Abstract
P pili are important virulence factors in uropathogenic Escherichia coli. The Cpx two-component signal transduction system controls a stress response and is activated by misfolded proteins in the periplasm. We have discovered new functions for the Cpx pathway, indicating that it may play a critical role in pathogenesis. P pili are assembled via the chaperone/usher pathway. Subunits that go ‘OFF-pathway’ during pilus biogenesis generate a signal. This signal is derived from the misfolding and aggregation of subunits that failed to come into contact with the chaperone in the periplasm. In response, Cpx not only controls the stress response, but also controls genes necessary for pilus biogenesis, and is involved in regulating the phase variation of pap expression and, potentially, the expression of a panoply of other virulence factors. This study demonstrates how the prototypic chaperone/usher pathway is intricately linked and dependent upon a signal transduction system.
Keywords: assembly/Cpx/P pili/regulation
Introduction
Microbial colonization is a key event in the early stages of most infectious diseases. Pyelonephritic Escherichia coli express P pili, which mediate binding to a globoside receptor in the human kidney (Leffler and Svanborg-Eden, 1980; Bock et al., 1985; Stromberg et al., 1990, 1991; Kuehn et al., 1992). This binding event has been shown to be critical in the ability of these bacteria to cause disease (Roberts et al., 1994).
The expression of P pili requires at least 11 genes organized in the pap gene cluster found on the chromosome of uropathogenic strains of E.coli (Hull et al., 1981). The pap gene cluster is found within pathogenicity islands (PAIs) (Hacker et al., 1990; Blum et al., 1995; Guyer et al., 1998). These PAIs contain genes for colonization (pili) and multiple virulence factors (hemolysin, cytotoxic necrotizing factor 1 and others), all linked, and sometimes duplicated, on the chromosome of uropathogenic strains of E.coli (Swenson et al., 1996; Kao et al., 1997).
P pili are composite structures consisting of a thick pilus rod and a thinner tip fibrillum. The rod is formed by a linear repeat of PapA subunits arranged as a right-handed helical cylinder having 3.28 subunits per turn (Baga et al., 1984; Gong and Makowski, 1992; Bullitt and Makowski, 1995). The tip fibrillum is composed of repeating PapE subunits arranged in an open helical configuration (Kuehn et al., 1992). Located at the distal end of the fibrillum is the adhesin PapG (Kuehn et al., 1992; Jacob-Dubuisson et al., 1993). The two proteins PapK and PapF serve as adaptors connecting the rod to the tip and the tip to the adhesin, respectively (Jacob-Dubuisson et al., 1993).
The expression of P pili is highly regulated. P pili undergo phase variation in which a bacterium can either express (phase ON) or not express (phase OFF) pili (Blyn et al., 1989). Phase variation may play an important role in pathogenesis, allowing the bacteria to sense its environment and express pili when needed, and turn off the expression when piliation is unnecessary and/or detrimental to the bacterium (van der Woude et al., 1996).
The pap gene cluster has a divergent promoter located between two genes that encode regulatory factors, PapB and PapI. The two promoters are denoted papBA and papI (Baga et al., 1985). Both promoters are regulated by many known factors including PapI (Kaltenbach et al., 1995), PapB (Baga et al., 1985; Forsman et al., 1989), leucine-responsive regulatory protein (Lrp) (van der Woude et al., 1995; Kaltenbach et al., 1998; Weyand and Low, 2000), deoxyadenosine methylase (DAM) (Blyn et al., 1990; Braaten et al., 1991, 1994; Nou et al., 1995), catabolite activator protein (CAP) (Forsman et al., 1992) and histone-like protein (HNS) (van der Woude et al., 1995; White-Ziegler et al., 1998).
The assembly of pili is a complex process involving competing productive and non-productive pathways. The assembly of P pili occurs via the highly conserved chaperone/usher pathway (Holmgren et al., 1992; Hung et al., 1996). Pilus subunits are immunoglobulin-like proteins that are missing the C-terminal G β-strand (Choudhury et al., 1999; Sauer et al., 1999). The absence of this strand creates a deep groove on the surface of the pilin that exposes the hydrophobic core. Biogenesis requires the prototype of the superfamily of periplasmic immunoglobulin-like chaperones, PapD (Normark et al., 1986; Hung et al., 1996). PapD donates its G1 β-strand to complete the fold of each subunit in a process called donor strand complementation (Choudhury et al., 1999; Sauer et al., 1999; Barnhart et al., 2000). The subunit groove occupied by the G1 β-strand also participates in subunit– subunit interactions in the pilus. Thus, donor strand complementation couples the folding of the subunit with the capping of its interactive groove (Choudhury et al., 1999; Sauer et al., 1999; Barnhart et al., 2000). During pilus assembly, which occurs at the outer membrane usher (Dodson et al., 1993; Saulino et al., 1998; Thanassi et al., 1998b), the N-terminal extension of an incoming subunit displaces the chaperone G1 β-strand and occupies the groove of the most recently incorporated subunit in a process called donor strand exchange. The mature pilus thus consists of an array of subunits, each of which contributes a strand to complete the fold of its neighbor (Choudhury et al., 1999; Sauer et al., 1999; Barnhart et al., 2000).
In the absence of a chaperone, subunits become predisposed to conformations that drive them ‘OFF-pathway’, resulting in non-productive interactions and their subsequent proteolytic degradation (Jones et al., 1997). Additionally, during pilus assembly, a fraction of the subunits fail to come into contact with the chaperone and are also diverted to the same OFF-pathway (Hung et al., 1999). This non-productive pathway activates two partially overlapping periplasmic stress responses, the two-component Cpx signal transduction pathway and the σE modulatory pathway, resulting in the activation of a panoply of new genes encoding periplasmic protein folding factors (DsbA and prolyl isomerases) and proteases (DegP) (Danese and Silhavy, 1997; Jones et al., 1997; Pogliano et al., 1997). DegP is the protease that degrades misfolded pilins, and DsbA is the enzyme that catalyzes the disulfide bond formation required for the assembly of P pili (Jacob-Dubuisson et al., 1994a; Jones et al., 1997).
The Cpx two-component signaling pathway consists of the inner-membrane histidine kinase CpxA (Raivio and Silhavy, 1997) and a cytoplasmic response regulator, CpxR (Dong et al., 1993). The activation of the Cpx signal transduction pathway is through a typical two-component regulatory system with CpxA being an autokinase, a CpxR kinase and a CpxR-P phosphatase (Raivio and Silhavy, 1997). In the activated state, the kinase:phosphatase ratio of CpxA is elevated (Raivio and Silhavy, 1997). The phosphorylation of CpxR enhances its binding upstream of target genes (Pogliano et al., 1997; Raivio and Silhavy, 1997), leading to transcriptional activation and elevated expression. The Cpx pathway responds not only to pilus subunits going OFF-pathway, but also to other stresses affecting the bacterial envelope, including overproduction of the novel outer membrane lipoprotein NlpE (Snyder et al., 1995), high pH (Nakayama and Watanabe, 1995) and an alteration in the lipid composition of the bacterial membrane (Mileykovskaya and Dowhan, 1997). Addition ally, the activation of the Cpx pathway is regulated by both auto-amplification and feedback inhibition mechanisms (Raivio et al., 1999). The cpxRA operon is itself a member of the Cpx regulon, so the expression of this operon is elevated in response to stresses to the bacterial envelope (Raivio et al., 1999). Feedback inhibition occurs when the small, periplasmic, Cpx-regulated molecule CpxP is overexpressed, for this leads to downregulation of the Cpx pathway in a manner that is CpxA dependent (Danese and Silhavy, 1998; Raivio et al., 1999).
In this study we investigated the involvement of the Cpx pathway in P pilus biogenesis. The analysis of the effect of a cpxR null mutation revealed an assembly defect resulting in shorter pili. This assembly defect was in part due to the role of Cpx in controlling the expression of factors that are important for efficient pilus assembly. In addition, we found that CpxR can positively affect the expression of the pap promoter.
Results
Short pili are assembled in the absence of cpxR
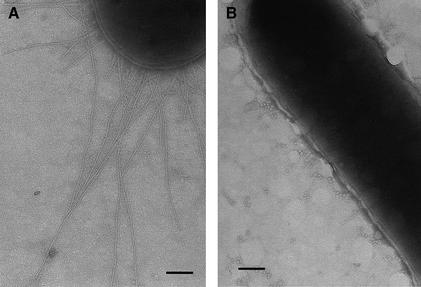
The involvement of the Cpx pathway in P pilus biogenesis was investigated by analyzing the effect of a cpxR null mutation on pilus expression. The pap gene cluster, under its natural promoter (pPAP5) (Lindberg et al., 1984), was expressed in a strain with a cpxR null allele (TR51). After induction of the pap promoter on tryptic soy agar (TSA), pili were isolated by MgCl2 precipitation and by Galα(1–4)Gal affinity chromatography. The cpxR null mutation resulted in a 5-fold decrease in the amount of pili that could be purified from equal cultures via MgCl2 precipitation (Figure 1A, lanes 5 and 6). However, no significant difference was observed between the wild-type strain and the cpxR– mutant in the amount of pili that could be isolated by Galα(1–4)Gal chromatography (Figure 1A, lanes 1 and 2). In addition, the hemagglutination (HA) titers, which compare relative amounts of adhesive pili but do not monitor total numbers of pili, were nearly identical between the two strains (Figure 1A, lanes 1 and 2; Table I). Since the MgCl2 purification procedure relies on a specific cross-linking of the pilus rods (comprised of PapA) (Soto et al., 1998), the results from this procedure reflect the relative amount of this pilus antigen assembled on the bacterial surface. In contrast, the purification of pili by Galα(1–4)Gal affinity chromatography depends on the binding of the tip-located PapG to the receptor (Hultgren et al., 1989). The presence of normal length rods (1–2 µM long) makes the binding and retention of PapG to the column inefficient due to the forces exerted by the rod (Soto et al., 1998). Therefore, this procedure selects for adhesive tip fibrillae joined to short rods. Based on this information, we deduced that the cpxR null mutation resulted in the production of shorter pili. To test this hypothesis, we examined the two strains by electron microscopy (EM) (Figure 1B and C) and found that the pili assembled in the absence of CpxR were very short (Figure 1C) compared with wild-type pili (Figure 1B).
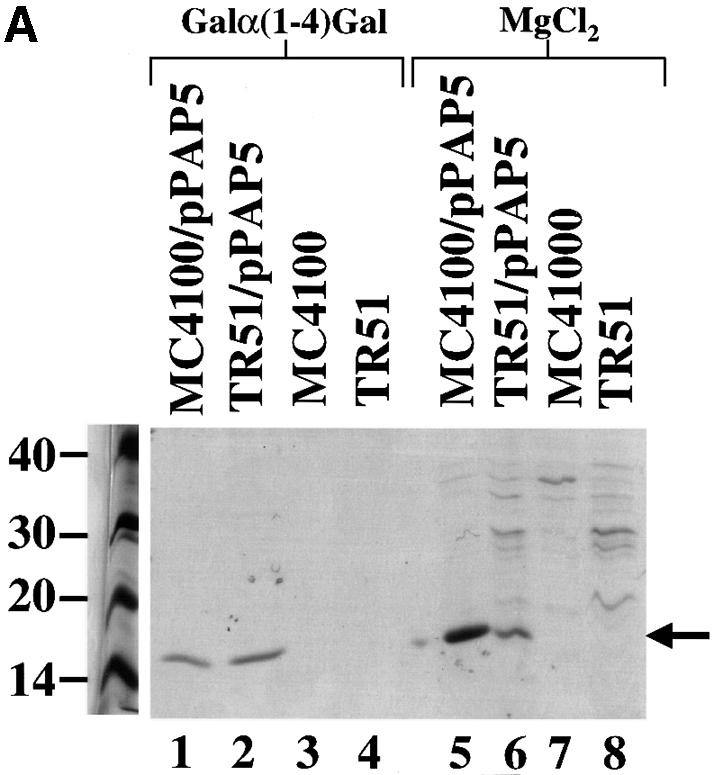
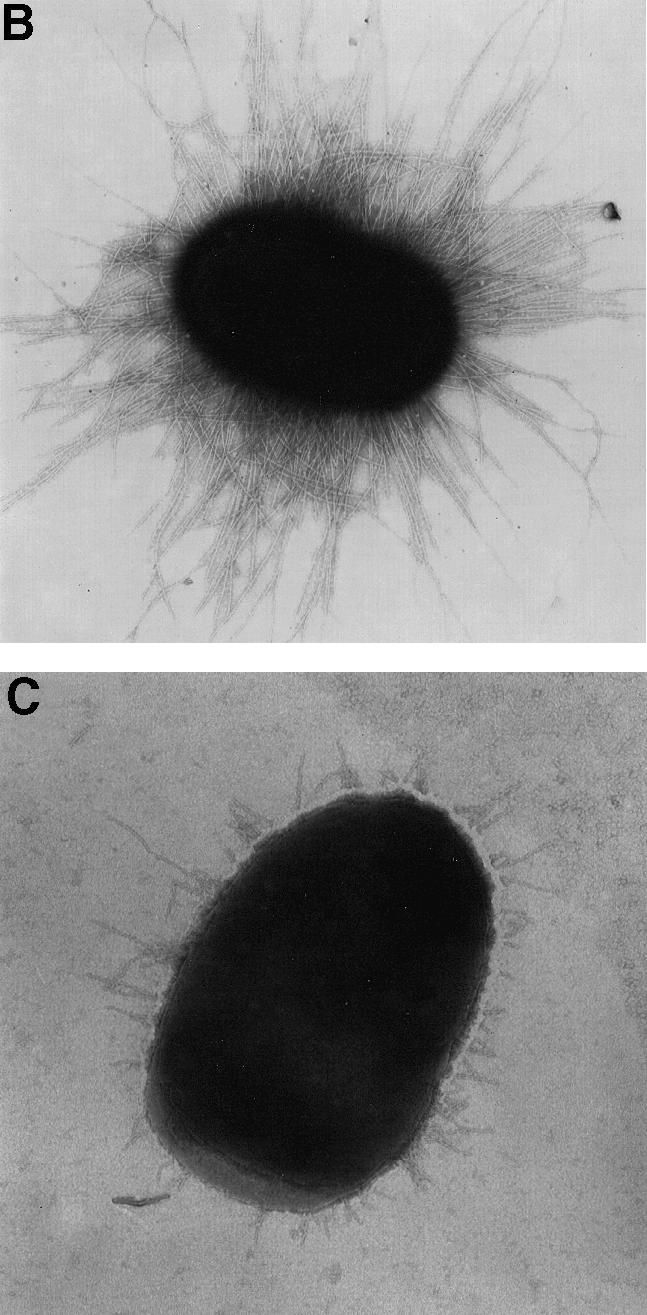
Fig. 1. Fewer and shorter P pili are assembled without the Cpx signaling pathway. (A) P pili were purified by Galα(1–4)Gal affinity chromatography in lanes 1–4 and by MgCl2 precipitation in lanes 5–8, run on SDS–PAGE and stained with Coomassie Blue. Pili were purified after 3 days passage on TSA plates from the following strains: wild-type strain alone, lanes 3 and 7 (MC4100); wild-type strain expressing P pili, lanes 1 and 5 (MC4100/pPAP5); cpxR null strain alone, lanes 4 and 8 (TR51); and cpxR null strain expressing P pili, lanes 2 and 6 (TR51/pPAP5). The arrow points to the PapA band. (B) Negative stain of wild-type cells expressing P pili (MC4100/pPAP5) at a magnification of 20 000. (C) Negative stain of cells with a cpxR null allele expressing P pili (TR51/pPAP5) at a magnification of 20 000.
Table I. Effects of cpx mutations and NlpE overproduction on P pili-mediated HA titers.
| Genotype | PhenotypeStrain/plasmid | HA titer |
|---|---|---|
| MC4100/pPAP5a | wild type/P pili (natural promoter) | 64 |
| TR51/pPAP5a | CpxR null/P pili (natural promoter) | 64 |
| TR14/pPAP5a | Cpx*/P pili (natural promoter) | 256 |
| TR20/pPAP5a | Cpx*/P pili (natural promoter) | 256 |
| MC4100/pRHU845a | wild type/P pili | 64 |
| MC4100/pRHU845/pLD404a | wild type/P pili/NlpE | 128 |
| MC4100/pRHU845b | wild type/P pili | 64 |
| MC4100/pRHU845/pLD404b | wild type/P pili/NlpE | 128 |
| MC4100/pRHU845c | wild type/P pili | 0 |
| MC4100/pRHU845/pLD404c | wild type/P pili/NlpE | 8 |
| MC4100/pFJ29d | wild type/P pili | 32 |
| TR51/pFJ29d | CpxR null/P pili | 32 |
| TR14/pFJ29d | Cpx*/P pili | 64 |
| TR20/pFJ29d | Cpx*/P pili | 32 |
The HA titer was quantitated after 3 days passage of the strains on TSA platesa, after overnight growth in shaking Luria broth without glucoseb or with 0.5% glucose addedc, or after growth to mid-logarithmic phased.
Cpx-controlled factors are necessary for efficient pilus biogenesis
We hypothesized that if the short pili phenotype observed above is independent of any regulation of the pap promoter, then pili produced from the cpxR null strain would still be short even when the pap operon is expressed from an inducible promoter. Thus, wild-type and cpxR null strains were transformed with pFJ29, which contains the pap operon behind an inducible trc promoter (Jacob- Dubuisson et al., 1994b). Pilus expression was examined by EM (Figure 2). It was found that the pili assembled in the absence of CpxR were very short (Figure 2B) when compared with wild-type pili (Figure 2A), but that the HA titers of the cpxR null mutant and the wild type were identical (Table I). These results argue that the short pili phenotype observed in the absence of the Cpx signaling pathway is an assembly defect and is independent of any regulation that the two-component system may exert on the pap promoter, arguing that Cpx-controlled factors facilitate pilus biogenesis.
Fig. 2. Short P pili are assembled without the Cpx signaling pathway when expressed behind an inducible promoter. (A) Negative stain of wild-type cells expressing P pili behind the trc promoter (MC4100/pFJ29) at a magnification of 50 000. (B) Negative stain of cells with a cpxR null allele expressing P pili behind the trc promoter (TR51/pFJ29) at a magnification of 50 000. The bar represents 200 nm.
Cpx* mutants increase piliation
Results presented above suggested that CpxR is involved in controlling the expression of factors that facilitate pilus biogenesis. Therefore, we investigated whether a constitutively active (phosphorylated) CpxR would increase the amount of isolatable pili compared with cells with a wild-type Cpx signaling system. This was investigated using gain-of-function Cpx* mutants (Cosma et al., 1995; Raivio and Silhavy, 1997) that contain mutations in cpxA. CpxA is a membrane-bound histidine kinase that, when activated, phosphorylates CpxR (Raivio and Silhavy, 1997). The Cpx* mutants in cpxA render them devoid of phosphatase activity and this gives an elevated kinase:phosphatase ratio (an increased level of phosphorylated CpxR), which increases expression of Cpx-regulated genes (Raivio and Silhavy, 1997). When the pap gene cluster was expressed behind the natural promoter (pPAP5) in two different cpx* backgrounds, the amount of isolatable pili increased relative to the wild-type background, as did the HA titers (Figure 3A, lane 1 versus 3 and 4; Table I). This effect was also observed in strain TR14 even when the pap operon was expressed from an inducible trc promoter, pFJ29 (Jacob-Dubuisson et al., 1994b) (Figure 3B, lane 1 versus lane 3; Table I). These results argue that an activated Cpx pathway may increase the efficiency of P pilus assembly by upregulating periplasmic protein folding factors that facilitate the biogenesis process (Jacob-Dubuisson et al., 1994a; Danese and Silhavy, 1997; Jones et al., 1997; Pogliano et al., 1997).
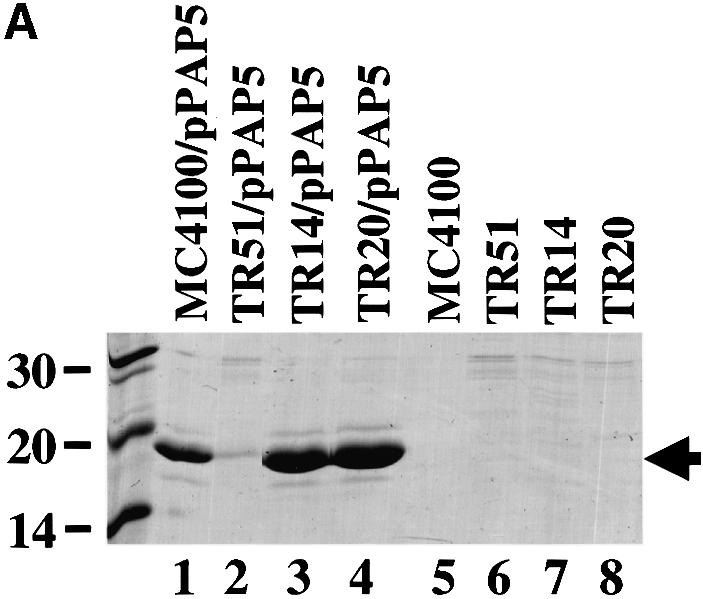
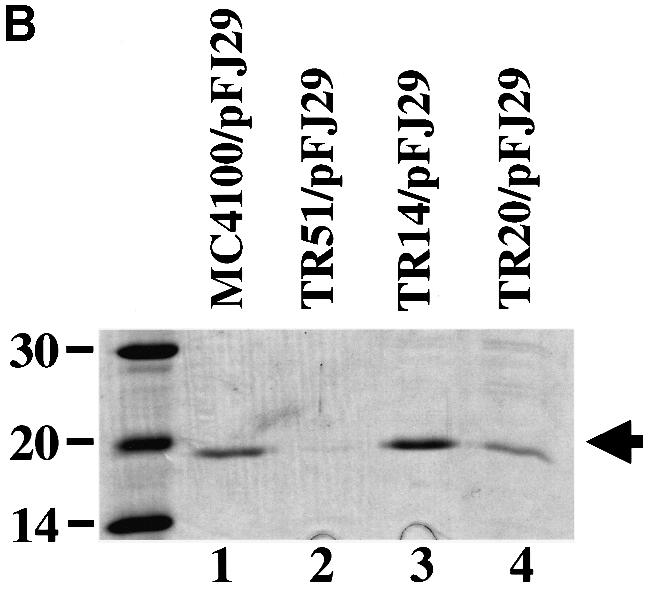
Fig. 3. P pilus assembly is increased in strains with signal-responsive cpx* mutations. (A) P pili were expressed from behind the natural promoter. MgCl2-precipitated pili were run on SDS–PAGE and stained with Coomassie Blue. Pili were purified after 3 days passage on TSA plates with the appropriate antibiotics from the following strains: wild type alone, lane 5 (MC4100), and expressing P pili, lane 1 (MC4100/pPAP5); cpxR null alone, lane 6 (TR51), and expressing P pili, lane 2 (TR51/pPAP5); TR14 alone, lane 7, and expressing P pili, lane 3 (TR14/pPAP5); and TR20 alone, lane 8, and expressing P pili, lane 4 (TR20/pPAP5). Verification of the PapA band was performed by western blot analysis using anti-pilus antiserum (data not shown). The arrow points to the PapA band. (B) P pili were expressed behind the trc promoter. MgCl2-precipitated pili were run on SDS–PAGE and stained with Coomassie Blue. Pili were purified after growth to mid-logarithmic phase in the following strains: wild type expressing P pili, lane 1 (MC4100/pFJ29); cpxR null expressing P pili, lane 2 (TR51/pFJ29); TR14 expressing P pili, lane 3 (TR14/pFJ29); TR20 expressing P pili, lane 4 (TR20/pFJ29). Verification of the PapA band was performed by western anlysis using anti-pilus antiserum (data not shown). The arrow points to the PapA band.
Signal sensed is independent of DegP
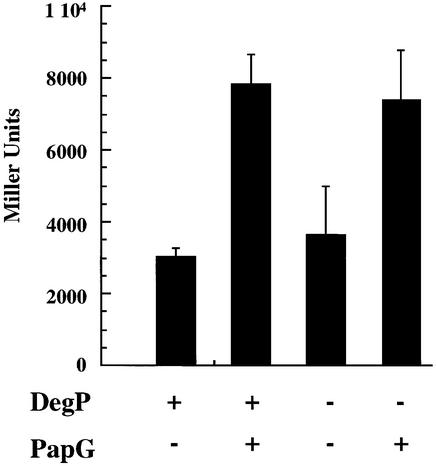
In the absence of an interaction with the chaperone, pilus subunits go OFF-pathway, i.e. they misfold and aggregate. OFF-pathway subunits have been shown to activate the Cpx pathway (Jones et al., 1997). Either the signal generated by OFF-pathway subunits could be due to direct interactions of misfolded subunits with either CpxA or factors that regulate CpxA, or the signal could be comprised of proteolytic fragments of OFF-pathway subunits degraded by the periplasmic protease DegP (Jones et al., 1997). The role of DegP in generating a signal that activates the Cpx pathway was investigated by using the cpxP–lacZ promoter fusion integrated into the chromosome of isogenic wild-type and degP null strains (KS272 and KS474) creating DH500 and DH501, respectively. The cpxP promoter has been shown to be specifically activated by the Cpx pathway (Danese and Silhavy, 1998). Since the PapG adhesin has been shown to highly activate the Cpx pathway (Jones et al., 1997), the expression of cpxP–lacZ was analyzed in the presence or absence of PapG in both strains. The expression of PapG in the wild-type and degP null strains, DH500 and DH501, induced the expression of the cpxP promoter 2.5-fold (Figure 4). This argues that OFF-pathway subunits generate a Cpx-activating signal prior to their proteolytic degradation by DegP.
Fig. 4. The signal generated by subunits going OFF-pathway is independent of the DegP protease. Wild-type and degP null strains (KS272 and KS474, respectively) with cpxP–lacZ fusions (DH500 and DH501, respectively) were analyzed. Cells were grown shaking in Luria broth, and PapG (pHJ8) or the vector control (pMMB66) was induced for expression during early logarithmic growth. Aliquots removed during mid-logarithmic growth and Miller assays were performed. The results shown on the graph are averages and standard deviations from three independent assays.
CpxR binding site in the pap operon
In addition to the assembly defect observed in the cpxR null mutant, an increase in the proportion of non-piliated cells was also observed by EM in the cpxR– mutant, suggesting that Cpx could affect the phase variation of P pilus expression. The average of two double blind experiments of counting piliated and non-piliated bacteria was 6% non-piliated for wild-type bacteria expressing P pili (MC4100/pPAP5) versus 18% non-piliated for the cpxR null strain (TR51/pPAP5). These differences were apparently not significant enough to be detected by HA titers since these strains produced similar HA titers (Table I). These results suggest that in addition to an assembly defect, the Cpx pathway may also regulate phase variation of P pili.
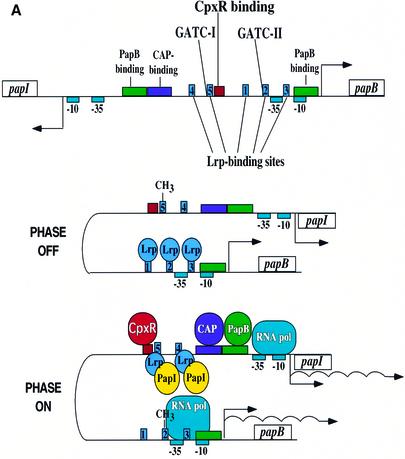
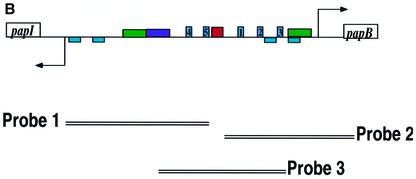
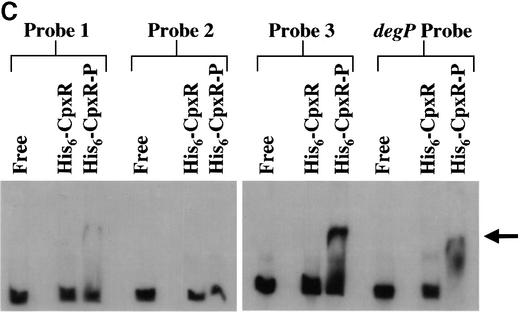
P pili undergo an ON–OFF phase variation that does not require any changes in the primary DNA sequences of the promoter (Blyn et al., 1989). Instead, a number of factors control phase variation by binding to regulatory sites within the promoter (Figure 5A) (van der Woude et al., 1996). PapI is a regulatory protein essential for the phase OFF–ON transition of the papBA promoter (Kaltenbach et al., 1995; van der Woude et al., 1996), which is the promoter for the entire pap operon (excluding papI). PapB, like CAP, can activate expression of papI, and it serves as a negative feedback regulator of papB (Forsman et al., 1989, 1992). There are two DAM methylation sites, GATC-I and GATC-II, and five Lrp binding sites (van der Woude et al., 1996). When the GATC-I site is methylated, Lrp is bound to Lrp binding sites 1, 2 and 3, and pap transcription is off (pilus expression is in phase OFF). Conversely, when GATC-I is not methylated or hemi-methylated, Lrp is able to bind to Lrp binding sites 4 and 5 as a Lrp–PapI complex. This titrates Lrp away from Lrp binding sites 1, 2 and 3, resulting in the methylation of GATC-II, which prevents rebinding of Lrp to these sites. As illustrated in Figure 5A, this activates transcription of the pap gene cluster (pilus expression is in phase ON) (Braaten et al., 1994; Kaltenbach et al., 1995; Nou et al., 1995; van der Woude et al., 1996). Within this highly regulated pap promoter region (Blyn et al., 1989; van der Woude et al., 1996) we identified several potential CpxR binding sites with the sequence 5′-GTAAA-3′. To determine whether CpxR bound specifically to the pap promoter region, we tested the ability of purified His6-CpxR protein to bind to DNA probes specific for the pap promoter (Figure 5B). Results from electrophoretic mobility shift assays (EMSAs) demonstrated that only the phosphorylated form of His6-CpxR (His6-CpxR-P) caused a gel shift and only with the DNA probe #3 (Figure 5C). These results argued that the DNA highlighted by the red box in the pap promoter region in Figure 5A contains at least part of a true binding site for CpxR-P.
Fig. 5. CpxR-P binds to the pap promoter. (A) Schematic of the pap promoter region (top) and the model of transcriptional inactivation, phase OFF (center), and activation, phase ON (bottom) (adapted from van der Woude et al., 1996). Phase variation is affected by the action of DAM on the adenines in the two DNA GATC sequences, designated GATC-I and GATC-II, contained within the Lrp binding sites 5 and 2, respectively (explained in text). Abbreviations: CAP, catabolite activator protein; Lrp, leucine-responsive regulatory protein. The involvement of CpxR is discussed in the text. (B) Schematic of strategy for DNA probes used in EMSAs. Only probe 3 contains the putative CpxR binding site; probes 1 and 2 do not. A positive control probe, DegP, encoding the previously identified CpxR binding site in the degP promoter was also used in the EMSAs. (C) EMSAs of His6-CpxR binding to the putative site in the pap promoter. His6-CpxR was purified and phosphorylated. Unphosphorylated (His6-CpxR) or phosphorylated His6-CpxR (His6-CpxR-P) was incubated with DNA probes from (B) and run on 4% acrylamide gels.
The cpx pathway can positively affect regulation of phase variation
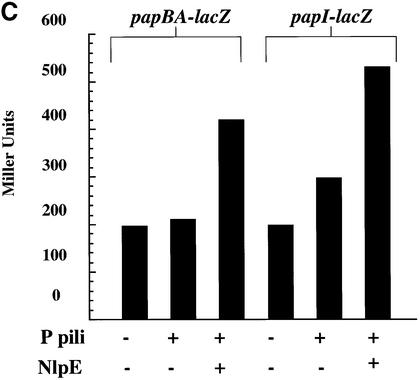
The hypothesis that Cpx is a regulator of phase variation was tested by investigating whether a specific induction of Cpx could override a phase OFF state and convert cells to phase ON. Expression of both the papI and papBA promoters is under catabolite repression (Baga et al., 1985; Forsman et al., 1992). Thus, cells grown in the presence of 0.5% glucose are in the phase OFF orientation (Baga et al., 1985; Forsman et al., 1992; Braaten et al., 1994; Nou et al., 1995; van der Woude et al., 1996): GATC-I is methylated; Lrp is bound to sites 1–3 overlapping the papBA promoter; consequently, the cells are non-piliated and HA negative (Figure 6A, lane 4; Table I). We investigated whether a specific induction of Cpx could result in pilus production even under catabolite-repressing conditions. Overproduction of NlpE is known to specifically activate Cpx (Snyder et al., 1995). Overproduction of NlpE in cells grown in 0.5% glucose resulted in HA-positive cells from which pili could be purified (Figure 6A, lanes 4 and 5; Table I). In addition, we found that after 2-fold passage on TSA plates or after growth in shaking Luria broth, the overproduction of NlpE increased the amount of isolatable pili by 4-fold (Figure 6B, lanes 1 and 2, and A, lanes 2 and 3). To further analyze whether transcription of the pap operon is upregulated by the expression of NlpE (the activation of the Cpx pathway), the activity of the papBA–lacZ and papI–lacZ fusions was monitored. The expression of NlpE increased the transcription from both promoters 2-fold (Figure 6C). The level of activity from the promoters was too low to detect in the presence of glucose. These results suggest that expression of NlpE, which specifically activates the Cpx pathway, results in an increased transcription from the pap promoter. Thus, the effect of stimulating pilus production under conditions of catabolite repression by expressing NlpE may be due, in part, to the ability of Cpx to positively affect pap transcription, although an increased efficiency of pilus biogenesis may also contribute to the effect.
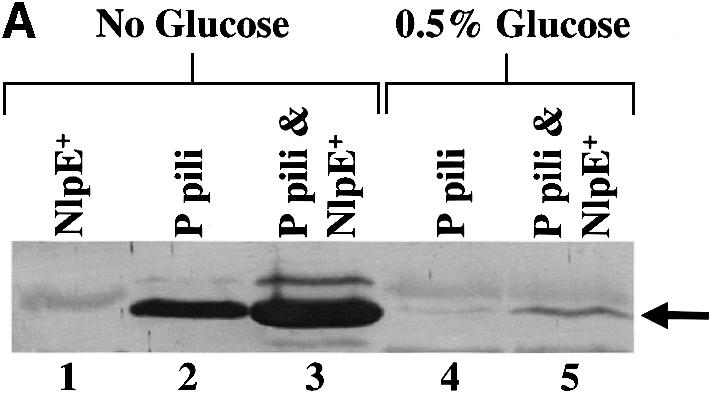
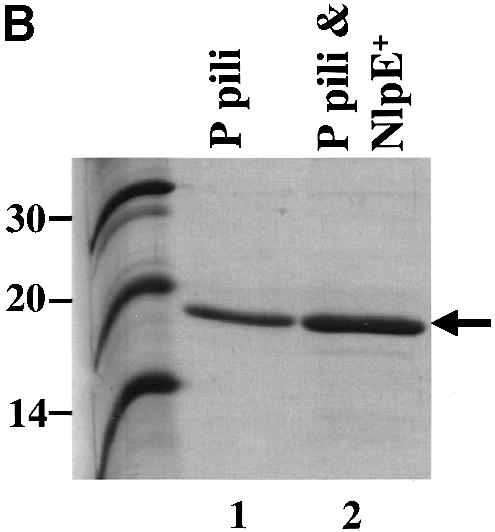
Fig. 6. Specific activation of the Cpx signaling pathway by overproduction of NlpE increases P pilus assembly and can relieve catabolite repression. (A) MgCl2-precipitated pili were run on SDS–PAGE and then immunoblotted by using anti-pilus antiserum. Pili were purified after overnight growth shaking in Luria broth with the appropriate antibiotics in the presence or absence of 0.5% glucose. Pili were purified without added glucose from the wild-type strain overexpressing NlpE, lane 1 (MC4100/pLD404); expressing P pili, lane 2 (MC4100/pRHU845); and expressing P pili while over expressing NlpE, lane 3 (MC4100/pRHU845/pLD404). Pili purified from cells grown with 0.5% glucose expressing only P pili, lane 4 (MC4100/pRHU845), and also overproducing NlpE, lane 5 (MC4100/pRHU845/pLD404). The arrow points to the PapA band. (B) MgCl2-precipitated pili were run on SDS–PAGE and then immunoblotted by using anti-pilus antiserum. Pili were purified after the bacteria had been passaged 3 days on TSA plates with the appropriate antibiotics. Pili were purified from the wild-type strain expressing only P pili, lane 1 (MC4100/pRHU845) or with the overproduction of NlpE, lane 2 (MC4100/pRHU845/pLD404). The arrow points to the PapA band. (C) The wild-type strain with papBA–lacZ and papI–lacZ expressing only P pili (CHJ101/pRHU845 and CHJ103/pRHU845, respectively) or with the overproduction of NlpE (CHJ101/pRHU845/pLD404 and CHJ103/pRHU845/pLD404) was analyzed. Aliquots were removed after overnight growth shaking in Luria broth with the appropriate antibiotics, and Miller assays were performed. The results shown on the graph are averages from two independent assays.
Discussion
The biogenesis of P pili is the prototype of the chaperone/usher secretion pathway used to assemble >26 architecturally diverse organelles in various Gram-negative bacteria (Hung et al., 1996; Thanassi et al., 1998a). Previous studies identified a relationship between P pilus assembly and the Cpx signaling pathway (Jones et al., 1997). The results reported here begin to reveal the more intimate connection that exists between pilus biogenesis, the two-component signaling system and pathogenesis.
Many periplasmic factors are regulated by the Cpx two-component regulatory system, including DegP, DsbA, PpiA and others (Danese and Silhavy, 1997; Pogliano et al., 1997). The signals known to activate the Cpx pathway include those stimuli that cause extra-cytoplasmic stresses such as high pH (Nakayama and Watanabe, 1995; Danese and Silhavy, 1998), alterations to the bacterial inner membrane (Mileykovskaya and Dowhan, 1997; Danese et al., 1998) and NlpE overexpression (Snyder et al., 1995). The first connection between P pilus biogenesis and the Cpx pathway was made when it was demonstrated that pilus subunits expressed in the absence of their cognate chaperone served as a potent activator of the Cpx pathway (Jones et al., 1997). In the present study, we discovered that the absence of a functional Cpx signaling pathway results in an assembly defect (short pili), which is presumably caused by the lack of Cpx-regulated factors necessary to facilitate pilus biogenesis. These might include the Cpx-regulated factors known to be involved in efficient pilus assembly, including the following: the protease DegP (Danese et al., 1995; Danese and Silhavy, 1997; Pogliano et al., 1997), which is involved in degrading misfolded proteins (Jones et al., 1997); the enzyme DsbA (Danese and Silhavy, 1997; Pogliano et al., 1997), which is required for assembly of P pili (Jacob-Dubuisson et al., 1994a); and other periplasmic folding factors that may be involved in pilus biogenesis, including PpiA (Danese and Silhavy, 1997; Pogliano et al., 1997).
The pap promoter is highly regulated by numerous factors, including PapB and PapI as well as Lrp, DAM, CAP and HNS (van der Woude et al., 1996). We discovered that CpxR binds to the pap promoter and affects the phase variation of pilus expression. The Cpx pathway is comprised of a membrane-bound sensor histidine kinase, CpxA, and a cytoplasmic response regulator, CpxR (Weber and Silverman, 1988; Dong et al., 1993). When phosphorylated, CpxR-P binds upstream of target genes to activate their expression (Pogliano et al., 1997; Raivio and Silhavy, 1997). The phosphorylated form of CpxR specifically bound to the pap promoter. The pap promoter is subject to an ON–OFF phase variation switch (Blyn et al., 1989, 1990). Thus, EM of cultures passaged on agar reveals both piliated and non-piliated cells due to phase variation. The knockout mutation in cpxR resulted in a 3-fold increase in the percentage of non-piliated cells, suggesting that Cpx plays a critical role in regulating the phase variation switch. Specifically, these results suggest that Cpx plays a role in maintaining the cells in a phase ON state of piliation. In contrast, bacteria grown in the presence of glucose are in a phase OFF state due to catabolite repression (Forsman et al., 1992). However, the activation of Cpx (using NlpE overexpression) in cells grown in the presence of glucose induced pilus expression—there were isolatable pili. The ability of NlpE overexpression to induce piliation under catabolite-repressing conditions is probably related in part to its ability to activate Cpx, which was shown to result in increased transcription of the pap promoter. Since the Cpx pathway can affect regulation of the pap operon, its positive effect on pap phase variation may be a contributing factor to increases in piliation in response to activation of Cpx, whether under catabolite-repressing conditions or not. The conversion of pilus expression from OFF to ON is thought to require two rounds of DNA replication to demethylate GATC-I, since there are no known demethylases (Braaten et al., 1994; van der Woude et al., 1996). Activation of Cpx via environmental cues may allow the bacteria to convert quickly from phase OFF to ON in a manner independent of demethylation. However, an increased efficiency of pilus biogenesis due to upregulation of assembly factors controlled by Cpx presumably contributes to the effect as well. The same effect was observed when P pili were expressed behind the natural promoter in strains with the cpx* mutations, which increase the ratio of kinase:phosphatase (an increased level of phosphorylated CpxR) (Raivio and Silhavy, 1997): the amount of isolatable pili increased. The increase in isolatable pili occurred in the cpx* strain TR14 even when P pili were expressed from the trc promoter, arguing that, in this case, increased efficiency of pilus biogenesis plays a significant role in increasing piliation.
The signal that is generated by OFF-pathway subunits is independent of the presence of the periplasmic protease DegP. It is known that subunits do not obtain their correct fold without the donation of the missing seventh C-terminal β-strand from the chaperone (Choudhury et al., 1999; Sauer et al., 1999; Barnhart et al., 2000). Together, these results suggest that the signal sensed by the Cpx pathway throughout assembly are subunits that are missing the information to fold correctly.
In conclusion, there exists an intricate connection between P pilus biogenesis and the Cpx pathway. During pilus biogenesis, a signal is generated by OFF-pathway subunits that activates Cpx (Jones et al., 1997; Hung et al., 1999). As a consequence, Cpx upregulates factors that facilitate pilus assembly (Danese and Silhavy, 1997; Jones et al., 1997; Pogliano et al., 1997). In addition, CpxR affects the phase variation of pap expression, favoring an ON state. Numerous environmental cues including temperature (Goransson and Uhlin, 1984; Blyn et al., 1989; White-Ziegler et al., 1998) and glucose (Baga et al., 1985; Forsman et al., 1992) influence the expression of pili. Environmental conditions in the urinary tract presumably favor the expression of pili at critical points during the pathogenic cascade (Kisielius et al., 1989; Lim et al., 1998). Interestingly, pilus assembly leads to the activation of Cpx, which in turn would serve to reinforce the commitment to make pili by activating periplasmic assembly factors and maintaining the ON state of phase variation. This would ensure that daughter cells remain piliated in order to facilitate the colonization of the epithelium and persistence in the urinary tract.
In addition, host–pathogen interactions in the urinary tract activate a cascade of innate defenses, such as an increase in the production of nitric oxide that may induce periplasmic stresses (Smith et al., 1996; Svanborg and Godaly, 1997; Mulvey et al., 2000; Mumtaz et al., 2000) and lead to an activation of Cpx. The activation of Cpx via pilus biogenesis and host–pathogen interactions serves to reinforce the commitment to produce pili, facilitating colonization of the urinary tract. In addition, Cpx may control the expression of a number of other virulence factors, as we have identified putative CpxR binding sites upstream of genes encoding hemolysin, cytotoxic necrotizing factor and type 1 pili (D.L.Hung and S.J.Hultgren, unpublished results). Thus, environmental cues that activate pilus expression may indirectly activate additional virulence factors due to the induction of Cpx by OFF-pathway subunits. In this way, Cpx may tie the expression of virulence factors to pilus biogenesis, which may be part of a mechanism in which microbial colonization is linked to the expression of a panoply of new genes important in the pathogenic cascade.
Materials and methods
Bacterial strains
The E.coli strain MC4100 was used as a host strain in all studies (Casadaban, 1976). TR51 (MC4100, cpxR::spc) was constructed by P1 transduction of the cpxR::spc allele (Danese et al., 1995) into MC4100 using standard techniques.
Strains TR14 and TR20 were constructed by P1 transduction of the cpxA106 and cpxA101 alleles into MC4100 metF::Tn10 strain, which fails to grow on minimal plates. Transductants that grew on minimal plates were selected and the presence of the cpx* allele was confirmed by testing for amikacin resistance as previously described (Cosma et al., 1995). Isolation and characterization of the various cpx* alleles were previously described (Cosma et al., 1995; Danese et al., 1995; Raivio and Silhavy, 1997). Specifically, TR14 (MC4100, cpxA106) and TR20 (MC4100, cpxA101) each contain a mutation in the cytoplasmic region of CpxA (Raivio and Silhavy, 1997).
DH500 and DH501 were created by P1 transduction of the cpxP–lacZ fusion (Danese and Silhavy, 1998) into KS272 and KS474 (KS272 degP::kan) (Strauch and Beckwith, 1988; Strauch et al., 1989; Jones et al., 1997), respectively, using standard techniques.
To create CHJ101 and CHJ103 the following oligonucleotides were used to PCR amplify pap promoter fragments from pPAP5 (Lindberg et al., 1984): PapBA, 5′-GGGGAATTCTTTCCCTCCATTATGCCTGT-3′ and 5′-GGGGGATCCATCGCGTATATTCAGCAAAA-3′; PapI, 5′-GGGGGATCCTTTCCCTCCATTATGCCTGT-3′ and 5′-GGGGAATTCATCGCGTATATTCAGCAAAA-3′. PCR amplification of promoter fragments was performed as previously described (Hung et al., 1999). EcoR1 and BamH1 sites were incorporated into the amplified fragments to allow cloning into pRS45 (kindly provided by Virginia Miller, WUMS, St Louis, MO). The promoter–LacZ fusions were moved from pRS45 onto λRS415 (kindly provided by Bob Simons, UCLA) following the protocol of Simons et al. (1987). MC4100 was lysogenized with λRS415-papBA and λRS45-papI resulting in CHJ101 and CHJ103, respectively.
Plasmid construction
The following plasmids have been described elsewhere. pPAP5 expresses the pap operon behind the natural promoter in the vector pBR322 (Lindberg et al., 1984). pRHU845 expresses the pap operon behind the natural promoter in the vector pACYC184 (Normark et al., 1983). pLD404 overexpresses NlpE (Snyder et al., 1995). pFJ29 expresses the pap operon behind the inducible trc promoter in the vector pACYC184 (Jacob-Dubuisson et al., 1994b). pHJ8 expresses PapG behind the tac promoter in the vector pMMB66 (Jones et al., 1997).
Hemagglutination assays
HA titers were performed as described (Slonim et al., 1992) after 3 days passage of the strains on TSA plates, or after overnight growth in shaking Luria broth with or without 0.5% glucose (see figure legends).
Pili preparations
Pili were prepared from cells expressing the pap operon behind the natural promoter (pPAP5) after 3 days passage on TSA plates. P pili were also purified from cells expressing the pap operon behind the trc promoter (pFJ29) after growth to mid-logarithmic phase, with no isopropyl-β-D-thiogalactopyranoside (IPTG) necessary for induction of the promoter since the trc promoter was very leaky. Equal amounts of cells by weight were divided into two aliquots for isolation of pili via two methods. P pili were precipitated using MgCl2 and NaCl as previously described (Jacob-Dubuisson et al., 1993). P pili were also affinity purified using Galα(1–4)Gal-Sepharose beads in a similar manner to that in which the PapD–PapG complex was purified (Hultgren et al., 1989).
Electron microscopy
Cells expressing P pili from pPAP5 were grown for 3 days on TSA plates to induce piliation. The bacteria expressing P pili from pFJ29 were grown overnight in Luria broth, subcultured (1:100) and grown to mid-logarithmic growth phase, with no IPTG necessary for induction of the promoter since the trc promoter was very leaky. Bacterial cells were fixed on grids for EM as described (Hultgren et al., 1985). The cells were stained on top of drops of 0.5% phosphotungstic acid (Sigma) for 25 s.
DNA binding studies
To create DNA probes 1–3 and the positive control probe DegP the following pairs of oligonucleotides were used to amplify the indicated regions of the pap promoter from pPAP5 (Lindberg et al., 1984) and the degP promoter from the chromosome of MC4100. The PCR products were cloned between the EcoR1 and BamH1 sites of pUC18 and each clone was verified by sequencing. pPAPBI840 encoding probe #1 contains a 202-bp segment of the pap promoter region (positions 840–1042 in DDBJ/EMBL/GenBank file ECPAPAK) amplified with primers 5′-GGGGAATTCACTGTAACAAAGTTTCTTCGA-3′ and 5′-GGGGGATCCAGCATAAAAGATCGTCTAAAT-3′. pPAPBI1057 encoding probe #2 contains a 200-bp segment of the pap promoter regions (positions 1057–1257 in DDBJ/EMBL/GenBank file ECPAPAK) amplified with primers 5′-GGGGGATCCACTTCATGATGCGCCATGTTT-3′ and 5′-GGGGAATTCCCATGATGTTTTTATCTGAGT-3′. pPAPBI970 encoding probe #3 contains a 200-bp segment of the pap promoter region (positions 970–1170 in DDBJ/EMBL/GenBank file ECPAPAK) amplified with primers 5′-GGGGAATTCTGTCACATTTTGTGTGTTATT-3′ and 5′-GGGGGATCCATTTAGTTTTTTATGTTGTAA-3′. pDEGP800 contains a 235-bp segment of the degP region (positions 69298–69533 in DDBJ/EMBL/GenBank file D26562) amplified with primers 5′-GGGGATCCGACCTCTATGCGTGGGATG-3′ and 5′-CCGAATTCGCCAAACCTAAACYCAGAGCC-3′, as described in Pogliano et al. (1997).
An N-terminal oligohistidine extension was added to CpxR and the protein was purified as described (Pogliano et al., 1997). His6-CpxR was phosphorylated by incubation of 300 pmol of protein for 1 h at 30°C with 100 mM Tris pH 7.0, 10 mM MgCl2, 125 mM KCl, 50 mM acetyl phosphate (lithium potassium salt, Sigma). EMSAs were run on 4% acrylamide gels at 4°C and performed as previously described (Lynch and Lin, 1996).
β-galactosidase assays
To assay the cpxP–lacZ fusion, bacteria were grown overnight in Luria broth, subcultured (1:100) and grown for 60 min. When required to induce expression of PapG from behind the tac promoter, IPTG was added to 0.5 mM to induce subunit protein. The cells were then grown shaking at 37°C to mid-logarithmic growth phase. Aliquots were removed and β-galactosidase assays were performed as described (Jones et al., 1997). To assay the papI–lacZ and papBA–lacZ fusions, bacteria were diluted 1:100 and aliquots were removed after overnight growth shaking in Luria broth with the appropriate antibiotics in the presence or absence of 0.5% glucose. β-galactosidase assays were performed as described (Jones et al., 1997).
Acknowledgments
Acknowledgements
We thank Chia-Suei Hung for his help. This work was supported by NIH training grant AI07172-19 (D.L.H.), a fellowship from the American Heart Association (T.L.R.), NIGMS grant GM34821 (T.J.S.), NIH Grant RO1DK51406 and Merit Grant 5R37AI2954910 (S.J.H.).
References
- Baga M., Normark,S., Hardy,J., O’Hanley,P., Lark,D., Olsson,O., Schoolnik,G. and Falkow,S. (1984) Nucleotide sequence of the gene encoding the papA pilus subunit of human uropathogenic Escherichia coli. J. Bacteriol., 157, 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baga M., Goransson,M., Normark,S. and Uhlin,B.E. (1985) Transcriptional activation of a Pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J., 4, 3887–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart M.M., Pinkner,J., Soto,G., Sauer,F.G., Langermann,S., Waksman,G., Frieden,C. and Hultgren,S.J. (2000) PapD-like chaperones provide the missing information for folding of pilin proteins. Proc. Natl Acad. Sci. USA, 97, 7709–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum G., Falbo,V., Caprioli,A. and Hacker,J. (1995) Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and α-hemolysin form the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol. Lett., 126, 189–195. [DOI] [PubMed] [Google Scholar]
- Blyn L.B., Braaten,B.A., White,Z.C., Rolfson,D.H. and Low,D.A. (1989) Phase-variation of pyelonephritis-associated pili in Escherichia coli: evidence for transcriptional regulation. EMBO J., 8, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyn L.B., Braaten,B.A. and Low,D.A. (1990) Regulation of pap pilin phase variation by a mechanism involving differential Dam methylation states. EMBO J., 9, 4045–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock K. et al. (1985) Specificity of binding of a strain of uropathogenic Escherichia coli to Galα(1–4)Gal-containing glycosphingolipids. J. Biol. Chem., 260, 8545–8551. [PubMed] [Google Scholar]
- Braaten B., Blyn,L.B., Skinner,B.S. and Low,D.A. (1991) Evidence for a methylation-blocking factor (mbf) locus involved in pap pilus expression and phase variation in Escherichia coli. J. Bacteriol., 173, 1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braaten B.A., Nou,X., Kaltenbach,L.S. and Low,D.A. (1994) Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E.coli. Cell, 76, 577–588. [DOI] [PubMed] [Google Scholar]
- Bullitt E. and Makowski,L. (1995) Structural polymorphism of bacterial adhesion pili. Nature, 373, 164–167. [DOI] [PubMed] [Google Scholar]
- Casadaban M.J. (1976) Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage λ and Mu. J. Mol. Biol., 104, 541–555. [DOI] [PubMed] [Google Scholar]
- Choudhury D., Thompson,A., Stojanoff,V., Langermann,S., Pinkner,J., Hultgren,S.J. and Knight,S.D. (1999) X-ray structure of the FimC-FimH chaperone–adhesin complex from uropathogenic Escherichia coli. Science, 285, 1061–1066. [DOI] [PubMed] [Google Scholar]
- Cosma C.L., Danese,P.N., Carlson,J.H., Silhavy,T.J. and Snyder,W.B. (1995) Mutational activation of the Cpx signal transduction pathway of Escherichia coli suppresses the toxicity conferred by certain envelope-associated stresses. Mol. Microbiol., 18, 491–505. [DOI] [PubMed] [Google Scholar]
- Danese P.N. and Silhavy,T.J. (1997) The σE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding systems in Escherichia coli. Genes Dev., 11, 1183–1193. [DOI] [PubMed] [Google Scholar]
- Danese P.N. and Silhavy,T.J. (1998) CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol., 180, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese P.N., Snyder,W.B., Cosma,C.L., Davis,L.J.B. and Silhavy,T.J. (1995) The Cpx two-component signal transduction pathway of Escherichia coli regulates degP transcription. Genes Dev., 9, 387–398. [DOI] [PubMed] [Google Scholar]
- Danese P.N., Oliver,G.R., Barr,K., Bowman,G.D., Rick,P.D. and Silhavy,T.J. (1998) Accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J. Bacteriol., 180, 5875–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson K.W., Jacob-Dubuisson,F., Striker,R.T. and Hultgren,S.J. (1993) Outer membrane PapC usher discriminately recognizes periplasmic chaperone–pilus subunit complexes. Proc. Natl Acad. Sci. USA, 90, 3670–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Iuchi,S., Kwan,H.-S., Lu,Z. and Lin,E.C.C. (1993) The deduced amino-acid sequence of the cloned cpxR gene suggests the protein is the cognate regulator for the membrane sensor, CpxA, in a two-component signal transduction system of Escherichia coli. Gene, 136, 227–230. [DOI] [PubMed] [Google Scholar]
- Forsman K., Goransson,M. and Uhlin,B.E. (1989) Autoregulation and multiple DNA interactions by a transcriptional regulatory protein in E.coli pili biogenesis. EMBO J., 8, 1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman K., Sonden,B., Goransson,M. and Uhlin,B.E. (1992) Antirepression function in Escherichia coli for the cAMP-cAMP receptor protein transcriptional activator. Proc. Natl Acad. Sci. USA, 89, 9880–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M. and Makowski,L. (1992) Helical structure of Pap adhesion pili from Escherichia coli. J. Mol. Biol., 228, 735–742. [DOI] [PubMed] [Google Scholar]
- Goransson M. and Uhlin,B.E. (1984) Environmental temperature regulates transcription of a virulence pili operon in E.coli. EMBO J., 3, 2885–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer D.M., Kao,J.S. and Mobley,H.L. (1998) Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis and Catheter-associated bacteriuria and from fecal samples. Infect. Immun., 66, 4411–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Bender,L., Ott,M., Wingender,J., Lund,B., Marre,R. and Goebel,W. (1990) Deletions and chromosomal regions coding for fimbrae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coli isolates. Microb. Pathog., 8, 213–225. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Kuehn,M.J., Brändén,C.-I. and Hultgren,S.J. (1992) Conserved imunoglobulin-like features in a family of periplasmic pilus chaperones in bacteria. EMBO J., 11, 1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R.A., Gill,R.E., Hsu,P., Minshaw,B.H. and Falkow,S. (1981) Construction and expression of recombinant plasmids encoding type 1 and d-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect. Immun., 33, 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S.J., Porter,T.N., Schaeffer,A.J. and Duncan,J.L. (1985) Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect. Immun., 50, 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S.J., Lindberg,F., Magnusson,G., Kihlberg,J., Tennent,J.M. and Normark,S. (1989) The PapG adhesin of uropathogenic Escherichia coli contains separate regions for receptor binding and for the incorporation into the pilus. Proc. Natl Acad. Sci. USA, 86, 4357–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung D.L., Knight,S.D., Woods,R.M., Pinkner,J.S. and Hultgren,S.J. (1996) Molecular basis of two subfamilies of immunoglobulin-like chaperones. EMBO J., 15, 3792–3805. [PMC free article] [PubMed] [Google Scholar]
- Hung D.L., Pinkner,J.S., Knight,S.D. and Hultgren,S.J. (1999) Structural basis of chaperone self-capping in P pilus biogenesis. Proc. Natl Acad. Sci. USA, 96, 8178–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob-Dubuisson F., Heuser,J., Dodson,K., Normark,S. and Hultgren,S.J. (1993) Initiation of assembly and association of the structural elements of a bacterial pilus depend on two specialized tip proteins. EMBO J., 12, 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob-Dubuisson F., Pinkner,J., Xu,Z., Striker,R., Padmanhaban,A. and Hultgren,S.J. (1994a) PapD chaperone function in pilus biogenesis depends on oxidant and chaperone-like activities of DsbA. Proc. Natl Acad. Sci. USA, 91, 11552–11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob-Dubuisson F., Striker,R. and Hultgren,S.J. (1994b) Chaperone-assisted self-assembly of pili independent of cellular energy. J. Biol. Chem., 269, 12447–12455. [PubMed] [Google Scholar]
- Jones C.H., Danese,P.N., Pinkner,J.S., Silhavy,T.J. and Hultgren,S.J. (1997) The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J., 16, 6394–6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach L.S., Braaten,B.A. and Low,D.A. (1995) Specific binding of PapI to Lrp–pap DNA complexes. J. Bacteriol., 177, 6449–6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach L., Braaten,B., Tucker,J., Krabbe,M. and Low,D. (1998) Use of a two-color genetic screen to identify a domain of the global regulator Lrp that is specifically required for pap phase variation. J. Bacteriol., 180, 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J.S., Stucker,D.M., Warren,J.W. and Mobley,H.L. (1997) Patho genicity island sequences of pyelonephritogenic Escherichia coli CFT973 are associated with virulent uropathogenic strains. Infect. Immun., 65, 2812–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisielius P.V., Schwan,W.R., Amundsen,S.K., Duncan,J.L. and Schaeffer,A.J. (1989) In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infections. Infect. Immun., 57, 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn M.J., Heuser,J., Normark,S. and Hultgren,S.J. (1992) P pili in uropathogenic E.coli are composite fibres with distinct fibrillar adhesive tips. Nature, 356, 252–255. [DOI] [PubMed] [Google Scholar]
- Leffler H. and Svanborg-Eden,C. (1980) Chemical identification of a glycosphingolipid receptor for Escherichia coli attaching to human urinary tract epithelial cells and agglutinating human erythrocytes. FEMS Microbiol. Lett., 8, 127–134. [Google Scholar]
- Lim J.K., Gunther,N.W., Zhao,H., Johnson,D.E., Keay,S.K. and Mobley,H.L. (1998) In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect. Immun., 66, 3303–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F.P., Lund,B. and Normark,S. (1984) Genes of pyelo nephritogenic E.coli required for digalactoside-specific agglutination of human cells. EMBO J., 3, 1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A.S. and Lin,E.C. (1996) Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J. Bacteriol., 178, 6238–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskaya E. and Dowhan,W. (1997) The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J. Bacteriol., 179, 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M.A., Schilling,J.D., Martinez,J.J. and Hultgren,S.J. (2000) Bad bugs and beleaguered bladders: interplay between uropathogenic E.coli and innate host defenses. Proc. Natl Acad. Sci. USA, 97, 8829–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz F.H., Khan,M.A., Thompson,C.S., Morgan,R.J. and Mikhailidis,D.P. (2000) Nitric oxide in the lower urinary tract: physiological and pathological implications. BJU Int., 85, 567–578. [DOI] [PubMed] [Google Scholar]
- Nakayama S. and Watanabe,H. (1995) Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J. Bacteriol., 177, 5062–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Lark,D., Hull,R., Norgren,M., Baga,M., O’Hanley,P., Schoolnik,G. and Falkow,S. (1983) Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect. Immun., 41, 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Baga,M., Goransson,M., Lindberg,F.P., Lund,B., Norgren,M. and Uhlin,B.E. (1986) Genetics and biogenesis of Escherichia coli adhesins. In Mirelman,D. (ed.), Microbial Lectins and Agglutinins: Properties and Biological Activity. Wiley Interscience, New York, NY, pp. 113–143.
- Nou X., Braaten,B., Kaltenbach,L. and Low,D.A. (1995) Differential binding of Lrp to two sets of pap DNA binding sites mediated by PapI regulates Pap phase variation in Escherichia coli. EMBO J., 14, 5785–5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J., Lynch,A.S., Belin,D., Lin,E.C.C. and Beckwith,J. (1997) Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev., 11, 1169–1182. [DOI] [PubMed] [Google Scholar]
- Raivio T.L. and Silhavy,T.J. (1997) Transduction of envelope stress in Escherichia coli by the cpx two-component system. J. Bacteriol., 179, 7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivio T.L., Popkin,D.L. and Silhavy,T.J. (1999) The Cpx envelope stress response is controlled by amplification and feedback inhibition. J. Bacteriol., 181, 5263–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J.A. et al. (1994) The Galα(1–4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl Acad. Sci. USA, 91, 11889–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F.G., Futterer,K., Pinkner,J.S., Dodson,K.W., Hultgren,S.J. and Waksman,G. (1999) Structural basis of chaperone function and pilus biogenesis. Science, 285, 1058–1061. [DOI] [PubMed] [Google Scholar]
- Saulino E.T., Thanassi,D.G., Pinkner,J. and Hultgren,S.J. (1998) Ramifications of kinetic partitioning on usher-mediated pilus biogenesis. EMBO J., 17, 2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R.W., Houman,F. and Kleckner,N. (1987) Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene, 53, 85–96. [DOI] [PubMed] [Google Scholar]
- Slonim L.N., Pinkner,J.S., Branden,C.I. and Hultgren,S.J. (1992) Interactive surface in the PapD chaperone cleft is conserved in pilus chaperone superfamily and essential in subunit recognition and assembly. EMBO J., 11, 4747–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.D., Wheeler,M.A., Foster,H.E.,Jr and Weiss,R.M. (1996) Urinary nitric oxide synthase activity and cyclic GMP levels are decreased with interstitial cystitis and increased with urinary tract infections. J. Urol., 155, 1432–1435. [PubMed] [Google Scholar]
- Snyder W.B., Davis,L.J., Danese,P.N., Cosma,C.L. and Silhavy,T.J. (1995) Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol., 177, 4216–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto G.E., Dodson,K.W., Ogg,D., Liu,C., Heuser,J., Knight,S., Kihlberg,J., Jones,C.H. and Hultgren,S.J. (1998) Periplasmic chaperone recognition motif of subunits mediates quaternary interactions in the pilus. EMBO J., 17, 6155–6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K.L. and Beckwith,J. (1988) An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc. Natl Acad. Sci. USA, 85, 1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K.L., Johnson,K. and Beckwith,J. (1989) Characterization of degP, a gene required for proteolysis in the cell envelope Escherichia coli at high temperature. J. Bacteriol., 171, 2689–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg N., Marklund,B.I., Lund,B., Ilver,D., Hamers,A., Gaastra,W., Karlsson,K.A. and Normark,S. (1990) Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Galα(1–4)Gal-containing isoreceptors. EMBO J., 9, 2001–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg N., Nyholm,P.-G., Pascher,I. and Normark,S. (1991) Saccharide orientation at the cell surface affects glycolipid receptor function. Proc. Natl Acad. Sci. USA, 88, 9340–9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg C. and Godaly,G. (1997) Bacterial virulence in urinary tract infection. Infect. Dis. Clin. North Am., 11, 513–529. [DOI] [PubMed] [Google Scholar]
- Swenson D.L., Bukanov,N.O., Berg,D.E. and Welch,R.A. (1996) Two pathogenicity islands in uropathogenic Escherichia coli J96: cosmid cloning and sample sequencing. Infect. Immun., 64, 3736–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanassi D.G., Saulino,E.T. and Hultgren,S.J. (1998a) The chaperone/usher pathway: a major terminal branch of the general secretory pathway. Curr. Opin. Microbiol., 1, 223–231. [DOI] [PubMed] [Google Scholar]
- Thanassi D.G., Saulino,E.T., Lombardo,M.-J., Roth,R., Heuser,J. and Hultgren,S.J. (1998b) The PapC usher forms an oligomeric channel: implications for pilus biogenesis across the outer membrane. Proc. Natl Acad. Sci. USA, 95, 3146–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Woude M.W., Kaltenbach,L.S. and Low,D.A. (1995) Leucine-responsive regulatory protein plays dual roles as both an activator and a repressor of the Escherichia coli pap fimbrial operon. Mol. Microbiol., 17, 303–312. [DOI] [PubMed] [Google Scholar]
- van der Woude M., Braaten,B. and Low,D. (1996) Epigenetic phase variation of the pap operon in Escherichia coli. Trends Microbiol., 4, 5–9. [DOI] [PubMed] [Google Scholar]
- Weber R.F. and Silverman,P.M. (1988) The cpx proteins of Escherichia coli K12. Structure of the cpxA polypeptide as an inner membrane component. J. Mol. Biol., 203, 467–478. [DOI] [PubMed] [Google Scholar]
- Weyand N.J. and Low,D.A. (2000) Regulation of Pap phase variation. Lrp is sufficient for the establishment of the phase off pap DNA methylation pattern and repression of pap transcription in vitro. J. Biol. Chem., 275, 3192–3200. [DOI] [PubMed] [Google Scholar]
- White-Ziegler C.A., Angus Hill,M.L., Braaten,B.A., van der Woude, M.W. and Low,D.A. (1998) Thermoregulation of Escherichia coli pap transcription: H-NS is a temperature-dependent DNA methylation blocking factor. Mol. Microbiol., 28, 1121–1137. [DOI] [PubMed] [Google Scholar]