Abstract
A set of catalysts for aminoacyl-tRNA synthesis is an essential component for translation. The RNA world hypothesis postulates that RNA catalysts could have played this role. Here we show an in vitro evolved precursor tRNA consisting of two domains, a catalytic 5′-leader sequence and an aminoacyl-acceptor tRNA. The 5′-leader sequence domain selectively self-charges phenylalanine on the 3′-terminus of the tRNA domain. This cis-acting ribozyme is susceptible to RNase P RNA, generating the corresponding 5′-leader segment and the mature tRNA. Moreover, the 5′-leader segment is able to aminoacylate the mature tRNA in trans. Mutational studies have revealed that C74 and C75 at the tRNA aminoacyl-acceptor end form base pairs with G71 and G70 of the trans-acting ribozyme. Such Watson–Crick base pairing with tRNA has been observed in RNase P RNA and 23S rRNA, suggesting that all three ribozymes use a similar mechanism for the recognition of the aminoacyl-acceptor end. Our demonstrations indicate that catalytic precursor tRNAs could have provided the foundations for the genetic coding system in the proto-translation system.
Keywords: aminoacyl tRNA synthetase/genetic code/in vitro selection/precursor tRNA/ribozyme
Introduction
Aminoacyl-tRNAs are essential components of the genetic coding system and are required for ribosomal protein synthesis in all living cells (Schimmel, 1987; Arnez and Moras, 1997; Ibba et al., 1997). In the modern translation system, the peptidyl-transferase machinery of the ribosome is a ribozyme, as revealed by X-ray structures (Nissen et al., 2000) and biochemical data (Noller et al., 1992; Zhang and Cech, 1997). Aminoacylation of tRNA, on the other hand, is catalyzed solely by protein enzymes, the aminoacyl-tRNA synthetases (ARSs). According to the RNA world hypothesis, however, the early translation system should have used ARS-like ribozymes for the aminoacylation of tRNAs or tRNA-like molecules (Piccirilli et al., 1992; Schimmel et al., 1993; Illangasekare et al., 1995; De Pouplana et al., 1998; Illangasekare and Yarus, 1999). (Note that tRNA-like molecule refers to a short RNA consisting of an aminoacyl-acceptor stem, which might evolutionarily be a precursor of modern tRNA, e.g. minihelix RNA containing the CCA 3′-terminus.) Despite the previous isolation of self-aminoacylating RNAs (Illangasekare et al., 1995; Jenne and Famulok, 1998; Illangasekare and Yarus, 1999), and even a trans-acting tRNA-charging ribozyme (Lee et al., 2000), there has not yet been a convincing demonstration of how an ARS-like ribozyme could have simultaneously evolved selectivity toward both a tRNA(-like) molecule and an amino acid. In this paper, we demonstrate that an in vitro evolved precursor tRNA (pre-tRNA) can catalyze the aminoacylation in cis with a remarkable amino acid selectivity. The 5′-leader segment of the pre-tRNA, generated by RNase P RNA cleavage, also exhibits tRNA aminoacylation in trans. This pre-tRNA may provide a simple solution for the dilemma of the selectivity issues raised above, since the catalytic domain is covalently linked to the tRNA substrate.
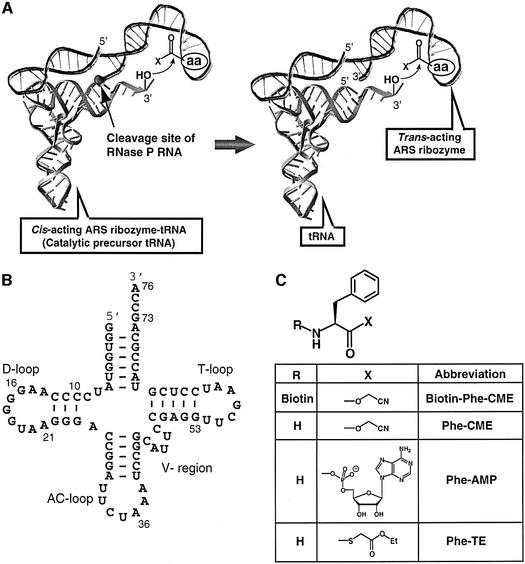
The concept of catalytic pre-tRNA arose from two considerations. First, in the modern biological system, mature tRNA is synthesized from pre-tRNA by successive endo- and exonuclease digestions. The maturation event of the 5′-end of tRNA is generally catalyzed by RNase P (Frank and Pace, 1998). In bacteria, the RNA component of RNase P is a stand-alone endonuclease, and thus, a ribozyme (Guerrier-Takada et al., 1983). As the ribozyme present in the contemporary biological system is considered to be a molecular fossil of the RNA world, it is plausible that modern day pre-tRNA maturation is also a remnant of this early time. Secondly, despite little conservation of the 5′-leader sequences of pre-tRNAs, RNase P universally excises all pre-tRNAs at the correct position. The apparent redundancy of 5′-leader sequences in modern translation led us to consider their possible role in proto-translation. If this 5′-leader sequence was once able to catalyze the 3′-aminoacylation of its attached tRNA segment, the gap between specific tRNAs and their cognate amino acids would be bridged (Figure 1A, left). Further processing of pre-tRNA by RNase P RNA might yield the corresponding trans-acting ARS ribozyme and the matured aminoacyl-tRNA, which could be used for rRNA-based protein synthesis (Figure 1A, right). Such catalytic 5′-leader sequences are unknown today; however, they might have been erased from the evolutionary records by the advent of protein ARSs. We therefore explored this possibility by employing in vitro evolution (Joyce, 1989; Bartel and Szostak, 1993; Gold et al., 1995; Wilson and Szostak, 1999) of a 5′-leader sequence in order to generate a ribozyme with tRNA aminoacylation activity.
Fig. 1. Schematic representation of a catalytic pre-tRNA. (A) Catalytic pre-tRNA with self-aminoacylation activity (left). The amino acid substrate (the amino acid side chain and leaving group are shown with aa and X, respectively) binds to the 5′-leader ribozyme domain, and the nucleophilic attack of the tRNA 3′-hydroxyl (indicated by a curved arrow) is accelerated. The cleavage site of RNase P RNA is shown by the straight arrow. Trans-acting ribozyme that aminoacylates the 3′-end of tRNA (right). (B) Secondary structure of artificial orthogonal suppressor tRNA (otRNA) used for the construction of the RNA pool. The base numbers of otRNA are assigned according to the tRNA numbering rule (Sprinzl et al., 1998). The abbreviations for tRNA loops are: AC, anticodon; V, variable; T, TΨC. (C) Chemical structure of the phenylalanyl substrates.
Results
Selection of active sequences
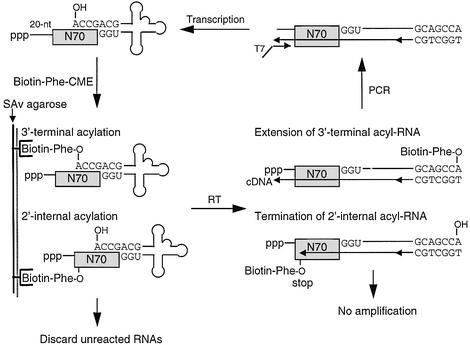
A pool of RNA sequences, consisting of 20-nucleotide (nt) constant and 70-nt random regions, was incorporated to the 5′-end of a tRNA sequence (Figure 2). With consideration toward future applications of ARS ribozymes in protein engineering, an artificial orthogonal suppressor tRNA (otRNA, Figure 1B) was chosen as the target tRNA (Liu et al., 1997). N-biotinyl-l-aminoacyl-cyanomethyl esters (Biotin-aa-CME) (Lee et al., 2000) were chosen as the aminoacyl donor substrates because the CME group has an ideal balance of activation and hydrolytic stability. It is also known that background acylation of RNA is negligible in the presence of 5 mM aminoacyl-CME at physiological pH (Suga et al., 1998; Lee et al., 2000). Furthermore, the CME group has a poor hydrogen-bonding capability, which ensures that the primary interaction of the substrate with the RNA will occur through the side chain of the amino acid substrate. These features of aminoacyl-CME allow us to select active RNA molecules with selectivity towards a desired amino acid. The biotin tag facilitates the isolation of active, i.e. aminoacylated, sequences on immobilized streptavidin (SAv). In this study, we utilized the phenylalanyl substrate (Biotin-Phe-CME, Figure 1C) as a representative example.
Fig. 2. Schematic representation of in vitro selection for self-aminoacylating pre-tRNAs. Abbreviations: N70, a random region with the length of 70 nt; SAv, streptavidin; RT, reverse transcription; cDNA, complementary DNA; T7, T7 RNA promoter.
The selection system is depicted in Figure 2. Agarose-immobilized SAv was used to isolate active sequences, which were tagged with biotin by self-aminoacylation (Lohse and Szostak, 1996; Lee et al., 2000). The aminoacylation reaction can potentially yield both the desired 3′-terminal aminoacylated RNAs and 2′-internally aminoacylated RNAs. In order to eliminate the latter, we applied stringent conditions during reverse transcription, so that its synthesis of complementary DNA is possibly terminated at 2′-internal aminoacylation sites (Lorsch et al., 1995; Lowe and Eddy, 1999). Thus, 3′-terminal aminoacylated RNAs are mainly reverse transcribed and amplified by PCR for the next round of selection.
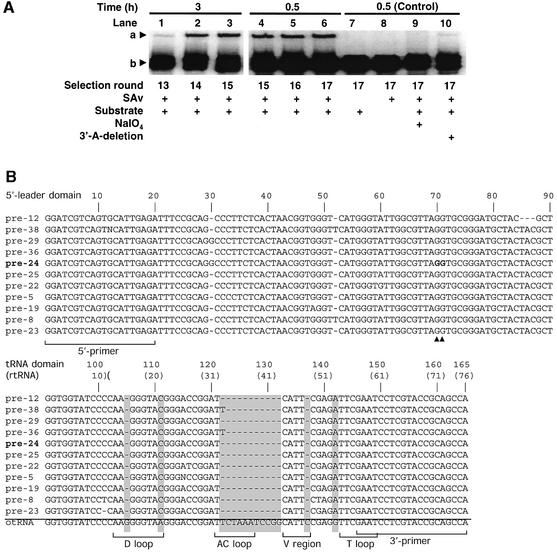
Fifteen rounds of selective amplification of the self-modifying RNA molecules in the pool yielded an enrichment of active sequences, which was confirmed by a SAv-dependent mobility gel shift assay (Figure 3A, lanes 1–3). Approximately 10% of the total input RNA molecules from round 15 showed aminoacylation after 3 h (lane 3). Two rounds of selection with shorter incubation times were employed in order to further enhance the activity in the pool (lanes 4–6). The absence of SAv or substrate resulted in loss of the retarded band (lanes 7 and 8), which is indicative of the self-aminoacylation of RNAs. Periodate oxidation of the 3′-terminal diol or deletion of the 3′-adenosine inhibited aminoacylation activity (lanes 9 and 10), confirming that the 3′-terminus is the aminoacylation site.
Fig. 3. Selection results. (A) Selection of catalytic pre-tRNAs. Autoradiogram showing self-aminoacylation activity as a function of selection cycle. One micromolar RNA and 1 mM Biotin-Phe-CME were used for the reaction in each selection round. a, Biotin-Phe-RNA complexed with SAv; b, RNA pool of each selection round. For periodate oxidation (lane 9), round 17 RNA was treated with 10 mM NaIO4 at 0°C for 1 h and ethanol precipitated prior to the aminoacylation reaction. Round 17 RNA lacking its 3′-A (lane 10) was prepared by transcription of a shortened template. (B) Sequence alignment of active clones isolated from round 17 RNA. In the tRNA domain, the observed deletions and mutations are highlighted by gray boxes, and this truncated tRNA domain sequence is referred to as rtRNA. For the alignment of the tRNA domain, the wild-type otRNA is shown together with the rtRNA sequences. The bases of the tRNA domain are numbered continuously from the 5′-leader domain sequence, and those of rtRNA in parentheses are numbered based on the rule of tRNA numbering (Sprinzl et al., 1998). G70 and G71 of the 5′-leader domain described in Figure 7B are shown by bold letters (their positions are indicated by triangles).
Thirty-six individual clones from the round 17 pool were screened for self-aminoacylation activity, and 11 clones exhibited appreciable activity. Alignment of their sequences revealed an ∼95% identity in the 5′-leader domains (Figure 3B, top), suggesting that all catalytic sequences diverged from one sequence in the original library. Although the sequence identity between the selected tRNA domain and the original otRNA is high, a small block of deletions in the anticodon (AC) region was observed (Figure 3B, bottom). This truncated tRNA sequence of the ribozyme is referred to as rtRNA.
Aminoacylation of the tRNA domain in cis
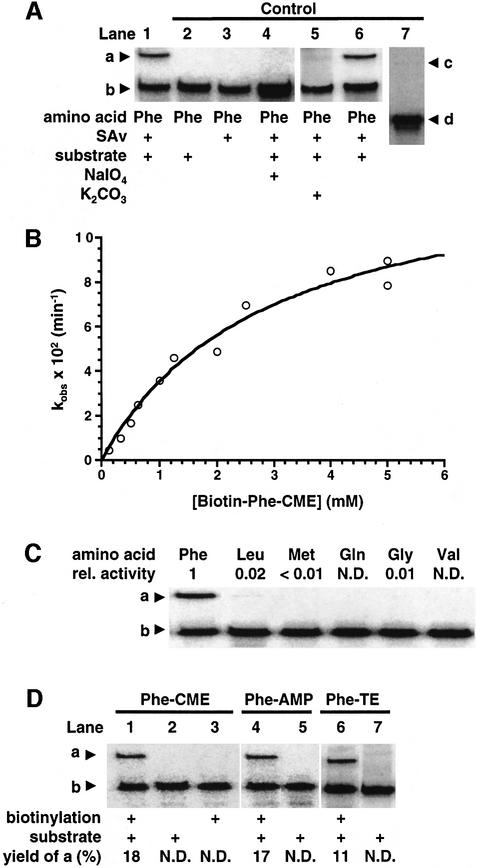
A representative clone, pre-24, was selected for further studies. The self-aminoacylation activity was confirmed by SAv-dependent mobility gel shift assay (Figure 4A, lanes 1–3). Periodate oxidation completely eliminated activity, indicating that the aminoacylation site is the 3′-end (lane 4). Mild base hydrolysis of aminoacyl-pre-24 with potassium carbonate resulted in loss of the retarded band, due to the specific hydrolysis of the ester bond between phenylalanine and pre-24 (lane 5). When re-exposed to the aminoacyl substrate, this deacylated pre-24 still showed full self-aminoacylation activity (lane 6). These control experiments are consistent with a 2′ or 3′ ester bond on the 3′-terminus as the only plausible linkage to the aminoacyl group.
Fig. 4. Self-aminoacylation activity of pre-24. a, Biotin-aminoacyl-pre-24 complexed with SAv; b, pre-24; c, Biotin-Phe-otRNA complexed with SAv; d, otRNA. (A) Self-aminoacylation of pre-24. Reactions were carried out in the presence of 1 µM RNA and 1 mM Biotin-Phe-CME (lanes 1, 2, 4, 5 and 6), 5 mM Biotin-Phe-CME (lane 7) or in the absence of substrate (lane 3). For periodate oxidation (lane 4), pre-24 was treated with 10 mM NaIO4 at 0°C for 1 h and ethanol precipitated prior to the aminoacylation reaction. For mild base hydrolysis of the aminoacyl group on pre-24 (lane 5), Biotin-Phe-pre-24 RNA (lane 1) was treated with 50 mM K2CO3 for 15 min at 37°C. The deacylated pre-24 from lane 5 was recovered by ethanol precipitation, then used for aminoacylation under the same conditions as lane 1 (lane 6). The incubation time was 30 min (lanes 1–6) or 3 h (lane 7). (B) Michaelis–Menten-like plot of the initial rates observed in self-aminoacylation of pre-24. The observed rate at each substrate concentration was determined by a slope of the linear region in a plot of time versus fraction of product with five time points. Data were fitted to the Michaelis–Menten non-linear regression curve, giving the values described in the text. (C) Amino acid selectivity of pre-24. Abbreviations: rel. activity, relative catalytic activity based on Biotin-Phe-CME; N.D., not detectable. Reactions were carried out in the presence of 1 µM RNA and 1 mM Biotin-aa-CME at 25°C for 2 h. (D) Comparison of self-aminoacylation activity of pre-24 using three distinct amino acid esters. Reactions were performed in the presence of 0.5 µM pre-24 and 5 mM Phe-CME (lane 1) or Phe-TE (lane 6) at 25°C for 30 min or 5 mM Phe-AMP (lane 4) on ice for 30 min. After aminoacylation, the biotinylation was performed as described in Materials and methods. Control experiments in the absence of substrate (lane 3) or biotinylation (lanes 2, 5 and 7) were performed.
A plot of initial rates of pre-24 self-aminoacylation as a function of substrate concentrations revealed Michaelis– Menten behavior with kinetic parameters of kcat = 0.13 ± 0.014 min–1 and Km = 2.8 ± 0.61 mM (Figure 4B). It should be noted that the solubility limit of the substrate constrains working concentrations to <5 mM in a buffer containing 5% ethanol. Although increasing ethanol concentration from 5 to 25% in the reaction buffer allowed us to carry out experiments using higher concentrations of substrate, it decreased kcat and increased Km. Therefore, the conditions shown in Figure 4B are optimized for ribozyme catalysis. To determine the rate acceleration offered by pre-24, we measured the rate of spontaneous aminoacylation of otRNA by Biotin-Phe-CME. After 3 h incubation of otRNA with 5 mM Biotin-Phe-CME, a 0.01% yield (which is our minimum detection limit) of Biotin-Phe-otRNA was observed (Figures 4A, lane 7 and 5C), giving an approximate value for kun of 5.5 × 10–7 min–1. Comparing this value with the kobs value obtained for pre-24 at 5 mM substrate (0.09 min–1) revealed a rate enhancement of 1.6 × 105-fold.
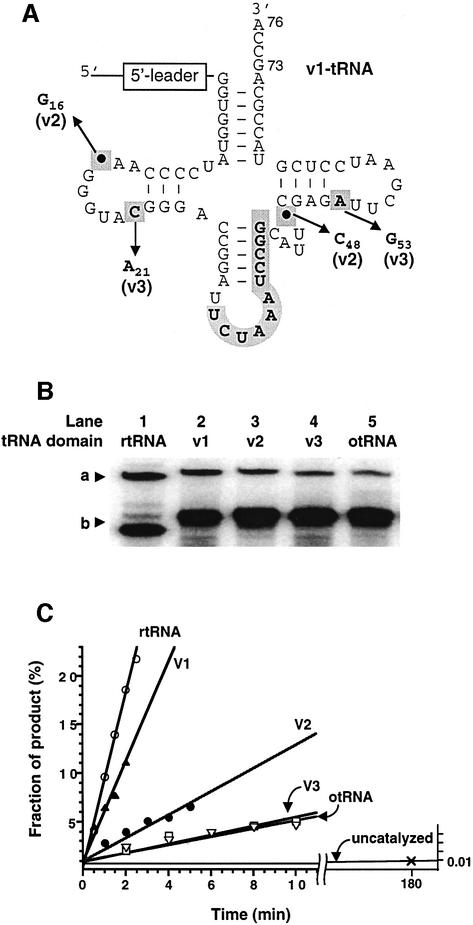
Fig. 5. Structure and self-aminoacylation activity of pre-24 and its variants. (A) Structure of otRNA variants introduced in the otRNA domain of pre-24. The mutations and deletions observed in rtRNA are highlighted by bold letters in gray boxes (see also Figure 1B). In order to distinguish the base numbering of tRNA from that of the 5′-leader domain, the subscript number is used for the assignment of tRNA bases according to the tRNA numbering rule (see Figure 3B). The structure of each variant is as follows: v1-tRNA (its structure is shown), in which the original anticodon region of otRNA was restored but the point mutations and deletions observed in rtRNA were maintained; v2-tRNA, in which the two point deletions were restored into v1-tRNA but the mutations were maintained; v3-tRNA, in which the two point mutations were restored into v1-tRNA but the deletions were maintained. (B) Comparison of self-aminoacylation activity of pre-24 and its variants with different tRNA domains. a, Biotin-Phe-pre-24 or variants complexed with SAv; b, pre-24 or variants. Reactions were carried out in the presence of 1 µM pre-24 (lane 1), variants (lanes 2–4) or pre-24otRNA (lane 5), incubating with 1 mM Biotin-Phe-CME at 25°C for 2 h. (C) Initial rates of self-aminoacylation of pre-24 and its variants. Reactions were carried out in the presence of 0.5 µM pre-24 (rtRNA) or its variants (v1–v3-tRNA and otRNA) incubated with 5 mM Biotin-Phe-CME at 25°C. The observed rate constants (kobs) are 0.089 min–1 (rtRNA), 0.051 min–1 (v1-tRNA), 0.012 min–1 (v2-tRNA), 0.0047 min–1 (v3-tRNA) and 0.0043 min–1 (otRNA).
The amino acid selectivity was investigated using five distinct Biotin-aa-CMEs (Figure 4C). Reaction rates for these amino acids were drastically reduced compared with phenylalanine, indicating that the ribozyme has a remarkable selectivity toward Biotin-Phe-CME. To define further the primary recognition element in the substrate, three other phenylalanyl esters (Figure 1C) were tested for activity (Figure 4D). Omission of biotin from the α-amino group, i.e. Phe-CME, showed self-aminoacylation activity (Figure 4D, lane 1). The absence of biotinylation (lane 2) or substrate (lane 3) for Phe-CME resulted in loss of the retarded band, indicating that the aminoacylation is necessary for the retarded band. Pre-24 also accommodated the adenylate (Phe-AMP) and a thioester (Phe-TE) in place of the CME leaving group (lanes 4 and 6). Although more detailed kinetic experiments for these substrates are currently under way, these preliminary results clearly indicate that the most critical recognition element of the substrate is the phenylalanyl side chain, not the biotinyl group or the leaving group.
Restoration of mutations and deletions in the tRNA domain
In order to gain insight into deletions and mutations observed in the selected otRNA domain (see bases highlighted in gray boxes in Figure 5A), four mutants of pre-24 were constructed. Restoring the original otRNA anticodon stem–loop into the rtRNA domain while maintaining the four point deletions and mutations in the loops (referred to as v1-tRNA, Figure 5A) resulted in only a 1.7-fold reduction of activity compared with rtRNA (Figure 5B, lanes 1 and 2, and C). This suggests that the anticodon region of otRNA does not contain critical recognition elements for the ribozyme domain. This would also explain the reason for the deletion of this region; unnecessary bases for activity could be randomly deleted by the selection-amplification processes.
Further restoration of the point deletions (v2-tRNA) and mutations (v3-tRNA) reduced activity by 7- and 19-fold, respectively (Figure 5B, lanes 3 and 4, and C). Complete replacement of the rtRNA sequence with the original otRNA (this particular catalytic pre-tRNA is referred to as pre-24otRNA) afforded a 20-fold reduction compared with pre-24 (Figure 5B, lane 5, and C). The similar reduction of activity observed for v3-tRNA and otRNA (lane 4 versus 5) indicates that the mutations play a more important catalytic role than the deletions. However, all changes from the initial otRNA sequence cooperatively affect the catalytic activity. S1 nuclease and Pb2+-dependent structural probing experiments on the mutant pre-24 with the v1-tRNA substitution showed that the cleavages of v1-tRNA domain appeared in or near the loop regions corresponding to the cloverleaf secondary structure, which are remarkably similar to those observed for otRNA alone (H.Saito and H.Suga, unpublished results). Thus, our results are consistent with the view that the v1-tRNA domain maintains its cloverleaf-like secondary structure (Figure 5A). We therefore conclude that the point mutations and deletions in the otRNA domain presumably maximize the interaction with the 5′-leader catalytic domain, resulting in significant enhancement of catalytic activity.
RNase P RNA cleavage of pre-tRNA and trans-aminoacylation of the mature tRNA by the 5′-leader ribozyme
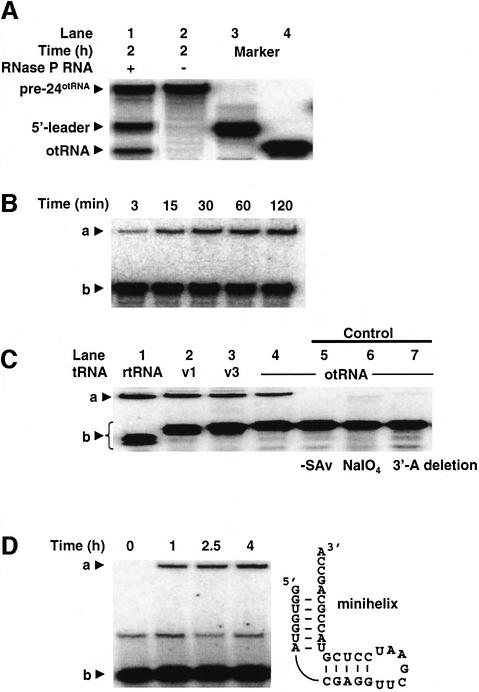
The catalytic activity preserved in pre-24otRNA led us to examine whether pre-24otRNA was susceptible to RNase P RNA scission (Figure 6A). Treatment of pre-24otRNA with Escherichia coli RNase P RNA produced two fragments of lengths corresponding to the 5′-leader segment and the mature otRNA (lanes 1 and 2, compared with in vitro transcripts of each fragment in lanes 3 and 4). Clearly, pre-24otRNA is susceptible to RNase P RNA hydrolysis. Thus, this in vitro evolved catalytic pre-tRNA can be segmented to the mature tRNA and 5′-leader fragment by the naturally occurring endonuclease ribozyme.
Fig. 6. RNase P RNA cleavage of pre-24otRNA and the 5′-leader ribozyme-catalyzed tRNA aminoacylation in trans. (A) Cleavage of pre-24otRNA by RNase P RNA. The 32P-body-labeled pre-24otRNA was treated with RNase P RNA for 2 h, resulting in the cleavage of 23% of pre-24otRNA (lane 1). The absence of RNase P RNA yielded no cleaved product (lane 2). The marker RNAs (5′-leader segment in lane 3 and otRNA in lane 4) were prepared by in vitro transcription using the corresponding DNA segments. (B) Trans-aminoacylation of otRNA. The autoradiogram displays the time course of 5′-leader ribozyme-catalyzed aminoacylation of 5′-32P-labeled otRNA. a, Biotin-Phe-otRNA complexed with SAv; b, otRNA. The RNase P-digested RNA fragments of pre-24otRNA (2 µM) were used for aminoacylation of 5′-32P-labeled otRNA (0.5 µM), giving kobs = 1.0 × 10–3 min–1. (C) Trans-aminoacylation of tRNA variants. rtRNA, v1, v3 and otRNA are the fragment of the tRNA domain described in Figures 1B and 5A. a, Biotin-Phe-otRNA or tRNA variants complexed with SAv; b, otRNA or tRNA variants. tRNA variants were prepared by in vitro transcription using the corresponding DNA segments. Controls: lane 5, the absence of SAv; lane 6, otRNA treated with NaIO4 was used for aminoacylation; lane 7, otRNA lacking its 3′-A was used for aminoacylation. (D) Trans-aminoacylation of a minihelix RNA. The autoradiogram depicts the time course of 5′-leader ribozyme-catalyzed aminoacylation of a minihelix RNA. a, Biotin-Phe-minihelix RNA complexed with SAv; b, minihelix RNA.
Next, we studied whether the 5′-leader fragment could aminoacylate the otRNA fragment in a trans fashion (Figure 1A, right). The 5′-leader and otRNA fragments generated by RNase P RNA digestion were treated with Biotin-Phe-CME (Figure 6B). The 5′-leader fragment does aminoacylate otRNA in trans. The in vitro transcribed 5′-leader fragment also exhibited trans-activity similar to that of the RNase P RNA-derived fragments (Figure 6C, lane 4), and control experiments confirmed 3′-terminal aminoacylation (lanes 5–7). The catalytic fragment also showed activity toward rtRNA, v1-tRNA and v3-tRNA in trans (Figure 6C, lanes 1–3). Thus, the RNase P RNA-digested 5′-leader fragment can fold independently into its functional structure, and act as a trans-acting aminoacylation enzyme (hereafter referred to as 5′-leader ribozyme).
We also tested the substrate properties of a minihelix RNA consisting of the acceptor–T stem–loop region of otRNA (Figure 6D). The minihelix RNA was also aminoacylated by the 5′-leader ribozyme, indicating that the anticodon loop is not essential for activity, but a 4-fold reduction of the observed rate as compared with otRNA suggests that the 5′-leader ribozyme interacts with additional elements in the full-length otRNA.
tRNA 3′-terminus recognition by the 5′-leader ribozyme
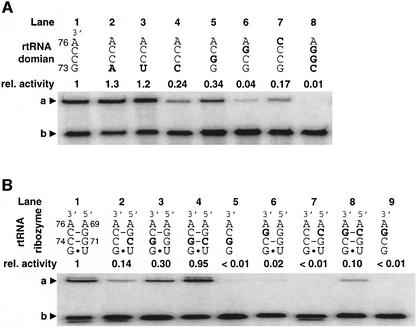
The CCA sequence commonly present in all tRNAs serves as an important recognition element for protein ARSs. The discriminator base at position 73, adjacent to the CCA sequence, also serves as a crucial identity element of tRNA in addition to other identity elements such as the anticodon (Schimmel, 1987; Hou, 1997; Beuning and Musier-Forsyth, 1999). We therefore wondered whether these bases also serve as important recognition elements for the 5′-leader ribozyme. Mutations were introduced into cis-acting pre-24, and these mutants were tested for activity (Figure 7A). The mutation of G73 to A73 or U73 increased activity slightly (lanes 2 and 3), whereas the mutation to C73 reduced activity by 5-fold (lane 4) (see Figures 3B and 5A for the numbering of rtRNA bases). This suggests that U of the ribozyme could be a counter base to the discriminator base at position 73, forming U-G73 or U-U73 wobble base pairs or a U-A73 Watson–Crick base pair (see below). The CCA sequence at the acceptor end is also critical for activity (lanes 5–7). Single mutations introduced in C74 and A76 in the CCA sequence mildly reduced activity by 3.3- and 5-fold (lanes 5 and 7), respectively, whereas the mutation in C75 gave a 25-fold reduction of activity (lane 6). The combination of the mutations at G73–C75 was detrimental for activity (lane 8).
Fig. 7. Mutational studies of the aminoacyl-acceptor end of tRNA and 5′-leader ribozyme. The introduced mutations are highlighted by bold letters. The base numbers of 5′-leader ribozyme and rtRNA are assigned according to Figure 3B. (A) Self-aminoacylation activity of pre-24 and its mutants containing mutations at the acceptor end of the tRNA domain. Abbreviations: rel. activity, relative catalytic activity based on wild-type pre-24. a, Biotin-Phe-pre-24 or its mutants complexed with SAv; b, pre-24 or its mutants. The mutations were introduced into the pre-24 DNA template by PCR site-directed mutagenesis using the corresponding 3′-primer. Self-aminoacylation was carried out in the presence of 1 mM Biotin-Phe-CME and 1 µM RNA at 25°C for 30 min. (B) Trans-aminoacylation activity and compensatory mutations of rtRNA and 5′-leader ribozyme. Abbreviations: rel. activity, relative catalytic activity based on the wild-type pair of rtRNA and 5′-leader ribozyme. a, Biotin-Phe-rtRNA or its mutant complexed with SAv; b, rtRNA or its mutant. Mutant rtRNA and 5′-leader ribozyme were independently transcribed in vitro, and the trans-aminoacylation was carried out in the presence of 1 µM mutant 5′-leader ribozyme and 0.5 µM mutant rtRNA at 25°C for 30 min.
During the course of structural studies of the 5′-leader ribozyme, Pb2+-dependent structural probing experiments showed that the cleavage sites at G70 and G71 of the 5′-leader ribozyme were protected by the addition of otRNA (H.Saito and H.Suga, unpublished result). Interestingly, the base adjacent to these tandem Gs is U72, which can form a G73-U72 wobble base pair. Based on these observations, we hypothesized that G71 and G70 of the 5′-leader ribozyme could form tandem Watson– Crick base pairs with C74 and C75 of tRNA. Therefore, we introduced mutations in both rtRNA and the 5′-leader ribozyme to see whether compensatory mutations could restore the catalytic activity (Figure 7B). The reactions were carried out in trans using 32P-labeled rtRNA or mutant rtRNAs in the presence of the 5′-leader ribozyme or its mutants. The mispairs introduced in the C74-G71 pair showed mild reductions in activity (lanes 2 and 3), while those in the C75-G70 pair are detrimental for activity (lanes 6 and 7). Most importantly, both compensatory mutations of these mispairs restored the activity: for G74-C71 to the wild-type level (lane 4), and for G75-C70 to 10% of the wild type (lane 8). These results imply that C74 and C75 in the tRNA most likely form Watson–Crick base pairs with G71 and G70, respectively, but the C75-G70 pair plays a more critical role in catalysis than simple base pairing.
Discussion
Comparison of the ARS-like ribozyme with naturally occurring ribozymes that interact with tRNA
The tandem G-C pairs of C74-G71 and C75-G70 are critical for catalytic activity as demonstrated in the mutational analysis. It should be noted that the compensatory substitution of the former base pair to the corresponding isosteric base pair completely restored activity, whereas the same substitution of the latter base pair yielded only partial restoration of activity. It is striking that such tandem G-C pairs involving C74 and C75 of tRNA have also been observed in two naturally occurring ribozymes, RNase P RNA and 23S rRNA (Kirsebom and Svärd, 1994; Samaha et al., 1995). Significantly, in the case of E.coli RNase P RNA, it has been demonstrated that the role of the C74-G293 pair is confined to a simple base-pairing interaction, whereas the C75-G292 pair plays a more important role in catalysis (Busch et al., 2000). Similarly, in the case of E.coli 23S ribosomal RNA, the compensatory mutation of C74-G2252 to the isosteric pair restores activity, whereas that of C75-G2251 to the isosteric pair fails to restore activity (Green et al., 1997) even though the X-ray structure of 50S ribosome confirms this Watson– Crick base pairing (Nissen et al., 2000). Although the 5′-leader ribozyme was isolated by in vitro evolution from a random sequence pool, it should be remarked that its strategy for the recognition of the tRNA 3′-terminus is very similar to that used in these natural ribozymes. This may imply that C74 and C75 of tRNA can be universally recognized by ribozymes, but C75 may serve as a critical recognition element involving catalytic activity.
Comparison of the ARS-like ribozyme with protein ARS
The 5′-leader ribozyme trans-aminoacylates not only the tRNA but also the minihelix RNA consisting of the acceptor–T stem–loop region. Interestingly, all ARSs critically recognize the discriminator base at position 73 (N73) (Hou, 1997), and some protein ARSs are able to charge amino acid on cognate minihelix RNAs (Hou and Schimmel, 1988; Schimmel, 1989; Frugier et al., 1992). Because of these observations, it has been proposed that an operational genetic code in the tRNA acceptor stem might be more ancient than the anticodon-based genetic code (Schimmel et al., 1993). The otRNA discriminator base at position G73 seems to interact with the 5′-leader ribozyme, presumably through the base-pair interaction with U72 in the ribozyme. Although unveiling the essential recognition elements of the 5′-leader ribozyme toward otRNA requires more detailed investigations, both the lack of interaction of ribozyme with the AC loop and the importance of the discriminator base for the recognition are consistent with the current hypothesis for the origin of the genetic coding system derived from the observations in protein ARSs. Thus, our demonstration further strengthens the view of an RNA-based aminoacylation system as the source of the genetic coding system.
A possible evolutionary pathway of the tRNA aminoacylation system
It has been postulated that tRNA or tRNA-like molecules are among the oldest RNAs surviving from the RNA world (Schimmel and Söll, 1997), and they might initially have functioned as genomic tags for RNA replication (Weiner and Maizels, 1987; Wientges et al., 2000). Furthermore, the structural similarities between some regions of group I introns and tRNA also suggest the importance of the tRNA-like structural motifs in the RNA world (Caprara et al., 1996). Therefore, it is reasonable to assume that tRNA(-like) molecules were already in existence when the proto-translation system started to evolve (De Pouplana et al., 1998). How could such tRNA(-like) molecules have been charged with amino acids in the early translation system? There are now a number of examples of RNA structural motifs that interact specifically with amino acids (Yarus, 1988; Famulok, 1994; Majerfeld and Yarus, 1994), and even aminoacylate a hydroxyl group (Illangasekare et al., 1995; Lohse and Szostak, 1996; Jenne and Famulok, 1998; Illangasekare and Yarus, 1999; Lee et al., 2000). However, it remains ambiguous how such active RNAs could have later evolved into aminoacyl-tRNA(-like) molecules or an aminoacylation system capable of charging amino acids onto tRNA(-like) molecules.
The catalytic pre-tRNA reported here may provide an explanation to this question (Figure 1A). Our demonstration has proved that a pre-tRNA can selectively self-aminoacylate its 3′-terminus. Because the catalytic domain is covalently attached to a specific tRNA, the selection toward the cognate tRNA is inherently set. Therefore, the pre-tRNA bears the essential selectivity toward both the amino acid and the tRNA. To generate the mature aminoacyl-tRNA, an RNase P-like ribozyme could have co-evolved with the catalytic pre-tRNA in order to separate the 5′-leader ribozyme from the tRNA portion. In this way, the aminoacylation and aminoacyl-adaptor functions of catalytic pre-tRNA could have diverged, allowing aminoacylated tRNA to become incorporated into protein synthesis. Thus, our demonstration indicates that catalytic pre-tRNAs can establish a direct relationship between amino acids and their acceptors in the early translation system. Since the catalytic pre-tRNA reported here also catalyzes aminoacylation on the minihelix RNA, and since present-day RNase P RNA also cleaves a 5′-leader sequence from minihelix RNA (McClain et al., 1987), the tRNAs in the scenario described above can simply be substituted with the tRNA-like molecules.
We have also shown that the catalytic pre-tRNA accepts phenylalanyl substrates with three distinct leaving groups: the adenylate, thio and cyanomethyl esters. Aminoacyl-AMPs are the natural aminoacyl donors in the present-day aminoacylation system. However, protein ARSs synthesize the aminoacyl-AMPs and charge on tRNA without releasing it to the solvent due to their intrinsic short half-life (instability). On the other hand, amino acid thioesters do not suffer from hydrolytic lability, unlike adenylates. These molecules could have been prebiotically accessible constituents of the ‘thioester world’ (de Duve, 1991), which might have co-existed with or pre-dated the RNA world. Our demonstration supports the idea that such thioesters could have been used as competent aminoacyl donors in the early RNA-based aminoacylation system.
In conclusion, we have demonstrated that an in vitro evolved pre-tRNA can catalyze its self-aminoacylation, and the 5′-leader sequence of this pre-tRNA can catalyze tRNA aminoacylation in trans. This ARS-like ribozyme has similar features to protein ARSs and naturally occurring ribozymes in terms of the strategy for the recognition of tRNA. The evolutionary pathway for tRNA aminoacylation is the most critical step in establishing the link between the genetic code and protein synthesis (Woese et al., 1966; Crick, 1968; Schimmel et al., 1993; Szathmáry, 1993; Ellington et al., 2000; Knight and Landweber, 2000; Yarus, 2000). Extensive genetic and functional searches of pre-tRNA and RNA molecules with tRNA-like structural features present in nature, combined with in vitro evolution, may shed further light on the evolutionary significance of pre-tRNA(-like) molecules, and thus on how the genetic code emerged in the early evolution of life.
Materials and methods
Pool construction
Four synthetic oligonucleotides were used for the pool construction: the random pool DNA template (5′-GGATCGTCAGTGCATTGAGA-N70-GGTGGTATCCCCAAGGGGTA-3′), the DNA template complementary to the orthogonal tRNAGln (5′-TGGCTGCGGTACGAGGATTCGAACCTCGGAATGCCGGATTTAGAAATCCGGTCCCTTACCCCTTGG GGATACCACC-3′), the 5′-primer containing T7 promoter sequence (5′-GGTAACACGCATATGTAATACGACTCACTATAGGATCGTCAGTGCATTGAGA-3′) and 3′-primer (5′-TGGCTGCGGTACGAGGATTC-3′). A large-scale Taq DNA polymerase extension of the DNA templates was performed under thermocycling conditions (95°C for 10 min, 55°C for 10 min and 72°C for 10 min). The full-length product was then amplified by seven cycles of large-scale PCR in the presence of the 5′- and 3′-primers. Four equivalents of the pool DNA with ∼1015 complexity were transcribed by T7 RNA polymerase in the presence of [α-32P]UTP and purified by 6% denaturing PAGE.
Synthesis of substrates
Biotin-Phe-CME and Boc-Phe-CME were synthesized by the same procedure as previously described (Suga et al., 1998). Synthesis of Phe-CME was carried out as follows: 9:1 trifluoroacetic acid (TFA)/anisole solution (500 ml) was added to Boc-Phe-CME (385 mg, 1.26 mmol) under argon atmosphere and the mixture was stirred at room temperature for 30 min. The solvent was removed in vacuo and ∼4 M hydrogen chloride in dioxane (4 ml) was added to the residue. The solution was concentrated in vacuo, and the addition of anhydrous ether to the residue yielded precipitate. The precipitate was dissolved in a minimal amount of MeOH, and to this solution anhydrous ether was added to reprecipitate the product. 1H NMR [400 MHz; d6-dimethyl sulfoxide (DMSO)] δ 8.66 (3H, s), 7.24–7.34 (5H, m), 5.08 (2H, s), 4.42 (1H, t, J = 6.6 Hz), 3.07–3.21 (2H, m). For amino acid selectivity study of pre-24, five Biotin-aa-CMEs (Leu, Met, Gln, Gly, Val) were synthesized by the same procedures as Biotin-Phe-CME.
Synthesis of phenylalanyl adenylate (Phe-AMP) was carried out by the same procedure as that described previously (Berg, 1958). 31P NMR analysis of the product indicated that the purity of Phe-AMP was ∼50% and the remaining by-product was unreacted AMP. The product was dissolved in water, and used for aminoacylation without further purification. 31P NMR (400 MHz; d6-DMSO) δ –0.12 (s, AMP), –7.63 (s, Phe-AMP).
Synthesis of phenylalanyl thioester (Phe-TE): N,N-bis(2-oxo-3-oxozolidiyl)phosphordiamitic chloride (238 mg, 0.94 mmol) was added to a solution of Boc-Phe (307 mg, 1.16 mmol) and triethylamine (175 ml, 2.26 mmol) in CH2Cl2 (10 ml). To this mixture, ethyl 2-mercapto acetate (100 ml, 0.91 mmol) was added slowly, and the reaction mixture was then stirred vigorously for 5 h at room temperature. The reaction was quenched by the addition of 20% NaHCO3 aqueous solution. After standard aqueous work-up, Boc-Phe-TE was isolated by column chromatography. 9:1 TFA/anisole solution (500 ml) was added to Boc-Phe-TE (200 mg, 0.30 mmol) under an argon atmosphere, and the mixture was stirred at room temperature for 30 min. The solvent was removed in vacuo and ∼4 M HCl in dioxane (4 ml) was added to form the hydrochloride salt. The solution was concentrated in vacuo, and the residue was dissolved in ether. The addition of petroleum ether to this solution resulted in the formation of precipitate, which was rinsed with ether and filtered to yield Phe-TE. 1H NMR (400 MHz; d6-DMSO) δ 8.47 (3H, s), 7.28–7.34 (5H, m), 4.47 (1H, s), 4.08–4.13 (2H, q, J = 7.2 Hz), 3.87 (2H, s), 3.11 (2H, d, J = 6.8 Hz), 1.19 (3H, t, J = 7.2 Hz).
Selection
Selection reactions were carried out under the following conditions: a mixture of 10 µM (first round only) or 1 µM RNA pool, 1 mM Biotin-Phe-CME, in EK buffer [50 mM N-(2-hydroxyethyl)piperazine-N′-(3-propanesulfonic acid); EPPS, 500 mM KCl pH 7.5], 100 mM MgCl2 and ethanol (25% of the total volume). The pool RNA was pre-incubated in EK buffer, heated at 95°C for 5 min and cooled to 25°C over 5 min. MgCl2 was then added, followed by a 5 min equilibration. The reaction was initiated by the addition of the substrate solution in ethanol, and incubated for 3 h at 25°C (30 min in the 15–17th rounds). The reaction was stopped by adding 2 vol of cold ethanol, and the RNA was ethanol precipitated twice. The RNA pellet was dissolved into EKE buffer (50 mM EPPS, 500 mM KCl, 5 mM EDTA pH 7.5), then incubated with 200 µl (1 ml for the first round) of streptavidin–agarose for 30 min at room temperature. Unbound RNAs were eluted with 20-resin volumes of the EKE buffer, 40-resin volumes of 4 M urea, then 10-resin volumes of water. The resin-bound RNAs were eluted by heating at 95°C for 10 min in the presence of 10 mM biotin pH 7. The collected RNAs were reverse transcribed using 100 U of MMLV reverse transcriptase (Promega) in the presence of 1 µM 3′-primer (5′-TGG CTGCGGTACGAGGATTC-3′), 125 µM dNTPs, 50 mM Tris–HCl, 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol (DTT) pH 8.3 at 42°C for 1 h (for rounds 1–14) or 10 min (for rounds 15–17). The cDNA was subjected to PCR followed by transcription under standard conditions. The transcribed RNAs were purified by 6% denaturing PAGE and used for the next round of selection.
Self-aminoacylation assay
The self-aminoacylation activity of pre-24 in the presence of Biotin-Phe-CME was assayed under conditions similar to those of the selection except that 12.5 mM KCl and 5% ethanol were used. At each time point, an aliquot of the reaction was ethanol precipitated twice, and the pellet was dissolved into 7 µl of MEUS buffer (25 mM MOPS, 5 mM EDTA, 8 M urea, 10 µM streptavidin pH 6.5). The resulting solution was analyzed by 10% polyacrylamide gel containing 4 M urea, running in a cold room in order to keep the gel temperature <20°C. Under these conditions, the streptavidin–biotin complex is stable, but the RNA is denatured. Mutations and deletions were introduced by PCR using synthetic 5′- and 3′-primers or synthetic cDNA templates containing the corresponding changes (PCR site-directed mutagenesis). For the amino acid selectivity (Figure 4C), 1 mM of each Biotin-aa-CME was used to ensure the solubility of all substrates. For substrates of Phe-CME, Phe-AMP and Phe-TE, self-aminoacylation reactions were carried out with the same procedures as those described above, except that the aminoacyl-RNA pellet was resuspended in an acidic EPPS buffer (0.3 M, pH 5.5). EPPS–KOH (0.3 M, pH 9.6) was then added to this solution, which brought the pH to 8.0. Immediately afterwards, the biotinylation reaction was initiated by the addition of 45 mM biotin-3-sulfo- N-hydroxylsuccinimide ester at 0°C, to bring the final concentration to 9 mM (Pütz et al., 1997). After 1 h, the reaction was terminated by ethanol precipitation twice. In the case of tRNA 3′-terminus mutational studies on pre-24 (Figure 7A), the wild-type molecules were substituted with the corresponding mutants.
RNase P RNA digestion
Escherichia coli RNase P RNA was in vitro transcribed using a PCR-amplified DNA template from the M1 gene of the pDW27 plasmid, then purified by 6% denaturing PAGE. The cleavage of pre-24otRNA (1 µM) by the RNase P RNA (1 µM) was carried out in 1 M NH4OAc, 50 mM MgCl2 and 0.1% SDS at 37°C (Ziehler and Engelke, 1996). After the reaction, the solution was ethanol precipitated twice and the resulting solution was analyzed by 10% denaturing PAGE. For the analysis of RNase P RNA digestion (Figure 6A), a 32P-body-labeled pre-24otRNA was used. For analysis of the trans-aminoacylation activity (Figure 6B), an unlabeled pre-24otRNA was cleaved, and the individual segments of otRNA and 5′-leader domain were purified by 10% denaturing PAGE. The tRNA segment was treated with calf intestinal alkaline phosphatase and then phosphorylated using T4 polynucleotide kinase in the presence of [γ-32P]ATP.
Trans-aminoacylation assay
The trans-aminoacylation activity was assayed under the same buffer conditions as self-aminoacylation. The reaction was initiated by the addition of 1 mM Biotin-Phe-CME. In Figure 6B, 5′-leader ribozyme and 5′-[32P]otRNA were dissolved into EKM buffer (50 mM EPPS, 12.5 mM KCl, 20 mM MgCl2) independently, heated at 70°C for 5 min and slowly cooled to 50°C. The two solutions were then mixed in the presence of 100 mM MgCl2, incubated at 50°C for 10 min, then slowly cooled to 25°C. In Figure 6C, in vitro transcripts of tRNA variants (each 2 µM) and 5′-leader ribozyme (6 µM) were used for this study. In Figure 6D, in vitro transcripts of minihelix RNA (3 µM) and 5′-leader ribozyme (4 µM) were used. The remaining procedures were the same as those described above. In the case of mutation studies on 5′-leader ribozyme and rtRNA (Figure 7B), the wild-type molecules were substituted with the corresponding mutants generated by the PCR site-directed mutagenesis. Before the reaction, 5′-leader ribozyme or its mutants (1 µM) and rtRNA or its mutants (0.5 µM) were heated at 95°C for 5 min and cooled to 25°C over 5 min. MgCl2 was then added, followed by a 5 min equilibration.
Acknowledgments
Acknowledgements
We thank all members of the Suga laboratory, particularly D.R.W.Hodgson and N.Lee for critical proof-reading and suggestions for the synthesis of substrates. We also thank P.Gollnick for the gift of M1 RNase P RNA gene, and J.W.Szostak and D.Wilson for their comments on the manuscript. H.Saito acknowledges K.Watanabe and the JSPS Research Fellowships for Young Scientists for generous support. This work was supported by the National Institutes of Health (GM59159) awarded to H.Suga.
References
- Arnez J.G. and Moras,D. (1997) Structural and functional considerations of the aminoacylation reaction. Trends Biochem. Sci., 22, 211–216. [DOI] [PubMed] [Google Scholar]
- Bartel D.P. and Szostak,J.W. (1993) Isolation of new ribozymes from a large pool of random sequences. Science, 261, 1411–1418. [DOI] [PubMed] [Google Scholar]
- Berg P. (1958) The chemical synthesis of amino acid adenylates. J. Biol. Chem., 253, 608–611. [PubMed] [Google Scholar]
- Beuning P.J. and Musier-Forsyth,K. (1999) Transfer RNA recognition by aminoacyl-tRNA synthetases. Biopolymers, 52, 1–28. [DOI] [PubMed] [Google Scholar]
- Busch S., Kirsebom,L.A., Notbohm,H. and Hartmann,R.K. (2000) Differential role of the intermolecular base-pairs G292-C75 and G293-C74 in the reaction catalyzed by Escherichia coli RNase P RNA. J. Mol. Biol., 299, 941–951. [DOI] [PubMed] [Google Scholar]
- Caprara M.G., Lehnert,V., Lambowitz,A.M. and Westhof,E. (1996) A tyrosyl-tRNA synthetase recognizes a conserved tRNA-like structural motif in the group I intron catalytic core. Cell, 87, 1135–1145. [DOI] [PubMed] [Google Scholar]
- Crick F.H.C. (1968) The origin of the genetic code. J. Mol. Biol., 38, 367–379. [DOI] [PubMed] [Google Scholar]
- de Duve C. (1991) Blueprint For a Cell: The Nature and Origin of Life. N. Patterson Publishers, Burlington, NC.
- De Pouplana L.R., Turner,R.J., Steer,B.A. and Schimmel,P. (1998) Genetic code origins: tRNAs older than their synthetases? Proc. Natl Acad. Sci. USA, 95, 11295–11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington A.D., Khrapov,M. and Shaw,C.A. (2000) The scene of a frozen accident. RNA, 6, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulok M. (1994) Molecular recognition of amino acids by RNA aptamers: an l-citrulline-binding RNA motif and its evolution into an l-arginine binder. J. Am. Chem. Soc., 116, 1698–1706. [Google Scholar]
- Frank D.N. and Pace,N.R. (1998) Ribonuclease P: unity and diversity in a tRNA processing ribozyme. Annu. Rev. Biochem., 67, 153–180. [DOI] [PubMed] [Google Scholar]
- Frugier M., Florentz,C. and Giegé,R. (1992) Anticodon-independent aminoacylation of an RNA minihelix with valine. Proc. Natl Acad. Sci. USA, 89, 3990–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Polisky,B., Uhlenbeck,O.C. and Yarus,M. (1995) Diversity of oligonucleotide functions. Annu. Rev. Biochem., 64, 763–797. [DOI] [PubMed] [Google Scholar]
- Green R., Samaha,R.R. and Noller,H.F. (1997) Mutations at nucleotides G2251 and U2585 of 23S rRNA perturb the peptidyl transferase center of the ribosome. J. Mol. Biol., 266, 40–50. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner,K., Marsh,T., Pace,N. and Altman,S. (1983) The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell, 35, 849–857. [DOI] [PubMed] [Google Scholar]
- Hou Y.-M. (1997) Discriminating among the discriminator bases of tRNAs. Chem. Biol., 4, 93–96. [DOI] [PubMed] [Google Scholar]
- Hou Y.-M. and Schimmel,P. (1988) A simple structural feature is a major determinant of the identity of a transfer RNA. Nature, 333, 140–145. [DOI] [PubMed] [Google Scholar]
- Ibba M., Curnow,A.W. and Söll,D. (1997) Aminoacyl-tRNA synthesis: divergent routes to a common goal. Trends Biochem. Sci., 22, 39–42. [DOI] [PubMed] [Google Scholar]
- Illangasekare M. and Yarus,M. (1999) Specific, rapid synthesis of Phe-RNA by RNA. Proc. Natl Acad. Sci. USA, 96, 5470–5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illangasekare M., Sanchez,G., Nickles,T. and Yarus,M. (1995) Aminoacyl-RNA synthesis catalyzed by an RNA. Science, 267, 643–647. [DOI] [PubMed] [Google Scholar]
- Jenne A. and Famulok,M. (1998) A novel ribozyme with ester transferase activity. Chem. Biol., 5, 23–34. [DOI] [PubMed] [Google Scholar]
- Joyce G.F. (1989) Amplification, mutation and selection of catalytic RNA. Gene, 82, 83–87. [DOI] [PubMed] [Google Scholar]
- Kirsebom L.A. and Svärd,S.G. (1994) Base pairing between Escherichia coli RNase P RNA and its substrate. EMBO J., 13, 4870–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R.D. and Landweber,L.F. (2000) Guilt by association: the arginine case revisited. RNA, 6, 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Bessho,Y., Wei,K., Szostak,J.W. and Suga,H. (2000) Ribozyme-catalyzed tRNA aminoacylation. Nature Struct. Biol., 7, 28–33. [DOI] [PubMed] [Google Scholar]
- Liu D.R., Magliery,T.J., Pastrnak,M. and Schultz,P.G. (1997) Engineering a tRNA and aminoacyl-tRNA synthetase for the site-specific incorporation of unnatural amino acids into proteins in vivo. Proc. Natl Acad. Sci. USA, 94, 10092–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse P.A. and Szostak,J.W. (1996) Ribozyme-catalysed amino-acid transfer reactions. Nature, 381, 442–444. [DOI] [PubMed] [Google Scholar]
- Lorsch J.L., Bartel,D.P. and Szostak,J.W. (1995) Reverse transcriptase reads through a 2′–5′ linkage and a 2′-thiophosphate in a template. Nucleic Acids Res., 23, 2811–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe T.M. and Eddy,S.R. (1999) A computational screen for methylation guide snoRNAs in yeast. Science, 283, 1168–1171. [DOI] [PubMed] [Google Scholar]
- Majerfeld I. and Yarus,M. (1994) An RNA pocket for an aliphatic hydrophobe. Nature Struct. Biol., 1, 287–292. [DOI] [PubMed] [Google Scholar]
- McClain W.H., Guerrier-Takada,C. and Altman,S. (1987) Model substrates for an RNA enzyme. Science, 238, 527–530. [DOI] [PubMed] [Google Scholar]
- Nissen P., Hansen,J., Ban,N., Moore,P.B. and Steitz,T.A. (2000) The structural basis of ribosome activity in peptide bond synthesis. Science, 289, 920–930. [DOI] [PubMed] [Google Scholar]
- Noller H.F., Hoffarth,V. and Zimniak,L. (1992) Unusual resistance of peptidyl transferase to protein extraction procedures. Science, 256, 1416–1419. [DOI] [PubMed] [Google Scholar]
- Piccirilli J.A., McConnell,T.S., Zaug,A.J., Noller,H.F. and Cech,T.R. (1992) Aminoacyl esterase activity of the Tetrahymena ribozyme. Science, 256, 1420–1424. [DOI] [PubMed] [Google Scholar]
- Pütz J., Wientges,J., Sissler,M., Giegé,R., Florentz,C. and Schwienhorst,A. (1997) Rapid selection of aminoacyl-tRNAs based on biotinylation of α-NH2 group of charged amino acids. Nucleic Acids Res., 25, 1862–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha R.R., Green,R. and Noller,H.F. (1995) A base pair between tRNA and 23S rRNA in the peptidyl transferase centre of the ribosome. Nature, 377, 309–314. [DOI] [PubMed] [Google Scholar]
- Schimmel P. (1987) Aminoacyl tRNA synthetases: general sheme of structure–function relationships in the polypeptides and recognition of transfer RNAs. Annu. Rev. Biochem., 56, 125–158. [DOI] [PubMed] [Google Scholar]
- Schimmel P. (1989) Parameters for the molecular recognition of transfer RNAs. Biochemistry, 28, 2747–2759. [DOI] [PubMed] [Google Scholar]
- Schimmel P. and Söll,D. (1997) When protein engineering confronts the tRNA world. Proc. Natl Acad. Sci. USA, 94, 10007–10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P., Giegé,R., Moras,D. and Yokoyama,S. (1993) An operational RNA code for amino acids and possible relationship to genetic code. Proc. Natl Acad. Sci. USA, 90, 8763–8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Horn,C., Brown,M., Ioudovitch,A. and Steinberg,S. (1998) Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res., 26, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H., Lohse,P.A. and Szostak,J.W. (1998) Structural and kinetic characterization of an acyl transferase ribozyme. J. Am. Chem. Soc., 120, 1151–1156. [DOI] [PubMed] [Google Scholar]
- Szathmáry E. (1993) Coding coenzyme handles: a hypothesis for the origin of the genetic code. Proc. Natl Acad. Sci. USA, 90, 9916–9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A.M. and Maizels,N. (1987) tRNA-like structures tag the 3′ ends of genomic RNA molecules for replication: Implications for the origin of protein synthesis. Proc. Natl Acad. Sci. USA, 84, 7383–7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wientges J., Pütz,J., Giegé,R., Florentz,C. and Schwienhorst,A. (2000) Selection of viral RNA-derived tRNA-like structures with improved valylation activities. Biochemistry, 39, 6207–6218. [DOI] [PubMed] [Google Scholar]
- Wilson D.S. and Szostak,J.W. (1999) In vitro selection of functional nucleic acids. Annu. Rev. Biochem., 68, 611–647. [DOI] [PubMed] [Google Scholar]
- Woese C.R., Dugre,D.H., Saxinger,W.C. and Dugre,S.A. (1966) The molecular basis for the genetic code. Proc. Natl Acad. Sci. USA, 55, 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M. (1988) A specific amino acid binding site composed of RNA. Science, 240, 1751–1758. [DOI] [PubMed] [Google Scholar]
- Yarus M. (2000) RNA-ligand chemistry: a testable source for the genetic code. RNA, 6, 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. and Cech,T.R. (1997) Peptide bond formation by in vitro selected ribozymes. Nature, 390, 96–100. [DOI] [PubMed] [Google Scholar]
- Ziehler W.A. and Engelke,D.R. (1996) Synthesis of small RNA transcripts with discrete 5′ and 3′ ends. Biotechniques, 20, 622–624. [DOI] [PubMed] [Google Scholar]