Abstract
The 207-kDa polyketide synthase (PKS) module (residues 1–1895) and the 143-kDa nonribosomal peptidyl synthetase (NRPS) module (1896–3163) of the 350-kDa HMWP1 subunit of yersiniabactin synthetase have been expressed in and purified from Escherichia coli in soluble forms to characterize the acyl carrier protein (ACP) domain of the PKS module and the homologous peptidyl carrier protein (PCP3) domain of the NRPS module. The apo-ACP and PCP domains could be selectively posttranslationally primed by the E. coli ACPS and EntD phosphopantetheinyl transferases (PPTases), respectively, whereas the Bacillus subtilis PPTase Sfp primed both carrier protein domains in vitro or during in vivo coexpression. The holo-NRPS module but not the holo-PKS module was then selectively aminoacylated with cysteine by the adenylation domain embedded in the HMWP2 subunit of yersiniabactin synthetase, acting in trans. When the acyltransferase (AT) domain of HMWP1 was analyzed for its ability to malonylate the holo carrier protein domains, in cis acylation was first detected. Then, in trans malonylation of the excised holo-ACP or holo-PCP3–TE fragments by HMWP1 showed both were malonylated with a 3:1 catalytic efficiency ratio, showing a promiscuity to the AT domain.
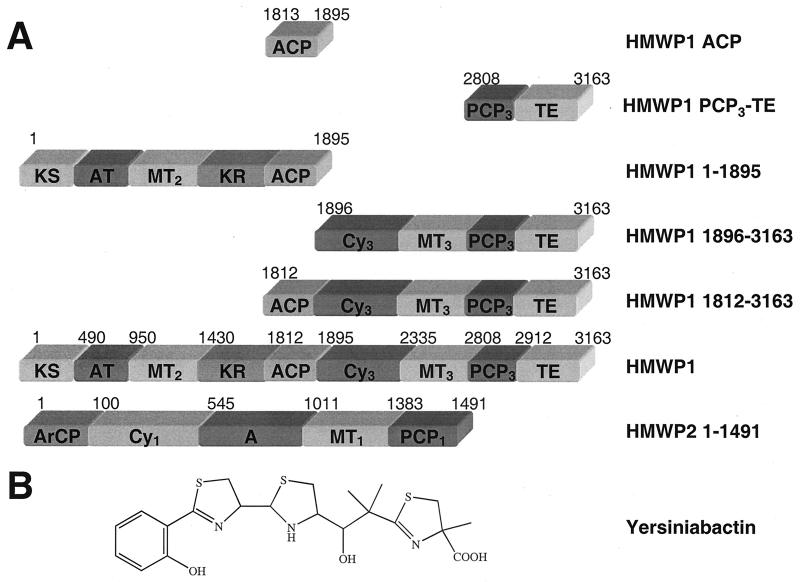
Yersiniabactin (Ybt) is an iron-chelating siderophore produced by the plague pathogen Yersinia pestis in iron-deficient environments and serves as a virulence factor to permit these disease-causing bacteria to grow effectively in animals and humans (1–4). Ybt is assembled by a three-subunit system, YbtE, HMWP2, and HMWP1 (Fig. 1A), comprising 17 predicted domains that function as a mixed nonribosomal peptidyl synthetase (NRPS)-polyketide synthase (PKS)-nonribosomal peptidyl synthetase assembly line (5). The four rings of Ybt (Fig. 1B) are derived from salicylate and three cysteines (5–8) with a malonyl CoA-derived t-butyl linker between the second and third five-membered heterocycles. In the 17 predicted domains of Ybt synthetase are five carrier proteins, three in HMWP2 and two in HMWP1, all of which have been shown (refs. 5, 6, 9, and this work) to be primed by phosphopantetheinylation and serve as the sites for acylation by salicyl (ArCP), cysteinyl [peptidyl carrier protein (PCP)1, PCP2, PCP3], or malonyl (acyl carrier protein, ACP) groups in Ybt chain growth.
Figure 1.
(A) HMWP1 fragments and HMWP2 1–1491 used in this study. All fragments were overexpressed in E. coli and purified as C-terminal hexahistidine-tagged fusions. Each domain, with amino acid residue numbers indicated above, is shown as a rectangle. (B) Structure of Yersiniabactin (Ybt).
Yersiniabactin is an example of a mixed nonribosomal peptide and polyketide, as are the natural products bleomycin (10), epothilone (11, 12), rapamycin (13), and FK506 (14). The molecular intersections of the NRPS and PKS assembly lines to produce these mixed products are of particular interest for both fundamental enzymology of natural product biosynthesis and for design and execution of combinatorial biosynthesis strategies. The HMWP1 subunit, 350 kDa with 9 predicted domains (5, 15) (Fig. 1A), contains a predicted PKS module, ketoacyl synthase (KS), acyltransferase (AT), methyltransferase (MT2), ketoacyl reductase (KR), and ACP in the first 207 kDa, followed by a 4-domain 143-kDa NRPS module, condensation/cyclization domain (Cy3), MT3, PCP3, and chain-terminating thioesterase (TE). In this work, we report the overproduction and purification of the PKS and the NRPS fragments of HMWP1 as well as the excised ACP and PCP3–TE fragments in soluble form in Escherichia coli, the posttranslational priming of each of the two carrier protein domains, and the subsequent selectivity of acylation (malonyl CoA) and aminoacylation (cysteinyl-AMP) by the AT domain of HMWP1 and the adenylation (A) domain of HMWP2, respectively.
Materials and Methods
Materials.
All chemicals were purchased from Sigma. Tris-(2-carboxyethyl)phosphine hydrochloride (TCEP) was purchased from Molecular Probes. DNA oligomers were purchased from Integrated DNA Technologies (Coralville, IA). 35S-labeled L-cysteine and [3H]CoASH were purchased from New England Nuclear. [2-14C]-Malonyl CoA was purchased from Moravek Biochemicals (Brea, CA). Wild-type, TE mutant (S2980A), and AT mutant (641A) of HMWP1 (15), PCP3-TE (9), Bacillus subtilis Sfp (16), and E. coli apo ACP (17), ACPS (17), and EntD (16) were prepared previously in our laboratory.
Cloning of HMWP1 Fragments.
The Y. pestis irp1 gene fragments corresponding to residues 1896–3163, 1813–1895 (the ACP fragment), 1812–3163, and 1–1895 (the PKS fragment) of HMWP1 were amplified from pSDR498.4 (2) by PCR by using Pfu polymerase (Stratagene). The first three PCR products were cloned into the NdeI/XhoI sites of pET22b vector to give pET22b-1896–3163, pET22b-ACP, pET22b-1812–3163. The last PCR product was cloned into the NcoI/BglII sites of pQE60 vector (Qiagen, Chatsworth, CA) to give pQE60-PKS. The DNA sequences of inserts were confirmed by sequencing. The three pET22b-based plasmids direct production of the protein fragments with C-terminal LEHHHHHH tags. Plasmids pET22b-ACP and pET22b-1896–3163 also add methionine, methionine, and glycine to the N termini of the ACP and 1896–3163 fragments, respectively. The plasmid pQE60-PKS directs production of the 1–1895 fragment with a C-terminal RSHHHHHH tag.
Overexpression and Purification of HMWP1 Fragments.
The above plasmids were separately transformed into E. coli strain BL21(DE3) (M15 for pQE60-PKS). For coexpression of the HMWP1 fragments with B. subtilis Sfp, the above plasmids were transformed individually into BL21 (DE3) or M15 containing a small plasmid pREP4-sfp encoding Sfp. The cells were grown at 37°C before induction and at 16–18°C for 8–12 h after induction with 75 μM isopropyl β-D-thiogalactopyranoside (IPTG). The ACP fragment was induced by 1 mM IPTG for 4 h at 37°C. Cells were then harvested and processed as described previously (15). The overexpressed proteins were purified by using superflow nickel resin (Qiagen), following our modified batch loading/column washing and eluting methods (15). After concentration, the proteins in the storage buffer (50 mM Tris-Cl, pH 8.0/1 mM TCEP/10 mM Mg2+/0.1 mM EDTA/10% glycerol) were flash frozen and stored at −80°C. Concentrations of the purified proteins were determined spectrophotometrically at 280 nm by using the calculated extinction coefficients 1278; 276,500; 217,880; and 230,660 M−1 cm−1 for the ACP, 1–1895, 1896–3163, and 1812–3163 fragments of HMWP1, respectively.
Assay for Phosphopantetheinylation of the Carrier Protein Domains of the HMWP1 Fragments.
A trichloroacetic acid (TCA) precipitation assay for the measurement of phosphopantetheinyl transferase (PPTase) activity as the covalent attachment of [3H]phosphopantetheine from [3H]CoASH to the carrier protein substrate at 37°C was performed as described previously (18, 19). The [3H]CoASH was the last component to be added. For both time courses and kcat and KM curves, the control reactions (no PPTases) were performed in parallel to offset the nonspecific radiolabeling of protein substrates. For determination of the apo percentage of the HMWP1 fragments and calibration of [3H]CoASH by using E. coli apo-ACP, the phosphopantetheinylation reactions were stopped by TCA after 4, 8, and 16 h at 37°C. Because the levels of radioactivity did not change during this time frame, the phosphopantetheinylation of the carrier proteins was considered to have reached maximum level.
Autoradiography Demonstrating Covalent Acylation of the Holo-HMWP1 Fragments.
Malonylation.
Each holo-HMWP1 fragment (1.5 μM) was mixed with [14C]-malonyl CoA (50 μM, 52 Ci/mol), TCEP (2.5 mM), and Tris-Cl (75 mM, pH 7.5) in the presence or absence of wild-type HMWP1 (0.3 μM), and the mixture (final volume 100 μl) was incubated at room temperature for 50 min. The reactions were quenched by 1 ml of 10% TCA. The proteins were pelleted, and each pellet was dissolved into 30 μl of gel-loading buffer (Stratagene). After adjusting the pH with 1 M Tris base, the mixture was loaded to a 3-mm 5.5% polyacrylamide gel and run at 80 volts. After the marker dye reached the bottom of the gel, the gel was stained, destained, soaked in the amplifier solution (Amersham Pharmacia) for 15 min, and dried on filter paper. The dried gel was exposed to Kodak x-ray film for 10 h at −80°C.
Cysteinylation.
HMWP1 holo fragments (1 μM each) were mixed with HMWP2 1–1491 (0.15 μM), L-[35S]cysteine (100 μM, 285 Ci/mol), TCEP (2 mM), MgCl2 (10 mM), and Tris-Cl (75 mM, pH 7.5) in the presence or absence of ATP (2 mM) (final volume 100 μl) and incubated at room temperature for 30 min before being quenched by 1 ml of 10% TCA. The samples, the PAGE gel, and autoradiography were handled as described above.
Measurement of Kinetic Parameters of Acylation of the Holo-HMWP1 Fragments in Trans.
Malonylation.
A preincubated solution of HMWP1 (TE mutant) (20 nM) and each holo protein substrate at increasing concentrations in the reaction buffer (75 mM Tris-Cl, pH 7.5/25 mM EDTA/2.5 mM TCEP) was mixed with [14C]-malonyl CoA (100 μM, 52 Ci/mol) at 22°C (final volume 100 μl). At different time points, 10-μl aliquots of the reaction mixture were quenched by 1 ml of 10% TCA. After addition of 25 μl of 2% BSA, the quenched mixture was centrifuged for 5 min at 13,200 × g to precipitate all proteins into one pellet. After three washes, each with 1 ml of 10% TCA, the pellet was dissolved into 150 μl of formic acid, and the solution was transferred to a scintillation vial containing 10 ml of scintillation fluid to count the radioactivity. The control reactions (no HMWP1) were performed in parallel to offset nonspecific radiolabeling of protein substrates. The observed rates extracted from the time courses were fit to the Michaelis–Menten equation to obtain the values of kcat and KM.
For the measurement of acylation stoichiometries, the acylation reactions (100 μl each) were quenched by 1 ml of 10% TCA after 2, 4.5, and 7 h at 22°C. Because the level of radioactivity did not change during this time domain, the covalent acylation of carrier proteins with [14C]-malonyl CoA was believed to have reached its maximum level.
Cysteinylation.
A preincubated solution of HMWP2 1–1491 (50 nM) and increasing concentrations of a holo protein substrate in reaction buffer (75 mM Tris-Cl, pH 7.5/10 mM MgCl2/1 mM TCEP/2 mM ATP) was mixed with L-[35S]cysteine (100 μM, 55.5 Ci/mol) at 37°C (final volume 100 μl). Aliquots of the reaction mixtures (17 μl each) were quenched at different times by 1 ml of 10% TCA. After addition of 5 μl of 2% BSA, each reaction mixture was processed as above. The control reactions (no ATP) were also performed in parallel. The observed rates of cysteinylation extracted from the time courses were fit to the Michaelis–Menten equation to yield the values of kcat and KM.
Results
Preparation of HMWP1 Fragments.
The nine-domain HMWP1 subunit of yersiniabactin synthetase contains two proposed carrier protein domains for the growing siderophore chain, the ACP domain (1813–1895) and the PCP3 domain (2808–2912, Fig. 1A). Both the ACP domain and the terminal bidomain PCP3-TE (2808–3163) of HMWP1 were prepared to test for subsequent priming and acylation selectivity. Analogously, the five-domain PKS module that comprises the first 1895 residues and the next four domains that comprise the NRPS module (1896–3163) of HMWP1 were prepared for the characterization of the ACP and PCP3 carrier domains in their modular context. A fifth HMWP1 fragment was the five-domain 1812–3163 construct that contains both ACP and PCP3 and allowed us to examine the two carrier protein domains simultaneously. Also shown in Fig. 1A are the full-length HMWP1 used as catalyst in some experiments below and the 1–1491 fragment of HMWP2 that contains the cysteine-specific adenylation domain used for loading the holo form of PCP3 (5). All of the HMWP1 fragments except the ACP fragment were C-terminal hexahistidine-tagged, expressed at low temperature (16–18°C) to yield soluble proteins and at low inducer IPTG concentration (75 μM) over a prolonged induction time (10–12 h) (15). The ACP fragment was overexpressed at 37°C and 1 mM IPTG for 4 h. After nickel affinity column chromatography (15) yields of the HMWP1, fragments were 4–7 mg/liter of E. coli culture.
Posttranslational Priming of the Apo Forms of ACP and PCP3 Domains.
The phosphopantetheinylation status of the ACP and PCP3 domains of the HMWP1 fragments as purified was assessed by the now standard assay (16) of incorporation of a radioactive phosphopantetheinyl moiety from [3H]-CoASH by a PPTase that will convert apo to holo carrier proteins. We have previously described two such PPTases from E. coli, the ACPS enzyme specific for the apo-ACP of E. coli fatty acid synthase (16, 20) and the EntD enzyme that is part of the enterobactin biosynthetic operon and primes the apo form of the PCP domain of EntF (16, 18). A third PPTase, the Sfp enzyme from surfactin-producing B. subtilis strains is more promiscuous and will work on a wide variety of apo-ACP and PCP domains (16, 19). All three PPTases were used with [3H]-CoASH to determine the fractional stoichiometry of phosphopantetheine incorporation with the HMWP1 fragments (Fig. 1A). Sfp was the most general priming catalyst and yielded the data of Table 1. Surprisingly the PCP3–TE bidomain was already almost fully in the holo form as judged by only 1% tritium incorporation based on concentration of the 40.4-kDa fragment. The specific activity of [3H]-CoASH was calibrated in parallel with E. coli apo-ACP (17) priming by ACPS. For comparison, the 10.5-kDa ACP fragment of HMWP1 was about 60% holo and 40% apo by this assay. Two ACP peaks were separable on HPLC in comparable ratios and gave the MS of 10498.8 and 10838.3 expected for the apo and holo 1813–1895 fragments, respectively. The total apo form percentages of 1–1895 (ACP), 1896–3163 (PCP3), and 1812–3163 (ACP and PCP3) were measured to be 5.2, 15.8, and 31%, respectively. In some subsequent preparations, the HMWP1 fragments were coexpressed in vivo with the sfp gene and yielded holo protein forms that incorporated no further tritium in these phosphopantetheinylation assays.
Table 1.
Kinetic parameters of phosphopantetheinylation of apo forms of the 1-1895 (ACP), 1896-3163 (PCP3), and 1812-3163 (ACP and PCP3) fragments by three different PPTases at 37°C
| Substrate | Enzyme | kcat, min−1 | KM, μM | kcat/KM (μM−1min−1) |
|---|---|---|---|---|
| 1-1895 | Sfp | 0.69 ± 0.03 | 0.026 ± 0.009 | 26.5 |
| ACPS | 1.6 ± 0.2 | 0.10 ± 0.04 | 16.0 | |
| EntD | 0.22 ± 0.07 | 1.9 ± 0.9 | 0.12 | |
| 1896-3163 | Sfp | 1.3 ± 0.2 | 0.6 ± 0.2 | 2.0 |
| EntD | 0.11 ± 0.01 | 0.46 ± 0.15 | 0.24 | |
| ACPS | No measurable phosphopantetheinylation | |||
| 1812-3163 | Sfp | 9.0 ± 0.5 | 1.1 ± 0.2 | 8.4 |
| ACPS | 12.4 ± 2.3 | 4.9 ± 1.5 | 2.5 | |
| EntD | 8.4 ± 1.3 | 3.1 ± 0.9 | 2.7 | |
The residual apo components of the 1–1895 and 1896–3163 were evaluated for kinetic efficiency of phosphopantetheinylation by the three purified PPTases. The data of Table 1 show that for the ACP-containing 1–1895 substrate the PCP-selective EntD was 136-fold less efficient (kcat/KM) than the ACP-selective ACPS enzyme and 228-fold lower than the promiscuous Sfp. In contrast, the apo-PCP3 domain in 1896–3163 was primed by both EntD and Sfp but was not recognized by ACPS. Finally, all three PPTases incorporated tritiated phosphopantetheine into the 1812–3163 fragment containing both the apo-ACP and apo-PCP3 domains, although it is likely only Sfp was efficiently priming both carrier proteins.
Covalent Malonylation of the Holo-ACP and Holo-PCP3 Domains in HMWP1 Fragments.
With the holo forms of the two carrier protein domains of HMWP1 ensured, the activity of the domains responsible for covalent loading of monomer substrates onto the posttranslationally introduced phosphopantetheine thiol could be assessed. First, HMWP1 was evaluated for self-acylation by [14C]-malonyl CoA mediated by the AT domain (residues 490–950), and radioactivity was incorporated. Then, the KS-AT bidomain fragment (residues 1–940), 1–1895, and full-length HMWP1 were examined separately in trans as sources of acyltransferase in the malonylation of the holo-ACP or PCP3-TE fragments with [14C]-malonyl CoA in TCA precipitation assay. Surprisingly, both holo-ACP and PCP3-TE were malonylated in the anticipated time and enzyme-dependent manner with the same order of enzymatic activity: HMWP1 >> 1–1895 > KS-AT (data not shown). The acyltransferase activity of HMWP1 was abolished by an AT domain active site mutation S641A (15).
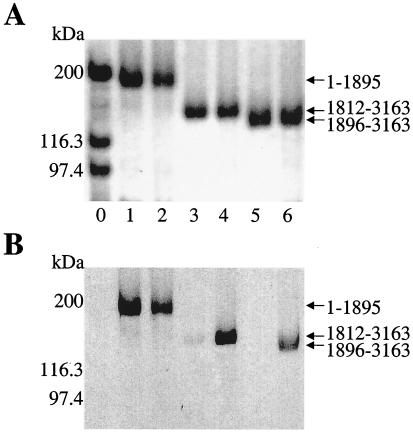
The ability of the AT domain to covalently acylate holo carrier protein domains in trans was further evaluated by SDS/PAGE and autoradiography, by using catalytic amounts of full-length HMWP1 and substrate levels of 1–1895, 1896–3163, and 1812–3163. With [14C]-malonyl CoA, radioactivity was incorporated into all three holo proteins (lanes 2, 4, and 6 in Fig. 2B). As expected, the 1–1895 fragment self-acylated (lane 1), but the other two did not in the absence of HMWP1 (lanes 3 and 5). The malonylation of 1896–3163 was somewhat surprising, given it has only the PCP3 domain. Fig. 2A confirmed the purity of the HMWP1 fragments.
Figure 2.
Demonstration of the covalent loading of the HMWP1 fragments with [14C]-malonyl CoA dependent on the acyltransferase activity of HMWP1. (A) Coomassie-stained gel of TCA-precipitated reaction mixtures. (B) Autoradiograph of the gel. All reaction mixtures contained one holo fragment of HMWP1 (1.5 μM) and [14C]-malonyl CoA (50 μM, 52 Ci/mol). Wild-type HMWP1 was omitted in the odd-numbered lanes and added (0.3 μM) in the even-numbered lanes except lane 0. Lane 0, molecular weight markers; lanes 1 and 2, holo-1–1895; lanes 3 and 4, holo-1812–3163; lanes 5 and 6, holo-1896–3163.
To compare the recognition of holo-ACP vs. holo-PCP3 domain by the AT domain of HMWP1 TE mutant S2980A, the kcat/KM values were obtained for the holo-ACP fragment and the holo-PCP3-TE fragment (data not shown) and summarized in Table 2. The use of HMWP1 mutant S2980A that fully retained the acyltransferase activity was to slow down any hydrolytic editing to remove the radioactive malonyl label (15). The two holo carrier protein domains are within 3-fold of each other for in trans malonylation catalytic efficiency, with substantial turnover numbers and about equivalent KM values. The bis-holo fragment 1812–3163 showed additive kinetic properties consistent with independent behavior of the two holo carrier protein domains when presented in cis in this fragment. The stoichiometry of 14C malonylation was also determined (Table 2), and 64% of the ACP sites and 74% of the PCP3-TE sites are acylatable. The 155% suprastoichiometric loading in 1812–3163 validates that both pantetheinyl prosthetic groups are available for malonyl thioester formation by the AT domain embedded in HMWP1.
Table 2.
Kinetic parameters of malonylation holo forms of the ACP, PCP3-TE, and 1812-3163 (ACP and PCP3) fragments in trans by HMWP1 TE mutant S2980A at 22°C
| Substrate | kcat, min−1 | KM, μM | kcat/KM, μM−1⋅min−1 | Stoichiometry |
|---|---|---|---|---|
| 1812-3163 | 296 ± 138 | 233 ± 129 | 1.27 | 1.55 |
| PCP3-TE | 84 ± 46 | 339 ± 226 | 0.25 | 0.74 |
| ACP | 202 ± 119 | 249 ± 169 | 0.81 | 0.64 |
Covalent Cysteinylation of Holo-PCP3 but Not Holo-ACP Domain by the A Domain of the HMWP2 Subunit.
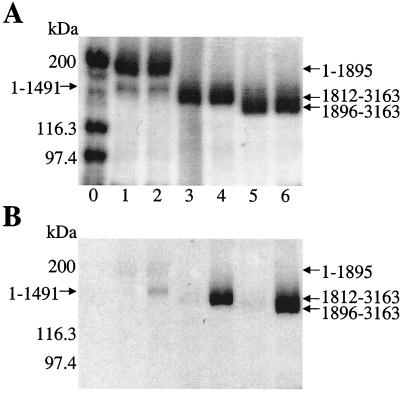
The recognition of the holo-ACP and holo-PCP3 domains of HMWP1 fragments by the single cysteine-activating adenylation domain (A domain) of the other subunit HMWP2 was next evaluated. The 1–1491 fragment of HMWP2 (Fig. 1B) was previously shown to have a fully active A domain. It was used with ATP and [35S]cysteine and the three fragments of HMWP1 listed in Fig. 3 and Table 3, first in a TCA precipitation assay to detect radiolabeled proteins, followed by SDS/PAGE and autoradiography. As shown in Fig. 3B, no label was incorporated into the PKS module of HMWP1 (1–1895), whereas ATP-dependent 35S-labeled 1896–3163 and 1812–3163 were produced, consistent with exclusive cysteinylation of the holo-PCP3 but not the holo-ACP domain. The bis holo 1812–3163 fragment and the holo-PCP3-TE fragment were then tested for saturation behavior in the 35S-cysteinylation assay (Table 3), and both gave Michaelis–Menten behavior, with catalytic efficiencies within 2-fold of each other. The kcat values of 33–61 min−1 and KM values of 10–40 μM show good recognition in trans.
Figure 3.
Demonstration of the covalent loading of the HMWP1 fragments with L-[35S]cysteine catalyzed by HMWP2 1–1491. (A) Coomassie-stained gel of TCA-precipitated reaction mixtures. (B) Autoradiograph of the gel. All reaction mixtures contained one holo-HMWP1 fragment (1 μM), HMWP1 1–1491 (0.15 μM), and [35S]cysteine (100 μM, 285 Ci/mol). ATP was omitted in the odd-numbered lanes and added (2 mM) in the even-numbered lanes except lane 0. Lane 0, molecular weight markers; lanes 1 and 2, holo-1–1895; lanes 3 and 4, holo-1812–3163; lanes 5 and 6, holo-1896–3163.
Table 3.
Kinetic parameters of cysteinylation of holo forms of the 1-1895 (ACP), PCP3-TE, and 1812-3163 (ACP and PCP3) fragments in trans by HMWP2 1-1491 at 37°C
| Substrate | kcatmin−1 | KM, μM | kcat/KM, μM−1·min−1 |
|---|---|---|---|
| 1812-3163 | 33 ± 1 | 10 ± 1 | 3.3 |
| PCP3-TE | 61 ± 13 | 40 ± 15 | 1.5 |
| 1-1895 | No measurable cysteinylation | ||
Discussion
Yersiniabactin synthetase from Y. pestis is an intriguing multimodular catalytic assembly line with 17 predicted domains spread over three subunits: YbtE (1 domain), HMWP2 (7 domains), and HMWP1 (9 domains). The structure of its siderophore product, Ybt, suggests its assembly from salicylate, three cysteines, malonate, and three S-adenosyl methionine-derived methyl groups. The 17 domains and their ordering account for the placement of the monomer units in the tetracyclic siderophore (Fig. 1B). Particularly intriguing is the 350-kDa HMWP1 subunit, which is divided into a five-domain PKS module and then a four-domain NRPS module, thought to be involved in addition of a malonyl unit and then a cysteinyl unit, respectively, into the Ybt assembly line. In this study, we report the overproduction and purification of the PKS module and NRPS module as soluble fragments from E. coli and their initial characterization for priming and acylations.
For NRPS and PKS enzymatic assembly lines to function in substrate activation and transfer and chain elongation, a serine side chain in each carrier protein domain must be posttranslationally primed with a CoASH-derived 4′-phosphopantetheine moiety (16, 21) to introduce the thiol that becomes acylated by either the incoming acyl CoA monomer (PKS module) or the incoming aminoacyl-AMP monomer (NRPS module). The HMWP1 subunit has one carrier protein domain (ACP) dedicated to the PKS portion and one peptidyl carrier protein domain (PCP3) in the NRPS portion of the assembly line. We have elsewhere noted the existence of apo-ACP selective and apo-PCP selective priming PPTases in E. coli and other bacteria (16, 18, 20). Our in vitro assays of the HMWP1 carrier protein domains with E. coli ACPS and E. coli EntD confirmed the strong preferential recognition of these ACP and PCP domains and that the nondiscriminating Sfp PPTase from B. subtilis (16, 19) is the preferred priming catalyst in vitro and will work in in vivo coexpression. It was somewhat surprising that substantial to almost exclusive posttranslational priming of some of the HMWP1 fragments occurred during expression, given the low fraction of priming reported by us (17, 22, 23) and others (24) for heterologous apo carrier protein domains, but this may well be due to the long induction period we used to obtain folded, soluble forms of HMWP1 and fragments.
Using Sfp coexpression in E. coli, we were able to obtain several milligrams of the holo forms of the PKS and NRPS modules from the HMWP1 subunit. This enabled initial evaluation of recognition of holo-ACP and holo-PCP3 by the two catalytic domains involved in acyl thioester and aminoacylthioester formation during Ybt chain growth.
The malonyl unit located between the thiazolidine and methylthiazoline rings of Ybt (Fig. 1B) is loaded by the second domain, AT, of the HMWP1 subunit in a typical PKS module operation. The covalent malonylation in cis was readily demonstrated on full-length HMWP1 and required a functional AT domain. When the ability of the AT domain to malonylate holo carrier proteins in trans was assayed, both the holo-ACP and the holo-PCP3-TE fragments were acylated, with saturation kinetics consistent with binding before catalysis, and only a 3-fold factor in catalytic efficiency in favor of the cognate holo-ACP domain. Whereas this lack of specificity could be an artifact of presenting the holo carrier proteins in trans rather than the physiological intrasubunit in cis presentation, this could also mean there is little if any AT and ACP protein–protein recognition. Perhaps propinquity and high local concentration is the prime determinant for regioselective malonylation of holo-ACP over holo-PCP3 in the HMWP1 subunit during Ybt chain growth. Elsewhere (15), we have recently noted the HMWP1 AT domain will promiscuously self-acylate with various noncognate acyl CoAs, and there is an efficient hydrolytic editing to clear misacylations that could be roadblocks to Ybt chain elongation. Perhaps malonyl-S-PCP3 mistakes are also hydrolytically corrected.
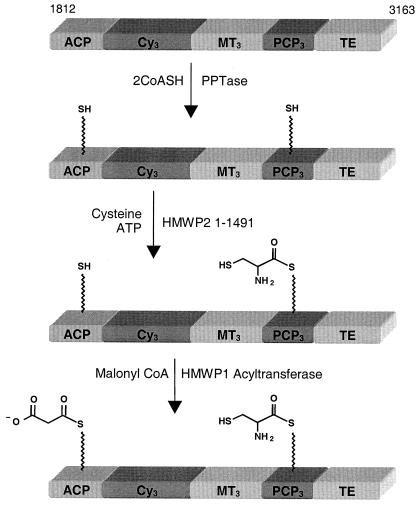
By contrast, the corresponding aminoacylation of the holo-PCP3 domain of the HMWP1 subunit occurs in trans in each turnover, from cysteinyl-AMP generated in the A domain on the HMWP2 subunit. We have previously noted that the 1–1491 fragment of HMWP2 can cysteinylate the excised PCP3 domain (5), but here we demonstrate exclusive regiospecificity, with no detectable aminoacylation of the holo-ACP domain in the HMWP1 1–1895 fragment. This argues for protein–protein recognition for the A domain to find the HS-pantetheinyl arm in PCP3 but not the same thiol prosthetic arm in ACP and may be another form of editing to ensure efficient Ybt chain growth. In the fragment 1812–3163, PPTase priming of both carrier proteins, followed by cysteinylation specifically at PCP3, then by malonylation on the holo-ACP, will yield the bis-acyl enzyme intermediate depicted in Fig. 4. This and other acylated forms of the ACP domain should allow future evaluation of functions of Cy3, MT3, and TE domains in HMWP1.
Figure 4.
Sequential phosphopantetheinylation, cysteinylation, and malonylation of the ACP and PCP3 domains in the 1812–3163 fragment of HMWP1.
The results described here on the PKS module and the NRPS module of the Ybt synthetase HMWP1 subunit form a base line for future studies on module interactions and the functional interfaces across these modules to permit acyl transfers, elongations, and the formation of hybrid PKS-NRPS natural products.
Acknowledgments
We thank Roger S. Flugel for generously providing E. coli ACPS and apo-ACP, Amy M. Gehring for B. subtilis Sfp and E. coli EntD, Robert D. Perry (University of Kentucky, Lexington, KY) for the pSDR498.4 plasmid, and Mohamed A. Marahiel (University of Marburg, Marburg, Germany) for the pREP4-Sfp plasmid. This work was supported in part by National Institutes of Health Grants AI 42736 (to C.T.W.) and T32 GMO7306. Z.S. is a Postdoctoral Fellow of the Jane Coffin Childs Memorial Fund for Medical Research.
Abbreviations
- ACP
acyl carrier protein
- AT
acyltransferase
- IPTG
isopropyl β-D-thiogalactopyranoside
- NRPS
nonribosomal peptidyl synthetase
- PCP
peptidyl carrier protein
- PKS
polyketide synthase
- TCEP
Tris-(2-carboxyethyl)phosphine hydrochloride
- TE
thioesterase
- Ybt
yersiniabactin
- TCA
trichloroacetic acid
- PPTase
phosphopantetheinyl transferase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.021537498.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.021537498
References
- 1.Jackson S, Burrows T W. Br J Exp Pathol. 1956;37:577–583. [PMC free article] [PubMed] [Google Scholar]
- 2.Fetherston J D, Lillard J W, Jr, Perry R D. J Bacteriol. 1995;177:1824–1833. doi: 10.1128/jb.177.7.1824-1833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry R D, Fetherston J D. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearden S W, Fetherston J D, Perry R D. Infect Immun. 1997;65:1659–1668. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gehring A M, DeMoll E, Fetherston J D, Mori I, Mayhew G F, Blattner F R, Walsh C T, Perry R D. Chem Biol. 1998;5:573–586. doi: 10.1016/s1074-5521(98)90115-6. [DOI] [PubMed] [Google Scholar]
- 6.Gehring A M, Mori I, Perry R D, Walsh C T. Biochemistry. 1998;37:11637–11650. doi: 10.1021/bi9812571. [DOI] [PubMed] [Google Scholar]
- 7.Suo Z, Walsh C T, Miller D A. Biochemistry. 1999;38:14023–14035. doi: 10.1021/bi991574n. [DOI] [PubMed] [Google Scholar]
- 8.Keating T A, Miller D A, Walsh C T. Biochemistry. 2000;39:4729–4739. doi: 10.1021/bi992923g. [DOI] [PubMed] [Google Scholar]
- 9.Keating T A, Suo Z, Ehmann D E, Walsh C T. Biochemistry. 2000;39:2297–2306. doi: 10.1021/bi992341z. [DOI] [PubMed] [Google Scholar]
- 10.Du L, Sánchez C, Chen M, Edward D J, Shen B. Chem Biol. 2000;7:623–642. doi: 10.1016/s1074-5521(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 11.Tang L, Shah S, Chung L, Carney J, Katz L, Khosla C, Julien B. Science. 2000;287:640–642. doi: 10.1126/science.287.5453.640. [DOI] [PubMed] [Google Scholar]
- 12.Molnar I, Schupp T, Ono M, Zirkle R, Milnamow M, Nowak-Thompson B, Engel N, Toupet C, Stratmann A, Cyr D D, et al. Chem Biol. 2000;7:97–109. doi: 10.1016/s1074-5521(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 13.Kleinkauf H, von Dohren H. J Antibiot. 1995;48:563–567. doi: 10.7164/antibiotics.48.563. [DOI] [PubMed] [Google Scholar]
- 14.Motamedi H, Cai S J, Shafiee A, Elliston K O. Eur J Biochem. 1997;244:74–80. doi: 10.1111/j.1432-1033.1997.00074.x. [DOI] [PubMed] [Google Scholar]
- 15.Suo, Z., Chen, H. & Walsh, C. T. (2001) Proc. Natl. Acad. Sci. USA, in press.
- 16.Lambalot R H, Gehring A M, Flugel R S, Zuber P, LaCelle M, Marahiel M A, Reid R, Khosla C, Walsh C T. Chem Biol. 1996;3:923–936. doi: 10.1016/s1074-5521(96)90181-7. [DOI] [PubMed] [Google Scholar]
- 17.Flugel R, Hwangbo Y, Lambalot R H, Cronan J E, Walsh C T. J Biol Chem. 2000;275:959–968. doi: 10.1074/jbc.275.2.959. [DOI] [PubMed] [Google Scholar]
- 18.Gehring A M, Bradley K A, Walsh C T. Biochemistry. 1997;36:8495–8503. doi: 10.1021/bi970453p. [DOI] [PubMed] [Google Scholar]
- 19.Quadri L E N, Weinreb P H, Lei M, Nakano M M, Zuber P, Walsh C T. Biochemistry. 1998;37:1585–1595. doi: 10.1021/bi9719861. [DOI] [PubMed] [Google Scholar]
- 20.Lambalot R H, Walsh C T. J Biol Chem. 1995;270:24658–24661. doi: 10.1074/jbc.270.42.24658. [DOI] [PubMed] [Google Scholar]
- 21.Cane D, Walsh C T, Khosla C. Science. 1998;282:63–68. doi: 10.1126/science.282.5386.63. [DOI] [PubMed] [Google Scholar]
- 22.Rusnak F, Sakaitani M, Drueckhammer D, Reichert J, Walsh C T. Biochemistry. 1991;30:2916–2927. doi: 10.1021/bi00225a027. [DOI] [PubMed] [Google Scholar]
- 23.Weinreb P H, Quadri L E N, Walsh C T, Zuber P. Biochemistry. 1998;37:1575–1584. doi: 10.1021/bi9719859. [DOI] [PubMed] [Google Scholar]
- 24.Crosby J, Sherman D H, Bibb M J, Revill W P, Hopwood D A, Simpson T J. Biochim Biophys Acta. 1995;1251:32–42. doi: 10.1016/0167-4838(95)00053-w. [DOI] [PubMed] [Google Scholar]