Abstract
Protein folding mediated by the Hsp70 family of molecular chaperones requires both ATP and the co-chaperone Hdj-1. BAG-1 was recently identified as a bcl-2-interacting, anti-apoptotic protein that binds to the ATPase domain of Hsp70 and prevents the release of the substrate. While this suggested that cells had the potential to modulate Hsp70-mediated protein folding, physiological regulators of BAG-1 have yet to be identified. We report here that the apoptotic regulator Scythe, originally isolated through binding to the potent apoptotic inducer Reaper, shares limited sequence identity with BAG-1 and inhibits Hsp70- mediated protein refolding. Scythe-mediated inhibition of Hsp70 is reversed by Reaper, providing evidence for the regulated reversible inhibition of chaperone activity. As Scythe functions downstream of Reaper in apoptotic induction, these findings suggest that Scythe/Reaper may signal apoptosis, in part through regulating the folding and activity of apoptotic signaling molecules.
Keywords: Hsp70 inhibition/Scythe/Reaper
Introduction
The Hsp70 chaperone proteins facilitate proper protein folding, prevent protein aggregation and assist in the assembly of multi-protein complexes. In this way, Hsp70 family members monitor and counteract the accumulation of potentially harmful misfolded polypeptides, particularly following exposure of the cell to stressful conditions (Hartl, 1996). Comprised of both constitutive and induced members, Hsp70 family members share the ability to recognize exposed hydrophobic patches on non-native proteins and promote their re-folding (Rassow et al., 1995; Rudiger et al., 1997). However, this protein folding requires, in addition to Hsp70/Hsc70, both ATP and a ‘co-chaperone’. The most intensively studied of these co-chaperones, Hdj-1, enhances ATP hydrolysis and concomitant release of the folded protein substrate (Hohfeld et al., 1995; Minami et al., 1996).
In vitro, purified Hsc70 releases non-native substrates in the presence of ATP. However, it was recently reported that Hsp70 can associate in vivo with BAG-1, a protein that prevents release of folded protein substrates, even in the presence of Hdj-1 and ATP (Hohfeld and Jentsch, 1997; Takayama et al., 1997; Demand et al., 1998; Stuart et al., 1998; Nollen et al., 2000). Indeed, BAG-1, the first reported negative regulator of Hsp70 function, forms ternary complexes with Hsp70, Hdj-1 and the substrate, maintaining the substrate in a partially folded, yet soluble state (Bimston et al., 1998; Luders et al., 2000a,b). In effect, without inhibiting Hsp70-mediated nucleotide hydrolysis, BAG-1 uncouples ATP hydrolysis from release of the folded substrate (Bimston et al., 1998).
Although its biochemical role in modulating Hsp70 function is clear, the precise biological function of BAG-1 is not known. Originally isolated as a bcl-2-interacting protein, BAG-1 was subsequently shown to associate with other signaling molecules, including Raf-1, the intracellular domain of the PDGF receptor and a number of steroid hormone receptors (Takayama et al., 1995; Zeiner and Gehring, 1995; Bardelli et al., 1996; Wang et al., 1996; Song et al., 2001). Intriguingly, under some circumstances, overexpression of BAG-1 was reported to have anti-apoptotic activity (Takayama et al., 1995). Structurally, BAG-1 does not have any particularly striking features; however, as originally described by Reed and colleagues, BAG family proteins do share a conserved C-terminal ∼50 amino acid motif dubbed the ‘BAG’ domain (Takayama et al., 1999). In addition, BAG-1 and several of its relatives have an N-terminal domain with a high degree of homology to ubiquitin. The functional significance of this homology is not yet clear.
We recently isolated an apoptotic regulator called Scythe, whose N-terminus, like that of BAG-1, bears marked homology to ubiquitin (Thress et al., 1998). Scythe acts downstream of Reaper, a small (65 amino acid) protein that was identified in a screen for apoptotic regulators in the fly, Drosophila melanogaster. Genetic evidence has implicated Reaper as an important mediator of apoptosis both during development and following DNA damage (White et al., 1994, 1996). Although Reaper homologs have not yet been identified in other systems, fly Reaper can induce apoptosis in human cells and can trigger biochemical hallmarks of apoptosis (mitochondrial cytochrome c release, caspase activation) in cell-free extracts prepared from Xenopus (Evans et al., 1997; McCarthy and Dixit, 1998). These data suggest that Reaper-responsive pathways are highly conserved.
Using recombinant Reaper as an affinity resin, Scythe was purified as a high-affinity Reaper interactor (Thress et al., 1998). As immunodepletion of Scythe from Xenopus egg cell-free extracts prevented both Reaper-induced cytochrome c release and caspase activation, it appeared that Scythe acted downstream of Reaper in the pathway of apoptotic induction. Further studies revealed that Scythe was actually a negative regulator of apoptosis, acting to sequester an as yet unidentified direct inducer of mitochondrial cytochrome c release (Thress et al., 1999). Upon binding of Reaper, Scythe released this factor(s), leading to mitochondrial cytochrome c release, caspase activation and full apoptosis. This series of events was recapitulated in a semi-purified system in that immunoprecipitates of Scythe, when washed extensively and incubated with Reaper, released a factor(s) capable of initiating cytochrome c release directly from purified mitochondria.
In experiments reported here, we show that the apparent similarities between BAG-1 and Scythe (the presence of an N-terminal ubiquitin domain, anti-apoptotic activity) are likely to be more than superficial. Indeed, we show that Scythe, like BAG-1, is a direct inhibitor of Hsp70 protein folding activity. Moreover, a BAG domain in Scythe mediates this inhibition. However, while the physiological means of reversing BAG-1-mediated Hsp70 inhibition are not known, we have found that Reaper can relieve Scythe-mediated repression of Hsp70. These data provide the first evidence for reversibility of Hsp70 inhibition by a co-chaperone ligand.
Results
Scythe bears structural similarity to BAG family proteins
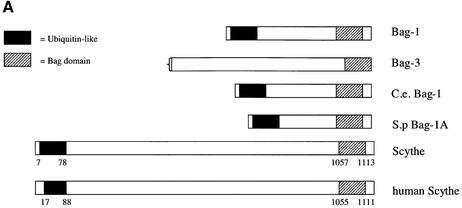
As both Scythe and BAG-1 are anti-apoptotic when overexpressed, and share, along with other BAG family proteins, an N-terminal ubiquitin-like domain, we were interested in the possibility that Scythe might also contain a BAG domain. Clustal alignments of BAG family members and both human and Xenopus Scythe proteins revealed candidate C-terminal BAG domains present in Scythe molecules from both species (Figure 1). While the overall similarity of the BAG domain across different proteins is ∼30%, four strictly conserved residues, found in all BAG family members, are also conserved in Scythe.
Fig. 1. Scythe structurally resembles BAG family proteins. (A) The domains of several BAG family members along with Xenopus and human Scythe showing the relative positions of the ubiquitin-like motif (black) and C-terminal ‘BAG’ domain (striped). The complete open reading frame of BAG-3 has yet to be fully sequenced. C.e., Caenorhabditis elegans; S.p., Schizosaccharomyces pombe. (B) Alignment of the C-terminal BAG domains of the proteins in (A). Dark gray and light gray shading indicate identical and similar residues, respectively.
Scythe binds to the ATPase domain of Hsc70/Hsp70 in a BAG domain-dependent fashion
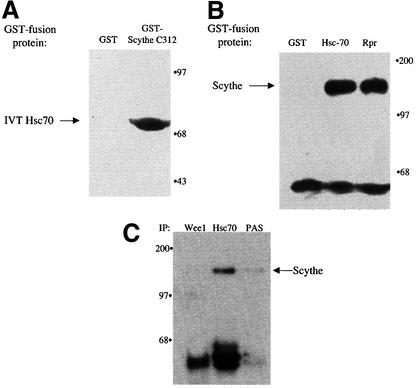
As described above, BAG-1 protein is unusual in its ability to inhibit the protein folding activity of Hsc70/Hsp70 family members; this inhibitory activity depends upon the presence of the BAG domain, which provides a direct binding site for Hsc70/Hsp70 proteins. The presence of a putative BAG domain on Scythe prompted us to examine whether it too might bind Hsc70/Hsp70 proteins. Xenopus egg extract was supplemented with radiolabeled, in vitro translated Hsc70 protein and incubated with Sepharose beads linked to either glutathione S-transferase (GST) or GST fused to the C-terminal half of Scythe (Scythe C312). After pelleting and extensive washing, these resins were examined by SDS–PAGE for the presence of bound Hsc70. As shown in Figure 2A, Hsc70 bound specifically to the Scythe resin. Similarly, Sepharose-linked GST–Hsc70 efficiently bound endogenous Xenopus Scythe from egg extracts, at levels comparable to those obtained using GST–Reaper as bait (Figure 2B). To demonstrate that endogenous Scythe and Hsc70 proteins were able to interact, we immunoprecipitated Hsc70 from Xenopus egg extracts and immunoblotted the samples with anti-Scythe sera. As shown in Figure 2C, endogenous Hsc70 and Scythe proteins co-immunoprecipitated, while Scythe did not associate with a control antibody (anti-Wee1) or protein A–Sepharose alone.
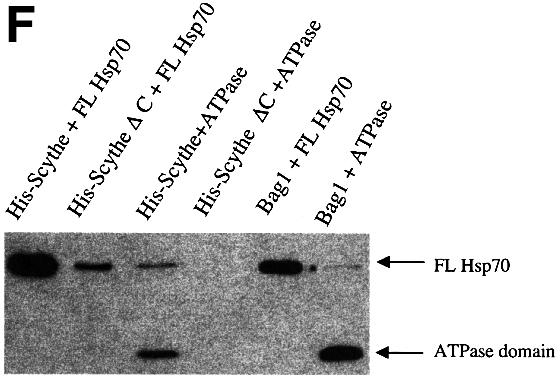
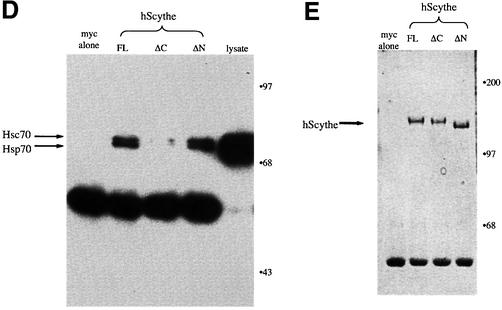
Fig. 2. Scythe binds Hsp70/Hsc70 in a BAG domain-dependent fashion. (A) In vitro translated Xenopus Hsc70 (IVT Hsc70) was added to extracts and incubated for 30 min at 4°C. GST or GST–Scythe C312 immobilized on glutathione–Sepharose beads was added to the extract and incubated for an additional 30 min. The beads were then washed three times with egg lysis buffer (ELB), resolved by SDS–PAGE and bands were visualized by autoradiography. (B) GST or the indicated GST fusion protein was immobilized on glutathione–Sepharose beads and incubated in the presence of Xenopus egg extract for 1 h at 4°C. The beads were then washed three times with ELB, resolved by SDS–PAGE and processed for western blotting with an anti-Scythe polyclonal antibody. (C) Antibodies against Xenopus Wee1 or Hsc70 were coupled to Protein A–Sepharose (PAS) beads and then incubated in Xenopus egg extract for 1 h at 4°C. Immunoprecipitates were washed three times with ELB, resolved by SDS–PAGE and processed for immunoblotting with an anti-Scythe polyclonal antibody. (D) 293T cells were transfected with 3 µg of either the indicated myc-tagged human Scythe construct (hScythe) or the parental myc plasmid alone (myc alone). Thirty-six hours after transfection, cells were lysed, centrifuged, and the supernatants incubated with a monoclonal myc antibody for 1 h at 4°C. PAS beads were then added and, after an additional 1 h incubation, the beads were pelleted, washed three times in lysis buffer, and bound proteins were resolved by SDS–PAGE. After western transfer, the blots were probed with an anti-Hsp70/Hsc70 monoclonal antibody. (E) The identical samples processed in (D) were run on a parallel SDS gel and proteins were stained with Coomassie Brilliant Blue. (F) His-tagged Scythe, His-tagged Scythe lacking the BAG domain (His-Scythe ΔC) or His-tagged BAG-1 was incubated in the presence of either full-length Hsp70 (FL Hsp70) or the ATPase domain of Hsp70 (ATPase) for 1 h at 4°C. His-tagged proteins were recovered using a nickel resin and bound proteins were separated by SDS–PAGE. After western transfer, the blots were probed with an anti-Hsp70 monoclonal antibody.
To determine whether Scythe–Hsc70 interactions could be observed between human proteins in intact cells, full-length myc-tagged human Scythe transfected into 293T cells was immunoprecipitated from cell lysates with anti-myc antibody and examined for the presence of bound Hsc70 protein. As anticipated, human Scythe and endogenous Hsc70 could, like their Xenopus counterparts, be co-precipitated. Importantly, as reported for BAG-1 protein, deletion of the BAG domain (hScythe ΔC; removal of the C-terminal 81 amino acids of Scythe), but not the N-terminal ubiquitin domain (hScythe ΔN) from Scythe completely abrogated binding to Hsc70 (Figure 2D), despite equal levels of expression of mutant and wild-type proteins (Figure 2E). Collectively, these data indicate that Scythe binds specifically to Hsc70/Hsp70 and that this binding is mediated by Scythe’s C-terminal BAG domain.
As Scythe, like BAG-1 protein, associates with Hsc70 through its BAG domain, we wished to determine whether Scythe also behaved like BAG-1 in its ability to interact specifically with the ATPase domain of Hsc70/Hsp70. Accordingly, we conducted a series of binding studies using His-tagged Scythe produced in baculovirus vectors and bacterially produced GST-tagged Hsp70 proteins. As was seen in the 293T lysates, full-length Hsp70 bound efficiently to full-length Scythe, while Scythe ΔC was greatly impaired in its ability to bind Hsp70 (Figure 2F). Importantly, full-length Scythe was also able to bind to the isolated ATPase domain of Hsp70, while deletion of the BAG domain from Scythe completely abrogated this association. These data are consistent with previously reported findings demonstrating that human Scythe can bind to a short sequence within the ATPase domain of the Hsp70-like protein, Stch (Kaye et al., 2000).
Scythe functions as a negative regulator of Hsp70 chaperone activity
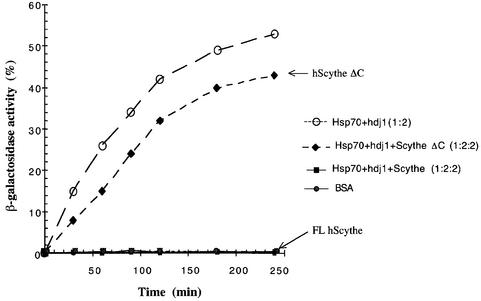
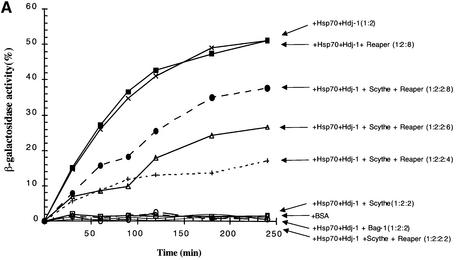
Among the group of co-chaperones/Hsp70 interactors, BAG-1 is the only protein reported to negatively regulate the protein folding ability of Hsc70/Hsp70 proteins (Takayama et al., 1997; Bimston et al., 1998; Nollen et al., 2000). Given the parallels between Scythe and BAG-1, we wished to determine whether Scythe might be a functional relative of BAG-1, able to negatively modulate the protein folding ability of Hsp70. Typically, in vitro protein folding assays examine the ability of Hsp70 chaperones to refold denatured test substrates. These assays appear to reflect faithfully the modulation of Hsp70 activity by both activators and inhibitors, even though the test substrates are not the true in vivo targets of the modulator–Hsp70 complex (Takayama et al., 1997; Nollen et al., 2000). Accordingly, we asked whether Scythe could alter the ability of Hsp70 to refold denatured β-galactosidase. Following denaturation in 6 M guanidine HCl, recombinant β-galactosidase was incubated in refolding buffer with purified recombinant Hsp70, ATP and the co-chaperone Hdj-1 in the presence or absence of baculovirus-produced Scythe protein. After various times of incubation, samples were assayed for β-galactosidase activity using the colorimetric substrate o-nitrophenyl-β-d-galactopyranoside. As reported previously, recombinant Hsp70/Hdj-1 allowed refolding of denatured β-galactosidase, with 50% recovery of enzymatic activity (Figure 3). However, when full-length Scythe was added to the assay at a 1:2 molar ratio with Hsp70, the Hsp70-mediated refolding of denatured β-galactosidase was completely inhibited (Figure 3). Importantly, the refolding assay was not appreciably inhibited by Scythe protein lacking the BAG domain (Scythe ΔC) (Figure 3). These data strongly suggest that Scythe has the previously unanticipated ability to act in a BAG-1-like manner to inhibit Hsp70-mediated protein refolding.
Fig. 3. Scythe functions as a negative regulator of Hsp70 chaperone activity. The inhibitory effect of full-length human Scythe (FL hScythe, 3.2 µM) and a truncated Scythe mutant lacking the ‘BAG’ domain (Scythe ΔC, 3.2 µM) on Hsp70-dependent refolding was examined by the percentage recovered activity of unfolded β-galactosidase (3.2 nM) diluted into a refolding buffer containing ATP (1 mM), Hsp70 (1.6 µM) and Hdj-1 (3.2 µM). As a control for spontaneous refolding, denatured β-galactosidase (3.2 nM) was diluted into refolding buffer containing 3.2 µM bovine serum albumin (BSA).
Reaper relieves Scythe-mediated inhibition of Hsp70
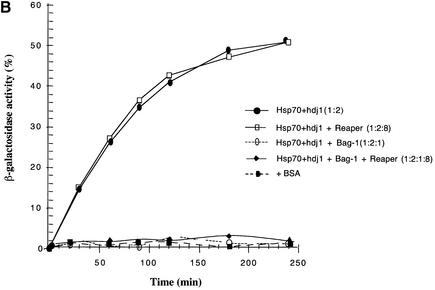
Although BAG-1 protein clearly inhibits Hsp70’s protein folding activity, its mode of regulation has yet to be elucidated. It has been postulated that BAG-1-mediated repression of Hsp70 must be reversible by as yet undiscovered ligands. As Scythe was originally identified as a Reaper ligand, we wished to determine whether Reaper might reverse Scythe’s antagonistic effect on Hsp70-mediated protein folding. To test this, we added increasing amounts of Reaper to the in vitro β-galactosidase refolding assay. As shown in Figure 4A, Reaper was able to relieve Scythe’s inhibition of Hsp70 refolding, with 80% reversal at a 1:4 (Scythe:Reaper) molar ratio. It is unclear whether this molar ratio reflects true complex stoichiometries because a significant fraction of bacterially produced Reaper protein may be insoluble/non-functional. Nonetheless, similar amounts of Reaper had no effect on BAG-1-mediated inhibition of Hsp70, indicating that the reversal was specific for Scythe (Figure 4B). These data demonstrate that Reaper binding provides an effective means of reversing Scythe’s inhibition of Hsp70 function.
Fig. 4. Reaper specifically relieves Scythe-mediated inhibition of Hsp70. (A) The experiment shown in Figure 3 was repeated with 3.2 µM human Scythe in the presence of increasing concentrations of Reaper (3.2–12.8 µM) to examine the reversal of Scythe-mediated inhibition on Hsp70-mediated refolding. (B) The inhibitory effect of BAG-1 (1.6 µM) on refolding was observed in the presence or absence of 12.8 µM Reaper, demonstrating that Reaper-induced reversal is specific for Scythe. As a control for spontaneous refolding, denatured β-galactosidase (3.2 nM) was diluted into refolding buffer containing 3.2 µM BSA.
Reaper inhibits the physical association of Scythe and Hsp70
Since a Scythe mutant unable to physically bind Hsp70 (ΔC Scythe) could not inhibit Hsp70-mediated protein refolding, we postulated that Reaper might act by rendering Scythe unable to bind Hsp70. To examine this issue, we conducted a series of protein binding studies using recombinant Reaper, Scythe and Hsp70 proteins. His-tagged Scythe was incubated with Hsp70 in the presence of increasing amounts of recombinant Reaper. As shown in Figure 5A, Reaper effectively inhibited the Scythe–Hsp70 interaction, while having a considerably less substantial effect on the BAG-1–Hsp70 association. These results are consistent with Reaper’s ability to reverse the functional effects of Scythe, but not BAG-1, on Hsp70-mediated protein re-folding. A similar reversal of Scythe–Hsc70 binding was observed when we added recombinant GST–Reaper protein, but not GST alone, to Scythe immunoprecipitates from 293 cells (Figure 5B). Collectively, these data demonstrate that Reaper binding to Scythe both displaces Hsc70 and reverses Scythe-mediated inhibition of Hsp70 function.
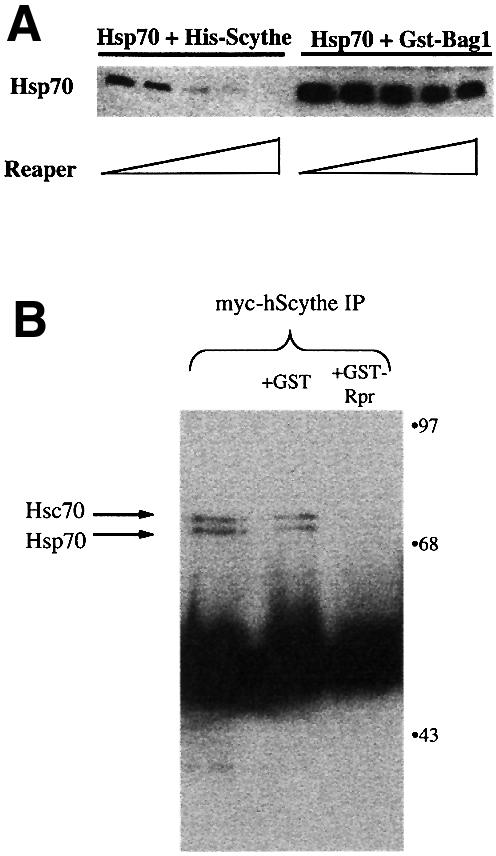
Fig. 5. Reaper specifically inhibits the physical association of Scythe and Hsp70. (A) His-Scythe (1 µM) or GST–BAG-1 (1 µM) was incubated with Hsp70 (1 µM) in refolding buffer. After complex formation, increasing concentrations (0, 2, 4, 8, 10 µM) of Reaper were added. Bound proteins were precipitated with either Ni+-agarose (His-Scythe) or glutathione–Sepharose (GST–BAG-1), washed, resolved by SDS–PAGE and processed for western blotting using Hsp70 monoclonal antibody 5a5. (B) 293T cells were transfected with 5 µg of myc-tagged human Scythe (myc-hScythe). Thirty-six hours after transfection, cells were lysed and centrifuged, and supernatants were incubated with recombinant GST or GST–Reaper (GST–Rpr) for 30 min at 4°C. Subsequently, the lysates were incubated with a monoclonal myc antibody for 1 h at 4°C. PAS beads were then added and, after an additional 1 h incubation, the beads were pelleted, washed three times in lysis buffer, and bound proteins were resolved by SDS–PAGE. After western transfer, the blots were probed with an anti-Hsc70 monoclonal antibody.
The BAG domain is required for sequestration of Scythe-associated cytochrome c-releasing activity
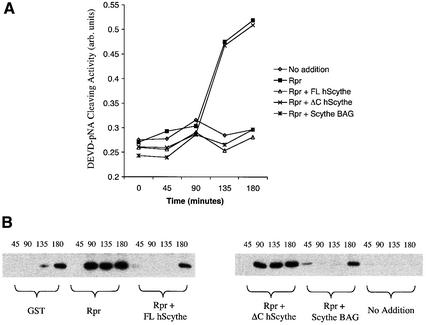
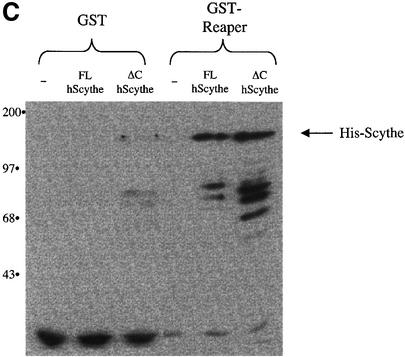
As described above, addition of recombinant Reaper to cell-free extracts of Xenopus eggs triggers a number of biochemical hallmarks of apoptosis, including mitochondrial cytochrome c release, caspase activation, and internucleosomal DNA cleavage and fragmentation of added nuclei. As we reported previously, addition of excess recombinant Scythe to Xenopus egg extracts inhibits Reaper-induced apoptosis. We therefore speculated that the exogenously added Scythe protein could re-sequester cytochrome c-releasing factors dissociated from endogenous Scythe by Reaper. In agreement with this interpretation, the material released from Scythe immunoprecipitates by Reaper could no longer induce cytochrome c release if first incubated with recombinant Scythe protein (Thress et al., 1999). In order to determine whether the ability of Scythe to interact with Hsc70 is important for its ability to sequester cytochrome c-releasing factor(s), egg extracts were supplemented with Reaper in combination with either wild-type human Scythe or Scythe unable to bind Hsc70 (ΔC Scythe). Under these conditions, the wild-type protein, but not the ΔC mutant Scythe, markedly dampened caspase activation and mitochondrial cytochrome c release in response to Reaper addition (Figure 6A and B). Importantly, while ΔC Scythe was unable to bind Hsc70 and inhibit Reaper-induced caspase activation, the ΔC and wild-type Scythe proteins bound Reaper to a similar extent (Figure 6C). Furthermore, the BAG domain of Scythe seems to be sufficient for these effects, as GST protein fused to the isolated BAG domain from Scythe (Scythe BAG) was as effective as excess full-length Scythe in abrogating Reaper-induced caspase activation and cytochrome c release (Figure 6A and B). These data suggest that the BAG domain, required for both Hsc70 binding and inhibition of Hsc70/Hsp70-mediated protein folding is also required for effective re-sequestration of the apoptosis-inducing factors released from endogenous Scythe by Reaper.
Fig. 6. BAG-deficient human Scythe is unable to protect against Reaper-induced apoptosis. (A) Recombinant Reaper (Rpr) protein (300 ng/µl) was added to Xenopus egg extracts in combination with equivalent amounts of recombinant full-length human Scythe (FL hScythe), BAG-deficient human Scythe (ΔC hScythe) or just the BAG domain of human Scythe (Scythe BAG). At the indicated times, 2 µl aliquots of extract were analyzed for caspase activity by cleavage of the artificial caspase substrate, DEVD-pNA. Following cleavage, released pNA was measured spectrophotometrically. (B) Samples were processed as in (A), but 15 µl aliquots were filtered through a 0.1 µM microfilter to remove particulate components, including mito chondria, and samples were processed for immunoblotting with an anti-cytochrome c monoclonal antibody. (C) Recombinant GST or GST–Reaper fusion proteins immobilized on glutathione–Sepharose beads were added to Xenopus egg extract and incubated at 4°C in the presence of equivalent amounts of either His-tagged full-length human Scythe (FL hScythe) or BAG-deficient Scythe (ΔC hScythe) proteins. After 1 h, the beads were pelleted, washed three times with ELB, resuspended in SDS sample buffer, and processed for immunoblotting using a monoclonal penta-His antibody.
Discussion
Scythe is a Reaper-interacting protein critical for Reaper-induced apoptosis in Xenopus egg extracts. In this report, we demonstrate that Scythe is also a modulator of Hsc70/Hsp70, able to inhibit chaperone-mediated protein folding. Reaper, in turn, inhibits this activity of Scythe. These findings raise the intriguing possibility that regulation of protein folding plays an important role in control of apoptosis by Reaper.
Reaper reversal of Scythe-mediated Hsp70 repression
Although BAG-1 was previously shown to inhibit Hsp70 function, a ligand able to associate with the BAG-1–Hsp70–substrate complex, dissociate BAG-1 and allow resumption of protein folding was only speculated (Bimston et al., 1998). Clearly, if BAG-1, or molecules like it, are to be considered viable regulators of protein homeostasis, the inhibition of Hsp70 must be reversible. In this report, we have identified Reaper as a ligand capable of reversing Scythe-mediated inhibition of Hsp70. Coincident with this reversal, Scythe is displaced from Hsp70, in accordance with the hypothetical scheme originally proposed for BAG-1. Interestingly, Reaper binds to Scythe at a site distinct from the BAG domain (between amino acids 235 and 312 of Scythe); therefore, the displacement of Hsc70 from Scythe is not the result of competitive binding of Reaper to the same site (C.Holley, K.Thress and S.Kornbluth, unpublished observations). Rather, we hypothesize that Reaper promotes a conformational change in Scythe, leading to dissociation of Hsp70.
Scythe–Hsp70 and apoptotic regulation
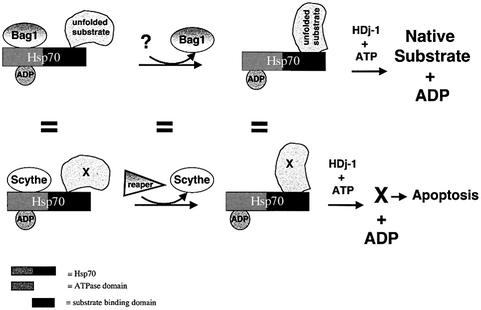
How does Scythe-mediated inhibition of Hsp70 protein folding relate to the ability of Scythe/Reaper to regulate apoptosis? Building on the model originally proposed for BAG-1 (Figure 7), we hypothesize that the cytochrome c-releasing factor(s) sequestered by Scythe (denoted as ‘X’ in Figure 7) is held in a soluble, partially folded state through binding to Hsc70–Scythe complexes. Although we do not yet know whether cytochrome c-releasing factors form direct contacts with both Scythe and Hsp70, Scythe, like BAG-1, binds to the ATPase domain of Hsp70 (Figure 2F), making the substrate-binding domain of Hsp70 potentially available for binding ‘X’ or similar molecules. Moreover, if Scythe functions mechanistically like BAG-1, it is likely that the contact between Hsc70/Hsp70 and ‘X’ is direct. Upon binding to Reaper, Scythe–Hsp70 complexes dissociate; according to our model, Hsp70 thus relieved of its inhibition then goes on to fold ‘X’, leading to cytochrome c release, caspase activation, etc.
Fig. 7. Model for Reaper/Scythe function. BAG-1 binds to the ATPase domain of Hsp70 and inhibits its ability to mediate protein folding. In the presence of a hypothetical ligand, BAG-1 is released from Hsp70, promoting release of native substrate. In the case of Scythe we hypothesize that a similar series of events occurs; however, Reaper serves as the ligand to trigger Scythe dissociation from the Hsp70 complex. According to this speculative model, ‘X’ is released in its native form and can then trigger mitochondrial cytochrome c release and caspase activation. The figure has been adapted from Bimston et al. (1998).
Superficially, Hsc70 seems to fit the description of ‘X’ itself, i.e. a protein bound to Scythe and dissociated by addition of Reaper. However, multiple experiments have failed to reveal any direct cytochrome c-releasing activity of recombinant Hsc70 protein (data not shown). Given the abundance of Hsp70 family members in the cell, this is not surprising. Nonetheless, the ability of Scythe to interact with Hsc70 appears to be important for its ability to sequester cytochrome c-releasing factor(s); a Scythe molecule able to interact with Reaper (data not shown), but lacking the BAG domain, could not behave like excess wild-type Scythe in preventing Reaper-induced apoptosis.
Although we have not yet definitively identified the Scythe-associated cytochrome c-releasing factors, the only factors thus far demonstrated to have direct cytochrome c-releasing activity are pro-apoptotic members of the bcl-2 family (Desagher et al., 1999; Gross et al., 1999; Shimizu et al., 1999). That ‘X’ may indeed be a bcl-2 family member is supported by several observations. First, the cytochrome c-releasing activity of ‘X’ released from Scythe can be abrogated by incubation with recombinant bcl-xL, an anti-apoptotic bcl-2 family member that can act through heterodimerization with its pro-apoptotic counterparts (Zha et al., 1997). Secondly, we have recently identified a relatively well conserved BH3 domain in the C-terminus of Scythe (M.Olson and S.Kornbluth, un published observations). BH domains (bcl-2-like heterodimerization domains), of which there are four types (BH1–BH4), are contiguous sequences shown to be critical for the association of bcl-2 family members (reviewed in Gross et al., 1999). Although BH3-containing proteins may, themselves, be cytochrome c-releasing factors, Scythe alone does not seem to have such an activity. We speculate, therefore, that the BH3 domain of Scythe is a docking site for a heterodimerized pro-apoptotic bcl-2 family member. This possibility is currently under investigation. However, since bcl-2 family members may form higher order multimers (e.g. for formation of pores in mitochondrial membranes), the role of Scythe-bound Hsc70 may, in this context, be to assemble higher order complexes, rather than to properly fold monomeric ‘X’ (Adams and Cory, 1998; Lewis et al., 1998).
Although our studies have thus far been confined to analysis of a Drosophila protein (Reaper) in a vertebrate system (Xenopus egg extracts), we note that the function of Scythe and its BAG domain may ultimately prove to be important in the context of fly apoptosis. As has recently been described, the Drosophila genome contains an apparent Scythe homolog (Adams et al., 2000; Jasny, 2000). Moreover, in preliminary studies we have found that in vitro translated fly Scythe can bind to fly Reaper (K.Thress and S.Kornbluth, unpublished).
Regulation of cellular signaling through modulation of Hsp70/Hsc70
Although Hsp70 family chaperones are abundant in the cell, proteins like BAG-1 and Scythe may confer substrate specificity on these proteins, promoting protein folding/assembly of bound substrates in a regulated manner. Held in an inactive or sequestered state, Hsc70 substrates bound to Scythe/BAG-like proteins could be tightly controlled by binding of specific ligands able to dissociate Scythe–Hsc70 or BAG-1–Hsc70 complexes. That these reactions would have the requisite specificity is highlighted by the fact that Reaper was able to reverse Scythe-mediated inhibition of Hsp70 protein folding, while having no effect on similar inhibition by BAG-1. As alluded to earlier, BAG-1 binds a number of cellular signaling molecules; it is not known whether Scythe is similarly diverse in its binding partners. Moreover, it is not clear whether distinct ligands might have differential effects on different BAG–substrate or Scythe–substrate complexes. Nonetheless, regulatory networks of ligand– co-chaperone–Hsc70 proteins as exemplified by Reaper–Scythe–Hsc70 offer a novel means to regulate the activity of cell signaling molecules critical for cell proliferation, cell death or cellular responses to stress.
Materials and methods
Preparation of Xenopus egg extracts
For induction of egg laying, mature female frogs were injected with 100 U of Pregnant Mare Serum Gonadotropin (PMSG) (Calbiochem) to induce oocyte maturation, followed by injection (3–28 days later) with human chorionic gonadotropin (HCG; USB). Fourteen to twenty hours after injection with HCG, eggs were harvested for extract production. Jelly coats were removed from eggs by incubation with 2% cysteine pH 7.8, washed three times in modified Ringers solution (1 M NaCl, 20 mM KCl, 10 mM MgSO4, 25 mM CaCl2, 5 mM HEPES pH 7.8, 0.8 mM EDTA), and then washed in ELB [250 mM sucrose, 2.5 mM MgCl2, 1.0 mM dithiothreitol (DTT), 50 mM KCl, 10 mM HEPES pH 7.4]. Eggs were packed by low speed centrifugation at 400 g. Following addition of aprotinin and leupeptin (final concentration 5 µg/ml), cytochalasin B (final concentration 5 µg/ml) and cycloheximide (final concentration 50 µg/ml), eggs were lysed by centrifugation at 10 000 g for 15 min. For nuclear formation, extracts were supplemented with demembranated sperm chromatin (1000 nuclei/µl) and an ATP-regenerating system (10 mM phosphocreatine, 2 mM ATP and 50 mg/ml creatine phosphokinase). Recombinant proteins added to extracts were diluted in XB buffer (50 mM sucrose, 100 mM KCl, 0.1 mM CaCl2, 1 mM MgCl2, 10 mM K–HEPES pH 7.7) and added at a concentration of 300 ng/µl, unless otherwise indicated.
Preparation of GST fusion proteins
Full-length Xenopus Hsc70 was PCR amplified using the following primers: 5′-GATCTCTAGACATGTCTAAGGGACCAGCAGTT-3′ and 5′-GATCCTCGAGTTAGTCAACCTCCTCAATAGT-3′. PCR fragments were cloned into the XbaI–XhoI sites of the expression vector Gex KG, a derivative of Gex 2T (Pharmacia) containing additional polylinker sites and a polyglycine insert, and transformed into the Topp 1 bacterial strain (Stratagene). Recombinant protein was produced as previously described (Evans et al., 1997). Full-length Drosophila Reaper and the C-terminal 312 amino acids of Scythe (Scythe C312) fused with GST were produced in a similar manner and constructed as described in Thress et al. (1998). GST fusions of human BAG-1 and Hsp70 were produced as previously described (Bimston et al., 1998).
Baculovirus production of human Scythe protein
Full-length human Scythe (FL hScythe) and a truncated hScythe lacking the C-terminal 81 amino acids (hScythe ΔC) were both PCR amplified to possess a C-terminal His6 tag and cloned into the XbaI–XhoI sites of the pFastBac vector. Protein was produced using the Bac-to-Bac Baculovirus Expression System (Gibco). Briefly, the resulting hScythe-pFastBac donor plasmids were transformed into DH10Bac Escherichia coli cells. Escherichia coli containing recombinant bacmid were cultured and recombinant bacmid DNA was recovered using a standard miniprep protocol. SF-9 insect cells were transfected with the bacmid DNA using CellFECTIN reagent (Gibco), incubated for 48 h at 27°C, and resulting recombinant baculovirus particles were harvested. Subsequently, SF-9 cells (2 × 106 cells/ml) were infected with baculovirus for 48 h, washed twice in phosphate-buffered saline and lysed by dounce homogenization in HBS [10 mM HEPES pH 7.5, 20 mM β-glycerolphosphate, 150 mM NaCl, 5 mM EGTA, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 10 µg/ml each of pepstatin, chymostatin and leupeptin]. Lysate was then centrifuged at 4°C for 10 min at 10 000 r.p.m., and the supernatant was incubated with 1 ml of Ni-NTA agarose (Qiagen) for 30 min at 4°C. The beads were washed in 50 vols of HBS and eluted with HBS containing 200 mM imidazole in five fractions of 500 µl each.
Cell culture and transfections
FL hScythe, hScythe lacking the N-terminal 87 amino acids (hScythe ΔN) or hScythe ΔC was PCR amplified and cloned into a modified pcDNA3.1 mammalian expression vector (Invitrogen) containing an in-frame C-terminal myc tag. 293T cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Sigma). Cells (5 × 105) were plated onto 60 mm dishes and 24 h later transfected with 3 µg of the appropriate myc-tagged hScythe construct or vector alone DNA using the Fugene transfection reagent (Roche biochemicals) as per manufacturer’s instructions.
Immunoprecipitations
Thirty-six hours after transfection, cells were harvested in lysis buffer (10 mM Tris pH 7.5, 50 mM NaCl, 5 mM EDTA, 1 mM PMSF, 2% Tween-20, 10% glycerol) and lysates were incubated with anti-myc monoclonal antinbody (Santa Cruz) for 2 h at 4°C. PAS beads were added to lysates, incubated for an additional 1 h, pelleted and washed three times in lysis buffer. Bound proteins were solubilized with sample buffer and resolved by SDS–PAGE. Resolved proteins were transferred to PVDF, blotted with an anti-Hsp70/Hsc70 monoclonal antibody (Affinity Bioreagents), incubated with HRP-linked goat anti-mouse secondary antibody and detected using the ECL system (Amersham).
Protein refolding assays
Protein refolding assays were conducted as previously described (Freeman et al., 1996).
DEVDase assay
To measure caspase activity, 3 µl of each sample were incubated with 90 µl of assay buffer (50 mM HEPES pH 7.5, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA, 10% glycerol) and the colorimetric substrate Ac-DEVD-pNA (final concentration 200 mM; Biomol Caspase-3 assay system) at 37°C. At various time points, absorbance was measured at 405 nm in a LabSystems MultiSkan MS microtiter plate reader.
Acknowledgments
Acknowledgements
Thanks to D.Lew for critical reading of the manuscript. This work was supported by grants from the NIH to S.K. (ROI GM56518 and ROI GM61919) and R.I.M. S.K. is a scholar of the Leukemia and Lymphoma Society.
References
- Adams J.M. and Cory,S. (1998) The Bcl-2 protein family: arbiters of cell survival. Science, 281, 1322–1326. [DOI] [PubMed] [Google Scholar]
- Adams M.D. et al. (2000) The genome sequence of Drosophila melanogaster. Science, 287, 2185–2195. [DOI] [PubMed] [Google Scholar]
- Bardelli A., Longati,P., Albero,D., Goruppi,S., Schneider,C., Ponzetto,C. and Comoglio,P.M. (1996) HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J., 15, 6205–6212. [PMC free article] [PubMed] [Google Scholar]
- Bimston D., Song,J., Winchester,D., Takayama,S., Reed,J.C. and Morimoto,R.I. (1998) BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J., 17, 6871–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demand J., Luders,J. and Hohfeld,J. (1998) The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol. Cell. Biol., 18, 2023–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S., Osen-Sand,A., Nichols,A., Eskes,R., Montessuit,S., Lauper,S., Maundrell,K., Antonsson,B. and Martinou,J.C. (1999) Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol., 144, 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E.K., Kuwana,T., Strum,S.L., Smith,J.J., Newmeyer,D.D. and Kornbluth,S. (1997) Reaper-induced apoptosis in a vertebrate system. EMBO J., 16, 7372–7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B.C. and Morimoto,R.I. (1996) The human cytosolic molecular chaperones hsp90, hsp70 (hsc70) and hdj-1 have distinct roles in recognition of a non-native protein and protein refolding. EMBO J., 15, 2969–2979. [PMC free article] [PubMed] [Google Scholar]
- Gross A., Yin,X.M., Wang,K., Wei,M.C., Jockel,J., Milliman,C., Erdjument-Bromage,H., Tempst,P. and Korsmeyer,S.J. (1999) Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem., 274, 1156–1163. [DOI] [PubMed] [Google Scholar]
- Hartl F.U. (1996) Molecular chaperones in cellular protein folding. Nature, 381, 571–579. [DOI] [PubMed] [Google Scholar]
- Hohfeld J. and Jentsch,S. (1997) GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1 [published erratum appears in EMBO J., 1998, 17, 847]. EMBO J., 16, 6209–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohfeld J., Minami,Y. and Hartl,F.U. (1995) Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell, 83, 589–598. [DOI] [PubMed] [Google Scholar]
- Jasny B.R. (2000) The universe of Drosophila genes. Science, 287, 2181. [DOI] [PubMed] [Google Scholar]
- Kaye F.J., Modi,S., Ivanovska,I., Koonin,E.V., Thress,K., Kubo,A., Kornbluth,S. and Rose,M.D. (2000) A family of ubiquitin-like proteins binds the ATPase domain of Hsp70-like Stch. FEBS Lett., 467, 348–355. [DOI] [PubMed] [Google Scholar]
- Lewis S., Bethell,S.S., Patel,S., Martinou,J.C. and Antonsson,B. (1998) Purification and biochemical properties of soluble recombinant human Bax. Protein Expr. Purif., 13, 120–126. [DOI] [PubMed] [Google Scholar]
- Luders J., Demand,J. and Hohfeld,J. (2000a) The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J. Biol. Chem., 275, 4613–4617. [DOI] [PubMed] [Google Scholar]
- Luders J., Demand,J., Papp,O. and Hohfeld,J. (2000b) Distinct isoforms of the cofactor BAG-1 differentially affect Hsc70 chaperone function. J. Biol. Chem., 275, 14817–14823. [DOI] [PubMed] [Google Scholar]
- McCarthy J.V. and Dixit,V.M. (1998) Apoptosis induced by Drosophila reaper and grim in a human system. Attenuation by inhibitor of apoptosis proteins (cIAPs). J. Biol. Chem., 273, 24009–24015. [DOI] [PubMed] [Google Scholar]
- Minami Y., Hohfeld,J., Ohtsuka,K. and Hartl,F.U. (1996) Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J. Biol. Chem., 271, 19617–19624. [DOI] [PubMed] [Google Scholar]
- Nollen E.A., Brunsting,J.F., Song,J., Kampinga,H.H. and Morimoto,R.I. (2000) Bag1 functions in vivo as a negative regulator of Hsp70 chaperone activity. Mol. Cell. Biol., 20, 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow J., Voos,W. and Pfanner,N. (1995) Partner proteins determine multiple functions of Hsp70. Trends Cell Biol., 5, 207–212. [DOI] [PubMed] [Google Scholar]
- Rudiger S., Buchberger,A. and Bukau,B. (1997) Interaction of Hsp70 chaperones with substrates. Nature Struct. Biol., 4, 342–349. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Narita,M. and Tsujimoto,Y. (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature, 399, 483–487. [DOI] [PubMed] [Google Scholar]
- Song J., Takeda,M. and Morimoto,R.I. (2001) Hsp70–BAG1 complex mediates a physiological stress signaling pathway that regulates Raf1/ERK and cell growth. Nature Cell Biol., in press. [DOI] [PubMed] [Google Scholar]
- Stuart J.K., Myszka,D.G., Joss,L., Mitchell,R.S., McDonald,S.M., Xie,Z., Takayama,S., Reed,J.C. and Ely,K.R. (1998) Characterization of interactions between the anti-apoptotic protein BAG-1 and Hsc70 molecular chaperones. J. Biol. Chem., 273, 22506–22514. [DOI] [PubMed] [Google Scholar]
- Takayama S., Sato,T., Krajewski,S., Kochel,K., Irie,S., Millan,J.A. and Reed,J.C. (1995) Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell, 80, 279–284. [DOI] [PubMed] [Google Scholar]
- Takayama S., Bimston,D.N., Matsuzawa,S., Freeman,B.C., Aime-Sempe,C., Xie,Z., Morimoto,R.I. and Reed,J.C. (1997) BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J., 16, 4887–4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S., Xie,Z. and Reed,J.C. (1999) An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J. Biol. Chem., 274, 781–786. [DOI] [PubMed] [Google Scholar]
- Thress K., Henzel,W., Shillinglaw,W. and Kornbluth,S. (1998) Scythe: a novel reaper-binding apoptotic regulator. EMBO J., 17, 6135–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thress K., Evans,E.K. and Kornbluth,S. (1999) Reaper-induced dissociation of a Scythe-sequestered cytochrome c-releasing activity. EMBO J., 18, 5486–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.G., Takayama,S., Rapp,U.R. and Reed,J.C. (1996) Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc. Natl Acad. Sci. USA, 93, 7063–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K., Grether,M.E., Abrams,J.M., Young,L., Farrell,K. and Steller,H. (1994) Genetic control of programmed cell death in Drosophila. Science, 264, 677–683. [DOI] [PubMed] [Google Scholar]
- White K., Tahaoglu,E. and Steller,H. (1996) Cell killing by the Drosophila gene reaper. Science, 271, 805–807. [DOI] [PubMed] [Google Scholar]
- Zeiner M. and Gehring,U. (1995) A protein that interacts with members of the nuclear hormone receptor family: identification and cDNA cloning. Proc. Natl Acad. Sci. USA, 92, 11465–11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha J., Harada,H., Osipov,K., Jockel,J., Waksman,G. and Korsmeyer,S.J. (1997) BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J. Biol. Chem., 272, 24101–24104. [DOI] [PubMed] [Google Scholar]