Abstract
The extracellular pathogen enteropathogenic Escherichia coli (EPEC) uses a type III secretion system to inhibit its uptake by macrophages. We show that EPEC antiphagocytosis is independent of the translocated intimin receptor Tir and occurs by preventing F-actin polymerization required for bacterial uptake. EPEC–macrophage contact triggered activation of phosphatidylinositol (PI) 3-kinase, which was subsequently inhibited in a type III secretion-dependent manner. Inhibition of PI 3-kinase significantly reduced uptake of a secretion-deficient mutant, without affecting antiphagocytosis by the wild type, suggesting that EPEC blocks a PI 3-kinase-dependent phagocytic pathway. EPEC specifically inhibited Fcγ receptor- but not CR3-receptor mediated phagocytosis of opsonized zymosan. We showed that EPEC inhibits PI 3-kinase activity rather than its recruitment to the site of bacterial contact. Phago cytosis of a secretion mutant correlated with the association of PI 3-kinase with tyrosine-phosphorylated proteins, which wild-type EPEC prevented. These results show that EPEC blocks its uptake by inhibiting a PI 3-kinase-mediated pathway, and translocates effectors other than Tir to interfere with actin-driven host cell processes. This constitutes a novel mechanism of phagocytosis avoidance by an extracellular pathogen.
Keywords: EPEC/F-actin/macrophage/phagocytosis/PI 3-kinase
Introduction
Enteropathogenic Escherichia coli (EPEC), a major cause of severe infantile diarrhoea in the developing world, colonizes the small intestinal mucosa and exerts its pathological effects by intimately attaching to the surface of enterocytes, causing histopathological alterations called attaching/effacing (A/E) lesions (Nataro and Kaper, 1998). A/E lesions result from the degeneration of the enterocyte brush border through the reorganization of the underlying cytoskeleton. This is triggered by bacteria adhering to the surface and leads to the localized loss of microvilli and the formation of actin-rich protrusions, known as pedestals, upon which the bacteria reside (Rosenshine et al., 1996b). Bacterial factors required for A/E lesion formation are all encoded within a 35 kb pathogenicity island called the locus of enterocyte effacement (LEE; Frankel et al., 1998). A/E lesion formation requires a type III secretion system, the type III-secreted proteins EspA, EspB and EspD, the outer membrane adhesin intimin and its translocated receptor, Tir (Frankel et al., 1998; DeVinney et al., 1999; Celli et al., 2000). Type III secretion systems are dedicated to the secretion of virulence factors and translocation of bacterial effector proteins directly into the host cell cytosol (Hueck, 1998). EspA forms surface filamentous organelles involved in protein translocation, which potentially link bacteria to the host cell surface (Knutton et al., 1998). EspB and EspD are translocated into the host cell and form a putative translocation pore in the membrane, through which bacterial effectors are injected (Taylor et al., 1998; Wolff et al., 1998; Kresse et al., 1999; Wachter et al., 1999). Upon contact with the host cell, EPEC translocates the intimin receptor Tir, which is tyrosine phosphorylated and inserted into the host cell membrane (Kenny et al., 1997; Kenny, 1999). Tir–intimin interaction promotes bacterial intimate attachment and ultimately leads to Tir-driven F-actin polymerization and pedestal formation (Goosney et al., 2000).
Current knowledge of the physiopathology of EPEC infections strongly indicates that A/E pathogens have an extracellular lifestyle (Nataro and Kaper, 1998). Although the molecular mechanisms of EPEC pedestal formation are still being elucidated (reviewed in Goosney et al., 1999b; Celli et al., 2000), the function of such cellular protrusions is not understood. In addition to allowing movement of adherent EPEC across the cellular surface (Sanger et al., 1996), such structures may prevent extracellular bacteria from being internalized by macropinocytic processes of epithelial cells. Interestingly, the rabbit form of EPEC, RDEC-1, has been shown to form A/E lesions on the surface of M cells in a rabbit infection model (Inman and Cantey, 1983). M cells are specialized epithelial cells located in the follicule-associated epithelium (FAE) of Peyer’s patches (Neutra et al., 1996). They actively transcytose enteric microorganisms and inert particles through the FAE, thus presenting them to underlying lymphoid cells and initiating the mucosal immune response. This suggests that A/E pathogens can prevent their uptake by blocking such phagocytic processes. By using macrophages as a model of EPEC interaction with phagocytic cells, we have shown recently that EPEC inhibits its uptake by J774A.1 or RAW264.7 murine macrophages. This requires a functional type III secretion system, including components of the translocation machinery EspA, EspB and EspD (Goosney et al., 1999a). However, the mechanisms employed by EPEC and the role of Tir–intimin interactions and pedestal formation are not clearly defined (Goosney et al., 1999a). Here we show that EPEC antiphagocytosis is a Tir-independent process, and occurs via the inhibition of phosphatidylinositol (PI) 3-kinase-dependent F-actin rearrangements that are required for bacterial uptake. This constitutes a novel mechanism of host cell cytoskeleton subversion by EPEC and the first report of inhibition of a PI 3-kinase-mediated pathway by a bacterial pathogen.
Results
EPEC antiphagocytosis is Tir independent
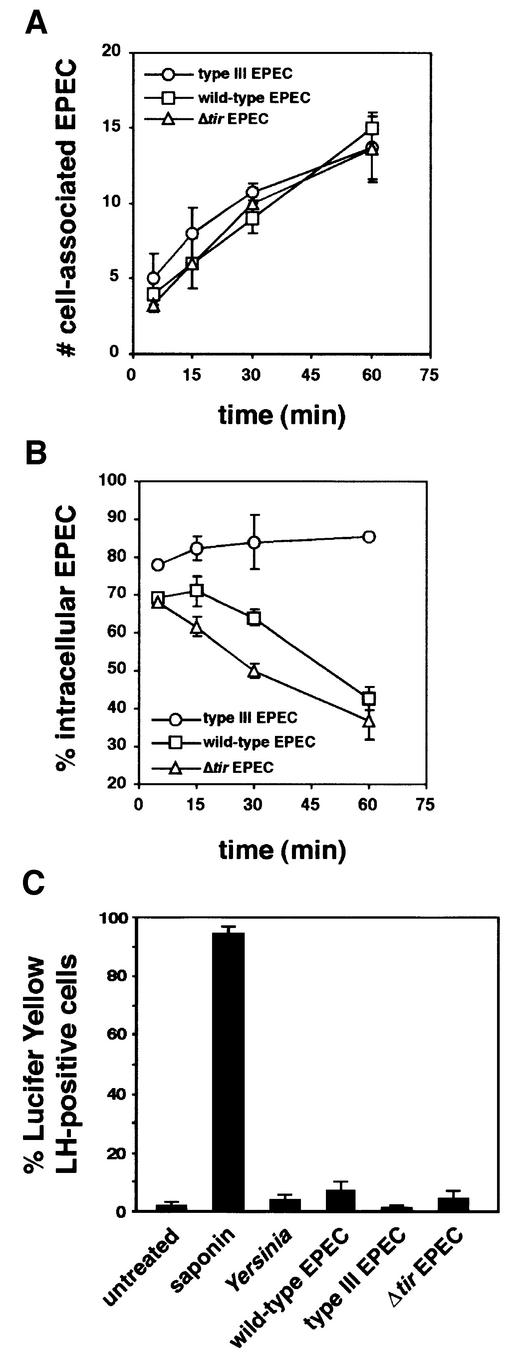
In previous studies, phagocytosis of EPEC strains was monitored by adding stationary phase bacteria to cultured macrophages. Under these conditions, the onset of EPEC antiphagocytosis occurred 2–3 h after addition (Goosney et al., 1999a), presumably due to the time required for bacterial growth and expression of EPEC virulence factors. In order to investigate further the mechanisms by which EPEC interrupts phagocytic processes, we sought to shorten the time to onset of EPEC-mediated antiphagocytosis, by culturing the bacteria, prior to infection, in Dulbecco’s modified Eagle’s medium (DMEM) at 37°C in 5% CO2. These conditions have been shown to induce the expression of the type III secretion system and bacterial effectors (Rosenshine et al., 1996a; Knutton et al., 1997). Quiescent LMmev macrophages were infected with early logarithmic phase bacteria. In a time course experiment, both the wild-type and the secretion-deficient EPEC strains interacted with macrophages in a similar manner, with an increasing number of cell-associated bacteria over a 60 min infection (Figure 1A). However, the secretion-deficient mutant was rapidly and continually phagocytosed (82.3 ± 3.1% intracellular bacteria after 15 min, 85.3 ± 0.6% after 60 min; Figure 1B), whereas internalization of wild-type EPEC decreased after 15 min, with a predominantly extracellular location after 60 min (42.7 ± 2.9% intracellular; Figure 1B). These results indicate that activation of EPEC virulence factors enables the bacteria to inhibit their phagocytosis with kinetics that allow for the simultaneous study of associated signalling events.
Fig. 1. Activated EPEC establishes Tir-independent antiphagocytosis within 60 min after interaction with LMmev macrophages. Macrophages were infected with early logarithmic phase wild-type, type III secretion-defective or Δtir EPEC strains for the indicated times. Samples were processed for immunofluorescent differential EPEC staining, and the number of cell-associated EPEC (A) and the percentage of intracellular bacteria (B) were determined as described in Materials and methods. (C) Lucifer Yellow exclusion assay on cells infected with Y.enterocolitica E 40 wild-type strain, the wild-type, type III secretion-deficient or Δtir EPEC strains shows no cytoxicity associated with Yersinia or EPEC antiphagocytosis. Treatment of cells with 0.005% saponin was used as positive control of pore formation. Data are means ± SEM from three to five independent experiments.
The role of Tir-driven pedestal formation in EPEC-mediated antiphagocytosis remains unclear, since a Tir mutant produced a phenotype intermediate between that of the wild-type and the secretion-deficient strains (Goosney et al., 1999a). This, however, was probably due to differences in the growth rates of the various strains used, whose genetic backgrounds are not identical (data not shown). We thus used a Δtir EPEC mutant, isogenic to both the wild-type and the type III secretion mutant strains (Gauthier et al., 2000), in order to investigate further the role of Tir-driven cytoskeletal rearrangements in EPEC antiphagocytosis. In a time course experiment, Δtir EPEC interacted with macrophages in a manner similar to both the wild-type and secretion-deficient strains (Figure 1A), indicating that interaction with macrophages does not require Tir-mediated intimate attachment. More interestingly, the percentage of intracellular Δtir EPEC over time followed a very similar pattern to that of the wild-type strain, with antiphagocytosis occurring after 60 min (37.0 ± 5.0% intracellular bacteria; Figure 1B). This clearly shows that EPEC antiphagocytosis does not require Tir and is thus independent of Tir-driven cytoskeletal rearrangements. Moreover, the requirement for a functional type III secretion system suggests that EPEC uses bacterial effector(s) other than Tir to block its phagocytosis.
To ensure that the decrease in uptake of type III secretion-competent EPEC was not the result of a non-specific cytotoxic effect due to a type III secretion-mediated pore-forming ability (Neyt and Cornelis, 1999), we examined pore formation in EPEC-infected macrophages using the Lucifer Yellow exclusion assay (Kirby et al., 1998; Neyt and Cornelis, 1999). Under infection conditions where both the wild-type and the Δtir EPEC induced antiphagocytosis for all infected cells (60 min; data not shown), only 7.0 ± 3.2% and 4.4 ± 2.9% of cells, respectively, were permeable to the dye, demonstrating very low numbers of damaged macrophages, as compared with the effect of 0.005% of the pore-forming detergent saponin (Figure 1C). Lucifer Yellow permeability was dependent upon the EPEC type III secretion system, since secretion-deficient bacteria induced permeability changes in 1.2 ± 1.0% of the cells, a level similar to that of uninfected cells (1.9 ± 1.5% positive cells; Figure 1C). Importantly, a wild-type Yersinia enterocolitica E40 strain, which inhibits its phagocytosis (see Figure 3C) using the YopH tyrosine phosphatase (Black and Bliska, 1997; Persson et al., 1997), induced Lucifer Yellow permeability in 4.0 ± 1.5% of cells (Figure 1C), a level comparable to that triggered by secretion-competent EPEC. Taken together, these results demonstrate that EPEC-mediated antiphagocytosis is a specific effect due to type III-translocated effector(s) and not type III secretion-dependent cytotoxicity.
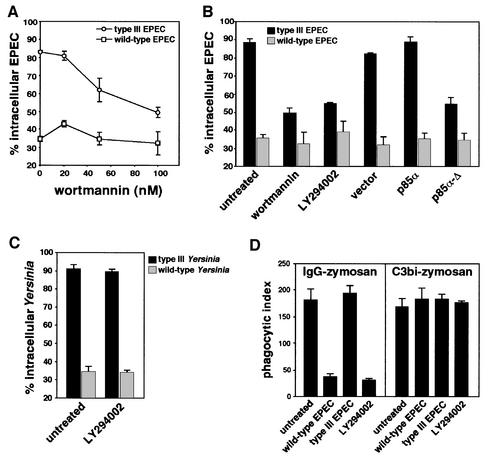
Fig. 3. Phagocytosis of secretion-deficient EPEC requires PI 3-kinase. (A) Effect of wortmannin on EPEC phagocytosis. Macrophages were treated with increasing concentrations of wortmannin, then infected for 60 min with either the secretion-deficient (type III EPEC) or the secretion-competent (wild-type EPEC) strains. Data are means ± SEM of the percentage of intracellular bacteria from three independent experiments. (B) LMmev macrophages were pre-treated with PI 3-kinase inhibitors wortmannin (100 nM) or LY294002 (25 µM), or transiently transfected with a tagged pEF-BOS vector expressing the rat CD2, with pEF-BOS-derived constructs expressing native or dominant-negative forms of p85α for 18 h. Macrophages were then infected for 60 min. Transfected cells were detected using mouse monoclonal anti-CD2 antibodies for vector transfectants and mouse monoclonal anti-p85α antibodies for p85α or p85α-Δ transfectants. The percentage of intracellular bacteria was scored only for transfected macrophages. (C) Phagocytosis assays of wild-type and type III secretion-deficient Y.enterocolitica strains in untreated or 25 µM LY294002-treated macrophages. Cells were infected for 40 min and phagocytosis was analysed as described in Materials and methods. (D) Effect of EPEC infection on phagocytosis of IgG- or C3bi-opsonized zymosan by macrophages. Cells were infected with EPEC for 60 min, or treated with 25 µM LY294002, then challenged with either IgG- or C3bi-opsonized zymosan as described in Materials and methods. All data are means ± SEM from 3–5 independent experiments.
EPEC prevents F-actin polymerization at the site of bacterial contact
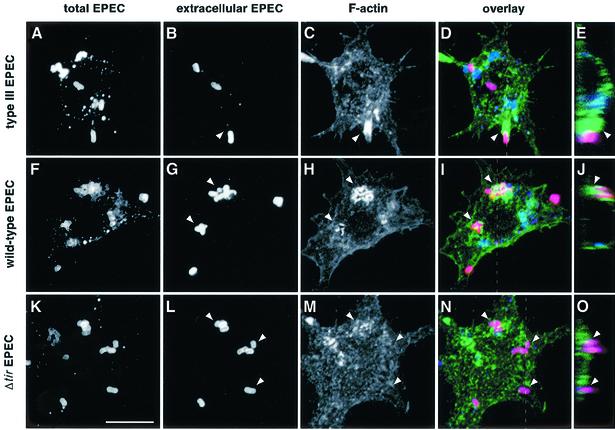
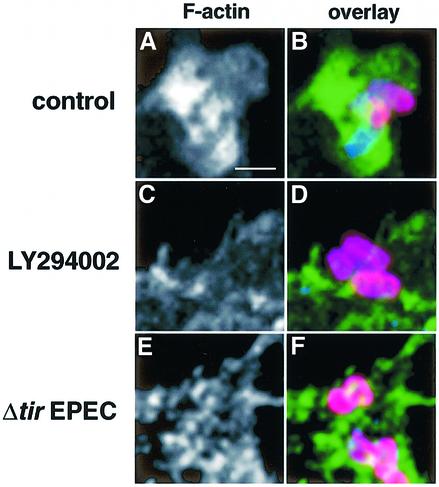
Phagocytic processes require cytoskeletal rearrangements that involve actin reorganization at the site of particle contact (Kwiatkowska and Sobota, 1999). EPEC has been shown to induce massive cytoskeletal reorganization at the surface of infected epithelial cells, as a result of Tir–intimin interactions (reviewed in Goosney et al., 1999b; Celli et al., 2000). We investigated whether EPEC also interferes with F-actin polymerization events at the surface of the macrophage, in order to block its phagocytosis. For this purpose, LMmev cells were infected for 60 min with different EPEC strains and F-actin was labelled using Alexa™488-conjugated phalloidin. F-actin rearrangements at the site of EPEC contact were subsequently analysed by confocal laser scanning microscopy. Infection with the type III secretion mutant (Figure 2A–E) led to a massive polymerization of F-actin around and underneath 75.0 ± 3.5% of cell-associated bacteria (arrowheads, Figure 2C–E), which demonstrates that phagocytosis of a secretion-deficient EPEC involves F-actin polymerization. Accordingly, treatment of macrophages with the F-actin depolymerizing agent, cytochalasin D, prior to infection completely abolished the uptake of the secretion mutant (see Figure 6E). Macrophages infected with wild-type EPEC had a majority of extracellular bacteria, consistent with EPEC antiphagocytic abilities (Figure 2F and G). Wild-type bacteria focused F-actin beneath them (78.7 ± 9.5% of bacteria; Figure 2H and I), although the accumulation pattern was strikingly different from that of the secretion mutant. This pattern is reminiscent of A/E lesion formation, where F-actin is polymerized in a very localized manner directly underneath cell-associated bacteria (Figure 2H–J). To confirm that F-actin rearrangements observed underneath extracellular wild-type EPEC were due to the initiation of Tir-driven pedestal formation, and not due to phagocytic events, we examined F-actin reorganization triggered by Δtir EPEC. This EPEC mutant, which cannot form pedestals (Kenny et al., 1997), remained extracellular (Figures 1B, and 2K and L), showed no significant F-actin rearrangement at the site of contact for 86.3 ± 0.6% of bacteria analysed (Figure 2M and N), but a diffuse clustering of cortical F-actin (Figure 2M, top left arrowhead, and O). Taken together, these results demonstrate that EPEC prevents polymerization of F-actin at the site of bacterial contact in a type III secretion-dependent manner, through a mechanism different from and independent of Tir-mediated pedestal formation.
Fig. 2. F-actin rearrangements at the site of EPEC adhesion to macrophages. LMmev macrophages were infected for 60 min with the secretion-deficient (A–E), wild-type (F–J) or the Δtir EPEC (K–O) strains. Samples were processed for confocal microscopy. (A, F and K) Staining of total cell-associated EPEC using a rabbit polyclonal anti-EPEC antiserum and Cy5-conjugated donkey anti-rabbit antibodies. (B, G and L) Staining of extracellular EPEC adhering to macrophages using a rabbit polyclonal anti-EPEC antiserum and Alexa™594-conjugated goat anti-rabbit antibodies. (C, H and M) F-actin staining using Alexa™488-conjugated phalloidin. (D, I and N). Three colour images obtained by merging the total bacteria, extracellular bacteria and F-actin projections. (E, J and O) Z cuts along the planes indicated by the dashed lines of (D), (I) and (N), respectively. Extracellular bacteria appear in pink, intracellular bacteria in blue, and F-actin in green. F-actin rearrangements underneath extracellular adhering bacteria are shown by arrowheads. Scale bar, 10 µm.
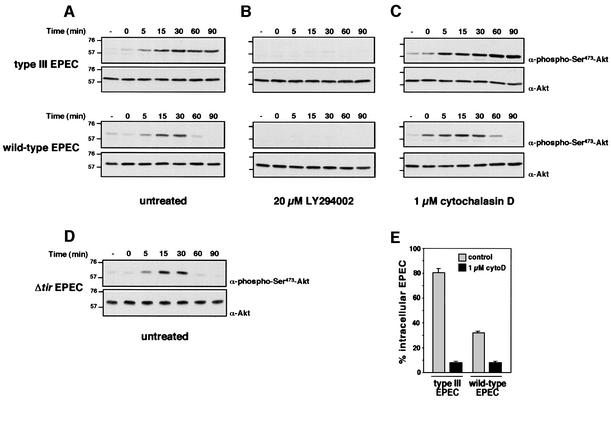
Fig. 6. EPEC inhibits contact-triggered PI 3-kinase-dependent Akt phosphorylation. LMmev macrophages were infected with different EPEC strains in time course experiments. Total cell lysates were resolved on an 8% SDS–polyacrylamide gel, transferred to nitrocellulose and probed with rabbit polyclonal anti-phospho-Ser473-Akt antibodies. Blots were stripped and reprobed with rabbit polyclonal anti-Akt antibodies to ensure equal protein loadings. Experiments were performed with untreated (A and D), 20 µM LY294002- (B) or 1 µM cytochalasin D-treated macrophages (C). Molecular masses are indicated in kDa. (E) Phagocytic assays of secretion-deficient or -competent EPEC strains in control or 1 µM cytochalasin D (cytoD)-treated macrophages after 60 min infection.
Phagocytosis of type III secretion-deficient EPEC requires a PI 3-kinase activity
The above results suggest that translocation of some bacterial effector protein(s) other than Tir probably interrupts phagocytic signalling pathways required for bacterial uptake. To delineate EPEC antiphagocytosis mechanisms further, we investigated signalling pathways possibly involved in EPEC phagocytosis. PI 3-kinases modulate many cytoskeleton-based cellular processes such as adhesion, spreading, macropinocytosis and phagocytosis (for a review see Wymann and Pirola, 1998). Specifically, activity of heterodimeric class IA p85-p110 PI 3-kinases is essential for macropinocytosis, pseudopod extension and phagocytic cup closure in FcγR-mediated phagocytosis (Araki et al., 1996; Cox et al., 1999). Given their involvement in cytoskeletal remodelling and in bacterial invasion (Ireton et al., 1996; Mecsas et al., 1998; Martinez et al., 2000), we investigated the potential role of PI 3-kinases in EPEC uptake by phagocytic cells. LMmev macrophages were treated with the PI 3-kinase inhibitor wortmannin prior to infection with EPEC, and phagocytic assays were performed as described. Phagocytosis of the secretion-deficient mutant was significantly decreased in a dose-dependent manner (P <0.05; Figure 3A). Similar results were obtained with either 100 nM wortmannin or 25 µM LY294002 (Figure 3B), strongly suggesting that uptake of the type III secretion mutant requires a PI 3-kinase activity. In contrast, the uptake of wild-type EPEC remained unchanged by wortmannin (Figure 3A) or LY294002 treatment (Figure 3B). Uptake of the type III secretion mutant of the antiphagocytic Y.enterocolitica E40 was not affected by inhibition of PI 3-kinase with LY294002 (Figure 3C), demonstrating that, unlike EPEC, Yersinia uptake by macrophages occurs via PI 3-kinase-independent phagocytic pathways.
To confirm these results and assess more specifically the role of class IA PI 3-kinases in EPEC phagocytosis, we transiently transfected LMmev macrophages with a dominant-negative form of the p85α-p110 PI 3-kinase regulatory subunit p85α-Δ, which lacks its p110-binding site (Dhand et al., 1994), and analysed the effect upon EPEC phagocytosis. In cells expressing p85α-Δ, uptake of the secretion mutant was significantly decreased (54.6 ± 3.4% intracellular bacteria versus 88.4 ± 2.2% in untransfected cells, P <0.05; Figure 3B). This effect was specific for the PI 3-kinase dominant-negative form since transfection with the same vector expressing the irrelevant rat CD2 molecule, or the native form of p85α, did not alter phagocytosis of the secretion mutant (Figure 3B). Both chemical inhibition of PI 3-kinases and functional inhibition of class IA PI 3-kinases with the dominant-negative p85α-Δ thus demonstrate that EPEC phagocytosis requires a class IA PI 3-kinase activity. Interestingly, the antiphagocytic effect of the wild-type strain was not affected by either PI 3-kinase inhibitors or expression of its dominant-negative form (Figure 3B).
EPEC inhibits FcγR- but not CR3-mediated phagocytosis of opsonized zymosan
Since EPEC inhibit their own uptake by macrophages, we investigated whether these bacteria can block other phagocytic mechanisms. We thus studied EPEC antiphagocytic abilities towards opsonized particles, by comparing the bacterial effect upon Fcγ receptor- and complement receptor CR3-mediated phagocytosis. In the absence of serum, macrophages were infected with either wild-type or secretion-deficient EPEC, left untreated or treated with the PI 3-kinase inhibitor LY294002. Cells were then challenged with either IgG- or C3bi-opsonized zymosan, and phagocytosis of opsonized particles was quantified as described in Materials and methods. While phagocytosis of secretion-deficient EPEC did not impair uptake of either IgG- or C3bi-opsonized zymosan as compared with untreated cells, infection with the wild-type strain dramatically inhibited IgG-opsonized zymosan uptake but not phagocytosis of C3bi-opsonized particles (Figure 3D). Uptake of IgG-opsonized zymosan was inhibited by LY294002, confirming the involvement of PI 3-kinase in this process (Araki et al., 1996; Cox et al., 1999). In contrast, CR3-mediated uptake of particles was insensitive to LY294002 (Figure 3D; E.Caron and A.Hall, personal communication), demonstrating its independence of PI 3-kinase. These results illustrate that EPEC can specifically inhibit phagocytic PI 3-kinase-dependent pathways triggered through the Fcγ receptors but not those activated upon engagement of the complement receptor CR3.
PI 3-kinase activity is required for F-actin polymerization at the site of bacterial contact
The above results show that phagocytosis of a secretion-deficient EPEC is associated with F-actin polymerization events at the site of bacterial contact, and requires a PI 3-kinase activity. To correlate F-actin-dependent phagocytic processes with PI 3-kinase activity, confocal microscopy was used to follow F-actin rearrangements during phagocytosis of the type III secretion EPEC after treatment with LY294002. Internalization of secretion-deficient bacteria by untreated macrophages was associated with massive F-actin rearrangements for 72.7 ± 8.3% of bacteria analysed (Figure 4A and B; see also Figure 2C and D). In contrast, LY294002-treated macrophages did not internalize secretion-deficient EPEC, and no significant F-actin polymerization was observed in the vicinity of adherent bacteria (74.0 ± 4.0% of analysed bacteria; Figure 4C and D). This demonstrates that inhibition of PI 3-kinase prevents F-actin-rich phagocytic cup formation and blocks EPEC phagocytosis. Interestingly, secretion-competent EPEC such as the Δtir mutant (Figure 4E and F) prevented F-actin phagocytic cup formation in a similar manner in the absence of PI 3-kinase inhibitor (see Figure 2M).
Fig. 4. Inhibition of PI 3-kinase activity prevents F-actin polymerization at the site of secretion-deficient EPEC contact. LMmev macrophages were treated with 25 µM LY294002 and infected for 60 min with the type III secretion EPEC mutant. Control represents cells with no inhibitor treatment. Samples were processed for confocal microscopy as described in Materials and methods. (A and B) Details of an untreated macrophage phagocytosing bacteria. Intracellular (blue) and extracellular bacteria (pink) are surrounded by massive F-actin polymerization (green). (C and D) Details of a LY294002-treated macrophage with extracellular adhering bacteria (pink). (E and F) Details of a Δtir EPEC-infected macrophage showing no F-actin polymerization underneath extracellular bacteria (pink). Scale bar, 1 µm.
PI 3-kinase is recruited at the site of EPEC contact with macrophages
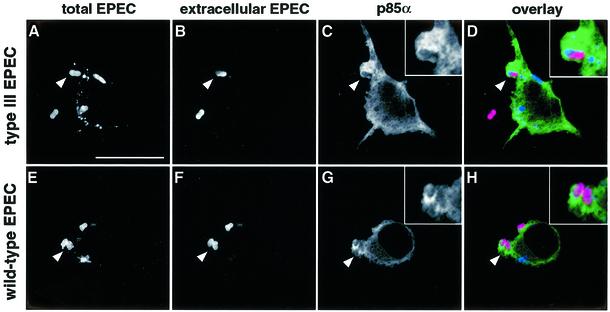
Class IA p85α-p110 PI 3-kinases are recruited to cellular membranes following interactions of the regulatory subunit p85α with phosphotyrosine motifs on activated receptors or adaptor molecules (Wymann and Pirola, 1998). Such interactions bring the catalytic p110 subunit to the membrane where it converts phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol-3,4,5-trisphosphate (PIP3) (Wymann and Pirola, 1998). To demonstrate further that class IA p85α-p110 PI 3-kinases are involved in EPEC phagocytosis, we investigated whether the enzyme is recruited to the site of bacterial contact. Since endogeneous levels of PI 3-kinase in LMmev macrophages are too low to detect by immunofluorescence (data not shown), we overexpressed the native form of p85α by transient transfection. Under these experimental conditions, overexpression of p85α did not interfere with EPEC phagocytosis (Figure 3B). Transfected macrophages were infected for 60 min and localization of PI 3-kinase was analysed by immunofluorescent labelling of the p85α subunit. PI 3-kinase was clearly revealed around and in the vicinity of secretion-deficient EPEC being phagocytosed (arrowhead, Figure 5A–D), demonstrating PI 3-kinase recruitment to this site. Recruitment of PI 3-kinase underneath extracellular wild-type bacteria (Figure 5F) was also observed (Figure 5G and H), although in a more restricted localization than secretion-deficient bacteria. Results identical to those for wild-type EPEC were obtained using Δtir EPEC (data not shown). Taken together, these results show that the induction of antiphagocytosis by secretion-competent EPEC is not dependent on inhibiton of PI 3-kinase recruitment to the site of contact.
Fig. 5. PI 3-kinase is recruited to the site of EPEC contact. LMmev macrophages were transiently transfected with a construct overexpressing the regulatory subunit of PI 3-kinase p85α. After 18 h transfection, cells were infected for 60 min with either the secretion-deficient (type III EPEC, A–D) or the secretion-competent (wild-type EPEC, E–H) EPEC strains. Samples were processed for confocal microscopy to localize PI 3-kinase. p85α-overexpressing cells were detected using mouse monoclonal anti-p85α followed by Alexa™488-conjugated goat anti-mouse antibodies. Total and extracellular EPEC were labelled as described in Figure 2. (A–D) A secretion-deficient bacterium (arrowhead) in the process of being phagocytosed (intracellular half blue, extracellular half pink; see close-up in D) is surrounded by dense p85α staining (green; C and D). (E–H) p85α staining (green; G and H) also localizes with extracellular (pink; H) secretion-competent bacteria (arrowheads, F and G). In a representative experiment, PI 3-kinase recruitment was detected for 88 and 84% of the secretion-deficient and wild-type bacteria analysed, respectively. Scale bar, 10 µm.
EPEC inhibits contact-triggered activation of PI 3-kinase
Given the above results, we hypothesized that EPEC may inhibit PI 3-kinase activity, rather than recruitment, in order to block uptake by macrophages. To investigate this, we assayed the activity of PI 3-kinase in EPEC-infected LMmev cells by monitoring the phosphorylation state of the PI 3-kinase downstream target Akt. Recruitment of this serine-threonine kinase to cellular membranes and subsequent phosphorylation at Thr308 and Ser473 residues is dependent upon the production of the PI 3-kinase lipid product, PIP3 (Marte and Downward, 1997). We thus analysed Ser473 phosphorylation of Akt in total cell lysates from EPEC-infected macrophages. Infection with the secretion-deficient EPEC led to increased phosphorylation of Akt, which was sustained for at least 90 min (Figure 6A). This phosphorylation was completely abolished by the PI 3-kinase inhibitor LY294002 (Figure 6B), further showing that phagocytosis of the secretion-deficient EPEC triggers activation of PI 3-kinase. Infection of macrophages with wild-type EPEC also triggered an increased PI 3-kinase-dependent Akt phosphorylation, but phosphorylation strikingly decreased after 30 min (Figure 6A and B). Similar kinetics of Akt phosphorylation were obtained with the Δtir EPEC (Figure 6D). Thus, secretion-competent EPEC inhibits PI 3-kinase activity with kinetics correlating with the onset of antiphagocytosis (see Figure 1B). To extend these results, we analysed Akt phosphorylation in cytochalasin D-treated macrophages, which prevented the uptake of both secretion-competent and -deficient EPEC by disrupting F-actin (Figure 6E). Cytochalasin D had no effect on the kinetics of Akt phosphorylation (Figure 6A and C), which shows that EPEC contact, and not uptake, is sufficient to trigger activation of PI 3-kinase in macrophages, which precedes F-actin polymerization. This also confirms that inhibition of PI 3-kinase activity is dependent upon the translocation of bacterial effector(s) from extracellular bacteria.
EPEC prevents PIP3-enriched phagocytic cup formation
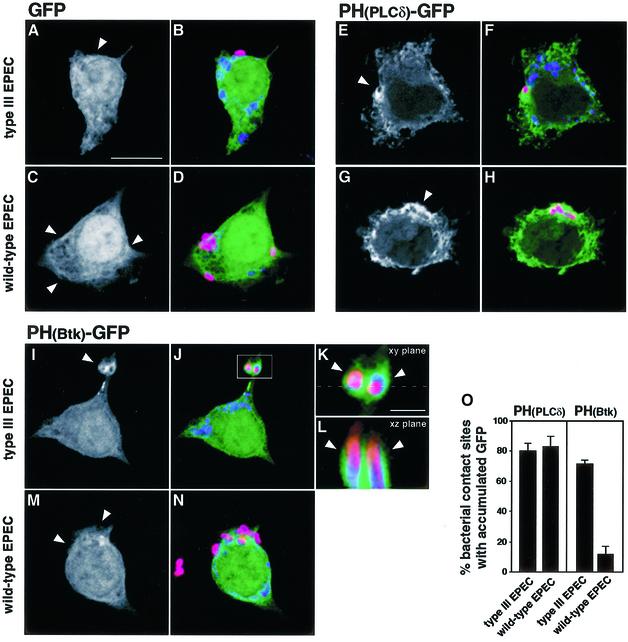
Our results showed that phagocytosis of EPEC is a process requiring PI 3-kinase, and that secretion-competent bacteria inhibit PI 3-kinase activity to prevent their phagocytic uptake. To complement the demonstration of PI 3-kinase recruitment, we wished to show direct evidence for PI 3-kinase activity at the site of bacterial contact. We therefore used fluorescence microscopy approaches to detect PI 3-kinase activity in situ. Pleckstrin homology (PH) domains bind preferentially to different phosphatidylinositol phosphates and can thus be used as probes for localized concentrations in membranes (Stauffer et al., 1998; Varnai and Balla, 1998; Gray et al., 1999; Varnai et al., 1999; Holz et al., 2000; Tall et al., 2000). The PH domain of phospholipase Cδ (PLCδ) binds preferentially to PIP2, the in vivo substrate of class IA PI 3-kinases (Varnai and Balla, 1998; Holz et al., 2000), while that of Btk, a member of the Tec family of non-receptor tyrosine kinases, binds to the PI 3-kinase lipid product PIP3 (Varnai et al., 1999). To evaluate the accumulation of PIP2 and PIP3 at the site of EPEC contact, we expressed these PH domains as fusions to green fluorescent protein (GFP) in macrophages. LMmev macrophages were transiently transfected with constructs expressing either the PH(PLCδ)–GFP or PH(Btk)–GFP fusion proteins, or a GFP control, and then infected with EPEC for 60 min. Macrophages transfected with the control GFP showed no accumulation of fluorescent signal at the site of contact of either the secretion-deficient or the wild-type strains (Figure 7A–D). In contrast, PH(PLCδ)–GFP accumulated at 80.3 ± 4.7% of sites of secretion-deficient EPEC phagocytosis (Figure 7E, F and O). Thus, EPEC phagocytosis is associated with a localized accumulation of PIP2. A similar accumulation (83.0 ± 6.6%; Figure 7O) was observed around extracellular wild-type bacteria (Figure 7G and H). PH(Btk)– GFP accumulated strongly in the vicinity of secretion-deficient bacteria being phagocytosed (71.3 ± 3.1% of sites analysed; Figure 7I–K and O). A Z-section of the projection obtained by confocal microscopy showed that PIP3 was localized in membrane projections surrounding the bacteria (Figure 7L). These results, which constitute in situ visualization of the PI 3-kinase activity at the site of EPEC phagocytosis, clearly demonstrate the involvement of this enzyme in the phagocytic process. In contrast, no PIP3 accumulation was observed where extracellular wild-type (Figure 7M and N) and Δtir bacteria (data not shown) adhered to the macrophages. Statistical analyses showed that only 11.3 ± 5.7% of adhering wild-type bacteria associated with accumulation of PIP3 (Figure 7O), which was significantly different from the PI 3-kinase product accumulation observed at the site of phagocytosis of secretion-deficient bacteria (see above; P <0.05). This demonstrates that secretion-competent EPEC locally inhibits the accumulation of the PI 3-kinase product PIP3 involved in the phagocytic processes.
Fig. 7. Accumulation of PIP2 and PIP3 at the site of EPEC contact with macrophages. LMmev macrophages were transiently transfected with pEGFP-N1 (A–D), pEGFP-N1-PH(PLCδ) (E–H) and pEGFP-N1-PH(Btk) (I–N) plasmids. After 18 h transfection, cells were infected for 60 min with either the secretion-deficient (type III EPEC, A, B, E, F and I–L) or the secretion-competent (wild-type EPEC, C, D, G, H, M and N) EPEC strains. Total and extracellular EPEC were labelled as described in Figure 2, and samples were analysed subsequently by confocal microscopy. Black and white projections show GFP fluorescence alone, while three-colour images are merged projections of GFP (green), total (blue) and extracellular (red) bacteria stainings. (A and B) Projection showing no GFP signal (arrowhead, A) around an extracellular secretion-deficient mutant (pink, B). (C and D) Projection showing no GFP signal (arrowheads, C) underneath extracellular wild-type bacteria (pink, D). (E and F) Projection showing PIP2 accumulation (arrowhead, E) around a secretion-deficient EPEC being phagocytosed (pink, F). (G and H) Projection showing PIP2 accumulation (arrowhead, G) in the vicinity of extracellular wild-type bacteria (pink, H). (I and J) Projection showing PIP3 accumulation (arrowhead, I) at the site of secretion-deficient EPEC contact (pink, J). (K) Magnification from (J) detailing PIP3 accumulation (arrowheads) in phagocytic cups around secretion-deficient EPEC being phagocytosed. (L) Z cut from (K), along the plane indicated by the dashed line, showing PIP3 accumulation (arrowheads) in membrane extensions around two secretion-deficient EPEC being phagocytosed (intracellular portions in blue, extracellular portions in pink). (M and N) Projection showing the absence of PIP3 accumulation (arrowhead, M) at the site of adherence of extracellular secretion-competent bacteria (pink, N). (O) Quantitation of PIP2 [PH(PLCδ) panel] and PIP3[PH(Btk) panel] accumulation at sites of type III secretion-deficient or wild-type EPEC contact. Scale bar, 10 µm, except in K and L, 1 µm.
EPEC induces dissociation of tyrosine-phosphorylated proteins from PI 3-kinase
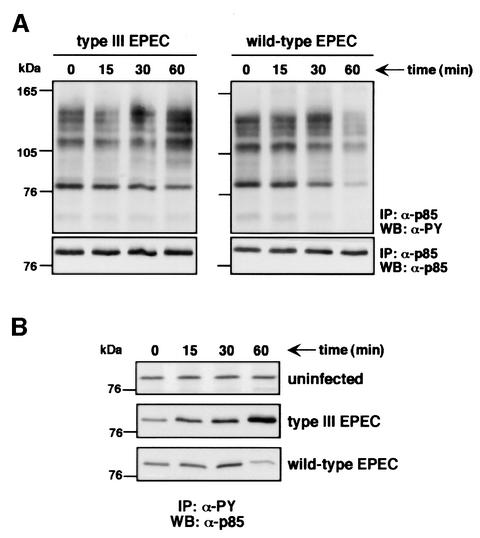
The interaction of secretion-competent EPEC with macrophages is associated with tyrosine dephosphorylation of host proteins, which temporally correlates with the onset of antiphagocytosis (Goosney et al., 1999a). Since our results describe a PI 3-kinase-mediated pathway as the target of EPEC antiphagocytosis, we examined PI 3-kinase association with tyrosine-phosphorylated (PY) proteins. PI 3-kinase complexes were immunoprecipitated from lysates of infected macrophages, and phosphotyrosine immunoblots were then performed to analyse the PY protein content of these immunoprecipitates. Infection with, and thus phagocytosis of, the secretion-deficient EPEC led to an increase of PY proteins immunoprecipitated with p85α over time (Figure 8A), which control immunoprecipitations with a rabbit pre-immune serum did not show (data not shown). The PY protein content of the PI 3-kinase complexes also increased in macrophages infected with the wild-type EPEC until 30 min post-infection (Figure 8A). However, the wild-type EPEC strain then caused a significant decrease of all the PY proteins associated with PI 3-kinase as shown at 60 min (Figure 8A). Consistently, reciprocal immunoprecipitations, in which the amounts of p85α associated with total PY proteins were analysed, showed a progressive increase of PI 3-kinase with time in secretion-deficient EPEC-infected cells, whereas this amount decreased after 30 min infection with the wild-type strain (Figure 8B). Under similar conditions, no detectable changes in the amount of PY protein-associated p85α were observed in uninfected controls (Figure 8B). Such kinetics correlate with the inhibition of PI 3-kinase activity (see Figure 6A) and the onset of antiphagocytosis (see Figure 1B). Thus, these experiments highlight that phagocytosis of secretion-deficient EPEC triggers association of PI 3-kinase with PY proteins, whereas antiphagocytosis mediated by secretion-competent EPEC correlates with the dissociation of these same PY proteins from the PI 3-kinase complex.
Fig. 8. PI 3-kinase association with phosphotyrosine (PY) proteins in EPEC-infected macrophages. (A) PY protein content in PI 3-kinase complexes from macrophages infected either with the secretion-deficient (type III EPEC) or the secretion-competent (wild-type EPEC) strains after the indicated times. PI 3-kinase complexes were immunoprecipitated from EPEC-infected macrophages using rabbit polyclonal anti-p85α antibodies as described in Materials and methods. Immunocomplexes were resolved on an 8% SDS–polyacrylamide gel, transferred to nitrocellulose and probed with mouse monoclonal anti-PY antibodies (upper panels). Blots were stripped and reprobed with rabbit polyclonal anti-p85α antibodies to ensure equal amounts of immunoprecipitated PI 3-kinase in the different samples (lower panels). (B) PY protein-associated PI 3-kinase in uninfected, secretion-deficient (type III EPEC) or secretion-competent (wild-type EPEC) EPEC-infected macrophages. PY proteins were immunoprecipitated using mouse monoclonal anti-PY (clone 4G10) antibody as described in Materials and methods. Immunoblots were probed with rabbit polyclonal anti-p85α antibodies. Time (min) refers to the duration of infection before immunoprecipitation.
Discussion
Many bacterial pathogens subvert the host cell machinery to gain entry into host cells. This often involves host cell cytoskeleton rearrangements, through the exploitation of pre-existing signalling pathways (Finlay and Falkow, 1997). The extracellular pathogen EPEC also manipulates the host cell cytoskeleton, but for purposes other than invasion, as exemplified by Tir-driven pedestal formation (Goosney et al., 2000). The ability of A/E pathogens to resist transcytosis by M cells (Inman and Cantey, 1983) suggests that these bacteria have evolved strategies of phagocytosis avoidance. Indeed, EPEC uses its type III secretion system to block its uptake by macrophages (Goosney et al., 1999a). In this work, we have characterized further the molecular mechanisms of EPEC antiphagocytosis. We have shown that EPEC antiphagocytosis is Tir independent and occurs by inhibition of the PI 3-kinase-dependent F-actin rearrangements required for bacterial uptake. PI 3-kinase regulates pseudopod extension and completion of phagocytic cup formation during FcγR-mediated phagocytosis (Araki et al., 1996; Cox et al., 1999). EPEC uptake by macrophages did not involve opsonin-dependent phagocytic receptors since experiments were performed in serum-free medium, without opsonization of the bacteria. This indicates that phagocytosis of secretion-deficient EPEC engages as yet uncharacterized opsonin-independent receptor(s), which also trigger PI 3-kinase-mediated signalling, and further suggests that PI 3-kinase is involved in phagocytic processes initiated by different receptors. Interestingly, EPEC also inhibits FcγR-mediated phagocytosis, which occurs through a PI 3-kinase-dependent pathway (Araki et al., 1996; Cox et al., 1999), but does not inhibit complement receptor CR3-mediated phagocytosis, which is independent of PI 3-kinase (E.Caron and A.Hall, personal communication; this work). This suggests that EPEC can specifically affect multiple PI 3-kinase-dependent phagocytic pathways, most probably by targeting common signal transduction steps. It also suggests that EPEC can exert inhibitory effects on other PI 3-kinase-dependent pathways, such as macropinocytosis (Araki et al., 1996; Zhou et al., 1998; Murray et al., 2000). EPEC antiphagocytic mechanisms could play important roles in EPEC pathogenesis at different stages of the infection. For instance, EPEC may use such mechanisms during the initial colonization of intestinal epithelium, to prevent macropinocytosis by enterocytes and thus establish itself extracellularly prior to Tir-mediated pedestal formation. We are currently investigating EPEC antiphagocytic properties on epithelial cells. EPEC antiphagocytosis may also account for the blockade of transcytosis observed at the surface of rabbit Peyer’s patches M cells (Inman and Cantey, 1983), a feature shared by no other bacterial pathogens (Neutra et al., 1996). Blocking uptake by M cells could reduce or delay the presentation of EPEC to immune cells from the FAE and alter the immune response to infection. This would thus favour colonization of the epithelium, a characteristic feature of EPEC infections (Nataro and Kaper, 1998). Recent observations in a mouse model of Citrobacter rodentium infection (B.A.Vallance, personal communication) indicate that A/E pathogens do contact phagocytic cells, such as macrophages and neutrophils, during later stages of infection, subsequent to epithelial barrier disruption and inflammation. It is likely that the antiphagocytic capabilities of A/E pathogens are required under such conditions, to prevent bacterial uptake and destruction. Furthermore, antiphagocytosis would also reduce EPEC antigen presentation by phagocytic cells. As a result, by limiting and/or delaying the triggered immune response and clearance of bacteria, antiphagocytosis would contribute to bacterial persistence.
Observations of F-actin rearrangements at the site of EPEC adherence to macrophages showed a type III secretion-dependent, but Tir-independent, inhibition of F-actin-rich phagocytic cup formation. In addition to providing clues to the molecular mechanisms of EPEC antiphagocytosis, this clearly shows that EPEC uses an additional mechanism of actin cytoskeleton subversion, other than Tir-driven pedestal formation (Goosney et al., 2000). EPEC antiphagocytic abilities are abolished by mutation of EspA, EspB and EspD (Goosney et al., 1999a). However, these three secreted proteins probably play essential roles in the translocation of EPEC effectors (Knutton et al., 1998; Wachter et al., 1999). It is thus difficult to distinguish whether these proteins have an additional effector function in EPEC antiphagocytosis, since their disruption affects the functioning of the translocation apparatus itself, much like type III secretion mutations. The LEE-encoded protein EspF, a proline-rich protein secreted through a type III secretion mechanism, is a potential candidate for an antiphagocytosis effector, since it is dispensable in EPEC pedestal formation (McNamara and Donnenberg, 1998). However, an espF deletion mutant still prevented its uptake by macrophages (data not shown). At present, EspA, EspB, EspD, EspF and Tir are the only known LEE-encoded type III-secreted proteins in EPEC. However, it is possible that other as yet undiscovered bacterial effector(s) are encoded elsewhere on the EPEC genome.
Given their involvement in both cytoskeletal remodelling and bacterial invasion of non-phagocytic cells (Ireton et al., 1996; Mecsas et al., 1998; Martinez et al., 2000), we investigated the potential role of PI 3-kinases in EPEC uptake by phagocytic cells. Chemical and competitive inhibition of class IA PI 3-kinase showed a requirement for this lipid kinase in EPEC phagocytosis. This was confirmed further by in situ visualization of PI 3-kinase recruitment and activity in membrane projections surrounding secretion-deficient bacteria. However, PI 3-kinase requirement was not absolute since its inhibition reduced phagocytosis by 50–55%. An explanation is the involvement of several PI 3-kinase-dependent and -independent phagocytic pathways in secretion-deficient EPEC uptake, which are initiated by one or different non-opsonic receptor(s).
Secretion-competent EPEC strains inhibited PI 3- kinase-dependent Akt phosphorylation. Since PI 3-kinase was detected immediately beneath extracellular bacteria, antiphagocytosis was not due to interference with PI 3-kinase recruitment to the site of bacterial adherence, an event that precedes and is essential to its activation (Wymann and Pirola, 1998). Consistently, the initial contact of EPEC with macrophages triggered a rapid activation of PI 3-kinase. However, after this initial contact period, secretion-competent EPEC inhibited PI 3-kinase activation with kinetics corresponding to the onset of antiphagocytosis. This inhibitory role is supported further by the absence of PIP3 generation at the site of adherence of secretion-competent bacteria. Such results clearly demonstrate that EPEC inhibits a PI 3-kinase-mediated pathway that is required for bacterial uptake by phagocytic cells.
Secretion-competent EPEC causes the dissociation of phosphotyrosine proteins associated with PI 3-kinase, which suggests that EPEC interferes with functional PI 3-kinase complexes to interrupt phagocytic signals. EPEC antiphagocytosis requires tyrosine dephosphorylation of host proteins (Goosney et al., 1999a). We have shown here that some of these targets are proteins associated with PI 3-kinase. One possibility is that EPEC translocates a bacterial protein tyrosine phosphatase (PTPase) into host cells to block phagocytosis. The enteropathogenic Yersinia effector YopH can specifically dephosphorylate the focal adhesion kinase (FAK) and p130Cas to interrupt β1-integrin-mediated uptake in non-phagocytic cells (Black and Bliska, 1997; Persson et al., 1997). An analogous EPEC PTPase could theoretically dephosphorylate proteins interacting with PI 3-kinase in macrophages to terminate the signals initiated upon bacterial contact. However, no PTPase activity similar to YopH has been detected in EPEC (Goosney et al., 1999a). Furthermore, Yersinia uptake by macrophages is independent of PI 3-kinase since, unlike EPEC, the PI 3-kinase inhibitor LY294002 did not decrease phagocytosis of a type III secretion mutant. This suggests that EPEC and Yersinia trigger different phagocytic pathways and thus probably use different antiphagocytic strategies. It should be noted that PI 3-kinase independence of secretion-deficient Yersinia phagocytosis by macrophages contrasts with findings that Yersinia invasion of epithelial cells is PI 3-kinase dependent (Mecsas et al., 1998; Schulte et al., 1998), probably reflecting different cell type-specific internalization pathways. Taken together, these observations illustrate that EPEC uses antiphagocytic mechanisms quite different from those of Yersinia species.
An EPEC effector injected into the host cell could prevent association of key regulatory proteins with PI 3-kinase, or inhibit the enzymatic activity of PI 3-kinase, either directly or indirectly. One hypothesis is that EPEC activates signalling pathways that negatively regulate PI 3-kinase activity, ultimately leading to the dissociation of PI 3-kinase complexes, as observed. For instance, such events could originate from the 3′-inositol phosphatase PTEN, a major tumour suppressor that converts the PI 3-kinase lipid product PIP3 into PIP2 and thus antagonizes PI 3-kinase-mediated signals (Vazquez and Sellers, 2000). Accumulation of PIP2 was observed at the site of phagocytosis of secretion-deficient EPEC and of extracellular secretion-competent bacteria. While PIP2 accumulation during phagocytic processes may reflect the involvement of this phosphoinositide in the dynamics of actin-rich structures at the cell surface (Tall et al., 2000), its localization around secretion-competent EPEC that have avoided phagocytosis could be due to the conversion of PIP3 by PTEN. Alternatively, the absence of PIP3 accumulation at the site of secretion-competent EPEC contact could reflect activation of a 5-inositol phosphatase, such as SHIP, which converts PIP3 into PI(3,4)P2 (Erneux et al., 1998). We are currently pursuing the mode of EPEC inhibition of PI 3-kinase-mediated signals to address these issues further.
Several bacterial pathogens require or purportedly activate PI 3-kinase-mediated signalling pathways to gain entry into non-phagocytic cells. For example, invasin-mediated entry of Yersinia into epithelial cells requires PI 3-kinase activity (Mecsas et al., 1998; Schulte et al., 1998), and InlB, a surface protein from Listeria monocytogenes, triggers activation of PI 3-kinase and subsequent bacterial entry (Ireton et al., 1996, 1999). Recently, type 1 pilus–mediated invasion of bladder epithelial cells by uropathogenic E.coli has been shown to require a PI 3-kinase activity associated with local cytoskeleton rearrangements (Martinez et al., 2000). In contrast, here we have shown that the extracellular pathogen EPEC inhibits PI 3-kinase-mediated pathways to prevent its uptake by phagocytic cells. This is the first report of a bacterial pathogen that can inhibit such signalling pathways, indicating that different pathogens have the ability to modulate PI 3-kinase activity to enhance their pathogenic lifestyles.
Materials and methods
Bacterial strains, cell lines and culture conditions
The bacterial strains used were the wild-type E2348/69 EPEC (Levine et al., 1978), the cfm14-2-1 escN type III secretion insertional mutant (Donnenberg et al., 1990) and a Δtir isogenic mutant of E2348/69 (Gauthier et al., 2000). They were grown in Luria–Bertani broth or on agar plates at 37°C. The Y.enterocolitica E40 wild-type strain (Sory et al., 1995) and its isogenic type III secretion yscN mutant (Woestyn et al., 1994) were grown in brain–heart infusion (BHI) broth or on agar plates at 30°C. The LMmev bone marrow-derived macrophage cell line was generated and immortalized as previously described (Radzioch et al., 1991) from C57BL/6J mev/+ wild-type mice and was maintained in DMEM (Gibco-BRL) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco-BRL) at 37°C in 5% CO2. Macrophages were used within passage numbers 6–15 to ensure no derivation.
Antibodies, plasmids and other materials
Rabbit polyclonal antiserum against rat p85α and monoclonal antibodies against phosphotyrosine (clone 4G10) were from Upstate Biotechnology. Monoclonal antibodies against human p85α were from Transduction Laboratories. Rabbit polyclonal antisera against phospho-Ser473-Akt and total Akt were from New England Biolabs. For immunoblotting, horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit IgG were from Sigma. For immunofluorescence labelling, Alexa™488-conjugated goat anti-rabbit, goat anti-mouse, Alexa™594-conjugated goat anti-rabbit and goat anti-mouse, Alexa™350-conjugated goat anti-rabbit IgG and Alexa™488-conjugated phalloidin were from Molecular Probes. Cy5-conjugated donkey anti-rabbit IgG was from Jackson ImmunoResearch Laboratories.
The cDNAs of bovine p85α, mutant p85α-Δ (lacking amino acids 479–513) (Dhand et al., 1994), and a pEF-BOS expression vector (Mizushima and Nagata, 1990) carrying the full-length rat CD2 cDNA, were kindly provided by Dr Doreen A.Cantrell (Imperial Cancer Research Fund, London, UK). Both p85α and p85α-Δ cDNAs were amplified by PCR using primers p85B (5′-CGGGATCCATGAGTGCCGAGGGGTACCAGTACCGGGCG-3′) and p85C (5′-CCATCGATTCACAGATCCTCTTCTGAGATGAGTTTTTGTTCTCGCCTCTGCTGCGCGTACACTGGGTAGGC-3′) introducing a C-terminal Myc tag. PCR products were cloned into pEF-BOS using BamHI and ClaI restriction sites. Recombinant plasmids were verified by sequencing. The GFP-expressing plasmid pEGFP-N1 was from Clontech. The pEGFP-N1 derivatives expressing GFP fusions to the PH domains of either PLCδ (Varnai and Balla, 1998) or Btk (Varnai et al., 1999) were kindly provided by Dr Tamas Balla (National Institutes of Health, Bethesda, MD).
Wortmannin and LY294002 were from Calbiochem. Cytochalasin D, human IgM and complement C5-deficient human serum were from Sigma.
Transient transfections of macrophages
Plasmid DNAs used for transient transfections of macrophages were purified using an EndoFree plasmid Maxi Kit (Qiagen). Macrophages (1.5 × 105) were seeded onto 12 mm glass coverslips in 10% FCS–DMEM 8 h prior to transfection. Transfections were performed using the FuGENE™6 transfection reagent (Roche Diagnostics), according to the manufacturer’s instructions, in 10% FCS–DMEM for 18 h at 37°C in 5% CO2, then processed for infection. Under these conditions, no toxicity of the FuGENE™6 reagent was observed using the trypan blue (0.08%; Gibco-BRL) exclusion assay. Typically, between 20 and 30% of the macrophages were transfected.
Infection procedures and phagocytic assays
EPEC strains were grown overnight in LB broth at 37°C, diluted 1:50 in pre-warmed DMEM and subcultured for 3 h at 37°C in 5% CO2. Early logarithmic phase bacteria were washed once with DMEM before infection. Yersinia strains were grown overnight at 30°C in BHI broth, then diluted 1:10 in BHI-OX (BHI supplemented with 4 mg/ml glucose, 20 mM MgCl2, 20 mM sodium oxalate), subcultured for 2 h at 30°C, then at 37°C for a further 2 h to induce the yop regulon (Neyt and Cornelis, 1999). Macrophages (1 × 105/well) were grown overnight in 10% FCS–DMEM then washed and incubated in serum-free DMEM to prevent any opsonization of infecting bacteria. Cells were infected at a multiplicity of infection (m.o.i.) of ∼200:1 for the required length of time at 37°C in 5% CO2. This theoretical m.o.i. actually led to numbers of cell-associated bacteria ranging from 10 to 20 per cell after 60 min infection. Cells were washed with phosphate-buffered saline (PBS) and then fixed in 2.5% paraformaldehyde pH 7.4, at 37°C for 10 min. Fixed cells were washed in PBS and blocked in 10% normal goat serum (NGS; Gibco-BRL)-PBS for 15 min at room temperature. Phagocytic assays were performed by differential staining of extracellular and total macrophage-associated EPEC. Extracellular bacteria were labelled prior to permeabilization using a rabbit anti-EPEC antiserum (Goosney et al., 1999a) followed by Alexa™488-conjugated goat anti-rabbit antibodies in NGS-PBS. Cells were then permeabilized with 0.2% saponin-NGS-PBS for 10 min at room temperature and total cell-associated EPEC were labelled using a rabbit anti-EPEC antiserum followed by Alexa™594-conjugated goat anti-rabbit antibodies in 0.2% saponin-NGS-PBS. For Yersinia experiments, bacteria were labelled using rabbit polyclonal antiserum against Y.enterocolitica serotype O:9 (Denka Seiken). For transfection experiments, staining of the transfected cells was performed after permeabilization with either mouse monoclonal antibodies against rat CD2 (pEF-BOS-CD2 transfection, Pharmingen) or monoclonal antibodies against human p85α (pEF-BOS-p85α or pEF-BOS-p85α-Δ transfections; Transduction Laboratories), followed by Alexa™594-conjugated goat anti-mouse antibodies. In this case, staining of total cell-associated EPEC was detected using Alexa™350-conjugated goat anti-rabbit antibodies, and extracellular bacteria were detected using Alexa™488-conjugated goat anti-rabbit antibodies. Numbers of extracellular and total cell-associated EPEC were scored for each of 50 cells per coverslip under a Zeiss Axioskop epifluorescence microscope using a 63× oil immersion objective. The ratio of extracellular to total cell-associated bacteria was calculated for each cell, from which the percentage intracellular bacteria was deduced. Statistical differences were considered significant for P values <0.05 using the Student’s t-test.
Opsonized zymosan phagocytosis assays
For FcγR-mediated phagocytosis, zymosan (1 mg/ml, Sigma) was opsonized with human IgG (25 mg/ml) for 2 h, then washed three times with PBS. IgG opsonization was confirmed by staining particles with Alexa™488-conjugated goat anti-human antibodies (Molecular Probes). For CR3-mediated phagocytosis, zymosan was C3bi-opsonized using human IgM and C5-deficient human serum (Sigma) as described previously (Caron and Hall, 1998). C3 deposition onto zymosan particles was verified by staining with goat anti-human C3 antibodies (Sigma).
Cells (1 × 105/well) were infected with EPEC strains as described above for 1 h at 37°C in 5% CO2, then washed extensively with PBS to remove non-adherent bacteria. IgG-zymosan was added to the infected cells in DMEM, which were left on ice for binding for 10 min, then placed at 37°C in 5% CO2 for 1 h. C3bi-zymosan was added directly to cells at 37°C and incubated further for 1 h. Cells were washed three times in PBS and fixed as described above. Since zymosan was readily visible by phase contrast microscopy, phagocytosis was analysed by detecting extracellular particles using Alexa™488-conjugated goat anti-human antibodies (IgG-zymosan; Molecular Probes), or anti-C3 antibodies followed by Cy2-conjugated donkey anti-goat antibodies (C3bi-zymosan; Jackson ImmunoResearch Laboratories) prior to permeabilization, while internalized particles remained unstained. Phagocytosis was scored by counting the number of internalized particles per 100 cells as a phagocytic index. In these experiments, the total number of zymosan particles (extracellular and intracellular) per 100 cells was similar under every condition tested (data not shown).
Lucifer Yellow LH exclusion experiments
Lucifer Yellow LH (Molecular Probes) exclusion experiments were performed as described previously (Kirby et al., 1998; Neyt and Cornelis, 1999) to evaluate pore formation in macrophage membranes by the EPEC type III secretion system.
Confocal laser scanning microscopy imaging
Specimens were observed on a Zeiss Axiovert S100VT equiped with a 63× oil immersion objective and attached to a Radiance Plus confocal microscope (BioRad). Images of 256 × 256 pixels (50 × 50 µm) were acquired using LaserSharp software (BioRad). Sections of 0.2 µm thickness (60–75 per image) were assembled into stacks and projected using the NIH Image 1.62 software. Projections were processed and merged using Adobe Photoshop.
Immunoprecipitations, Akt phosphorylation assay and immunoblotting
Approximately 8 × 106 cells were seeded into 150 mm dishes in 10% FCS–DMEM for 8 h, starved overnight in 0.5% FCS–DMEM then incubated for 3 h in serum-free DMEM. Cells were infected with EPEC strains at an m.o.i. of 200:1 and washed three times with ice-cold PBS. Cells were solubilized in 1 ml of ice-cold immunoprecipitation buffer [1% NP-40, 50 mM Tris–HCl pH 7, 2.5 mM EDTA, 1 mM NaF, 4 mM sodium orthovanadate, 1× complete protease inhibitor cocktail (Roche Diagnostics)]. Bacteria and cellular debris were removed from lysates by centrifugation for 10 min at 15 000 g, and 500 µg of clarified lysates were incubated at 4°C for 4 h with 5 µg of specific antibodies (rabbit polyclonal anti-p85α or mouse monoclonal anti-PY, clone 4G10). Protein A– (for rabbit IgG) or protein G– (for mouse IgG) Sepharose beads (Pharmacia Biotech) were blocked with bovine serum albumin (BSA) prior to addition to the samples, and then incubated for a further 2 h. Immunocomplexes were washed three times in immunoprecipitation buffer, then dissociated by boiling for 5 min in SDS sample buffer. For Akt phosphorylation assays, 1 × 106 serum-starved macrophages were infected for the required length of time, washed with PBS, then immediately lysed in boiling SDS sample buffer. Protein samples were resolved on an 8% SDS–polyacrylamide gel and transferred to nitrocellulose membranes (Trans-Blot; Bio-Rad) using a semi-dry apparatus. Membranes were blocked overnight in 4% BSA (for anti-phosphotyrosine detection) or 5% non-fat milk (for all other antibodies), 0.1% Tween-20, Tris-buffered saline (TBS). Primary and secondary antibodies were diluted appropriately in the corresponding blocking buffer and proteins were detected using the enhanced chemiluminescence-based detection kit (ECL Plus, Amersham).
Acknowledgments
Acknowledgements
We wish to thank Leigh Knodler, John Brumell, Bruce Vallance, Olivia Steele-Mortimer and Mike Gold for critical reading of the manuscript and helpful discussions. We also gratefully acknowledge Doreen Cantrell for the kind gift of bovine p85α and p85α-Δ cDNA, and Tamas Balla for the gift of the PH(PLCδ)–GFP and PH(Btk)–GFP constructs. This work was supported by a Howard Hughes International Research Scholar Award and an operating grant from the Canadian Institutes of Health Research (CIHR) to B.B.F. M.O. is a member of a CIHR group in host–pathogen interactions, a FRSQ Junior 2 scholar and a Burroughs Wellcome Fund awardee in molecular parasitology. J.C. was supported by a postdoctoral fellowship from the Fondation pour la Recherche Médicale.
References
- Araki N., Johnson,M.T. and Swanson,J.A. (1996) A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol., 135, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D.S. and Bliska,J.B. (1997) Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J., 16, 2730–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E. and Hall,A. (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science, 282, 1717–1721. [DOI] [PubMed] [Google Scholar]
- Celli J., Deng,W. and Finlay,B.B. (2000) Enteropathogenic Escherichia coli (EPEC) attachment to epithelial cells: exploiting the host cell cytoskeleton from the outside. Cell. Microbiol., 2, 1–9. [DOI] [PubMed] [Google Scholar]
- Cox D., Tseng,C.C., Bjekic,G. and Greenberg,S. (1999) A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem., 274, 1240–1247. [DOI] [PubMed] [Google Scholar]
- DeVinney R., Gauthier,A., Abe,A. and Finlay,B.B. (1999) Enteropathogenic Escherichia coli: a pathogen that inserts its own receptor into host cells. Cell. Mol. Life Sci., 55, 961–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand R. et al. (1994) PI 3-kinase: structural and functional analysis of intersubunit interactions. EMBO J., 13, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M.S., Calderwood,S.B., Donohue-Rolfe,A., Keusch,G.T. and Kaper,J.B. (1990) Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect. Immun., 58, 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C., Govaerts,C., Communi,D. and Pesesse,X. (1998) The diversity and possible functions of the inositol polyphosphate 5-phosphatases. Biochim. Biophys. Acta, 1436, 185–199. [DOI] [PubMed] [Google Scholar]
- Finlay B.B. and Falkow,S. (1997) Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev., 61, 136–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel G., Phillips,A.D., Rosenshine,I., Dougan,G., Kaper,J.B. and Knutton,S. (1998) Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol., 30, 911–921. [DOI] [PubMed] [Google Scholar]
- Gauthier A., de Grado,M. and Finlay,B.B. (2000) Mechanical fractionation reveals structural requirements for enteropathogenic Escherichia coli Tir insertion into host membranes. Infect. Immun., 68, 4344–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosney D.L., Celli,J., Kenny,B. and Finlay,B.B. (1999a) Enteropathogenic Escherichia coli inhibits phagocytosis. Infect. Immun., 67, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosney D.L., de Grado,M. and Finlay,B.B. (1999b) Putting E.coli on a pedestal: a unique system to study signal transduction and the actin cytoskeleton. Trends Cell Biol., 9, 11–14. [DOI] [PubMed] [Google Scholar]
- Goosney D.L., DeVinney,R., Pfuetzner,R.A., Frey,E.A., Strynadka,N.C. and Finlay,B.B. (2000) Enteropathogenic E.coli translocated intimin receptor, Tir, interacts directly with α-actinin. Curr. Biol., 10, 735–738. [DOI] [PubMed] [Google Scholar]
- Gray A., Van Der Kaay,J. and Downes,C.P. (1999) The pleckstrin homology domains of protein kinase B and GRP1 (general receptor for phosphoinositides-1) are sensitive and selective probes for the cellular detection of phosphatidylinositol 3,4-bisphosphate and/or phosphatidylinositol 3,4,5-trisphosphate in vivo. Biochem. J., 344, 929–936. [PMC free article] [PubMed] [Google Scholar]
- Holz R.W., Hlubek,M.D., Sorensen,S.D., Fisher,S.K., Balla,T., Ozaki,S., Prestwich,G.D., Stuenkel,E.L. and Bittner,M.A. (2000) A pleckstrin homology domain specific for phosphatidylinositol 4,5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J. Biol. Chem., 275, 17878–17885. [DOI] [PubMed] [Google Scholar]
- Hueck C.J. (1998) Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev., 62, 379–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman L.R. and Cantey,J.R. (1983) Specific adherence of Escherichia coli (strain RDEC-1) to membraneous (M) cells of the Peyer patch in Escherichia coli diarrhea in the rabbit. J. Clin. Invest., 71, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Payrastre,B., Chap,H., Ogawa,W., Sakaue,H., Kasuga,M. and Cossart,P. (1996) A role for phosphoinositide 3-kinase in bacterial invasion. Science, 274, 780–782. [DOI] [PubMed] [Google Scholar]
- Ireton K., Payrastre,B. and Cossart,P. (1999) The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J. Biol. Chem., 274, 17025–17032. [DOI] [PubMed] [Google Scholar]
- Kenny B. (1999) Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol. Microbiol., 31, 1229–1241. [DOI] [PubMed] [Google Scholar]
- Kenny B., DeVinney,R., Stein,M., Reinscheid,D.J., Frey,E.A. and Finlay,B.B. (1997) Enteropathogenic E.coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell, 91, 511–520. [DOI] [PubMed] [Google Scholar]
- Kirby J.E., Vogel,J.P., Andrews,H.L. and Isberg,R.R. (1998) Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol., 27, 323–336. [DOI] [PubMed] [Google Scholar]
- Knutton S., Adu-Bobie,J., Bain,C., Phillips,A.D., Dougan,G. and Frankel,G. (1997) Down regulation of intimin expression during attaching and effacing enteropathogenic Escherichia coli adhesion. Infect. Immun., 65, 1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Rosenshine,I., Pallen,M.J., Nisan,I., Neves,B.C., Bain,C., Wolff,C., Dougan,G. and Frankel,G. (1998) A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J., 17, 2166–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresse A.U., Rohde,M. and Guzman,C.A. (1999) The EspD protein of enterohemorrhagic Escherichia coli is required for the formation of bacterial surface appendages and is incorporated in the cytoplasmic membranes of target cells. Infect. Immun., 67, 4834–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowska K. and Sobota,A. (1999) Signaling pathways in phagocytosis. BioEssays, 21, 422–431. [DOI] [PubMed] [Google Scholar]
- Levine M.M., Bergquist,E.J., Nalin,D.R., Waterman,D.H., Hornick,R.B., Young,C.R. and Sotman,S. (1978) Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet, 1, 1119–1122. [DOI] [PubMed] [Google Scholar]
- Marte B.M. and Downward,J. (1997) PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem. Sci., 22, 355–358. [DOI] [PubMed] [Google Scholar]
- Martinez J.J., Mulvey,M.A., Schilling,J.D., Pinkner,J.S. and Hultgren,S.J. (2000) Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J., 19, 2803–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara B.P. and Donnenberg,M.S. (1998) A novel proline-rich protein, EspF, is secreted from enteropathogenic Escherichia coli via the type III export pathway. FEMS Microbiol. Lett., 166, 71–78. [DOI] [PubMed] [Google Scholar]
- Mecsas J., Raupach,B. and Falkow,S. (1998) The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol. Microbiol., 28, 1269–1281. [DOI] [PubMed] [Google Scholar]
- Mizushima S. and Nagata,S. (1990) pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res., 18, 5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J., Wilson,L. and Kellie,S. (2000) Phosphatidylinositol-3′ kinase-dependent vesicle formation in macrophages in response to macrophage colony stimulating factor. J. Cell Sci., 1132, 337–348. [DOI] [PubMed] [Google Scholar]
- Nataro J.P. and Kaper,J.B. (1998) Diarrheagenic Escherichia coli. Clin. Microbiol. Rev., 11, 142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neutra M.R., Frey,A. and Kraehenbuhl,J.P. (1996) Epithelial M cells: gateways for mucosal infection and immunization. Cell, 86, 345–348. [DOI] [PubMed] [Google Scholar]
- Neyt C. and Cornelis,G.R. (1999) Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol., 33, 971–981. [DOI] [PubMed] [Google Scholar]
- Persson C., Carballeira,N., Wolf-Watz,H. and Fallman,M. (1997) The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J., 16, 2307–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzioch D., Hudson,T., Boule,M., Barrera,L., Urbance,J.W., Varesio,L. and Skamene,E. (1991) Genetic resistance/susceptibility to mycobacteria: phenotypic expression in bone marrow derived macrophage lines. J. Leukoc. Biol., 50, 263–272. [DOI] [PubMed] [Google Scholar]
- Rosenshine I., Ruschkowski,S. and Finlay,B.B. (1996a) Expression of attaching/effacing activity by enteropathogenic Escherichia coli depends on growth phase, temperature and protein synthesis upon contact with epithelial cells. Infect. Immun., 64, 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Ruschkowski,S., Stein,M., Reinscheid,D.J., Mills,S.D. and Finlay,B.B. (1996b) A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J., 15, 2613–2624. [PMC free article] [PubMed] [Google Scholar]
- Sanger J.M., Chang,R., Ashton,F., Kaper,J.B. and Sanger,J.W. (1996) Novel form of actin-based motility transports bacteria on the surfaces of infected cells. Cell Motil. Cytoskel., 34, 279–287. [DOI] [PubMed] [Google Scholar]
- Schulte R., Zumbihl,R., Kampik,D., Fauconnier,A. and Autenrieth,I.B. (1998) Wortmannin blocks Yersinia invasin-triggered internalization, but not interleukin-8 production by epithelial cells. Med. Microbiol. Immunol. (Berl.), 187, 53–60. [DOI] [PubMed] [Google Scholar]
- Sory M.P., Boland,A., Lambermont,I. and Cornelis,G.R. (1995) Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl Acad. Sci. USA, 92, 11998–12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer T.P., Ahn,S. and Meyer,T. (1998) Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr. Biol., 8, 343–346. [DOI] [PubMed] [Google Scholar]
- Tall E.G., Spector,I., Pentyala,S.N., Bitter,I. and Rebecchi,M.J. (2000) Dynamics of phosphatidylinositol 4,5-bisphosphate in actin-rich structures. Curr. Biol., 10, 743–746. [DOI] [PubMed] [Google Scholar]
- Taylor K.A., O’Connell,C.B., Luther,P.W. and Donnenberg,M.S. (1998) The EspB protein of enteropathogenic Escherichia coli is targeted to the cytoplasm of infected HeLa cells. Infect. Immun., 66, 5501–5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P. and Balla,T. (1998) Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol., 143, 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P., Rother,K.I. and Balla,T. (1999) Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton’s tyrosine kinase pleckstrin homology domain visualized in single living cells. J. Biol. Chem., 274, 10983–10989. [DOI] [PubMed] [Google Scholar]
- Vazquez F. and Sellers,W.R. (2000) The PTEN tumor suppressor: an antagonist of phosphoinositide 3-kinase signaling. Biochim. Biophys. Acta, 1470, M21–M35. [DOI] [PubMed] [Google Scholar]
- Wachter C., Beinke,C., Mattes,M. and Schmidt,M.A. (1999) Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol. Microbiol., 31, 1695–1707. [DOI] [PubMed] [Google Scholar]
- Woestyn S., Allaoui,A., Wattiau,P. and Cornelis,G.R. (1994) YscN, the putative energizer of the Yersinia Yop secretion machinery. J. Bacteriol., 176, 1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff C., Nisan,I., Hanski,E., Frankel,G. and Rosenshine,I. (1998) Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol. Microbiol., 28, 143–155. [DOI] [PubMed] [Google Scholar]
- Wymann M.P. and Pirola,L. (1998) Structure and function of phosphoinositides 3-kinases. Biochim. Biophys. Acta, 1436, 127–150. [DOI] [PubMed] [Google Scholar]
- Zhou K., Pandol,S., Bokoch,G. and Traynor-Kaplan,A.E. (1998) Disruption of Dictyostelium PI3K genes reduces [32P]phosphatidylinositol 3,4 bisphosphate and [32P]phosphatidylinositol trisphosphate levels, alters F-actin distribution and impairs pinocytosis. J. Cell Sci., 111, 283–294. [DOI] [PubMed] [Google Scholar]