Abstract
The enhanced stress resistance exhibited by starved bacteria represents a central facet of virulence, since nutrient depletion is regularly encountered by pathogens in their natural in vivo and ex vivo environments. Here we explore the notion that the regular stress responses, which are mediated by enzymatically catalyzed chemical transactions and promote endurance during the logarithmic growth phase, can no longer be effectively induced during starvation. We show that survival of bacteria in nutrient-depleted habitats is promoted by a novel strategy: finely tuned and fully reversible intracellular phase transitions. These non-enzymatic transactions, detected and studied in bacteria as well as in defined in vitro systems, result in DNA sequestration and generic protection within tightly packed and highly ordered assemblies. Since this physical mode of defense is uniquely independent of enzymatic activity or de novo protein synthesis, and consequently does not require energy consumption, it promotes virulence by enabling long-term bacterial endurance and enhancing antibiotic resistance in adverse habitats.
Keywords: biocrystallization/DNA segregation/Dps/starvation/virulence
Introduction
The intrinsic chemical and physical vulnerability of DNA molecules (Lindahl, 1993), and the lethal effects caused by unrepaired DNA lesions even when they occur at low frequency, highlight the need for particularly efficient DNA protection mechanisms. Eukaryotes maintain the integrity of their DNA through two distinct pathways. Detrimental chemicals are neutralized and damage is repaired through enzymatically catalyzed chemical processes. These biochemical pathways are amplified by the tight nucleosomal assembly that provides a highly effective structural protection against DNA-modifying agents (Ljungman and Hanawalt, 1992). Such a lasting mode of DNA protection is, however, absent in prokaryotes, which lack nucleosomal organization and whose chromatin is characterized by a significantly lower ratio of non-specific DNA-binding proteins to DNA than that found in eukaryotes (Kellenberger and Arnold, 1992).
In bacterial natural habitats, exposure to DNA-damaging factors such as oxidating and alkylating agents, radicals or UV irradiation is often accompanied by nutrient depletion. The outcome of DNA lesions sustained during starvation is particularly severe, since rapidly growing bacteria contain several copies of their chromosome whereas only one copy is usually found in stationary-state cells (Givskov et al., 1994). Owing to this lack of redundancy, DNA lesions in starved bacteria can not be repaired through homologous recombination pathways that are highly effective in actively growing cells. Moreover, recombination and excision DNA repair pathways require rapid synthesis of numerous enzymes, whose exquisitely regulated activities are heavily ATP dependent (Lin and Sancar, 1992). The extravagance of DNA defense pathways is underscored by the adaptive response to DNA alkylation, where an entire protein molecule is expended in order to repair a single damaged base (Samson, 1992). Whereas such imposing requirements can be effectively met as long as nutrients are plentiful, they can hardly be deployed in starved cells, where enzymes are rapidly and progressively degraded (Stadtman, 1992), and de novo protein synthesis as well as energy-generating processes are seriously impaired (Huisman et al., 1996). Thus, basic kinetic and thermodynamic considerations point towards a fundamental enigma: how is the broad and constitutive DNA protection that characterizes stationary-state bacteria achieved?
Starved Escherichia coli cells produce a non-specific DNA-binding protein termed Dps (Almiron et al., 1992; Altuvia et al., 1994), which accumulates to a very large amount and constitutes the major component of the chromatin in late stationary-phase bacteria (Azam et al., 1999). Within DNA–Dps complexes, the stability of Dps is dramatically enhanced relative to the stability of the free protein (Almiron et al., 1992), and DNA is effectively protected (Martinez and Kolter, 1997). Close Dps homologs were identified in distantly related bacteria (Chen and Helmann, 1995; Pena et al., 1995), implying that this protein maintains a general and crucial function. We have reported recently that interaction between purified Dps and DNA results in the rapid formation of extremely stable DNA–Dps co-crystals, and that crystalline assemblies of similar morphology are present in starved E.coli cells that overexpress Dps (Wolf et al., 1999).
In this study we show that DNA packaging and segregation within DNA–Dps micro-crystalline assemblies occur in wild-type bacteria and result in a generic, starvation-dependent DNA protection. The packaging is found to proceed through a non-enzymatic and fully reversible phase transition that is tightly regulated by the intracellular concentration of polyvalent cations. DNA protection in bacteria that lack Dps is shown to derive from a different mode of phase transition: DNA collapse into a liquid-crystalline phase. On the basis of these observations, we raise the notion that DNA protection in starved bacteria relies mainly on physical, rather than chemical, processes. Since these transactions do not require enzymatic activity or de novo protein synthesis, and hence do not entail energy consumption, they can be effectively deployed during prolonged and severe starvation.
Results
DNA protection in starved bacteria: DNA–Dps co-crystals
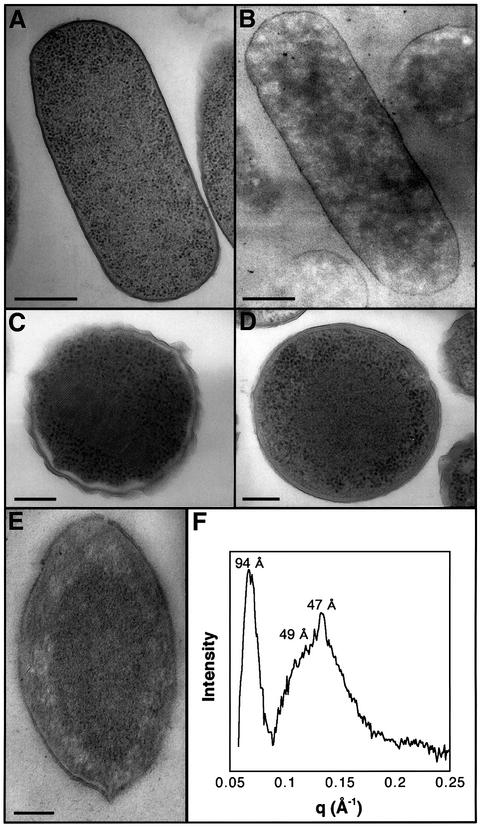
The chromatin in electron micrographs of actively growing wild-type bacteria is demarcated as amorphous ribosome-free spaces that are irregularly spread over the cytoplasm (Figure 1A and B). Such an extensive and apparently random chromatin distribution is consistent with a state of high metabolic activity, reflecting the multitude of ongoing DNA transactions (Hobot et al., 1985; Robinow and Kellenberger, 1994). A strikingly different morphology is exhibited in starved E.coli cells. Two days following the onset of a stationary state, bacteria that overexpress the Dps protein as well as wild-type cells exhibit a prominent demixing of the chromatin and the ribosomes, with the ribosomes localized at the periphery of the cytoplasm. Whereas DNA–Dps co-crystals are formed in starved bacteria that overexpress Dps (Figure 1C), such structures are only seldom detected in starved wild-type cells. In the majority of these cells, a dense mass, from which the ribosomes are completely excluded, is observed in the center of the cytoplasm (Figure 1D). Notably, this massive reorganization is also exhibited by bacteria treated with chloramphenicol, a protein-synthesis blocker, 2 h following the onset of the stationary state. To assess the composition of the dense mass, which is formed irrespective of the growth medium (LB or M63), the DNA-specific stain osmium-ammine (Vazquez et al., 1995) was applied to electron microscopy sections of starved bacteria. In contrast to the extensive DNA spreading indicated by this method in actively growing cells (Figure 1B), starved bacteria exhibit heavy staining that exclusively co-localizes with the central dense mass (Figure 1E), thus indicating massive DNA segregation within this region.
Fig. 1. Electron microscopy and X-ray scattering patterns of E.coli cells. (A) Wild-type E.coli at mid-logarithmic phase. The dark particles are ribosomes. The ribosome-free spaces contain chromatin. (B) Escherichia coli at mid-logarithmic phase, stained solely with the DNA-specific reagent osmium-ammine-SO2. The irregular spreading of chromatin over the cytoplasm is indicated. (C) Dps-overproducing cell induced at mid-logarithmic phase and further incubated for 48 h. (D) Wild-type E.coli incubated for 48 h following the onset of the stationary phase. (E) Same as (D), but stained with the DNA-specific reagent osmium-ammine-SO2. Samples were prepared by the cryo-fixation method. Scale bars are 400 nm (A and B) and 150 nm (C–E). (F) X-ray scattering patterns from intact wild-type E.coli cells, presented as a difference profile, which is obtained by subtracting the scattering curve of mid-logarithmic phase bacteria, in which no bands are discerned, from the scattering profile of E.coli cells incubated for 48 h following the onset of stationary phase.
We have shown previously that ordered intracellular structures can be detected and characterized by X-ray scattering measurements conducted on intact cells (Reich et al., 1994). In order to gain insight into the structural features of the dense central region detected in stationary-state bacteria, we applied this non-invasive technique to actively growing and starved E.coli cells. Starved wild-type bacteria exhibit two X-ray bands: a relatively narrow band that corresponds to a spacing of 94 Å, and a broad band in which two maxima, at 49 and 47 Å, could be discerned (Figure 1F). Similar diffraction patterns are revealed by stationary-state bacteria that slightly overproduce the Dps protein, whereas actively growing cells or starved bacteria incubated for 2 h in fresh media do not exhibit any X-ray diffraction maxima.
The diffraction patterns are consistent with a DNA–Dps structure in which Dps and DNA form stacked alternat ing layers. According to this model, the diffraction at 94 Å corresponds to the intra-layer spacing between Dps dodecamers whose diameter is ∼90 Å (Grant et al., 1998). The broad band centered at 47–49 Å is suggested to represent a superposition of a second order Dps–Dps diffraction and DNA–DNA spacing. Notably, similar DNA–DNA spacings were detected in tightly packed DNA assemblies in vitro (Radler et al., 1997) and in vivo (Reich et al., 1994). On the basis of the X-ray scattering patterns, the intracellular DNA staining and the findings that Dps is the most abundant DNA-binding protein in starved bacteria and the predominant protein bound to DNA when DNA is isolated from such bacteria (Azam et al., 1999; our unpublished observations), we propose that the central region in stationary wild-type cells consists of tightly packed DNA–Dps microcrystals.
DNA–Dps co-crystallization is tuned by doubly charged ions
Bacteria that are induced to overexpress the Dps protein early in their logarithmic phase continue to proliferate at an unperturbed growth rate, although Dps accumulates in these cells to large amounts (Martinez and Kolter, 1997). This observation is in marked contrast to the effects of other DNA-binding proteins such as H-NS and SASP, whose overexpression in actively growing E.coli leads to a massive DNA condensation and rapid loss of viability (Setlow et al., 1991; Spurio et al., 1992). We find that actively growing Dps-overexpressing cells do not reveal any discrete X-ray scattering peaks, and their morphology is indistinguishable from that of actively growing wild-type bacteria. Thus, the formation of DNA–Dps crystals or microcrystals, as well as the segregation of the nucleoid and the ribosomes, is a growth phase-dependent phenomenon. Two interrelated questions are raised by these findings. The first involves the nature of the signal that specifically induces the formation of ordered DNA–Dps complexes in stationary-state bacteria. The second question concerns the actual mode of DNA–Dps interaction. This issue is raised by the observation that Dps does not reveal any recognizable DNA-binding motifs (Almiron et al., 1992; Grant et al., 1998), and particularly by the finding that the surface of the Dps dodecameric assembly, which is the plausible DNA-binding species, is dominated by negative charges (Grant et al., 1998), as are DNA molecules.
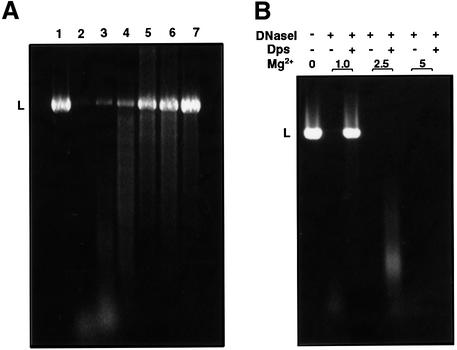
In order to address these issues, we studied the factors that affect in vitro DNA–Dps co-crystallization. The process was found to be highly dependent upon Mg2+ ions. In a solution containing 20 mM NaCl and 1.0 mM divalent ion chelator EDTA, neither DNA–Dps co-aggregates nor crystalline structures could be detected, whereas large and highly ordered crystals were rapidly formed when the EDTA concentration was reduced to 0.4 mM (Figure 2A). Only small crystals could be observed when Mg2+ was added to a final concentration of 1 mM, and no assemblies were obtained when the concentration of the doubly charged ion was raised to 3.0 mM (Figure 2B and C). Notably, elevated concentrations of either EDTA (presumed to chelate Mg2+ ions that remain bound to the Dps during its purification) or of Mg2+ not only inhibit the formation of the co-crystals but also act to destroy pre-existing ordered complexes, thus demonstrating the reversibility of the co-crystallization process. The salient effects exerted by Mg2+ ions upon DNA–Dps interaction are further indicated by DNA protection studies. When DNA is exposed to DNase I following incubation with Dps, progressive DNA protection is observed as the Dps:DNA ratio is increased (Figure 3A). Yet, when Mg2+ ions are included in the reaction mixture in increasing amounts, DNA protection is progressively eliminated and is completely abolished at 5.0 mM Mg2+ (Figure 3B). The dependency of DNA–Dps interaction and DNA protection upon Mg2+ ions is unaffected by monovalent ions in the concentration range 20–100 mM.
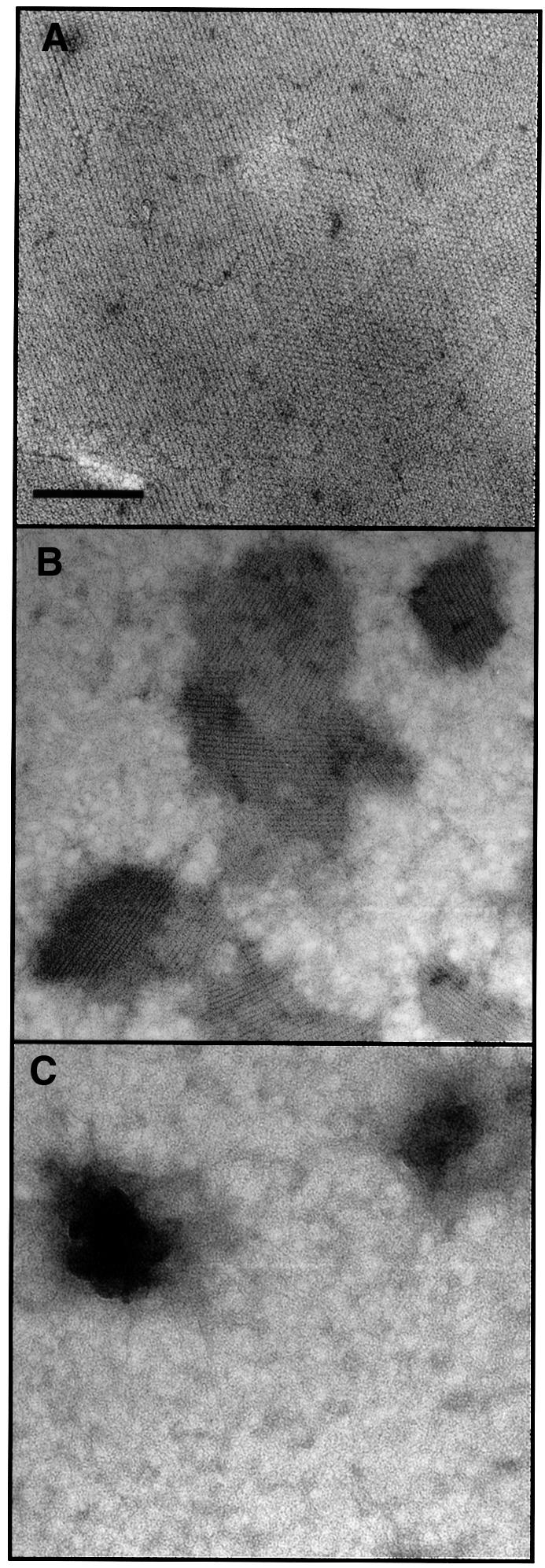
Fig. 2. Effects of Mg2+ on DNA–Dps co-crystallization. Dps and closed- circular DNA (at a protein:DNA weight ratio of 1:5) were incubated for 10 min in 10 mM Tris pH 7.0, 20 mM NaCl, 0.4 mM EDTA, with the following Mg2+ concentrations: (A) no Mg2+ added, large DNA–Dps co-crystals (as the assembly shown here, which spans the whole field of the micrograph) are regularly detected; (B) 1.0 mM, only small ordered complexes are obtained; (C) 3.0 mM, no co-crystalline assemblies can be detected under such conditions. The magnification in (A), (B) and (C) is identical; scale bar is 150 nm.
Fig. 3. Effects of Mg2+ on Dps-mediated DNA protection. (A) Dps-mediated DNA protection. Lane 1, linear DNA (pBluescript, 2958 bp, linearized with EcoRI); lane 2, linear DNA treated with DNase I (1 U, 5 min at room temperature); lanes 3–7, DNase I treatment of Dps–DNA complexes at the following Dps:DNA weight ratios: 0.5, 1.0, 2.0, 3.0 and 5.0, respectively. (B) Mg2+ effects. Dps–DNA complexes (5:1 w/w protein:DNA ratio) with or without added Mg2+ were treated with DNase I. The Mg2+ row designates the concentration of Mg2+ in mM; the Dps and DNase I rows designate the presence or absence of these substances in the reaction mixtures. Note that nuclease activity is preserved at the whole range of [Mg2+] used, as indicated by control experiments consisting of DNA, DNase I and Mg2+ at various concentrations, but no Dps (left lane of each [Mg2+]).
Thus, both DNA–Dps co-crystallization and Dps-related DNA protection depend upon the specific concentration of Mg2+ ions. In addition to substantiating the notion that protection is directly related to DNA–Dps co-crystallization, these observations provide a clue to the mode by which the negatively charged Dps dodecamers interact with DNA. We propose that DNA–Dps complex formation is mediated through multiple ion bridges that are maintained by doubly charged cations. Significantly, such ion bridges can be formed only in a particular range of Mg2+ concentration. Above or below this range, both DNA and Dps dodecamers exhibit surfaces that are predominantly positively or negatively charged, respectively, and hence electrostatically repel each other.
We proceeded to examine the effects of both Mg2+ and Fe2+ ions as well as of the polyvalent cation spermidine upon the morphology of starved E.coli cells. As described above, stationary-state bacteria assemble their DNA in DNA–Dps micro co-crystals that segregate in the central part of the cytoplasm and are conspicuously separated from the ribosomes. In sharp contrast, no DNA–Dps dense assembly or DNA–ribosome separation could be detected in cells that were starved for 2 days in media supplemented with 5 mM Mg2+, 2.5 mM spermidine or 10 µM Fe2+. Similarly, no crystalline structures were observed under these conditions in starved bacteria that overproduce Dps. In both cases, the morphology is indistinguishable from that revealed by actively growing cells. In addition, starved wild-type or Dps-overproducing bacteria incubated in magnesium-, spermidine- or iron-enriched media do not reveal any discrete X-ray scattering peaks, indicating that such conditions prevent the formation of ordered DNA–Dps complexes.
On the basis of these results, and the observation that the concentration of free Mg2+ ions within E.coli cells in depleted media is essentially identical to that in the environment (Hurwitz and Rosano, 1967), we claim that extracellular divalent cations provide an on–off signal for intracellular DNA–Dps co-crystallization. We do not assign a signaling role to polyvalent species such as spermidine, since the concentration of these species that is required to affect the in vivo assembly is too high to be physiologically relevant. Thus, as long as the media contain nutrients that sustain fast and active growth, including divalent ions in relatively high concentrations, DNA–Dps complex formation is prevented due to electrostatic repulsion. Yet, bacterial proliferation in a defined in vivo or ex vivo environment leads to a progressive depletion of divalent ions due to rapid consumption or host defense activities. As the concentration of the ions decreases below a threshold value, ion bridges between DNA and Dps dodecamers can be formed, resulting in binding. When fresh nutrients, including divalent ions, are supplied, the reverse process occurs, leading to the release of DNA from the DNA–Dps co-crystals. This correlation provides a straightforward interpretation of the finding that Dps overexpression during logarithmic phase neither interferes with bacterial proliferation nor leads to the formation of ordered DNA–Dps complexes.
DNA protection in starved dps– bacteria: DNA cholesteric phase
Three-day-starved wild-type E.coli have been shown to be essentially unaffected by relatively high doses of oxidating agents, such as 45 mM H2O2 for 40 min, whereas identical treatment led to a rapid loss of viability of more than seven orders of magnitude in 3-day-starved dps-knockout bacteria (Almiron et al., 1992). We find, however, that 6-day-starved dps-knockout cells exposed to H2O2 exhibit a viability loss of less than two orders of magnitude, while wild-type bacteria remain unaffected. This observation, which indicates a large acquisition of an effective Dps-independent mode of protection following prolonged starvation, prompted us to study the morphology of dps-knockout cells.
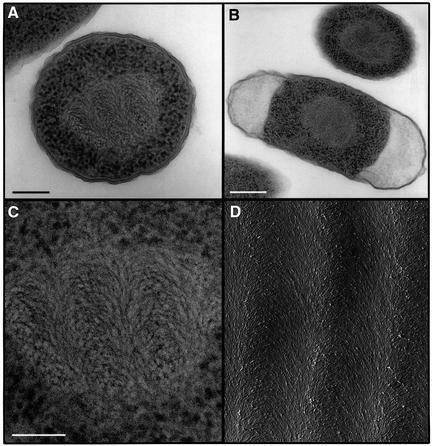
The morphological features of actively growing dps– bacteria are indistinguishable from those revealed by actively growing wild-type E.coli. While 5-day-old dps– bacteria still exhibit the same morphology, a dramatically different structure is revealed after an additional day. As observed for stationary wild-type E.coli, 6-day-old dps– cells exhibit a total separation of the ribosomes, localized at the periphery of the cell, from the DNA, which occupies the central part of the cytoplasm (Figure 4A–C). Yet, the morphology of the DNA in the starved mutant differs conspicuously from that revealed by starved wild-type cells, assuming a configuration of parallel rows of nested arcs. Very similar DNA patterns were detected previously in slowly growing bacteria and in primitive algae (Livolant, 1991), and were suggested to represent a cholesteric liquid crystalline DNA organization (Leforestier and Livolant, 1993). In this arrangement, DNA segments are ordered in pseudoplanes that rotate with respect to each other to form a helicoidal structure. A projection into a plane of an oblique section of such an organization yields parallel rows of nested arcs (Leforestier and Livolant, 1993), as indeed is observed in electron micrographs depicting an obliquely fractured in vitro cholesteric phase obtained from pure DNA molecules (Figure 4D).
Fig. 4. Electron microscopy of Dps– cells and of an in vitro DNA cholesteric phase. (A and B) Dps– cells from cultures incubated for 6 days following the onset of a stationary phase, exhibiting a cholesteric DNA organization. Dps– bacteria harvested following shorter incubation periods revealed morphological patterns identical to those exhibited by wild-type E.coli at mid-logarithmic phase. (C) Magnification of the central region of the cell depicted in (A). Scale bars in (A), (B) and (C) are 150, 300 and 100 nm, respectively. (D) Electron micrograph (provided by Dr A.Leforestier) of an in vitro cholesteric phase of pure DNA molecules (146 bp; 200 mg/ml). The sample has been freeze-fractured following cryo-fixation at an oblique angle to the cholesteric stratification (Leforestier and Livolant, 1993). The nested arcs that characterize the cholesteric phase are detected in both the in vivo and in vitro samples.
Purified DNA molecules undergo a spontaneous transition from isotropic to liquid crystalline phases upon alteration of the solvent properties or when the DNA concentration approaches that found in living systems (Livolant, 1991; Leforestier and Livolant, 1993; Sikorav et al., 1994; Pelta et al., 1996). The tight DNA packaging within a cholesteric organization has been shown to substantially reduce the accessibility of DNA molecules to a variety of damaging factors, including irradiation, radicals and nucleases (Weinberger et al., 1988; Spotheim-Maurizot et al., 1992; Newton et al., 1996). Thus, formation of a DNA liquid-crystalline phase in starved Dps– cells is proposed to provide an alternative pathway for a generic, structurally based DNA protection. This notion is strongly supported by the observation that the DNA reorganization detected in dps– cells coincides with the enhanced resistance to H2O2 that is acquired by these cells. The reorganization is unaffected by the extracellular concentration of doubly charged cations, but is accelerated by the presence of polyamines in the growth medium. Whereas a cholesteric DNA phase is detected in Dps– cells following 5–6 days of ‘regular’ starvation, bacteria supplemented with 0.2 mM spermidine at the onset of the stationary phase reveal a cholesteric organization after only 4 days.
As is the case for wild-type bacteria, the morphological traits detected in starved Dps– cells are completely reversible: the morphology of 6-day-old cells incubated in fresh medium for 6 h is identical to that of actively growing bacteria. Yet, 6-day-old Dps– cells form detectable colonies on Luria–Bertani (LB) plates only 2–3 h after colonies of stationary-phase wild-type bacteria can be detected. Since the growth rates of the two strains in either rich or poor media are identical, this observation implies that wild-type cells are capable of resuming growth following long-term starvation faster than Dps– bacteria.
Discussion
Attenuated efficiency of enzymatically mediated DNA protection during starvation
All currently known prokaryotic defense pathways rely on inducible mechanisms that are activated by specific signals. Since inducible responses need to cope with assaults that are already present, they must evolve and reach full capacity at the highest degree of speed and efficiency. In order to meet this requirement, bacterial stress strategies contain elaborate integrated circuits and branched regulatory cascades that enable a burst-like induction of one or several regulons as a response to a particular assault. Activation of inducible stress responses is thus associated with massive and rapid metabolic readjustments that require de novo synthesis of numerous proteins, some to very large quantities. Such metabolic alterations are fundamentally incompatible with a state of prolonged starvation, during which the supply of exogenous substrates is limited or non-existent. The ability of starved bacteria to resist environmental assaults is, therefore, assigned to a maintenance mode that is mediated by a group of proteins whose synthesis is triggered at the onset of the stationary phase and proceeds for several hours (Hengge-Aronis, 1993, 1996; Huisman et al., 1996; Dukan and Nyström, 1999). In order for a maintenance strategy to be effective, two fundamental requirements must be met. Enzymes must remain functional throughout the duration of the stationary phase, and the energy required for the chemical processes that they catalyze must be available.
Neither of these requirements can be fulfilled effectively during prolonged periods of starvation. A growing body of evidence points to an accelerated oxidation and degradation of proteins during stationary phase. Significantly, proteins that are involved directly in bacterial stress responses, including the heat shock protein DnaK and the DNA-binding protein H-NS, were found to be particularly susceptible to oxidative damage (Dukan and Nyström, 1998, 1999). Thus, proteins are continuously depleted as starvation proceeds, leading to a severely and progressively impaired ability of the cells to maintain stress responses. Even more imposing are considerations that pertain to energy supplies. Environmental assaults are generally met through enzymatically catalyzed chemical reactions that act to neutralize detrimental agents or to repair damaged cellular components. For these reactions to be effective, their intrinsic thermodynamic equilibrium must, in most cases, be perturbed towards desired products. This bias is commonly achieved by coupling the reaction to the hydrolysis of high-energy species such as ATP. Yet, in stationary-phase bacteria, the main source of high-energy compounds is the degradation of endogenous components: membranes and ribosomes (Nyström et al., 1996). Thus, unless tightly regulated, enzymatic repair activities may, by themselves, lead to an irreversible loss of cell integrity or of essential cellular functions (Nyström et al., 1996). It follows that inducible chemically dependent defense pathways that are highly effective during active growth or short periods of nutrient depletion are fundamentally incompatible with prolonged starvation.
Intracellular structural phase transitions and DNA protection
We find that when inducible defense strategies that are based on enzymatically catalyzed chemical transactions can no longer be deployed effectively, DNA protection is dominated by fully reversible and finely tuned physical transactions: intracellular phase separation and structural phase transitions. These processes result in ordered DNA structures whose condensed organization limits accessibility to detrimental factors. In wild-type stationary-state bacteria, the abundant Dps protects DNA through DNA–Dps co-crystallization. The Dps is uniquely suitable to promote this mode of protection. The exceptional propensity of the protein to co-crystallize with DNA (Wolf et al., 1999), along with the dramatically enhanced stability of both DNA and Dps following complex formation (Almiron et al., 1992), indicate that the assembly can outlast prolonged starvation and highly adverse conditions.
Revealing a predominantly negatively charged surface (Grant et al., 1998), the Dps dodecamer can not bind directly to DNA molecules. Indeed, we find that both the in vitro and in vivo DNA–Dps co-crystallization, as well as Dps-related DNA protection, are strictly dependent upon the presence and precise concentration of doubly charged cations. Dications are thus proposed to act as ion bridges, which, due to electrostatic considerations, can be formed only within a particular concentration range of the divalent species. This notion is supported by the observations that intracellular DNA–Dps complexes are not formed upon expression—or overexpression—of Dps during logarithmic growth, when divalent ions are abundant, and that both the in vitro and in vivo DNA–Dps co-crystals are very rapidly disrupted upon addition of dications. Notably, the highly specific concentrations of the divalent ions that are required for the formation or disruption of the DNA–Dps complexes, and the fast kinetics of these ion-dependent processes even in extensively starved bacteria, strongly imply a direct electrostatic role of the dications in the binding process. Moreover, the pronounced similarity between the well-defined in vitro DNA–Dps co-crystals and the intracellular structures (Wolf et al., 1999) indicates that the in vivo co-crystals of these negatively charged species do not contain an additional macromolecule, acting as an intermediate.
DNA–Dps interaction through ion bridges that are mediated by divalent ions allows for an exquisite regulation of both the co-crystallization and the DNA release processes: slight alterations in the intracellular concentrations of the divalent species determine the direction of the phase transition between a dispersed and a crystalline DNA organization. Clearly, such an electrostatic mode of regulation could not have been accomplished with a positively charged DNA-binding protein. The proposed role of divalent ions as an on–off switch is of fundamental physiological significance: upon bacterial infection, the host actively reduces the availability of iron ions in the phagosomes as part of its defense strategy against pathogens (Mahan et al., 1996). Moreover, it has been suggested that phagosome maturation is accompanied by a decrease in Mg2+ ions within the phagosomal milieu to very low levels, and that this decrease acts as a primary environmental signal for the activation of the PhoPQ virulence system in Salmonella (Vescovi et al., 1996). As such, the protection strategy presented in this study provides a new aspect to the notion that pathogenic bacteria have evolved effective means to exploit the host defense mechanisms (Finlay and Cossart, 1997).
The morphology of bacterial chromatin is determined by the interplay of two factors: macromolecular crowding, which promotes DNA condensation, and coupled transcription–translation processes that act as an expansion force (Woldringh et al., 1995). DNA collapse into a cholesteric organization in starved dps– bacteria can be interpreted in terms of such a balance. Specifically, following prolonged starvation, transactions in which DNA is involved are limited. Moreover, since Dps is the dominant DNA-binding protein in starved wild-type bacteria (Azam et al., 1999), its absence in stationary-state Dps– cells and the continuous degradation of other proteins (Dukan and Nyström, 1998) lead to a particularly low and progressively decreasing protein:DNA ratio in the chromatin of these cells. Thus, at a given time during the stationary state, the balance between DNA condensation and expansion forces is tipped towards condensation, leading to a spontaneous DNA collapse into a tightly packed cholesteric organization. The finding that the addition of positively charged polyamines to the growth medium accelerates the formation of the intracellular cholesteric phase in stationary Dps– cells is consistent with the fact that these species promote an isotropic–cholesteric DNA transition in vitro (Sikorav et al., 1994; Pelta et al., 1996). Notably, phosphate starvation has been shown to effect enhanced degradation of threonine and arginine to spermidine, resulting in the accumulation of this polyamine in stationary-state bacteria (Gérard et al., 1999).
DNA reorganization into a cholesteric phase is accompanied by a segregation of the chromatin and the ribosomes. This phenomenon can be directly attributed to entropic effects. The entropic cost of packing spherical particles (i.e. ribosomes) with rod-like molecules is large enough to exceed the entropic cost of total demixing (Madden and Herzfeld, 1993; Minsky et al., 1997). Consequently, a concentrated cholesteric phase of the DNA rods is formed, which completely excludes ribosomes. Similarly, ribosomes are excluded from the tightly packed DNA–Dps co-crystals that are formed in stationary wild-type bacteria. As such, the morphological reorganization exhibited by starved wild-type and by Dps– cells can be considered as a manifestation of intracellular phase separation and phase transition processes.
The structural transitions into crystalline or liquid-crystalline DNA morphologies that promote DNA protection during starvation do not require de novo protein synthesis or enzymatically catalyzed chemical transformations, and hence require no input of energy. Thus, these transactions can be effectively induced during prolonged starvation. The abundance of close Dps homologs in diverse bacteria implies that this physical mode of DNA protection is general and crucial. An elegant facet of this strategy concerns the signals that control the phase transitions. Fluctuation of divalent cation concentrations in the natural in vivo or ex vivo habitats, as well as alterations of the protein:DNA ratio or of the intracellular amounts of polyamines, are intrinsic features of starvation (Gérard et al., 1999) or of the host defense pathways against pathogens (Mahan et al., 1996; Vescovi et al., 1996). Moreover, the very short time span over which the structural transitions occur in all cells implies that gradual changes in the concentrations of divalent ions, polyamines or any other potential signals do not elicit the transitions until a threshold is reached by either an increase or decrease in the value of the signal. Thus, starvation-dependent signals act as an on–off switch of the intracellular phase transitions that can be rapidly elicited and, most significantly, swiftly reversed when nutrients are supplied. These considerations raise two additional points. The faster resumption of growth revealed by wild-type cells relative to Dps– bacteria following long-term starvation implies that DNA protection through DNA–Dps co-crystallization can be more rapidly and effectively adjusted than that achieved by means of a cholesteric organization and is, as such, advantageous. Moreover, DNA protection through structural sequestration is conceptually akin to the constitutive protection exhibited by sporulating bacteria. Sporulation is, however, associated with an early and total commitment: once initiated, it proceeds to its end, irrespective of changes in the environmental conditions (Parker et al., 1996). In this respect, defense through structural phase transitions is superior to sporulation as it does not commit the cells.
Finally, since DNA protection by means of structural phase transitions is independent of enzymatically catalyzed chemical transactions, it is likely to be unperturbed by antibiotics that function through interference with enzymatic activity. In light of the intimate correlation between starvation and virulence, this study points to the possibility that application of agents that perturb intracellular phase transitions in conjunction with antibiotics may promote the efficiency of antimicrobial treatments.
Materials and methods
Electron microscopy
Wild-type E.coli (ZK126), dps-knockout cells (Almiron et al., 1992) or Dps overproducer (ZK126 dps::cm; Martinez and Kolter, 1997) were grown in LB or M63 medium at 37°C for the time periods indicated in the figure legends. Bacteria were fixed by ultra-fast freezing in liquid ethane and cryo-substituted as described (Hobot et al., 1985). Samples were embedded in Epon; thin sections were stained with 1% uranyl acetate and examined on a Philips CM12 electron microscope operating at 100 kV. Three independent experiments were conducted for each set of conditions, and in each experiment >1000 cell slices were screened. Notably, similar morphologies were detected in bacteria fixed by either cryo or chemical methods. Since these two fixation modes proceed through fundamentally different mechanisms (Hobot et al., 1985), this similarity indicates that the morphologies observed are genuine.
Intracellular localization of DNA was performed with the DNA-specific stain osmium-ammine-SO2 (Vazquez et al., 1995). Grid-mounted thin sections of Epon-embedded bacteria were floated on 5 N HCl for 30 min at room temperature, washed with distilled water and treated with osmium ammine-B (Polysciences) in 8 N acetic acid and 40 mM sodium metabisulfite for 1 h at 37°C. Sections were then rinsed thoroughly with distilled water, dried and studied without additional staining. Experiments aimed at the intracellular localization of Dps with anti-Dps antibody failed. This failure is assigned to the high degree of similarity between Dps and bacterioferritin (Grant et al., 1998), which resulted in cross-reactions of the antibody and hence in a poor signal-to-noise ratio of the in situ labeling procedure.
X-ray scattering measurements of intact cells
Specimens consisted of bacterial pellets prepared from 3 ml cultures. In order to minimize radiation-induced damage and to obtain a higher density of bacteria within them, pellets were treated with 2% glutaraldehyde in 0.1 M sodium cacodylate pH 7.4 at room temperature. Two or three independently prepared samples of each category were examined. X-ray measurements were carried out at the National Synchrotron Source, Brookhaven National Laboratory. A multi-wire position-sensitive detector was used for data acquisition, with exposure times of 5 min. Background subtraction and cylindrical averaging of the two-dimensional data about the pattern center in order to obtain a one-dimensional plot of intensity versus scattering vector were accomplished using software written by M.Capel (Brookhaven National Laboratory).
DNA protection assay
Dps was allowed to interact with linear DNA (2 µg of pBluescript, 2958 bp, linearized with EcoRI) for 1 h at room temperature in 10 mM NaCl, 0.5 mM EDTA, 10 mM Tris pH 7.2; the protein:DNA ratio and MgCl2 concentrations are indicated in Figure 3. Following incubation, the complex was treated for 5 min with 1 U of DNase I at 37°C. Reactions were stopped by incubation at 75°C for 10 min, followed by treatment with proteinase K (50 µg/ml), 5 mM MgCl2, 2% SDS and 0.3 M sodium acetate for 1 h at 37°C. The protein was extracted with phenol, DNA was precipitated and loaded on a 1% agarose gel.
Acknowledgments
Acknowledgements
We thank Amélie Leforestier for the electron micrograph of the in vitro DNA cholesteric phase. We also thank Benjamin Geiger, Adam Heller, Frederick C.Neidhardt, Thomas Nyström, Conrad L.Woldringh and Michael Yarmolinsky for critical reading of the manuscript and helpful comments. This work was supported by the Israel Science Foundation founded by the Academy of Sciences and Humanities, and by the Minerva Foundation, Germany.
References
- Almiron M., Link,A.J., Furlong,D. and Kolter,R. (1992) A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev., 6, 2646–2654. [DOI] [PubMed] [Google Scholar]
- Altuvia S., Almiron,M., Huisman,G., Kolter,R. and Storz,G. (1994) The dps promoter is activated by OxyR during growth and by IHF and σ S in stationary phase. Mol. Microbiol., 13, 265–272. [DOI] [PubMed] [Google Scholar]
- Azam A.T., Iwata,A., Nishimura,A., Ueda,S. and Ishihama,A. (1999) Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol., 181, 6361–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. and Helmann,J.D. (1995) Bacillus subtilis MrgA is a Dps(PexB) homologue: evidence for metalloregulation of an oxidative-stress gene. Mol. Microbiol., 18, 295–300. [DOI] [PubMed] [Google Scholar]
- Dukan S. and Nyström,T. (1998) Bacterial senescence: stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental induction of the heat shock regulon. Genes Dev., 12, 3431–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukan S. and Nyström,T. (1999) Oxidative stress defense and deterioration of growth-arrested Escherichia coli cells. J. Biol. Chem., 274, 26027–26032. [DOI] [PubMed] [Google Scholar]
- Finlay B.B. and Cossart,P. (1997) Exploitation of mammalian host cell functions by bacterial pathogens. Science, 276, 718–725. [DOI] [PubMed] [Google Scholar]
- Gérard F., Dri,A.M. and Moreau,P.L. (1999) Role of Escherichia coli RpoS, LexA and H-NS global regulators in metabolism and survival under aerobic, phosphate-starvation conditions. Microbiology, 145, 1547–1562. [DOI] [PubMed] [Google Scholar]
- Givskov M., Eberl,L. and Molin,S. (1994) Responses to nutrient starvation in Pseudomonas putida KT2442: two-dimensional electrophoretic analysis of starvation- and stress-induced proteins. J. Bacteriol., 176, 4816–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant R.A., Filman,D.J., Finkel,S.E., Kolter,R. and Hogle,J.M. (1998) The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nature Struct. Biol., 5, 294–303. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R. (1993) Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell, 72, 165–168. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R. (1996) Regulation of gene expression during entry to stationary phase. In Neidhardt,F.C. et al. (eds), Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press, Washington, DC, pp. 1497–1512.
- Hobot J.A., Villiger,W., Escaig,J., Maeder,M., Ryter,A. and Kellenberger,E. (1985) Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J. Bacteriol., 162, 960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman G.W., Siegele,D.A., Zambrano,M.M. and Kolter,R. (1996) Morphological and physiological changes during stationary phase. In Neidhardt,F.C. et al. (eds), Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press, Washington, DC, pp. 1672–1682.
- Hurwitz C. and Rosano,C.L. (1967) The intracellular concentration of bound and unbound magnesium ions in Escherichia coli. J. Biol. Chem., 242, 3719–3722. [PubMed] [Google Scholar]
- Kellenberger E. and Arnold,S.G.B. (1992) Chromatins of low-protein content: special features of their compaction and condensation. FEMS Microbiol. Lett., 79, 361–370. [DOI] [PubMed] [Google Scholar]
- Leforestier A. and Livolant,F. (1993) Supramolecular ordering of DNA in the cholesteric liquid crystalline phase: an ultrastructural study. Biophys. J., 65, 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.J. and Sancar,A. (1992) (A)BC excinuclease: the Escherichia coli nucleotide excision repair enzyme. Mol. Microbiol., 6, 2219–2224. [DOI] [PubMed] [Google Scholar]
- Lindahl T. (1993) Instability and decay of the primary structure of DNA. Nature, 362, 709–715. [DOI] [PubMed] [Google Scholar]
- Livolant F. (1991) Ordered phases of DNA in vivo and in vitro. Physica A, 176, 117–137. [Google Scholar]
- Ljungman M. and Hanawalt,P.C. (1992) Efficient protection against oxidative DNA damage in chromatin. Mol. Carcinog., 5, 264–269. [DOI] [PubMed] [Google Scholar]
- Madden T.L. and Herzfeld,J. (1993) Crowding-induced organization of cytoskeletal elements: I. Spontaneous demixing of cytosolic proteins and model filaments to form filament bundles. Biophys. J., 65, 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan M.J., Slauch,J.M. and Mekalanos,J.J. (1996) Environmental regulation of virulence gene expression in Escherichia, Salmonella and Shigella spp. In Neidhardt,F.C. et al. (eds), Escherichia coli and Salmonella: Cellular and Molecular Biology. ASM Press, Washington DC, pp. 2803–2815.
- Martinez A. and Kolter,R. (1997) Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J. Bacteriol., 179, 5188–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minsky A., Ghirlando,R. and Reich,Z. (1997) Nucleosomes: a solution to a crowded intracellular environment? J. Theor. Biol., 188, 379–385. [DOI] [PubMed] [Google Scholar]
- Newton G.L., Aguilera,J.A., Ward,J.F. and Fahey,R.C. (1996) Polyamine-induced compaction and aggregation of DNA—a major factor in radioprotection of chromatin under physiological conditions. Radiat. Res., 145, 776–780. [PubMed] [Google Scholar]
- Nyström T., Larsson,C. and Gustafsson,L. (1996) Bacterial defense against aging: role of the Escherichia coli ArcA regulator in gene expression, readjusted energy flux and survival during stasis. EMBO J., 15, 3219–3228. [PMC free article] [PubMed] [Google Scholar]
- Parker G.F., Daniel,R.A. and Errington,J. (1996) Timing and genetic regulation of commitment to sporulation in Bacillus subtilis. Microbiology, 142, 3445–3452. [DOI] [PubMed] [Google Scholar]
- Pelta J.J., Durand,D., Doucet,J. and Livolant,F. (1996) DNA mesophases induced by spermidine: structural properties and biological implications. Biophys. J., 71, 48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena M.M., Burkhart,W. and Bullerjahn,G.S. (1995) Purification and characterization of a Synechococcus sp. strain PCC 7942 polypeptide structurally similar to the stress-induced Dps/PexB protein of Escherichia coli. Arch. Microbiol., 163, 337–344. [DOI] [PubMed] [Google Scholar]
- Radler J.O., Koltover,I., Salditt,T. and Safinya,C.R. (1997) Structure of DNA–cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science, 275, 810–814. [DOI] [PubMed] [Google Scholar]
- Reich Z., Wachtel,E.J. and Minsky,A. (1994) Liquid-crystalline mesophases of plasmid DNA in bacteria. Science, 264, 1460–1463. [DOI] [PubMed] [Google Scholar]
- Robinow C. and Kellenberger,E. (1994) The bacterial nucleoid revisited. Microbiol. Rev., 58, 211–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson L. (1992) The suicidal DNA repair methyltransferases of microbes. Mol. Microbiol., 6, 825–831. [DOI] [PubMed] [Google Scholar]
- Setlow B., Hand,A.R. and Setlow,P. (1991) Synthesis of a Bacillus subtilis small, acid-soluble spore protein in Escherichia coli causes cell DNA to assume some characteristics of spore DNA. J. Bacteriol., 173, 1642–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorav J.L., Pelta,J. and Livolant,F. (1994) A liquid crystalline phase in spermidine-condensed DNA. Biophys. J., 67, 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spotheim-Maurizot M., Garnier,F., Sabattier,R. and Charlier,M. (1992) Metal ions protect DNA against strand breakage induced by fast neutrons. Int. J. Radiat. Biol., 62, 659–666. [DOI] [PubMed] [Google Scholar]
- Spurio R., Durrenberger,M., Falconi,M., La Teana.A., Pon,C.L. and Gualerzi,C.O. (1992) Lethal overproduction of the Escherichia coli nucleoid protein H-NS: ultramicroscopic and molecular autopsy. Mol. Gen. Genet., 231, 201–211. [DOI] [PubMed] [Google Scholar]
- Stadtman E.R. (1992) Protein oxidation and aging. Science, 257, 1220–1224. [DOI] [PubMed] [Google Scholar]
- Vazquez N.G., Biggiogera,M. and Echeverria,O.M. (1995) Activation of osmium ammine by SO2-generating chemicals for EM Feulgen-type staining of DNA. Eur. J. Histochem., 39, 101–106. [PubMed] [Google Scholar]
- Vescovi E.G., Soncini,F.C. and Groisman,E.A. (1996) Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell, 84, 165–174. [DOI] [PubMed] [Google Scholar]
- Weinberger S., Berman,C. and Minsky,A. (1988) Ordered DNA–polypeptide complexes of extreme chirality: effects of polypeptide handedness on DNA long-range asymmetry. J. Am. Chem. Soc., 110, 8231–8232. [Google Scholar]
- Woldringh C.L., Jensen,P.R. and Westerhoff,H.V. (1995) Structure and partitioning of bacterial DNA: determined by a balance of compaction and expansion forces? FEMS Microbiol. Lett., 131, 235–242. [DOI] [PubMed] [Google Scholar]
- Wolf S.G., Frenkiel,D., Arad,T., Finkel,S.E., Kolter,R. and Minsky,A. (1999) DNA protection by stress-induced biocrystallization. Nature, 400, 83–85. [DOI] [PubMed] [Google Scholar]