Abstract
Plo1-associated casein kinase activity peaked during mitosis before septation. Phosphatase treatment abolished this activity. Mitotic Plo1 activation had a requirement for prior activation of M-phase promoting factor (MPF), suggesting that Plo1 does not act as a mitotic trigger kinase to initiate MPF activation during mitotic commitment. A link between Plo1 and the septum initiating network (SIN) has been suggested by the inability of plo1Δ cells to septate and the prolific septation following plo1+ overexpression. Interphase activation of Spg1, the G protein that modulates SIN activity, induced septation but did not stimulate Plo1-associated kinase activity. Conversely, SIN inactivation did not affect the mitotic stimulation of Plo1-associated kinase activity. plo1.ts4 cells formed a misshapen actin ring, but rarely septated at 36°C. Forced activation of Spg1 enabled plo1.ts4 mutant cells, but not cells with defects in the SIN component Sid2, to convert the actin ring to a septum. The ability of plo1+ overexpression to induce septation was severely compromised by SIN inactivation. We propose that Plo1 acts before the SIN to control septation.
Keywords: cell cycle/mitosis/polo kinase/Schizosaccharomyces pombe/SIN
Introduction
Commitment to mitosis is controlled by the protein kinase complex called M-phase promoting factor (MPF) (Ohi and Gould, 1999). MPF comprises a catalytic subunit p34cdc2/Cdk1 and its binding partner cyclin B. MPF is inhibited during interphase by Wee1- and Mik1-related kinases, which phosphorylate key residues of p34cdc2. The phosphatases Cdc25/Pyp3 can remove these phosphates to produce active MPF. Commitment to mitosis can be viewed as the balance of activity between Cdc25 and Wee1-related molecules. Studies of cell cycle control in Xenopus oocyte extracts have identified a positive feedback loop whereby a trigger level of active MPF catalyses the conversion of the remaining MPF in the extract to the active state. This activation involves phosphorylation of Cdc25 by p34cdc2 and at least one other kinase (Hoffmann et al., 1993; Izumi and Maller, 1993, 1995). The co-purification of the polo-like kinase (Plk) Plx1 with Cdc25 in Xenopus extracts suggests an involvement of Plks in this activation loop (Kumagai and Dunphy, 1996). Moreover, phosphorylation of Cdc25 during the amplification loop or in vitro by Plx1 enables the protein to be recognized by the monoclonal antibody MPM-2. MPM-2 recognizes a phospho epitope found on Cdc25 following amplification loop activation in extracts (Kuang et al., 1994).
Although MPF can directly regulate some mitotic processes, other conserved kinases such as Plks control many mitotic events. The Drosophila polo mutant was identified because of the propensity of cells to form monopolar rather than bipolar spindles. Subsequent analyses of Plks in diverse systems suggested that they regulate multiple and distinct mitotic events in every round of cell division (Glover et al., 1998; Nigg, 1998). In addition to a role in the MPF positive feedback loop, these functions include centrosome maturation, spindle formation, anaphase promoting complex (APC) regulation, several aspects of cytokinesis and a crucial role in mitotic exit.
In Schizosaccharomyces pombe, only one Plk, Plo1, has been identified to date (Ohkura et al., 1995). Plo1 function is required for spindle formation and cytokinesis. The first stage of cytokinesis in fission yeast is the generation of a cortical ring in the cell equator during prophase (Le Goff et al., 1999). This contains several components, including F-actin and Mid1/Dmf1. Mid1/Dmf1 resides in the nucleus during interphase, but forms a ring around the cortex that predicts the site of F-actin ring formation. The export of Mid1/Dmf1 from the nucleus coincides with its phosphorylation and remodelling into an equatorial ring (Sohrmann et al., 1996). Cells that lack Mid1/Dmf1 function form a highly disorganized and misplaced structure instead of a tight F-actin ring at the cell equator (Chang et al., 1996; Sohrmann et al., 1996). This phenotype is highly reminiscent of some plo1ts mutants (Bähler et al., 1998). The ability of Mid1/Dmf1 and Plo1 to interact in the two-hybrid system, and the inability of Mid1/Dmf1 to leave the nucleus in plo1ts mutants, suggested that Plo1 may directly phosphorylate Mid1/Dmf1 to regulate ring formation (Bähler et al., 1998). Strains lacking Plo1 altogether do not form a cortical F-actin ring, although it is unclear whether this is a variation on the mid1/dmf1 mutant phenotype or a further requirement for Plo1 during an earlier stage of ring formation (Ohkura et al., 1995).
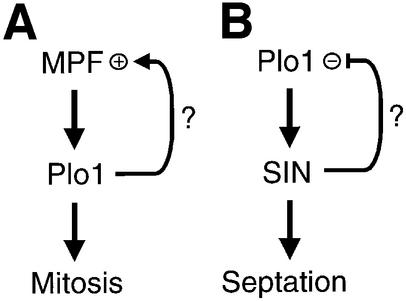
Constriction of the cortical F-actin ring is co-ordinated with the generation of the septum at the end of anaphase. Cells lacking plo1+ cannot form a septum, while plo1+ overexpression in interphase drives inappropriate septation (Ohkura et al., 1995). Overproduction of the small GTP binding protein Spg1 or the protein kinase Cdc7 also induces multiple rounds of septation (Fankhauser and Simanis, 1994; Schmidt et al., 1997). Spg1 activity is regulated by a bipartite GAP protein composed of the products of the byr4+ and cdc16+ genes (Furge et al., 1998; Le Goff et al., 1999). Spg1 is found on the spindle pole bodies (SPBs) throughout the cell cycle. GTP-bound Spg1 recruits Cdc7 to the SPB (Sohrmann et al., 1998), which then activates a downstream network containing at least two more kinases and induces septation (Le Goff et al., 1999). There is a striking similarity between this Spg1 ‘septum initiating network’ (SIN) of S.pombe and the ‘mitotic exit network’ (MEN) of Saccharomyces cerevisiae (Balasubramanian et al., 2000; Cerutti and Simanis, 2000). All but one of the SIN components identified to date have counterparts that function in a similar manner in the MEN. It is currently unclear whether Plo1 regulates septation by acting before, after or in parallel with the SIN.
Like other Plks, Plo1 associates with the division apparatus. It is recruited to the medial ring, SPB and spindle upon mitotic commitment, and shows a high affinity for the SPB until the onset of anaphase (Bähler et al., 1998; Mulvihill et al., 1999). Spindle and medial ring association is lost early in anaphase B, and the intensity of Plo1 association with the SPB diminishes considerably during the early stages of anaphase B. SPB association requires the activity of Cdc25 and Cdc2, but no SIN components. As Plo1 protein levels remain constant throughout the cell cycle, post-translational modification of Plo1 or the provision of a Plo1 docking site seems to regulate Plo1–SPB interactions (Mulvihill et al., 1999).
We used an in vitro assay to monitor changes in Plo1-associated kinase that accompany cell cycle progression and found a mitotic peak in Plo1-associated kinase activity. Mitotic activation involves phosphorylation and is reliant upon prior activation of Cdc25 and Cdc2. We conclude with experiments suggesting that Plo1 primarily regulates septation by acting before the SIN.
Results
In vitro Plo1-associated kinase activity
Cell extracts were prepared from cdc25-22 cells in which either a wild-type plo1+ (IH1558) or plo1.K69R (IH1559) mutant gene had been integrated at the leu1 locus. plo1.K69R carries a point mutation that is predicted to abolish kinase activity (Hanks and Hunter, 1995). In both cases, the integrated gene was tagged at its N-terminus by fusion to three copies of the haemagglutinin (HA) epitope recognized by the antibody 12CA5 (Field et al., 1988), and expression was regulated by the thiamine-repressible nmt41 promoter. The wild-type gene tagged in this way can substitute for plo1+ (Mulvihill et al., 1999). Expression of the kinase mutant did not arrest growth (see below). The tagged proteins accumulated to levels that were comparable to the abundance of endogenous Plo1 (see below).
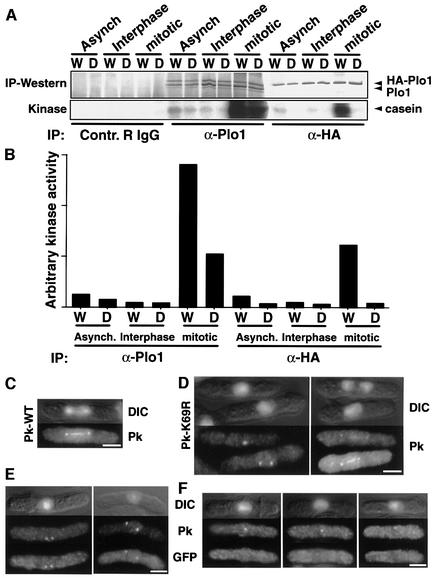
Tagged, but not untagged Plo1 was precipitated when 12CA5 was used to pull down HA–Plo1 or HA– Plo1.K69R from whole-cell extracts. This shows that the tagged molecules did not associate with the untagged, endogenous protein under these conditions. Casein kinase activity was detected in 12CA5 precipitates from mitotic cell extracts expressing HA–Plo1, but not from identical extracts of cells expressing HA–Plo1.K69R, despite the precipitation of similar quantities of HA-tagged Plo1 protein in each case (Figure 1A and B). This shows that the activity we are monitoring is dependent on Plo1 activity. HA–Plo1-associated kinase activity of extracts prepared from mitotic cells considerably exceeded that of the kinase prepared from an asynchronous culture, which, in turn, was greater than the activity detected in cells that were in the G2 phase of the cell cycle. When the polyclonal anti-Plo1 antibody HN184 was used instead of 12CA5 for immunoprecipitation, both HA-tagged and endogenous Plo1 were pulled down. Mitotic extracts of strains expressing either HA–Plo1.K69R or HA–Plo1 had high kinase activity. The kinase activity in cells expressing HA–Plo1.K69R was due to the presence of the untagged endogenous Plo1 protein in the reaction. Immuno precipitation with serum from an unimmunized rabbit did not precipitate any casein kinase activity (Figure 1A). These data demonstrated that a casein kinase activity is associated with Plo1.
Fig. 1. Plo1.K69R has compromised mitotic kinase activity but still associates with the mitotic apparatus. (A) Cell extracts were prepared from cdc25.22 mutants harbouring either HA-tagged wild-type plo1+ (strain IH1558, indicated by W) or mutant plo1.K69R (strain IH1559, indicated by D) in pINT541 integrated at the leu1 locus. Cells were induced to express HA–Plo1 (W or D) for 17 h (Asynch, asynchronous cells) and then shifted to the restrictive temperature of 36°C for 4 h to arrest cell cycle progression at the G2/M boundary (Interphase). A portion of the arrested culture was returned to 25°C for 40 min to produce a population of mitotic cells (mitotic). Non-specific rabbit IgG, the polyclonal anti-Plo1 antibody HN184 or the 12CA5 monoclonal antibody was used to precipitate the different versions of Plo1. Kinase assay reactions of the precipitates were blotted onto nitrocellulose membranes, monitored for 32P labelling of casein (kinase) and probed with HN184 (IP–western) to determine Plo1 levels in each reaction. (B) The specific activity of the kinase in each condition was plotted after calculating the kinase activity per unit protein. (C–F) Immunofluorescence images. Each block represents different images of the same cells. The staining in each particular panel is written at its side. DIC indicates combined DAPI/DIC images. Anti-PK immunofluorescence staining of IH1857 (C) and IH1858 (D). (E and F) Combined anti-GFP and anti-PK immunofluorescence staining of spores arising from mating IH1857 with IH1845. Bar, 2.5 µm.
Plo1.K69R associates with the mitotic apparatus in plo1+ and plo1Δ cells
The generation of the plo1.K69R allele enabled us to ask whether the association of Plo1 with the spindle and the SPB during mitosis required Plo1 activity. We expressed N-terminally Pk-tagged plo1+ or plo1.K69R genes in the diploid strain IH1847. Both tagged proteins showed normal localization patterns (Figure 1C and D). The lack of association between endogenous untagged and tagged Plo1 in the immunoprecipitation reactions in Figure 1A may suggest that it is unlikely that PK-Plo1.K69R is recruited to the spindle by binding to endogenous Plo1. The endogenous molecule could, however, direct spindle association of PK-Plo1.K69R by phosphorylating it or modifying a docking protein. We therefore localized PK-Plo1K69R in plo1.d1 cells in which the genomic plo1+ open reading frame has been replaced by the ura4+ gene (hence referred to as plo1Δ). As plo1+ is essential, the plo1Δ deletion allele was propagated in a heterozygous plo1Δ/plo1+ diploid and the phenotype analysed in germinating haploid plo1Δ spores. The heterogeneous phenotypes of germinating plo1Δ cells suggest that some Plo1 is carried over from the diploid parent into the spores and that a phenotype only emerges upon exhaustion of this parental protein (Ohkura et al., 1995). We therefore generated a parental diploid in which the plo1+ gene was fused to green fluorescent protein (GFP) (Bähler et al., 1998). This allowed us to use GFP antibodies to monitor the levels of the ‘parental Plo1.GFPS65T protein’ at the same time as localizing PK-Plo1.K69R with a monoclonal antibody that recognizes the PK epitope.
Germination of a spore mix containing diploid plo1Δ/plo1.GFPS65T and haploid plo1Δ spores in media lacking leucine and uracil selected for growth of cells containing both the plasmid-borne plo1.NPKK69R and the genomic plo1Δ alleles. Anti-GFP and anti-PK immunofluorescence signals were simultaneously seen on SPBs in dividing cells (Figure 1E). In contrast, many apparently interphase cells in the same preparation had strong PK but no anti-GFP SPB staining (Figure 1F). Thus, the affinity of PK-Plo1K69R for the SPB did not appear to be affected by reducing the abundance of the wild-type protein below our detection limits.
Plo1-associated in vitro kinase activity peaks during mitosis
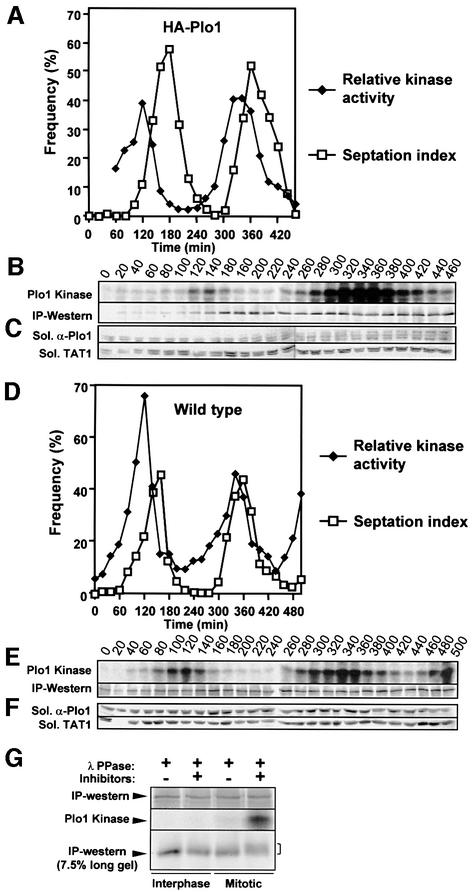
Before examining the cell cycle regulation of Plo1 activity in great detail, it was necessary to determine the similarity between the profile of kinase activity seen for HA–Plo1 with 12CA5 antibodies and that seen when the endogenous protein was precipitated by HN184. HA-tagged Plo1-associated activity was assessed in a culture of otherwise wild-type cells (IH1314) that had been synchronized with respect to cell cycle progression by centrifugal elutriation. To minimize any adverse consequences of protein overproduction upon the validity of the kinase assay data in this particular experiment, plo1.NHA expression was manipulated so that the levels of the tagged protein approached and did not exceed those of the endogenous wild-type protein at the end of the experiment. The panels in Figure 2B show two read-outs of the same gel. The upper panel shows phosphorylation of casein, whilst the blots in the lower panel show the amount of Plo1 in each kinase reaction. As with all figures in this study, the plotted kinase activity was calculated by dividing the measured kinase activity by the relative amount of Plo1 precipitated. A sharp peak in Plo1-associated kinase activity was seen during mitosis in both cell cycles (Figure 2A and B).
Fig. 2. Plo1-associated kinase activity peaks in mitosis. (A–C) Other wise wild-type cells (IH1314) were induced to express HA–Plo1 for 13 h and then synchronized with respect to cell cycle progression by elutrient centrifugation size selection at time 0. (A) An aliquot was taken at each time point and processed for a quantitative kinase assay using 12CA5 as in Figure 1. (B) The kinase assay (Plo1 kinase) and HN184 immunoblot of the reaction (IP–western) were used to calculate (A). (C) A parallel aliquot was used to determine the levels of Plo1 and tubulin in the soluble fraction. HA–Plo1 initially appeared at ∼60 min and continued to accumulate as the experiment progressed. (D–F) The wild-type strain (IH365) was synchronized and processed as for IH1314 in (A–C) using HN184 to pull down Plo1. Soluble Plo1 protein levels were normalized for variation between samples by determining the ratio of signals from anti-Plo1 and anti-α-tubulin (TAT1) from the same western blot. Three independent sets of blotting data from two independent experiments, each covering two complete cell cycles, showed that levels of soluble Plo1 did not vary during cell cycle progression (data not shown). (G) Interphase or mitotic Plo1 immunocomplexes were prepared from cdc25.22 cultures as for Figure 1A and treated with λ phosphatase with or without inhibitors before processing in a kinase assay (upper panels) and running on a large 7.5% gel (bottom panel).
To determine whether the kinase activity associated with the endogenous protein peaked similarly in mitosis, HN184 was used to precipitate Plo1 from a size-selected wild-type culture (IH365). After correcting for the levels of Plo1 in each assay, the oscillating activity profile was indistinguishable from that with the HA-tagged protein (Figure 2D and E), indicating that the polyclonal antibody gives an accurate measure of Plo1-associated kinase activity. As we have previously shown for the entire population of Plo1 (Mulvihill et al., 1999), soluble Plo1 levels remained constant throughout the cell cycle (Figure 2C and F). Thus, fluctuations in Plo1 activity can not be due to fluctuations in Plo1 abundance. We therefore asked whether the phosphorylation status of Plo1 affected its activity. Active, mitotic Plo1 was incubated with λ phosphatase before its activity was monitored in a kinase assay. Phosphatase treatment virtually abolished Plo1 activity and condensed the broad Plo1 band, which is seen upon running the kinase reactions on large SDS–PAGE gels, to a tighter, more compact band (Figure 2G, compare lanes 3 and 4 of IP–western). Kinase activity was not inhibited when phosphatase inhibitors were included in the phosphatase treatment buffer (Figure 2G). These data show that Plo1-associated kinase activity peaks during mitosis and that this activation may arise from mitotic phosphorylation.
Plo1 is activated after MPF
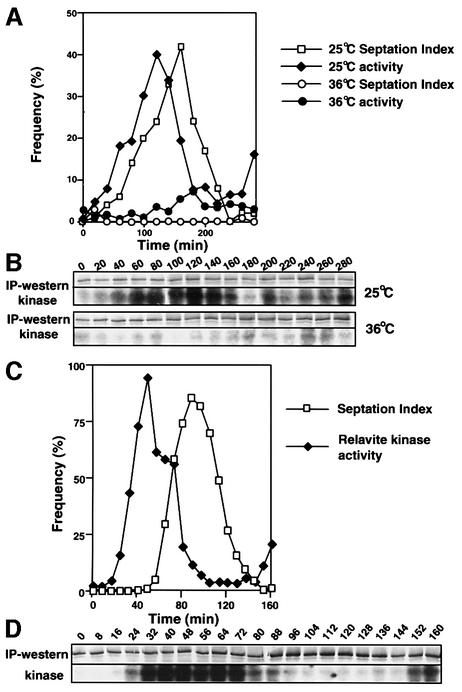
The kinase activity plots in Figure 2 suggested that Plo1 activity rises early in mitosis. Given the debate about the role played by Plks in MPF activation, we asked whether Plo1 was activated before or after MPF. We used cdc2.33 mutant cells to address this point because they have temperature-sensitive p34cdc2 H1-kinase activity (Moreno et al., 1989). Early G2 cdc2-33 cells were collected at 25°C and half of the culture was immediately shifted to 36°C to inactivate p34cdc2 function. The other half was kept at 25°C. Plo1-associated activity peaked before septation in the 25°C culture (Figure 3A and B). In contrast, no rise in activity was seen in cells arrested at the cdc2 execution point by incubation at 36°C (Figure 3A and B). This dependency of Plo1-associated activity upon MPF activation was confirmed by cooling part of the culture that had been incubated at 36°C back down to 25°C, after 150 min at the restrictive temperature, to restore p34cdc2.33 function (time 0 in Figure 3C and D). Plo1-associated activity rose dramatically after the return to 25°C (Figure 3C and D; the degree of activation is clearly apparent when one considers that the 0 time point in Figure 3C is between the 140 and 160 min time points in Figure 3B, 36°C).
Fig. 3. Plo1-associated kinase activation requires MPF activity. A cdc2.33 culture that had been grown at 25°C was synchronized with respect to cell cycle progression by elutrient centrifugation before being split into two separate cultures. One was immediately incubated at 36°C whilst the other was kept at 25°C. (A and B) Samples from the 36 and 25°C cultures were processed to assess Plo1-associated kinase activity. Before plotting the data for the figure, the Plo1 kinase assay at each temperature (B) was adjusted according to the amount of Plo1 in the kinase assay. (C and D) At 150 min, half of the 36°C culture was rapidly cooled to 25°C and processed as for (A) and (B).
Given that phosphorylation appears to regulate Plo1-associated kinase activity (Figure 2G) and the plo1+ nucleotide sequence predicts a single p34cdc2 consensus phosphorylation site (Nigg, 1993), we mutated this threonine residue to alanine to ask whether the dependency of Plo1 activity upon MPF function was due to direct phosphorylation of this site by MPF. The plo1.T14A mutant was integrated into the genome at the leu1+ locus fused to three HA epitope tags at its N-terminus and regulated by the nmt41 promoter. The kinase activity of 12CA5 precipitates from the strain expressing HA– Plo1.T14A was indistinguishable from that of the strain expressing HA–Plo1 (Figure 4). As plo1.T14A can suppress the plo1Δ growth defect (not shown), we conclude that MPF is unlikely to stimulate Plo1-associated kinase by phosphorylating threonine 14.
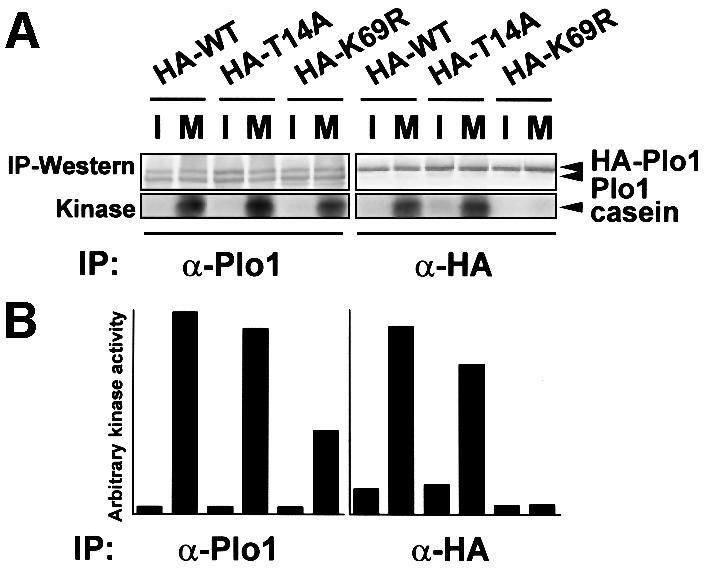
Fig. 4. Mutation of the p34cdc2 consensus phosphorylation site in Plo1 does not block activation of in vitro kinase activity. (A) Cell extracts were prepared as for Figure 1 from cdc25.22 mutants harbouring HA-tagged wild-type plo1+ (strain IH1558) (HA–WT), HA-tagged plo1.K69R (strain IH1559) (HA–K69R) or HA-tagged plo1.T14A (strain IH1724) (HA–T14A). I, interphase extract; M, mitotic extract. In each strain, the tagged gene was integrated at the leu1 locus under the control of the nmt41 promoter using pINT541. Kinase assays were performed as for Figure 1. (B) The kinase activity per unit protein for each assay.
Plo1 activation and the timing of mitotic events
Established procedures were used to generate cultures that have highly synchronous mitoses in order to relate changes in Plo1-associated kinase activity to distinct mitotic events more precisely. Cells were synchronized by size selection, followed by temporary cell cycle arrest at the cdc25 execution point. Because Cdc25 triggers mitosis by removing an inhibitory phosphate from p34cdc2, cells are unable to divide while Cdc25 activity is missing. Restoring Cdc25 activity results in rapid commitment to mitosis. Using elutriation to select a G2 population at the start of the experiment and limiting the arrest at the G2/M boundary to 20 min results in mitotic synchrony of ∼70% (Hagan and Yanagida, 1997).
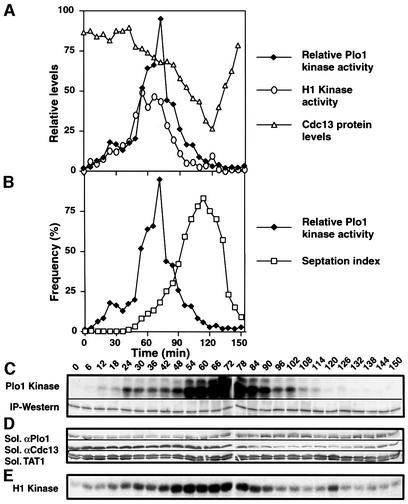
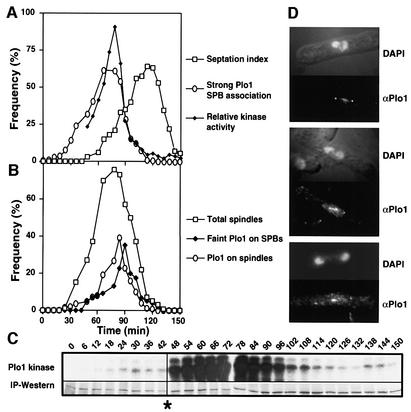
Early G2 temperature-sensitive cdc25.22 cells were cultured at 36°C for 160 min, before the return to 25°C. Samples were then taken every 6 min to monitor Plo1 activity (Figure 5C), Plo1 and Cdc13 (cyclin B) protein levels (Figure 5D), and histone H1 kinase activity (Figure 5E). Plo1-associated activity was slightly higher 24 min after release than at the surrounding points, suggesting that there may be an early activation of Plo1, which is distinct from the following, major phase of Plo1-associated kinase activity (Figure 5A, C and E). The start of the second phase of Plo1 activation at 42 min coincided with the initiation of Cdc13 destruction. Cdc13 destruction accelerated at 84 min and continued at this rate until 120 min. The cessation of Cdc13 destruction coincided precisely with the return of Plo1-associated activity to its basal, starting level. The reduction in Plo1 affinity for the SPB and its loss from the spindle also coincided precisely with the return of Plo1-associated kinase activity to its starting, basal level (Figure 6), suggesting that this marks a major, early anaphase control point in mitotic progression.
Fig. 5. Plo1-associated kinase activity in a highly synchronous mitosis. A cdc25.22 culture was synchronized by centrifugal elutriation and the early G2 cells were shifted to 36°C for 160 min before returning to 25°C at time 0. Samples were processed to assay Plo1 (A–C) and H1 kinase activity (A and E) and blotted to monitor Plo1 and Cdc13 levels in soluble extracts (A and D) as for Figure 2. The raw data of the Plo1-associated kinase assay [(C), Plo1-associated kinase] were normalized for the amount of Plo1 protein in each reaction [(C), IP–western] and are presented in (A) and (B). Plo1 and Cdc13 levels were calculated by the ratio of the signal from these polyclonal antibodies to that arising from blotting with the anti-tubulin antibody TAT1. Each western blot was performed on a strip cut from the same master blot. These blots were only performed once, but similar data were obtained in parallel experiments (Figure 6).
Fig. 6. Maximal Plo1-associated kinase activity coincides with maximal SPB association. The experiment described in the legend for Figure 5 was repeated and one sample was processed for anti-Plo1 (HN1814) immunofluorescence microscopy. (A and B) Graphical representation of the indicated features of the culture. (C) There was a uniform error, which reduced the activity of the first eight kinase assays; however, the trend seen in Figure 5 of an early peak is still apparent. (D) Anti-Plo1 staining 90 min after return to 25°C.
Plo1 activation and SIN activity
Overproduction of the small G protein Spg1 or mutation of its bipartite Cdc16/Byr4 GAP complex activates the SIN and drives septation in interphase cells (Minet et al., 1979; Fankhauser et al., 1993; Song et al., 1996; Schmidt et al., 1997). As the septation phenotypes associated with manipulation of plo1+ levels suggest an intimate relationship between Plo1 and SIN function, we asked whether Plo1-associated kinase activity might be regulated by the SIN. Specifically, Plo1-associated activity was followed as the SIN was activated in interphase cells (Cerutti and Simanis, 1999).
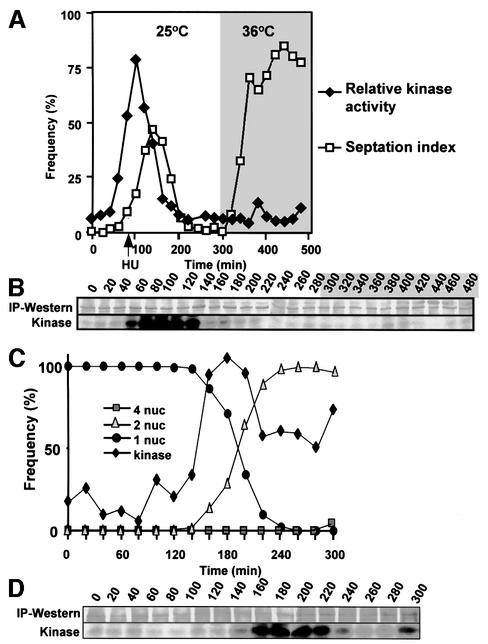
The inhibitor of DNA synthesis hydroxyurea was added to a cell-cycle-synchronized cdc16-116 culture 60 min after these early G2 cells had been isolated by centrifugal elutriation. Because these cells were maintained at the permissive temperature of 25°C, they went through one mitosis before arresting cell cycle progression in the next S phase. Once the cells were arrested in S phase, the temperature of this culture was shifted to 36°C to inactivate Cdc16 and so activate Spg1 and drive septation (Figure 7A). Although Plo1 associated with the SPB as a peak of Plo1-associated kinase activity preceded the first peak of septation following synchronization, no SPB association (data not shown) or Plo1-associated kinase activity accompanied the prolific septation arising from Cdc16 inactivation in G1/S phase (Figure 7A and B).
Fig. 7. The dependency of Plo1 activation upon SIN function. Plo1-associated kinase was assayed and the specific activity was determined as for Figure 1. Septation indices and the number of nuclei per cell were both scored after DAPI/calcofluor staining. (A and B) G2 cdc16-116 cells were isolated by centrifugal elutriation at time 0 and cultured at 25°C. Sixty minutes later, hydroxyurea (HU) was added to the culture (arrow in A). The temperature was shifted to 36°C at 290 min. The grey shading indicates the period at 36°C. (A) Quantification of the kinase data in (B) and the septation index. (C and D) IH1751 (spg1.B8 cdc7.A20) cells were grown at 25°C before small G2 cells were isolated by centrifugal elutriation at time 0 and half the culture was incubated at 36°C and the other at 25°C. The profile and extent of kinase activation at 36 and 25°C were very similar (not shown).
We next asked whether the activation of Plo1-associated kinase activity was affected when SIN function was abolished. To ensure maximal SIN inactivation, we used a strain containing ts– mutations in both the G protein Spg1 and its effector kinase Cdc7. The kinetics of kinase activation in spg1.B8 cdc7.A20 mutant cells (Figure 7C and D) was comparable to that seen in wild-type cells (Figure 2D). The reduction in kinase activity as cells left mitosis was not as dramatic as that seen in wild-type backgrounds, suggesting that SIN activity may down-regulate Plo1 during mitotic exit. The main conclusions we draw from these experiments are that SIN activation in interphase cells did not stimulate Plo1-associated kinase activity and that mitotic activation of the kinase was not affected by blocking SIN function.
Activation of Spg1 overcomes the sin– phenotype of plo1.ts4
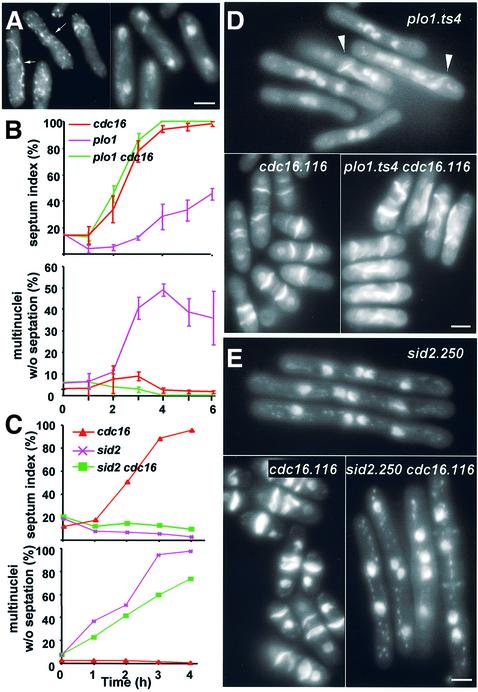
The ability of inappropriate Spg1 activation to drive septation without stimulating Plo1-associated kinase activity suggested that an active SIN may not require Plo1 to drive septation. If this were the case, activation of the SIN alone should be sufficient to bypass an actin ring activation defect of a plo1 mutant. We therefore activated the SIN with a cdc16.116 mutation in a plo1.ts4 background. At 36°C, plo1.ts4 cells mimicked a sin– defect because they formed a highly misshapen medial F-actin ring but rarely used it to generate a septum (Figure 8A, B and D). If Plo1 acted before the SIN, the plo1.ts4 mutation should not perturb the ability of the cdc16.116 mutation to drive septation. If, however, Plo1 acts after, or is a part of the SIN, the plo1.ts4 mutation should compromise Cdc16-induced septation.
Fig. 8. SIN activation suppresses the sin– phenotype of plo1.ts4 but not sid2.250 mutants. (A) An asynchronous culture of plo1.ts4 cells was shifted to 36°C for 3 h and stained with TRITC–phalloidin. All anaphase cells (n = 140) contained an F-actin ring, although it was generally misshapen or misplaced (left panel, arrows). (D) DAPI/calcofluor staining showed that whilst some cells in the population use the defective F-actin rings to generate septa (arrowheads), most do not and go through multiple cell cycles without cytokinesis. (B and C) Asynchronous early log phase mutant cultures were shifted from 25 to 36°C at t = 0 and stained with DAPI/calcofluor to score the septation index (upper panel) and frequency of cells with multiple nuclei and no septa (lower panel). (D and E) Representative micrographs of calcofluor staining of each culture 5 h (D) or 4 h (E) after the shift to 36°C. Bar, 2.5 µm.
Asynchronous single plo1.ts4 and cdc16.116, and double plo1.ts4 cdc16.116 cultures were grown to early log phase at 25°C before shifting the temperature to 36°C. The septation profile of the plo1.ts4 cdc16.116 mutant mirrored that of the single cdc16.116 mutant (Figure 8B and D). In contrast, cdc16.116 could not compensate for the ts– septation deficiency of the sin– mutant sid2.250 (Balasubramanian et al., 1998) (Figure 8C and E). Thus, independent SIN activation bypassed the septation defect of plo1.ts4.
plo1+ overexpression can only induce septation when the SIN is functional
While the data suggested that Plo1 is unlikely to act after Spg1 in the regulation of septation, Plo1 is required to use the actin ring for septation (Figure 8A and D). Thus, Plo1 either acts in concert with the SIN and is rendered redundant by strong activation of the SIN, or acts before the SIN in a progressive decision to septate. To differentiate between these possibilities, we asked whether an active SIN was required for the prolific septation that follows plo1+ overexpression (Ohkura et al., 1995).
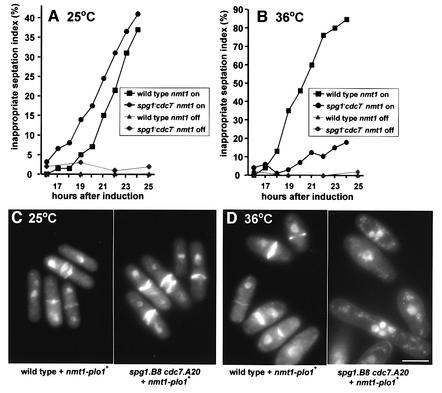
plo1+ expression was induced in an spg1.B8 cdc7.A20 double mutant or an isogenic wild-type culture for 15 h at 25°C. The culture was then split into two, and one half was maintained at 25°C while the other was incubated at 36°C. The accumulation of septa following plo1+ overexpression was unaffected by the spg1.B8 cdc7.A20 mutations at 25°C (Figure 9A and C). In contrast, inactivation of the SIN at 36°C severely compromised the ability of plo1+ overexpression to induce septation (Figure 9B and D). We conclude that plo1+ overexpression cannot overcome a defective SIN to induce septation and that Plo1 overproduction predominantly induces septation by activating the SIN.
Fig. 9. SIN function is required for the induction of septation by plo1+ overexpression. spg1.B8 cdc7.A20 pHN204 and an isogenic spg1+cdc7+ pHN204 strain were grown to early log phase in EMM2 + thiamine at 25°C, washed three times in EMM2 – thiamine, split into two, and one half was resuspended in EMM2 + thiamine while the other half was resuspended in EMM2 – thiamine to give early log phase cultures 15 h later. Each of the cultures was then further divided; one half was incubated at 25°C, the other at 36°C. DAPI/calcofluor staining (lower panels) identified inappropriate septation events (cells with multiple septa, or a single septum and a single nucleus). (A and C) 25°C; (B and D) 36°C. Although the double mutant displayed a minor septation defect at 25°C, the kinetics of septation following plo1+ induction was very similar to that in a wild-type background. All micrographs were captured and printed at the same scale. The widening of the cell girth during plo1+ overexpression may indicate that excess Plo1 inhibits the re-establishment of tip growth following mitosis (Mitchison and Nurse, 1985). This is consistent with genetic interactions between plo1ts and pom1 mutants (Bähler et al., 1998). Bar, 2.5 µm.
Discussion
We have used an in vitro kinase assay in cultures from wild-type and mutant strains to show that Plo1-associated kinase activity is very low in interphase cells and peaks during mitosis. This mitotic activation is likely to involve phosphorylation as the kinase activity was essentially abolished by phosphatase treatment. The activity of the kinase may not be required for its association with the SPB.
Polo kinase and mitotic commitment
Microinjection of antibodies to Plk into untransformed mammalian cells blocks mitotic commitment (Lane and Nigg, 1996), suggesting that Plks play a key role in regulating the onset of mitosis. The demonstration that Xenopus Plk, Plx1, binds, phosphorylates and activates Cdc25C (Kumagai and Dunphy, 1996) led to the proposal that Plx1 may act as the initial trigger to activate MPF via activation of Cdc25 (Kumagai and Dunphy, 1996; Qian et al., 1999). An alternative interpretation has been that Plx1 functions during the positive feedback loop to amplify this initial trigger MPF kinase to precipitate commitment to mitosis (Abrieu et al., 1998; Karaiskou et al., 1999). Two sets of data support the latter role. First, depletion of Plx1 from Xenopus oocytes delays mitotic commitment in a way that can be overcome by addition of excess Cdc25C (Qian et al., 1998a). Secondly, Plx1 activity is not required in Xenopus oocyte extracts for mitotic commitment per se; rather, its presence ensures abrupt non-linear kinetics of MPF activation (Karaiskou et al., 1999).
The lack of Plo1-associated kinase activity in cell-cycle-arrested cdc2.33 and cdc25.22 mutants argues against a role for Plo1 as the initial, MPF-activating, trigger kinase in S.pombe. If Plo1 were the primary trigger that initiates MPF activation, we would have expected Plo1 to be already active at these mutant arrest points. The dependency of Cdc25 activation upon prior activation of p34cdc2 in S.pombe (Kovelman and Russell, 1996) suggests that, like Xenopus, mitotic commitment in S.pombe is regulated by a positive feedback loop. Thus, if Plo1 does play a role in mitotic commitment in S.pombe, our data favour a role in this positive feedback loop (Figure 10A).
Fig. 10. Plo1 function during mitotic commitment and exit. Plo1 activation during mitotic commitment requires MPF activity. It is currently unclear whether Plo1 functions in the positive feedback loop that controls commitment to mitosis in S.pombe (A). Plo1 acts before the SIN. Some data suggest that the SIN may down-regulate Plo1 activity (B).
Mutation of the sole MPF consensus phosphorylation site in Plo1 did not block mitotic activation of Plo1, suggesting that MPF is unlikely to activate Plo1 by direct phosphorylation. Phosphorylation of Plo1 is, however, required to stimulate/maintain mitotic activity as treatment of mitotic Plo1 precipitates with λ phosphatase abolished kinase activity. The Ste20-related kinase xPlkk has been identified through its ability to phosphorylate and activate Plx1 (Qian et al., 1998b). Whilst several Ste20-related kinases can be found in many systems, including S.pombe (data not shown), it is currently unclear how universal regulation by xPlkk-related kinases will be and whether it applies to mitotic as well as meiotic systems.
In addition to promoting Plo1 activity by triggering a phosphorylation cascade, MPF may activate Plo1 by regulating partner or docking proteins. It may be significant in this respect that Plo1 is prematurely recruited to the interphase SPB in cells that contain a mutation in the SPB component Stf1/Cut12, which confers the ability to bypass the requirement for Cdc25 function (Bridge et al., 1998; Mulvihill et al., 1999).
Plo1 activity and mitotic progression
The profile of Plo1 activity in highly synchronous mitotic cultures suggested that there may be distinct phases in Plo1 activation or that discrete subpopulations execute distinct roles at different stages of mitosis. It may be significant that Cdc13 degradation started just after the first phase of Plo1 activation, and that the rate of Cdc13 destruction accelerated when Plo1-associated kinase activity peaked at metaphase. A similar biphasic breakdown of cyclin in S.cerevisiae has been attributed to successive activation of Cdc20 and Cdh1 (Yeong et al., 2000). The relationship between Plo1 activity and APC function awaits further analysis, but it is clear that, unlike mammalian Plk (Golsteyn et al., 1995; Hamanaka et al., 1995; Lee et al., 1995) and budding yeast Cdc5p (Charles et al., 1998; Shirayama et al., 1998), Plo1 is not degraded towards the end of mitosis (Mulvihill et al., 1998).
Plo1 acts before SIN in the regulation of septation
The central role played by the small GTP protein Spg1 in controlling septation has been effectively demonstrated by the prolific septation in interphase cells arising from Spg1 overproduction, or inactivation of its Cdc16/Byr4 GAP complex (Minet et al., 1979; Fankhauser et al., 1993; Fankhauser and Simanis, 1994; Song et al., 1996; Schmidt et al., 1997). Plo1 is also required for septum formation and can similarly drive septation in interphase cells when overproduced (Ohkura et al., 1995).
A number of observations suggested that the major role for Plo1 in regulating septation is executed before the SIN. First, inappropriate SIN activation in interphase cells did not stimulate Plo1-associated kinase activity and mitotic activation of the kinase was not affected by blocking SIN function. Thus, Plo1’s role in regulating septation is unlikely to lie downstream of the SIN. Secondly, Spg1 activation could induce septation in cells containing a plo1.ts mutation that compromised the use of the F-actin ring for septation. Finally, a functional SIN was required for the prolific septation following Plo1 overproduction. These data could suggest that Plo1 acts in concert with the SIN to induce septation, but that SIN activation can override the Plo1 requirement. Alternatively, Plo1 could induce septation by acting before the SIN and so be required for SIN activation. The recruitment of Cdc7 to the interphase SPB upon Plo1 overproduction (Mulvihill et al., 1999) favours the latter model and places Plo1 above the SIN in the regulation of septation (Figure 10B). Positioning Plo1 before the SIN also explains why no plo1 mutants were found among 514 sin– mutants in a screen for suppressors of a cdc16Δ strain and why a screen for mutants that required elevated levels of Plo1 for survival identified multiple sin genes (V.Simanis, personal communication; Cullen et al., 2000). It is now important to establish whether Plo1 directly phosphorylates a component of the Spg1 switch, or whether the effect is indirect, for example, by forcing the APC to prematurely target the destruction of a SIN inhibitor. It will also be important to study the feedback between the SIN and Plo1. Inactivation of Plo1-associated kinase activity during mitotic exit was less dramatic in spg1.B8 cdc7.A20 than in wild-type cells. Similarly, the affinity of Plo1 for the post-mitotic SPB is elevated in cdc7.A20 mutants (Mulvihill et al., 1999). A simple explanation for these observations is that the SIN may down-regulate Plo1 during mitotic exit (Figure 10).
Materials and methods
Cell culture and genetic manipulations
Schizosaccharomyces pombe cells were grown in EMM2 (Moreno et al., 1991). EMM2 was supplemented with 4 µM thiamine to repress genes regulated by the nmt1 promoter (Basi et al., 1993; Maundrell, 1993). Synchronous cultures were generated by centrifugal elutriation (Mulvihill et al., 1999).
Strains used are listed in Table I. The QuikChange site-directed mutagenesis kit (Stratagene) was used with the appropriate oligonucleotides and p41plo1.NHA (Mulvihill et al., 1999) as the substrate to mutate Lys69 to arginine (plo1.NHAK69R) and so create p41plo1.NHAK69R. plo1.NHAK69R was cloned into pINT541 and the construct was integrated in the genome at the leu1 locus to create IH1354. The same approach was used to generate IH1720 in which the plo1.NHAT14A mutant gene was expressed from the leu1 locus under the control of the same, intermediate strength nmt41 promoter. p41plo1.NPK and p41plo1.NPKK69R were created by replacing the AgeI–NdeI fragment of p41plo1.NHA and p41plo1.NHAK69R, respectively, with the AgeI–NdeI fragment from pREP41PkN (Craven et al., 1998). IH1847 was mated with IH1845 to generate haploid plo1.d1 spores, which harbored the p41plo1.NPKK69R plasmid. These spores were treated with helicase, 30% ethanol and washed before being germinated in EMM2 media containing adenine and histidine.
Table I. Strains used in this study.
| Strain number | Genotype | Source |
|---|---|---|
| IH164 | h– cdc2.33 | Nurse et al. (1976) |
| IH365 | h– ura4.d18 leu1.32 | laboratory stock |
| IH403 | h+/h– ade6-M210/ade6-M216 plo1.d1/plo1 leu1.32/leu1.32 ura4.d18/ura4.d18 | Ohkura et al. (1995) |
| IH634 | h– cdc25.22 ura4.d18 leu1.32 his2 | Nurse et al. (1976) |
| IH635 | h– cdc25.22 ura4.d18 | Nurse et al. (1976) |
| IH1297 | h– cdc16.116 ura4.d18 | Nurse et al. (1976) |
| IH1314 | h– leu1::pint5(ura4+)- plo1.NHA ura4.d18 | Mulvihill et al. (1999) |
| IH1354 | h– leu1::pint5(ura4+)- plo1.NHAK69R ura4.d18 | this study |
| IH1558 | h– cdc25.22 leu1::pint5(ura4+)- plo1.NHA ura4.d18 | this study |
| IH1559 | h– cdc25.22 leu1::pint5(ura4+)- plo1.NHAK69R ura4.d18 | this study |
| IH1720 | h+ leu1::pint5(ura4+)- plo1.NHAT14A ade6.M216 ura4.d18 | this study |
| IH1724 | h+ cdc25.22 leu1::pint5(ura4+)- plo1.NHAT14A ura4.d18 | this study |
| IH1740 | h– cdc7.A20 spg1.B8 leu1.32 ura4.d18 | this study |
| IH1750 | h– leu1.32 ura4.d18 pHN204 | this study |
| IH1751 | h– cdc7.A20 spg1.B8 leu1.32 ura4.d18 pHN204 | this study |
| IH1755 | h– plo1.GFPS65T | Bähler et al. (1999) |
| IH1829 | h– cdc16.116 sid2.250 ura4.d18 ade6-M216 | this study |
| IH1830 | h+ cdc16.116 ura4.d18 leu1.32 | this study |
| IH1831 | h– sid2.250 ura4.d18 | Balasubramanian et al. (1998) |
| IH1845 | h– ade6-M216 leu1.32 plo1.GFPS65T ura4.d18 | this study |
| IH1846 | h– ade6-M210 leu1.32 plo1.d1 ura4.d18 p41plo1.NHA | this study |
| IH1847 | h+/h+ ade6-M210/ade6-M216 plo1.d1/plo1.GFPS65T leu1.32/leu1.32 ura4.d18/ura4.d18 | this study |
| IH1857 | h+/h+ ade6-M210/ade6-M216 plo1.d1/plo1.GFPS65T leu1.32/leu1.32 ura4.d18/ura4.d18 p41plo1.NPK | this study |
| IH1858 | h+/h+ ade6-M210/ade6-M216 plo1.d1/plo1.GFPS65T leu1.32/leu1.32 ura4.d18/ura4.d18 p41plo1.NPKK69R | this study |
| IH1859 | h+/h+ ade6-M210/ade6-M216 plo1.d1/plo1.GFPS65T leu1.32/leu1.32 ura4.d18/ura4.d18 pREP81 | this study |
Biochemical manipulations
Soluble cell extracts were prepared and blotted as described previously (Mulvihill et al., 1999). Affinity-purified IH3 antibodies (Takeuchi and Yanagida, 1993) were used to detect Cdc13. For one kinase reaction, 4 × 108 cells were washed with 1 ml of STOP buffer and snap-frozen as pellets in liquid nitrogen to be stored at –80°C until use. Pellets were resuspended in 100 µl of IP buffer [50 mM HEPES pH 7.5, 100 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 µg/ml aprotinin, 1 µg/ml pepstatin, 1 µg/ml leupeptin, 20 mM β-glycerophosphate, 0.1 mM Na3VO4, 50 mM NaF] and disrupted by agitation with 1 ml of acid-washed glass beads in a Hybaid Ribolyser for 3 s at speed 6.5. Cell debris was removed from the cell extract by a 10 min spin in a microcentrifuge. Protein A–Sepharose beads (Amersham-Pharmacia) were washed with IP buffer. Ten microlitres of packed beads were loaded either with 5 µl of anti-Plo1 antisera (HN184; Ohkura et al., 1995) or 50 µg of anti-HA monoclonal antibody (12CA5; Boehringer Manheim) in 100 µl of IP buffer + 0.1 M lysine, 10 µg/ml bovine serum albumin at 4°C for 1 h. After four washes with IP buffer, 10 µl of beads were mixed with 100 µl of extract for 1 h at 4°C, washed twice with wash buffer A (50 mM HEPES pH 7.5, 500 mM NaCl, 1 mM EDTA, 1% Triton X-100), once with wash buffer B (50 mM HEPES pH 7.5, 1 mM EDTA) and once with CK buffer [20 mM HEPES pH 7.5, 150 mM KCl, 10 mM MgCl2, 2 mM dithiothreitol (DTT), 1 mM EGTA] and resuspended in 6.3 µl of CK buffer + 3 µCi of [γ-32P]ATP (5000 Ci/mmol; Amersham), 160 µM ATP, 0.5 µg/ml heparin, 7.5 µg casein. After incubation for 20 min at 30°C, 5 µl of sample buffer were added, the mix run on 10% SDS–PAGE and blotted to a nitrocellulose membrane. The membranes were probed with HN184 and developed with alkaline phosphatase-conjugated secondary antibodies. The relative level of Plo1 (Plo1L) in each lane was calculated by measuring the pixel density of a scanned membrane image with NIH image software. The radioactivity of casein (Plo1P) was quantified with a Fujix BAS2000 phosphoimager. Kinase activity per unit Plo1 in each reaction was calculated as Plo1P ÷ Plo1L. Histone H1 kinase assays were as described in Creanor and Mitchison (1996). Phosphatase treatment of HN184 immunocomplexes was initiated by two washes with pre-AP buffer [PAP; 50 mM Tris–HCl pH 7.5, 0.1 mM EDTA, 5 mM DTT, 1 mM PMSF, 15 mM p-nitrophenyl phosphate (pNPP)] or pre-API buffer (PAP + 10 mM Na3VO4, 50 mM NaF, 60 mM β-glycerophosphate). The beads were then resuspended in either AP buffer [50 mM Tris–HCl pH 7.5, 0.1 mM EDTA, 5 mM DTT, 0.01% Brij 35, 2 mM MnCl2, 15 mM pNPP, 100 U of λ phosphatase (New England Biolabs)] or API buffer (AP + 10 mM Na3VO4, 50 mM NaF, 50 mM EDTA, 60 mM β-glycerophosphate) and incubated at 30°C for 30 min. The beads were then washed four times with CKI (CK buffer + 10 mM Na3VO4, 50 mM NaF, 50 mM EDTA) and once in CK buffer. After resuspension in CK, the sample was split in two and half used for kinase assay while 15 mM pNPP was added to the other half. The lack of a pNPP reaction showed that phosphatase activity had not been carried through into the kinase reaction.
Fluorescence microscopy
DAPI staining of nuclei and calcofluor staining to score septation indices were as described in Moreno et al. (1991). Cells were fixed with 2.5% glutaraldehyde to score condensed chromosomes (Toda et al., 1981). Plo1 (with HN184), PK/GFP [with MAb336 (Serotec) and antibodies cited in Sawin et al., 1999] and tubulin [with TAT1 (Woods et al., 1989)] immunofluorescence and phalloidin staining were carried out according to Marks and Hyams (1985) and Mulvihill et al. (1999).
Acknowledgments
Acknowledgements
We thank Dr Viesturs Simanis for strains, Professor Keith Gull for TAT1, Dr K.Sawin for anti-GFP antibodies, Dr Viesturs Simanis, Hiroyuki Ohkura and colleagues in Manchester and Dr Ohkura’s group for stimulating discussions. This work was supported by Cancer Research Campaign (CRC) Grants SP2219/0102 and SP2219/0601.
References
- Abrieu A., Brassac,T., Galas,S., Fisher,D., Labbe,J.C. and Doree,M. (1998) The polo-like kinase Plx1 is a component of the MPF amplification loop at the G(2)/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci., 111, 1751–1757. [DOI] [PubMed] [Google Scholar]
- Bähler J., Steever,A.B., Wheatley,S., Wang,Y.L., Pringle,J.R., Gould,K.L. and McCollum,D. (1998) Role of polo kinase and Mid1p in determining the site of cell division in fission yeast. J. Cell Biol., 143, 1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M.K., McCollum,D., Chang,L., Wong,K.C.Y., Naqvi,N.I., He,X.W., Sazer,S. and Gould,K.L. (1998) Isolation and characterization of new fission yeast cytokinesis mutants. Genetics, 149, 1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian M.K., McCollum,D. and Surana,U. (2000) Tying the knot: linking cytokinesis to the nuclear cycle. J. Cell Sci., 113, 1503–1513. [DOI] [PubMed] [Google Scholar]
- Basi G., Schmid,E. and Maundrell,K. (1993) TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene, 123, 131–136. [DOI] [PubMed] [Google Scholar]
- Bridge A.J., Morphew,M., Bartlett,R. and Hagan,I.M. (1998) The fission yeast SPB component Cut12 links bipolar spindle formation to mitotic control. Genes Dev., 12, 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti L. and Simanis,V. (1999) Asymmetry of the spindle pole bodies and spg1p GAP segregation during mitosis in fission yeast. J. Cell Sci., 112, 2313–2321. [DOI] [PubMed] [Google Scholar]
- Cerutti L. and Simanis,V. (2000) Controlling the end of the cell cycle. Curr. Opin. Genet. Dev., 10, 65–69. [DOI] [PubMed] [Google Scholar]
- Chang F., Woollard,A. and Nurse,P. (1996) Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J. Cell Sci., 109, 131–142. [DOI] [PubMed] [Google Scholar]
- Charles J.F., Jaspersen,S.L., Tinker-Kulberg,R.L., Hwang,L., Szidon,A. and Morgan,D.O. (1998) The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr. Biol., 8, 497–507. [DOI] [PubMed] [Google Scholar]
- Craven R.A., Griffiths,D.J.F., Sheldrick,K.S., Randall,R.E., Hagan,I.M. and Carr,A.M. (1998) Vectors for the expression of tagged proteins in Schizosaccharomyces pombe. Gene, 221, 59–68. [DOI] [PubMed] [Google Scholar]
- Creanor J. and Mitchison,J.M. (1996) The kinetics of the B cyclin p56cdc13 and the phosphatase p80cdc25 during the cell cycle of the fission yeast Schizosaccharomyces pombe. J. Cell Sci., 109, 1647–1653. [DOI] [PubMed] [Google Scholar]
- Cullen C.F., May,K.M., Hagan,I.M., Glover,D.M. and Ohkura,H. (2000) A new genetic method for isolating functionally interacting genes: high plo1+-dependent mutants and their suppressors define genes in mitotic and septation pathways in fission yeast. Genetics, 155, 1521–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C. and Simanis,V. (1994) The Cdc7 protein kinase is a dosage-dependent regulator of septum formation in fission yeast. EMBO J., 13, 3011–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C., Marks,J., Reymond,A. and Simanis,V. (1993) The S. pombe cdc16 gene is required both for maintenance of p34cdc2 kinase activity and regulation of septum formation—a link between mitosis and cytokinesis. EMBO J., 12, 2697–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa,J., Broek,D., Macdonald,B., Rodgers,L., Wilson,I.A., Lerner,R.A. and Wigler,M. (1988) Purification of a ras-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition. Mol. Cell. Biol., 8, 2159–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furge K.A., Wong,K., Armstrong,J., Balasubramanian,M. and Albright,C.F. (1998) Byr4 and Cdc16 form a two component GTPase activating protein for the Spg1 GTPase that controls septation in fission yeast. Curr. Biol., 8, 947–954. [DOI] [PubMed] [Google Scholar]
- Glover D.M., Hagan,I.M. and Tavares,A. (1998) Polo kinases: a team that plays throughout mitosis. Genes Dev., 12, 3777–3787. [DOI] [PubMed] [Google Scholar]
- Golsteyn R.M., Mundt,K.E., Fry,A.M. and Nigg,E.A. (1995) Cell-cycle regulation of the activity and subcellular localization of Plk1, a human protein kinase implicated in mitotic spindle function. J. Cell Biol., 129, 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I. and Yanagida,M. (1997) Evidence for cell cycle-specific, spindle pole body-mediated, nuclear positioning in the fission yeast Schizosaccharomyces pombe. J. Cell Sci., 110, 1851–1866. [DOI] [PubMed] [Google Scholar]
- Hamanaka R., Smith,M.R., O’Connor,P.M., Maloid,S., Mihalic,K., Spivak,J.L., Longo,D.L. and Ferris,D.K. (1995) Polo-like kinase is a cell cycle-regulated kinase activated during mitosis. J. Biol. Chem., 270, 21086–21091. [DOI] [PubMed] [Google Scholar]
- Hanks S.K. and Hunter,T. (1995) The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J., 9, 576–596. [PubMed] [Google Scholar]
- Hoffmann I., Clarke,P.R., Marcote,M.J., Karsenti,E. and Draetta,G. (1993) Phosphorylation and activation of human Cdc25-C by Cdc2 cyclin-B and its involvement in the self-amplification of MPF at mitosis. EMBO J., 12, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T. and Maller,J.L. (1993) Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol. Biol. Cell, 4, 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T. and Maller,J. (1995) Phosphorylation and activation of Xenopus Cdc25 phosphatase in the absence of Cdc2 and Cdk2 kinase activity. Mol. Biol. Cell, 6, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaiskou A., Jessus,C., Brassac,T. and Ozon,R. (1999) Phosphatase 2A and Polo kinase, two antagonistic regulators of Cdc25 activation and MPF auto-amplification. J. Cell Sci., 112, 3747–3756. [DOI] [PubMed] [Google Scholar]
- Kovelman R. and Russell,P. (1996) Stockpiling of Cdc25 during a DNA-replication checkpoint arrest in Schizosaccharomyces pombe. Mol. Cell. Biol., 16, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J., Ashorn,C.L., Gonzalea-Kuyvenhoven,M. and Penkala,J.E. (1994) cdc25 is one of the MPM-2 antigens involved in the activation of maturation-promoting factor. Mol. Biol. Cell, 5, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy,W.G. (1996) Purification and molecular-cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science, 273, 1377–1380. [DOI] [PubMed] [Google Scholar]
- Lane H.A. and Nigg,E.A. (1996) Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol., 135, 1701–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff X., Utsig,S. and Simanis,V. (1999) Controlling septation in fission yeast: finding the middle and timing it right. Curr. Genet., 35, 571–584. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Yuan,Y.O., Kuriyama,R. and Erikson,R. (1995) Plk is an M-phase specific protein kinase and interacts with a kinesin-like protein. Mol. Cell. Biol., 15, 7143–7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. and Hyams,J.S. (1985) Localization of F-actin through the cell-division cycle of Schizosaccharomyces pombe. Eur. J. Cell Biol., 39, 27–32. [Google Scholar]
- Maundrell K. (1993) Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene, 123, 127–130. [DOI] [PubMed] [Google Scholar]
- Minet M., Nurse,P., Thuriaux,P. and Mitchison,M. (1979) Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe. J. Bacteriol., 137, 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison J.M. and Nurse,P. (1985) Growth in cell length in the fission yeast Schizosaccharomyces pombe. J. Cell Sci., 75, 357–376. [DOI] [PubMed] [Google Scholar]
- Moreno S., Hayles,J. and Nurse,P. (1989) Regulation of p34cdc2 protein kinase during mitosis. Cell, 58, 361–372. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar,A. and Nurse,P. (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol., 194, 795–823. [DOI] [PubMed] [Google Scholar]
- Mulvihill D.P., Petersen,J., Ohkura,H., Glover,D.M. and Hagan,I.M. (1999) Plo1 kinase recruitment to the spindle pole body and its role in cell division in Schizosaccharomyces pombe. Mol. Biol. Cell, 10, 2771–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E.A. (1993) Cellular substrates of p34(cdc2) and its companion cyclin-dependent kinases. Trends Cell Biol., 3, 296–301. [DOI] [PubMed] [Google Scholar]
- Nigg E.A. (1998) Polo-like kinases: positive regulators of cell division from start to finish. Curr. Opin. Cell Biol., 10, 776–783. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux,P. and Nasmyth,K. (1976) Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet., 146, 167–178. [DOI] [PubMed] [Google Scholar]
- Ohi R. and Gould,K. (1999) Regulating the onset of mitosis. Curr. Opin. Cell Biol., 11, 267–273. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Hagan,I.M. and Glover,D.M. (1995) The conserved Schizosaccharomyces pombe kinase Plo1, required to form a bipolar spindle, the actin ring and septum, can drive septum formation in G1 and G2 cells. Genes Dev., 9, 1059–1073. [DOI] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E., Li,C. and Maller,J.L. (1998a) Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol. Cell. Biochem., 18, 4262–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E. and Maller,J.L. (1998b) Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science, 282, 1701–1704. [DOI] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E. and Maller,J.L. (1999) Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol. Cell. Biol., 19, 8625–8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K., Hajibagheri,M.A.N. and Nurse,P. (1999) Mis-segregation of cortical identity in a fission yeast PAK mutant. Curr. Biol., 9, 1335–1338. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Sohrmann,M., Hofmann,K., Woolard,A. and Simanis,V. (1997) The Spg1 GTPase is an essential dosage-dependent inducer of septum formation in Schizosaccharomyces pombe. Genes Dev., 11, 1519–1534. [DOI] [PubMed] [Google Scholar]
- Shirayama M., Zachariae,W., Ciosk,R. and Nasmyth,K. (1998) The polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J., 17, 1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrmann M., Fankhauser,C., Brodbeck,C. and Simanis,V. (1996) The Dmf1/Mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev., 10, 2707–2719. [DOI] [PubMed] [Google Scholar]
- Sohrmann M., Schmidt,S., Hagan,I. and Simanis,V. (1998) Asymmetric segregation on spindle poles of the Schizosaccharomyces pombe septum-inducing protein kinase Cdc7p. Genes Dev., 12, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K., Mach,K.E., Chen,C.V., Reynolds,T. and Albright,C.F. (1996) A novel suppressor of ras1 in fission yeast, byr4, is a dosage-dependent inhibitor of cytokinesis. J. Cell Biol., 133, 1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M. and Yanagida,M. (1993) A mitotic role for a novel fission yeast protein kinase Dsk1 with cell-cycle stage dependent phosphorylation and localization. Mol. Biol. Cell, 4, 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Yamamoto,M. and Yanagida,M. (1981) Sequential alterations in the nuclear chromatin region during mitosis of the fission yeast Schizosaccharomyces pombe—video fluorescence microscopy of synchronously growing wild-type and cold-sensitive cdc mutants by using a DNA-binding fluorescent probe. J. Cell Sci., 52, 271–287. [DOI] [PubMed] [Google Scholar]
- Woods A., Sherwin,T., Sasse,R., Macrae,T.H., Baines,A.J. and Gull,K. (1989) Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci., 93, 491–500. [DOI] [PubMed] [Google Scholar]
- Yeong F.M., Lim,H.H., Padmashree,C.G. and Surana,U. (2000) Exit from mitosis in budding yeast: biphasic inactivation of Cdc28-Clb2 mitotic kinase and the role of Cdc20. Mol. Cell, 5, 501–511. [DOI] [PubMed] [Google Scholar]