers,E.W. and Lipman,D.J. (1990) Basic local alignment search tool. J. Mol. Biol., 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Madden,T.L., Schaffer,A.A., Zhang,J., Zhang,Z., Miller,W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenstrom P. (1999) Effectors for the Rho GTPases. Curr. Opin. Cell Biol., 11, 95–102. [DOI] [PubMed] [Google Scholar]
- Boguski M.S. and McCormick,F. (1993) Proteins regulating Ras and its relatives. Nature, 366, 643–654. [DOI] [PubMed] [Google Scholar]
- Bourne H.R., Sanders,D.A. and McCormick,F. (1990) The GTPase superfamily: a conserved switch for diverse cell functions. Nature, 348, 125–132. [DOI] [PubMed] [Google Scholar]
- Browning D.D., The,T. and O’Day,D.H. (1995) Comparative analysis of chemotaxis in Dictyostelium using a radial bioassay method: protein tyrosine kinase activity is required for chemotaxis to folate but not to cAMP. Cell. Signal., 7, 481–489. [DOI] [PubMed] [Google Scholar]
- Bucher P., Karplus,K., Moeri,N. and Hofmann,K. (1996) A flexible motif search technique based on generalized profiles. Comp. Chem., 20, 3–23. [DOI] [PubMed] [Google Scholar]
- Bush J., Franek,K. and Cardelli,J. (1993) Cloning and characterization of seven novel Dictyostelium discoideum rac-related genes belonging to the rho family of GTPases. Gene, 136, 61–68. [DOI] [PubMed] [Google Scholar]
- Bush J., Temesvari,L., Rodriguez-Paris,J., Buczynski,G. and Cardelli,J. (1996) A role for a Rab4-like GTPase in endocytosis and in regulation of contractile vacuole structure and function in Dictyostelium discoideum. Mol. Biol. Cell, 7, 1623–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang T.H., Xu,X., Kaartinen,V., Heisterkamp,N., Groffen,J. and Bokoch,G.M. (1995) Abr and Bcr are multifunctional regulators of the Rho GTP-binding protein family. Proc. Natl Acad. Sci. USA, 92, 10282–10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C.Y., Lee,S., Briscoe,C., Ellsworth,C. and Firtel,R.A. (2000) Role of Rac in controlling the actin cytoskeleton and chemotaxis in motile cells. Proc. Natl Acad. Sci. USA, 97, 5225–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coso O.A., Chiariello,M., Yu,J.C., Teramoto,H., Crespo,P., Xu,N., Miki,T. and Gutkind,J.S. (1995) The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell, 81, 1137–1146. [DOI] [PubMed] [Google Scholar]
- Daley G.Q., Van Etten,R.A. and Baltimore,D. (1990) Induction of chronic myelogenous leukemia in mice by the P210Bcr/abl gene of the Philadelphia chromosome. Science, 247, 824–830. [DOI] [PubMed] [Google Scholar]
- de Hostos E.L., Bradtke,B., Lottspeich,F., Guggenheim,R. and Gerisch,G. (1991) Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein β subunits. EMBO J., 10, 4097–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontier M., Hocht,P., Mintert,U. and Faix,J. (2000) Rac1 GTPases control filopodia formation, cell motility, endocytosis, cytokinesis and development in Dictyostelium. J. Cell Sci., 113, 2253–2265. [DOI] [PubMed] [Google Scholar]
- Faix J., Clougherty,C., Konzok,A., Mintert,U., Murphy,J., Albrecht,R., Muhlbauer,B. and Kuhlmann,J. (1998) The IQGap-related protein DGAP1 interacts with Rac and is involved in the modulation of the F-actin cytoskeleton and control of cell motility. J. Cell Sci., 111, 3059–3071. [DOI] [PubMed] [Google Scholar]
- Gabriel D., Hacker,U., Kohler,J., Muller-Taubenberger,A., Schwartz,J.M., Westphal,M. and Gerisch,G. (1999) The contractile vacuole network of Dictyostelium as a distinct organelle: its dynamics visualized by a GFP marker protein. J. Cell Sci., 112, 3995–4005. [DOI] [PubMed] [Google Scholar]
- Gerald N., Dai,J., Ting-Beall,H.P. and De Lozanne,A. (1998) A role for Dictyostelium racE in cortical tension and cleavage furrow progression. J. Cell Biol., 141, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaven J.A., Whitehead,I.P., Nomanbhoy,T., Kay,R. and Cerione,R.A. (1996) Lfc and Lsc oncoproteins represent two new guanine nucleotide exchange factors for the Rho GTP-binding protein. J. Biol. Chem., 271, 27274–27381. [DOI] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Hall A.L., Schlein,A. and Condeelis,J. (1988) Relationship of pseudopod extension to chemotactic hormone-induced actin polymerization in amoeboid cells. J. Cell. Biochem., 37, 285–299. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Morris,C. and Groffen,J. (1989) Abr, an active Bcr-related gene. Nucleic Acids Res., 17, 8821–8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Jenster,G., ten Hoeve,J., Zovich,D., Pattengale,P.K. and Groffen,J. (1990) Acute leukaemia in Bcr/Abl transgenic mice. Nature, 344, 251–253. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Kaartinen,V., van Soest,S., Bokoch,G.M. and Groffen,J. (1993) Human Abr encodes a protein with GAPrac activity and homology to the DBL nucleotide exchange factor domain. J. Biol. Chem., 268, 16903–16906. [PubMed] [Google Scholar]
- Hofmann K., Bucher,P., Falquet,L. and Bairoch,A. (1999) The PROSITE database, its status in 1999. Nucleic Acids Res., 27, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G.E., Allen,W.E. and Ridley,A.J. (1998) The Rho GTPases in macrophage motility and chemotaxis. Cell Adhes. Comm., 6, 237–245. [DOI] [PubMed] [Google Scholar]
- Katan M. and Allen,V.L. (1999) Modular PH and C2 domains in membrane attachment and other functions. FEBS Lett., 452, 36–40. [DOI] [PubMed] [Google Scholar]
- Larochelle D.A., Vithalani,K.K. and De Lozanne,A. (1996) A novel member of the rho family of small GTP-binding proteins is specifically required for cytokinesis. J. Cell Biol., 133, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle D.A., Vithalani,K.K. and De Lozanne,A. (1997) Role of Dictyostelium RacE in cytokinesis—mutational analysis and localization studies by use of green fluorescent protein. Mol. Biol. Cell, 8, 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludbrook S.B., Eccleston,J.F. and Strom,M. (1997) Cloning and characterization of a RhoGAP homolog from Dictyostelium discoideum. J. Biol. Chem., 272, 15682–15686. [DOI] [PubMed] [Google Scholar]
- Manstein D.J., Schuster,H.-P., Morandini,P. and Hunt,D.M. (1995) Cloning vectors for the production of proteins in Dictyostelium discoideum. Gene, 162, 129–134. [DOI] [PubMed] [Google Scholar]
- Minden A., Lin,A., Claret,F.X., Abo,A. and Karin,M. (1995) Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell, 81, 1147–1157. [DOI] [PubMed] [Google Scholar]
- Neuhaus E.M., Horstmann,H., Almers,W., Maniak,M. and Soldati,T. (1998) A universal ethane-freezing/methanol-fixation procedure highly improves preservation of structure and antigenicity in immuno fluorescence and pre-embedding immunoelectron microscopy. J. Struct. Biol., 121, 326–342. [DOI] [PubMed] [Google Scholar]
- Olson M.F., Ashworth,A. and Hall,A. (1995) An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science, 269, 1270–1272. [DOI] [PubMed] [Google Scholar]
- Parsons T.D., Coorssen,J.R., Horstmann,H. and Almers,W. (1995) Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron, 15, 1085–1096. [DOI] [PubMed] [Google Scholar]
- Peracino B. et al. (1998) G protein β subunit-null mutants are impaired in phagocytosis and chemotaxis due to inappropriate regulation of the actin cytoskeleton. J. Cell Biol., 141, 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting C.P., Schultz,J., Milpetz,F. and Bork,P. (1999) SMART: identification and annotation of domains from signalling and extracellular protein sequences. Nucleic Acids Res., 27, 229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak A., Fedorov,R., Alexandrov,K., Albert,S., Goody,R.S., Gallwitz,D. and Scheidig,A.J. (2000) Crystal structure of the GAP domain of Gyp1p: first insights into interaction with Ypt/Rab proteins. EMBO J., 19, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi M.J. and Scarlata,S. (1998) Pleckstrin homology domains: a common fold with diverse functions. Annu. Rev. Biophys. Biomol. Struct., 27, 503–528. [DOI] [PubMed] [Google Scholar]
- Ridley A.J. (1997) Signalling by Rho family proteins. Biochem. Soc. Trans., 25, 1005–1010. [DOI] [PubMed] [Google Scholar]
- Ridley A.J. and Hall,A. (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell, 70, 389–399. [DOI] [PubMed] [Google Scholar]
- Rivero F., Albrecht,R., Dislich,H., Bracco,E., Graciotti,L., Bozzaro,S. and Noegel,A.A. (1999) RacF1, a novel member of the Rho protein family in Dictyostelium discoideum, associates transiently with cell contact areas, macropinosomes, and phagosomes. Mol. Biol. Cell, 10, 1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Hannah,M.J. and Huttner,W.B. (1997) Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J. Cell Biol., 137, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J., Milpetz,F., Bork,P. and Ponting,C.P. (1998) smart, a simple modular architecture research tool—identification of signaling domains. Proc. Natl Acad. Sci. USA, 95, 5857–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seastone D.J., Lee,E., Bush,J., Knecht,D. and Cardelli,J. (1998) Overexpression of a novel rho family GTPase, RacC, induces unusual actin-based structures and positively affects phagocytosis in Dictyostelium discoideum. Mol. Biol. Cell, 9, 2891–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soisson S.M., Nimnual,A.S., Uy,M., Barsagi,D. and Kuriyan,J. (1998) Crystal structure of the Dbl and pleckstrin homology domains from the human son of sevenless protein. Cell, 95, 259–268. [DOI] [PubMed] [Google Scholar]
- Steyer J.A., Horstmann,H. and Almers,W. (1997) Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature, 388, 474–478. [DOI] [PubMed] [Google Scholar]
- Tan E.C., Leung,T., Manser,E. and Lim,L. (1993) The human active breakpoint cluster region-related gene encodes a brain protein with homology to guanine nucleotide exchange proteins and GTPase-activating proteins. J. Biol. Chem., 268, 27291–27298. [PubMed] [Google Scholar]
- Tapon N. and Hall,A. (1997) Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr. Opin. Cell Biol., 9, 86–92. [DOI] [PubMed] [Google Scholar]
- Vojtek A.B. and Cooper,J.A. (1995) Rho family members: activators of MAP kinase cascades. Cell, 82, 527–529. [DOI] [PubMed] [Google Scholar]
- Voncken J.W. et al. (1995) Increased neutrophil respiratory burst in Bcr-null mutants. Cell, 80, 719–728. [DOI] [PubMed] [Google Scholar]
- Voncken J.W., Kaartinen,V., Groffen,J. and Heisterkamp,N. (1998) Bcr/Abl associated leukemogenesis in Bcr null mutant mice. Oncogene, 16, 2029–2032. [DOI] [PubMed] [Google Scholar]
- Watts P.J. and Ashworth,J.M. (1970) Growth of myxamoebae of the cellular slime mold Dictyostelium discoideum in axenic culture. Biochem. J., 119, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienke D.C., Knetsch,M.L.W., Neuhaus,E.M., Reedy,M.C. and Manstein,D.J. (1999) Disruption of a dynamin homologue affects endocytosis, organelle morphology, and cytokinesis in Dictyostelium discoideum. Mol. Biol. Cell, 10, 225–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittinghofer A., Scheffzek,K. and Ahmadian,M.R. (1997) The interaction of Ras with GTPase-activating proteins. FEBS Lett., 410, 63–67. [DOI] [PubMed] [Google Scholar]