Abstract
Vascular endothelial growth factor receptor-3 (VEGFR-3) has an essential role in the development of embryonic blood vessels; however, after midgestation its expression becomes restricted mainly to the developing lymphatic vessels. The VEGFR-3 ligand VEGF-C stimulates lymphangiogenesis in transgenic mice and in chick chorioallantoic membrane. As VEGF-C also binds VEGFR-2, which is expressed in lymphatic endothelia, it is not clear which receptors are responsible for the lymphangiogenic effects of VEGF-C. VEGF-D, which binds to the same receptors, has been reported to induce angiogenesis, but its lymphangiogenic potential is not known. In order to define the lymphangiogenic signalling pathway we have created transgenic mice overexpressing a VEGFR-3-specific mutant of VEGF-C (VEGF-C156S) or VEGF-D in epidermal keratinocytes under the keratin 14 promoter. Both transgenes induced the growth of lymphatic vessels in the skin, whereas the blood vessel architecture was not affected. Evidence was also obtained that these growth factors act in a paracrine manner in vivo. These results demonstrate that stimulation of the VEGFR-3 signal transduction pathway is sufficient to induce specifically lymphangiogenesis in vivo.
Keywords: angiogenesis/lymphangiogenesis/vascular endothelial growth factors (VEGFs)/VEGF receptors
Introduction
Growth of new blood and lymphatic vessels by the processes of angiogenesis and lymphangiogenesis requires the activation of specific signal transduction pathways in endothelial cells. These signals are at least in part mediated by members of the vascular endothelial growth factor (VEGF) family via their receptors (VEGFRs) on the surface of endothelial cells. The members of the mammalian VEGF family known to date are VEGF, placenta growth factor (PlGF), VEGF-B, VEGF-C and VEGF-D. They show significant identity at the level of amino acid sequence, but are strikingly different in terms of their mechanisms of regulation, expression patterns and receptor binding profiles (reviewed by Eriksson and Alitalo, 1999). The prototype VEGF regulates vasculogenesis, haematopoiesis and vascular permeability, and is implicated in many physiological and pathological processes (Ferrara, 1999). Like the VEGFs, the three known VEGFRs are differentially expressed. In adult tissues, VEGFR-1 and VEGFR-2 localize predominantly to blood vascular endothelial cells, whereas VEGFR-3 is expressed mainly in lymphatic endothelia (for references see Veikkola et al., 2000).
VEGF-C and VEGF-D, which are the only known ligands for VEGFR-3, are produced as precursor proteins with N- and C-terminal propeptides flanking the VEGF homology domain (VHD; Joukov et al., 1996; Lee et al., 1996; Orlandini et al., 1996; Yamada et al., 1997; Achen et al., 1998). The secreted factors undergo proteolytic processing, resulting in the cleavage of the propeptides and increased affinity for VEGFR-2 (Joukov et al., 1997; Stacker et al., 1999a). The fully processed or mature forms of VEGF-C and VEGF-D consist of the VHD, which acts as a ligand for both VEGFR-2 and VEGFR-3 (Joukov et al., 1997; Achen et al., 1998). Mature VEGF-C and VEGF-D are mitogenic and chemotactic for endothelial cells in culture and angiogenic in vivo (Lee et al., 1996; Joukov et al., 1997; Achen et al., 1998; Cao et al., 1998; Witzenbichler et al., 1998; Marconcini et al., 1999). Importantly, VEGF-C has been shown to induce lymphangiogenesis in transgenic mouse skin and in mature chick chorioallantoic membrane (Jeltsch et al., 1997; Oh et al., 1997). Replacing the second of the eight conserved, characteristically spaced cysteine residues in the VHD (Cys156) with a serine residue in recombinant VEGF-C resulted in a mutant factor (VEGF-C156S), which is a selective agonist of VEGFR-3 (Joukov et al., 1998). VEGF-C156S induced autophosphorylation of VEGFR-3 but not VEGFR-2 in transfected cells, but its activity in primary endothelial cells was not tested.
Transgenic overexpression of VEGF and VEGF-C has yielded important data on their vascular effects (Jeltsch et al., 1997; Detmar et al., 1998), but these studies have not clarified the roles of specific VEGFRs in angiogenesis versus lymphangiogenesis due to the fact that both VEGF and VEGF-C bind more than one receptor. On the other hand, the in vivo studies of VEGFR function have been hampered by the embryonic death of the knockout mice (Fong et al., 1995; Shalaby et al., 1995; Dumont et al., 1998). We wanted to assess the potential in vivo function of VEGF-D and to determine the role of VEGFR-3-specific signals in lymphangiogenesis. For these studies we generated transgenic mouse strains expressing VEGF-D or VEGF-C156S in the skin under the human keratin 14 (K14) promoter. Here we show that VEGF-D is lymphangiogenic. Importantly, stimulation of only VEGFR-3 by VEGF-C156S was sufficient for generating the hyperplastic lymphatic phenotype, demonstrating that a single receptor tyrosine kinase mediates signals sufficient for lymphatic vascular growth.
Results
Receptor specificity of VEGF-C156S and VEGF-D
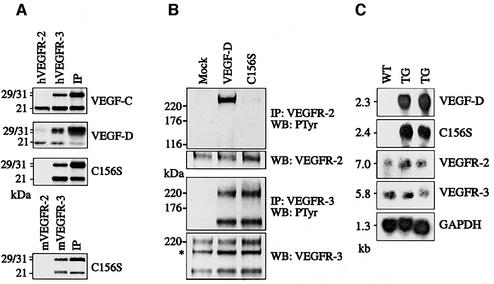
We have previously shown that human VEGF-C and VEGF-D bind to soluble human VEGFR-2 and VEGFR-3 (Joukov et al., 1997; Achen et al., 1998) and that VEGF-C156S only binds to human VEGFR-3 (Joukov et al., 1998). Human VEGF-D and VEGF-C156S were assessed for their ability to bind to mouse VEGFRs using soluble fusion proteins where the extracellular domains of mouse VEGFR-2 or VEGFR-3 are fused to the immunoglobulin (Ig) γ-chain Fc domain. VEGF-D was found to bind to both mouse VEGFR-2 and VEGFR-3, whereas VEGF-C156S and mouse VEGF-D bound only to mouse VEGFR-3 (Figure 1A; M.E.Baldwin, B.Catimel, E.Nice, S.Roufail, N.E.Hall, K.L.Stenvers, K.Alitalo, S.A.Stacker and M.G.Achen, in preparation). Therefore, the receptor binding patterns of human VEGF-D and VEGF-C156S are retained in mice. In order to test whether the receptor binding pattern is reflected in VEGFR activation by ligand-induced dimerization in primary dermal microvascular endothelial cells, we stimulated the cells with the purified factors followed by immunoprecipitation of VEGFR-2 and VEGFR-3 and anti-phosphotyrosine immunoblotting analysis. Tyrosyl autophosphorylation of VEGFR-2 was stimulated by VEGF-D but not by VEGF-C156S, whereas VEGFR-3 autophosphorylation was stimulated by both of these ligands (Figure 1B). The differential receptor binding of these factors thus leads to specific receptor activation in primary endothelial cells.
Fig. 1. Receptor specificity and expression of the transgene-encoded proteins. (A) Receptor binding analysis of metabolically labelled VEGF-C, VEGF-D and VEGF-C156S using soluble human or mouse VEGFR-2 and VEGFR-3. (B) Immunoprecipitation and phosphotyrosine analysis of VEGFR-2 and VEGFR-3 after stimulation of primary dermal microvascular endothelial cells with starvation medium, VEGF-D or VEGF-C156S. The asterisk denotes an intracellular VEGFR-3 precursor, which is not phosphorylated upon stimulation. (C) Transgene mRNA expression in K14-VEGF-D and K14-VEGF-C156S mice, and the levels of VEGFR-2 and VEGFR-3 mRNA were determined by northern blotting and hybridization of total skin RNA with the specific probes. WT, wild type; TG, transgenic mouse.
Generation and analysis of K14-VEGF-D and K14-VEGF-C156S mice
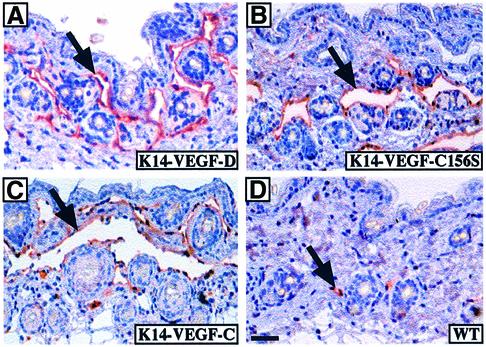
We next wanted to analyse the effects of VEGF-D and VEGF-C156S in vivo in transgenic mice. For this, human VEGF-D and VEGF-C156S cDNAs were cloned into the K14 vector, which directs transgene expression to the basal cells of the epidermis, and the expression constructs were injected into fertilized mouse oocytes. The resulting mouse lines were analysed for the expression of the transgene-encoded growth factors and their receptors, VEGFR-2 and VEGFR-3, by northern blotting of total skin RNA (Figure 1C). While both transgenes were abundantly expressed in the skin, the levels of VEGFR-2 and VEGFR-3 mRNAs were low and seemingly unaffected by transgene expression. K14-VEGF-D and K14-VEGF-C156S mice appeared healthy and their growth and reproductive rates were normal. Histological examination of the skin of both K14-VEGF-D and K14-VEGF-C156S mice revealed large spaces in the upper dermis lacking connective tissue elements, lined by a thin layer of endothelium but devoid of red blood cells (Figure 2). These spaces were reminiscent of the enlarged lymphatic vessels seen in K14-VEGF-C mice (Figure 2C; Jeltsch et al., 1997) and they were often found in the proximity of the hair follicles, where transgene expression was strongest.
Fig. 2. Lymphatic hyperplasia induced by transgene overexpression. Tissue sections from K14-VEGF-D (A), K14-VEGF-C156S (B), K14-VEGF-C (C) or wild-type (D) skin were immunostained for mouse VEGFR-3. Transgenic skin contains large spaces lined with a VEGFR-3-positive endothelium (A–C), whereas only a few lymphatic capillaries are seen in wild-type skin (D). Scale bar in (D) for (A–D), 50 µm.
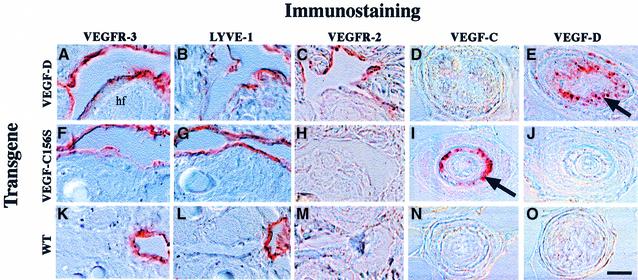
Immunohistochemical analysis revealed that the endothelial cells lining the large spaces were positive for the panendothelial marker platelet endothelial cell adhesion molecule-1 (PECAM-1) (not shown). These cells were also positive for VEGFR-3 and for lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) (Figure 3), both of which are known to provide relatively specific antigenic markers for the lymphatic endothelium in normal tissues (Jussila et al., 1998; Banerji et al., 1999; Partanen et al., 1999). Interestingly, in K14-VEGF-D mice the endothelium stained weakly positive for VEGFR-2, whereas corresponding structures in K14-VEGF-C156S and wild-type mice were VEGFR-2 negative (Figure 3C, H and M). Very weak staining for von Willebrand factor, and no staining for type IV collagen or smooth muscle actin (data not shown), confirmed the lack of blood endothelial markers, a basal lamina and a pericyte/smooth muscle cell layer, which are all hallmarks of blood vascular structures.
Fig. 3. Immunohistochemical analysis of transgenic skin. High magnification via Nomarski optics. Note that the VEGFR-3-positive endothelial cells associated with the hair follicles (hf) in both wild-type and transgenic skin are also positive for the lymphatic marker LYVE-1 (A, B, F, G, K and L). Also, the endothelium lining the hyperplastic vessels was positive for VEGFR-2 in the K14-VEGF-D mice (C), whereas the hyperplastic vessels of the K14-VEGF-C156S mice and wild-type lymphatic capillaries were negative (H and M). Hair follicles stained for VEGF-C (D, I and N) and VEGF-D (E, J and O). Scale bar in (O) for (A–O), 15 µm.
To verify transgene expression at the protein level, skin sections were stained using antibodies against VEGF-D and VEGF-C. In the hair follicles of K14-VEGF-D mice the outer root sheath cells stained positive for VEGF-D (Figure 3E, arrow), whereas no staining was observed in the hair follicles of K14-VEGF-C156S or wild-type mice (Figure 3J and O). In K14-VEGF-C156S mice, strong staining for VEGF-C was observed in the same cells (Figure 3I).
Selective hyperplasia of lymphatic, but not blood vessels in transgenic skin
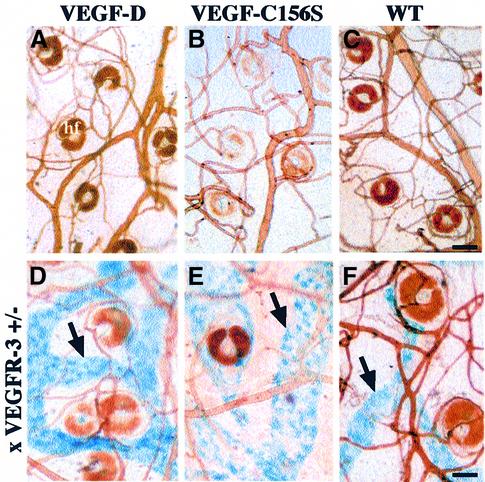
In order to analyse the dermal blood vascular phenotype of K14-VEGF-D and K14-VEGF-C156S mice, whole-mount tissue preparations of transgenic and wild-type ear skin were studied after vascular perfusion with biotin-labelled Lycopersicon esculentum lectin. When injected intravenously, this lectin binds to the surface of the endothelial cells allowing visualization of all blood vascular structures (Thurston et al., 1999). The blood vessels of K14-VEGF-D and K14-VEGF-C156S mice appeared normal in this analysis, as no differences from wild-type vessels could be found (Figure 4A–C). In order to visualize the lymphatic vessels in whole-mount preparations, the transgenic mice were crossed with heterozygous mutant VEGFR-3+/LacZ mice (Dumont et al., 1998). In these mice, one allele coding for VEGFR-3 has been disrupted by insertion of the LacZ coding sequence, allowing the localization of VEGFR-3 expression by staining for β-galactosidase activity, which gives a blue signal (Dumont et al., 1998). The dermal lymphatic vessels in K14-VEGF-D × VEGFR-3+/LacZ and K14-VEGF- C156S × VEGFR-3+/LacZ compound heterozygous mice were considerably enlarged in comparison with the lymphatic vasculature in VEGFR-3+/LacZ mice (Figure 4D–F). These phenotypes demonstrate that VEGF-D and the VEGFR-3-specific ligand VEGF-C156S induce selective hyperplasia of the lymphatic but not blood vasculature when overexpressed under the K14 promoter.
Fig. 4. Whole-mount analysis of skin blood and lymphatic vasculature. Blood vessels were visualized by injecting the mice intravenously with biotin-labelled L.esculentum lectin followed by vascular perfusion (A–F). Lymphatic vessels were stained blue (arrows) in the skin of K14-VEGF-D and K14-VEGF-C156S mice crossed with heterozygous mutant VEGFR-3+/LacZ mice (D–F). Scale bar in (C) for (A–C), 75 µm; in (F) for (D–F), 65 µm.
Functional analysis of the hyperplastic lymphatic vessels
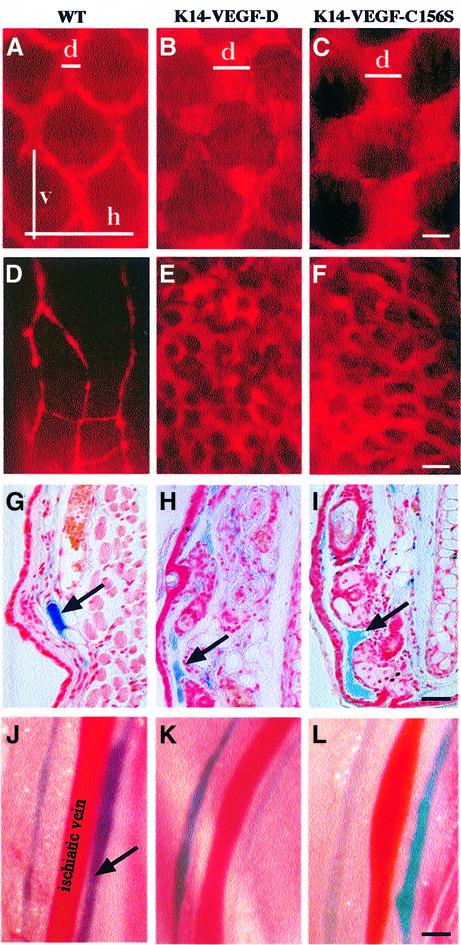
In order to determine whether the enlarged lymphatic capillaries in K14-VEGF-D and K14-VEGF-C156S skin were functional, we investigated lymphatic fluid uptake and transport in the skin after intradermal injections of TRITC-conjugated dextran, ferritin or Evans Blue dye. When fluorescent dextran was injected into the skin it was rapidly taken up by the superficial lymphatic capillary network in both normal and transgenic mice, as revealed by the fluorescence of these vessels in the tail and ear (Figure 5A–F). In K14-VEGF-D and K14-VEGF-C156S mice the lymphatic capillary network appeared vastly dilated when compared with the wild-type controls. To quantitate the degree of dilation, we measured the average lymphatic capillary diameter as well as the horizontal and vertical mesh sizes of the tail lymphatic capillary network in transgenic and wild-type mice. While the diameter of the lymphatic capillaries in K14-VEGF-D and K14-VEGF-C156S mice was approximately 3-fold larger than in wild-type mice, the horizontal and vertical mesh sizes were not significantly altered (Table I; see also Figure 5A–C).
Fig. 5. Functional analysis of the lymphatic vessels of normal and transgenic skin. The lymphatic capillaries were visualized by an intradermal injection of TRITC-conjugated dextran into the tail (A–C) or ear (D–F). Ferritin was used as a marker for lymphatic colloidal transport in histological sections from the ear (arrows in G–I). Lymphatic vessels adjacent to the ischiatic vein after injection of Evans Blue into the hind footpads (J–L). Scale bar in (C) for (A–C), 170 µm; in (F) for (D–F), 270 µm; in (I) for (G–I), 80 µm; and in (L) for (J–L), 1250 µm. d, diameter; h, horizontal mesh size; v, vertical mesh size.
Table I. Structural parameters of lymphatic vessel network in the tail.
| K14-VEGF-D | K14-VEGF-C156S | Wild type | |
|---|---|---|---|
| Diameter (mean µm ± SD) | 184.6 ± 20.8 | 168.6 ± 18.2 | 69.5 ± 25.9 |
| Horizontal mesh size (mean µm ± SD) | 600.0 ± 65.2 | 613.9 ± 64.7 | 591.7 ± 78.0 |
| Vertical mesh size (mean µm ± SD) | 393.6 ± 75.0 | 390.2 ± 49.3 | 369.7 ± 49.9 |
| No. of animals | 3 | 3 | 3 |
| P value (Mann–Whitney test) | 0.05 | 0.05 |
While the lymphatic capillaries of the tail were organized in a regular hexagonal pattern, in the ear they had a more variable architecture. Injections of fluorescent dextran into the ears of wild-type mice showed that normally the lymphatic capillaries are thin and relatively far apart from each other (see Figure 5D). However, in K14-VEGF-D and K14-VEGF-C156S mice the dextran spread more widely and diffusely within the skin. To understand whether the observed pattern reflects lymphatic vessel leakiness and thus spread of the marker thoughout ear interstitium, we injected ferritin intradermally into the ear. Ferritin can be used as a lymph marker and visualized in histological sections by staining with Prussian Blue (Leu et al., 2000). Microscopic observation revealed the blue iron compound to be contained within the lymphatic vessels in both transgenic and wild-type mice, demonstrating that the hyperplastic lymphatic capillaries of K14-VEGF-D and K14-VEGF-C156S mice are not abnormally leaky (Figure 5G–I). Staining of serial sections with endothelial markers revealed the presence of an intact lymphatic endothelium lining the lymphatic spaces in the transgenic mice (data not shown).
The superficial lymphatic capillaries of the skin drain via connecting vessels into the deeper collecting lymphatic channels. The route of lymphatic transport from the skin can be marked by an intradermal injection of Evans Blue dye and by following the appearance of the dye in the deep collecting lymphatic channels. Upon injection of Evans Blue into the hind limb footpads of wild-type, K14-VEGF-D or K14-VEGF-C156S mice the lymphatic vessels on both sides of the ischiatic vein were rapidly stained blue in all of the mice (Figure 5J–L). Therefore, fluid transport from the skin did not appear to be impaired in K14-VEGF-D and K14-VEGF-C156S mice despite the hyperplasia of the superficial lymphatic capillaries.
Paracrine effects of the K14-VEGF-D and K14-VEGF-C156S transgene products
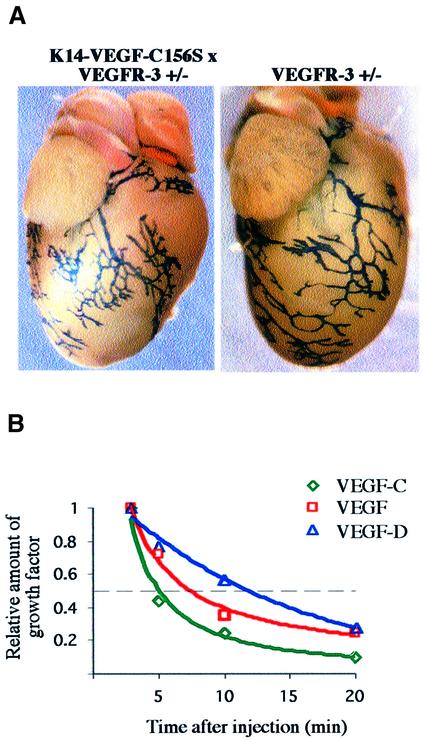
To find out whether transgenic overexpression of VEGF-D and VEGF-C156S had effects beyond the skin, internal organs in the compound heterozygous K14-VEGF-D × VEGFR-3+/LacZ and K14-VEGF-C156S × VEGFR-3+/LacZ mice were stained for β-galactosidase activity. When the whole-mount lymphatic staining patterns of various internal organs were compared with VEGFR-3+/LacZ mice, no obvious differences were found. As an example, Figure 6A shows the pericardial lymphatic vessels of a K14-VEGF-C156S × VEGFR-3+/LacZ and a VEGFR- 3+/LacZ mouse. Although variable in architecture, there were no consistent differences in these vessels between the normal and transgenic mice.
Fig. 6. Lack of a visceral phenotype and a short systemic half-life of transgene encoded proteins. (A) Pericardial lymphatic vessels of a K14-VEGF-C156S × VEGFR-3+/LacZ compound heterozygous and a VEGFR-3+/LacZ mouse were analysed by β-galactoside staining. (B) Relative amounts of VEGF-C, VEGF-D and VEGF in mouse circulation as a function of time after an intravenous injection of recombinant protein. The dashed line denotes 1/2 of the maximum growth factor concentration just after injection.
One explanation for the absence of an internal organ phenotype was to assume that effective concentrations of VEGF-D or VEGF-C156S did not reach these target organs. To determine whether the K14-transgene product can be detected in mouse circulation, we compared serum concentrations of VEGF-D in K14-VEGF-D and wild- type mice of different ages by a specific enzyme-linked immunosorbent assay (ELISA). The antigen could not be detected in any of the samples, although the detection level of this assay as determined by the use of purified recombinant protein was <0.1 pM. In transgenic skin lysates the average VEGF-D concentration was 4.5 pM, whereas no VEGF-D could be detected in the skin of age-matched control mice. As reference tissues, we tested lysates from transgenic and wild-type heart and lung, but no differences between wild-type and transgenic mice were detected.
To measure the systemic half-life of recombinant VEGF-C and VEGF-D proteins, anaesthetized wild-type mice were given an intravenous injection of 1 µg of VEGF-D or VEGF-C, and blood was drawn at different time points after the injection. As determined by ELISA, the half-life of both VEGF-D and VEGF-C in mouse circulation was <15 min (Figure 6B), and by 30 min the factors had been cleared below the assay detection level. For comparison, we also injected recombinant VEGF into the circulation and measured its clearance rate. VEGF disappeared in a similar manner to VEGF-D and VEGF-C with a half-life of ∼8 min and complete clearance by 30 min (Figure 6B). Co-injections of VEGF-C and VEGF-D, or VEGF-C together with VEGF, yielded similar clearance rates to those for the single factors (data not shown). As the total blood volume in mice constitutes 5–6% of body weight (1–1.5 ml in mice of 20–25 g), the blood concentrations of the factors after an injection of 1 µg of recombinant protein should range from 16 to 23 nM, i.e. to be well above the reported binding constants of VEGF-D and VEGF-C to VEGFR-2 (560 and 410 pM, respectively) and to VEGFR-3 (200 and 135 pM; Joukov et al., 1997; Stacker et al., 1999a). The injected ligand can thus be considered to saturate the receptor, and the clearance of VEGF-D and VEGF-C from the circulation after factor co-injection was thus probably not due to binding to their specific receptors on blood vessel endothelia. In conclusion, the lymphatic hyperplasia phenotype may be restricted to the skin due to the rapid clearance of the transgene products from the circulation via a receptor-independent mechanism.
Soluble VEGFR-3 blocks the lymphatic hyperplasia in the skin
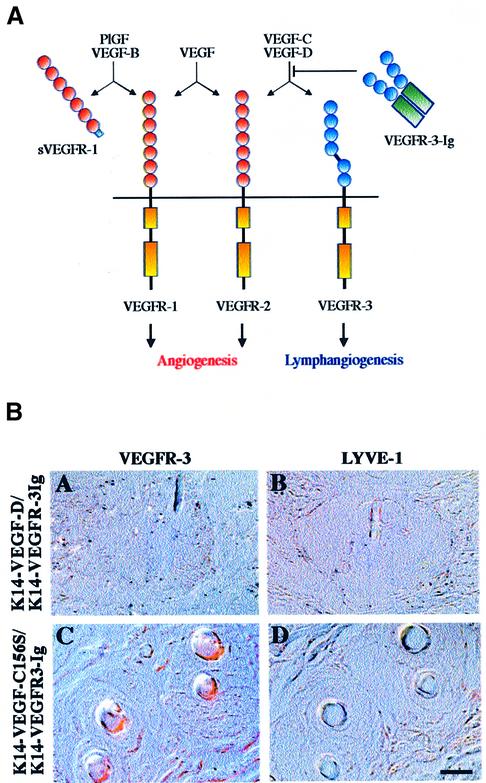
Recombinant soluble VEGFR-1 or VEGFR-2 can inhibit both physiological and pathological angiogenesis, such as retinal neovascularization, corpus luteum angiogenesis or tumour growth (Aiello et al., 1995; Ferrara et al., 1998; Goldman et al., 1998; Kong et al., 1998; Lin et al., 1998; Takayama et al., 2000). We wanted to test whether the lymphatic hyperplasia in the skin of K14-VEGF-D and K14-VEGF-C156S mice can be neutralized by soluble VEGFR-3. We therefore mated the mice with K14-VEGFR-3-Ig transgenic mice expressing a soluble chimeric protein consisting of the ligand binding portion of the extracellular part of VEGFR-3 joined to the Fc domain of Ig γ-chain (Figure 7A; Makinen et al., 2001). When the skin of the double transgenic mice was examined histologically, lymphatic vessels were no longer seen although transgene expression remained high, as confirmed by northern blotting (Figure 7B and data not shown). Therefore, the soluble VEGFR-3 is capable of inhibiting VEGF-D- and VEGF-C156S-induced lymphatic hyperplasia in vivo.
Fig. 7. Lymphatic hyperplasia is neutralized in double transgenic mice expressing soluble VEGFR-3-Ig. (A) Schematic presentation of the binding patterns of the transgene-encoded proteins and endogenous VEGF-VEGFR family members in the skin. (B) Immunohistochemical staining of K14-VEGF-D × VEGFR-3-Ig and K14-VEGF-C156S × VEGFR-3-Ig double transgenic skin for VEGFR-3 and LYVE-1 (A–D). Note the lack of lymphatic vessels. Scale bar in (D) for (A–D), 15 µm.
Discussion
Until now, VEGF-C has been the only growth factor known to target the lymphatic vascular compartment in vivo. VEGF-D is closely related to VEGF-C in structure, and together they are the only known natural ligands for VEGFR-3 (Joukov et al., 1996; Achen et al., 1998). Despite the fact that VEGF-D binds to VEGFR-2 and has been reported to be angiogenic (Marconcini et al., 1999), transgenic overexpression of VEGF-D led to lymphatic hyperplasia but no angiogenesis. In addition, the VEGFR-3-selective mutant factor VEGF-C156S also induced lymphatic hyperplasia, showing that the necessary and sufficient signals for the growth of lymphatic vessels are transduced via VEGFR-3.
It is unlikely that the lymphatic hyperplasia in K14-VEGF-D and K14-VEGF-C156S mice would be a secondary consequence of an increased production of lymphatic fluid in response to increased microvascular permeability, as both VEGF-D and VEGF-C156S have been reported to be inactive in the Miles vascular permeability assay (Joukov et al., 1998; Stacker et al., 1999b). The hyperplasia appeared to result from both increased endothelial cell proliferation in the existing lymphatic vessels and from the formation of additional lymphatic vessels, as we observed greater numbers of enlarged lymphatic capillaries in the ear skin of transgenic mice when compared with wild-type mice. This is consistent with the result from the K14-VEGF-C mice where the lymphatic hyperplasia was associated with an increased endothelial cell proliferation rate (Jeltsch et al., 1997).
The enlarged lymphatic capillaries were located in the upper dermis in association with the hair follicles. This localization correlates well with the published K14 promoter expression in the basal cell layer of the epidermis and in the outer root sheaths of the hair follicles (Byrne et al., 1994). The lymphatic nature of these vessels was confirmed by their staining for both LYVE-1 and VEGFR-3, two antigenic markers for the lymphatic endothelium (Jussila et al., 1998; Banerji et al., 1999). Importantly, blood vessels in K14-VEGF-D and K14-VEGF-C156S mice were VEGFR-3 negative. Our results thus indicate that VEGF-D and VEGF-C156S retain their lymphatic specificity in vivo. Interestingly, the hyperplastic lymphatic capillaries of K14-VEGF-D mice were weakly positive for VEGFR-2, whereas those of K14-VEGF-C156S mice and the lymphatic capillaries of wild-type mice expressed no VEGFR-2. This result may reflect a positive feedback loop where VEGFR-2, which is normally expressed only in trace amounts in the endothelia of the lymphatic capillaries, is upregulated upon ligand binding and receptor activation.
High-resolution, whole-mount imaging techniques were used to study the blood vessel morphology of K14-VEGF-D and K14-VEGF-C156S mice, but no differences between wild-type and transgenic mice were found. Even though VEGFR-3 is expressed in blood vessel endothelia early in embryonic development, by embryonic day 13.5–14.5 when the K14 promoter expression becomes widespread in the skin keratinocytes (Byrne et al., 1994), VEGFR-3 has already been downregulated in blood vessels (Kaipainen et al., 1995). In concert with the VEGFR-3 developmental expression pattern, the binding sites of VEGF and VEGF-C in mouse embryos overlap early in the development, but diverge to target the blood vascular and lymphatic compartments, respectively, as the lymphatic vessels develop (Lymboussaki et al., 1999). VEGFR-3 has been shown to be upregulated on blood vascular endothelium in tumours and in chronic wounds (Partanen et al., 1999; Valtola et al., 1999; Paavonen et al., 2000). In these pathological conditions and in tissue ischaemia, VEGF-C and VEGF-D could thus promote angiogenesis. Tissue proteolytic activity in such processes is also enhanced, possibly contributing to the proteolytic processing of both VEGF-C and VEGF-D and formation of their VEGFR-2 binding forms (Achen et al., 1998; Stacker et al., 1999a). The lack of blood vascular effects of VEGF-D in the present model could thus result from both its incomplete proteolytic processing in the skin and from the low levels of VEGFR-2 present on quiescent blood vascular endothelium (our unpublished data).
The in vivo morphology of the K14-VEGF-D and K14-VEGF-C156S lymphatic capillaries was abnormal. In the tail, the characteristic honeycomb-like pattern was preserved, but the transgenic vessel diameter was approximately three times that of the wild-type mice. In the ears of K14-VEGF-D and K14-VEGF-C156S mice, the superficial lymphatic vascularity was increased in comparison with wild-type mice. The hyperplastic lymphatic capillaries were, however, functional, in that they were capable of liquid uptake and transport to the deep collecting lymphatic vessels. Also, these hyperplastic vessels did not appear to be abnormally leaky, as shown by the ferritin transport experiments.
In contrast to the superficial lymphatic vessels, the collecting vessels and visceral lymphatic vessels of K14-VEGF-D and K14-VEGF-C156S mice were seemingly unaffected by transgene overexpression, and no transgene-encoded growth factors could be detected circulating in the bloodstream. The absence of the growth factors in the circulation possibly results from their distribution to the pericellular matrix of the dermis and from their short systemic half-lives, probably due to clearance by the liver, the spleen and the kidneys, as determined by analysis of iodinated VEGF and VEGF-C injected into rat circulation (K.Paavonen and K.Alitalo, unpublished results). Lack of systemic vascular effects in K14-VEGF-D and K14-VEGF-C156S mice suggests that these molecules could be useful in the development of local gene therapy. Local induction of lymphatic capillary growth is of clinical interest as failure to regenerate lymphatic vessels (e.g. after surgery) results in secondary lymphoedema. Targeted delivery of VEGF-D or VEGF-C156S by gene transfer could perhaps also be used to induce lymphatic regeneration after injury.
Primary lymphoedema, a rare early-onset autosomal dominant disorder of the lymphatic system, was recently linked to mutations in the VEGFR-3 tyrosine kinase domain (Karkkainen et al., 2000). Disruption of VEGFR-3 signalling by receptor inactivating mutations or by soluble VEGFR-3 results in lymphatic hypoplasia, underlining the importance of VEGFR-3-mediated signals for the maintenance of lymphatic function after embryonic development (Karkkainen et al., 2000; Makinen et al., 2001). Other growth factor/receptor families are also likely to participate in the formation and maintenance of the lymphatic vessels. The Tie-1 receptor tyrosine kinase was detected on the hyperplastic lymphatic capillaries of K14-VEGF-C mice (Jeltsch et al., 1997), and targeted disruption of the angiopoietin-2 gene in mice results in a complete absence of the lymphatic vessels (C.Suri, M.Witte and G.Yancopoulos, personal communication). However, our results demonstrate for the first time that VEGFR-3 signalling alone is sufficient for the generation of all the necessary secondary signals for the induction of growth of functional lymphatic vessels in vivo. One important conclusion from these results is thus that specific inhibitors of VEGFR-2 that are used to block angiogenesis (see Veikkola et al., 2000) may not suffice to inhibit lymphangiogenesis and possibly associated metastasis in human tumours (Mandriota et al., 2001; Skobe et al., 2001; Stacker et al., 2001).
Materials and methods
Receptor binding and activation analysis
293T cells were transfected with expression constructs encoding human full-length VEGF-C, VEGF-D or VEGF-C156S using the calcium phosphate precipitation method. Twenty-four hours after transfection the cells were washed twice with phosphate-buffered saline (PBS) and incubated in methionine- and cysteine-deficient MEM for 60 min. The cells were then pulse-labelled for 30 min in medium containing 100 µCi/ml [35S]Met/[35S]Cys (Promix, Amersham), subsequently washed with PBS and chased in Dulbecco’s modified Eagle’s medium supplemented with 0.2% bovine serum albumin (BSA) and 0.2 mM each of non-radioactive cysteine and methionine. After a chasing period of 8 h, the conditioned medium was harvested, supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF), 4 µg/ml leupeptin and 0.1 U/ml aprotinin, and cleared by centrifugation. Endogenous VEGF was depleted using monoclonal anti-human VEGF antibodies (R&D Systems) and protein G–Sepharose. The binding of VEGF-C, VEGF-D and VEGF-C156S to the human VEGF receptors was assessed by precipitation using soluble recombinant proteins consisting of the first three Ig-like loops of VEGFR-2 or VEGFR-3 fused to the Fc portion of human IgG (Achen et al., 1998). The fusion proteins (200 ng) were incubated with 1 ml of the pulse–chase-labelled conditioned medium at +4°C for 2 h in the binding buffer (0.5% BSA, 0.02% Tween-20 and 1 µg/ml heparin). The complexes were then precipitated with protein A– Sepharose and washed twice with the binding buffer and once with 20 mM Tris pH 7.4. The bound proteins were analysed by SDS–PAGE. The binding of human VEGF-C156S to mouse receptors was tested using 200 ng of recombinant soluble mouse VEGFR-2 (R&D Systems) or 1 ml of conditioned medium from cells transfected with a construct encoding the first three Ig-like loops of mouse VEGFR-3 fused to the Fc portion of IgG.
Receptor stimulation was carried out using passage 4–6 human dermal microvascular endothelial cells (Promocell). Subconfluent cells were starved overnight in microvascular endothelial cell growth medium (Promocell) containing hydrocortisone (1 µg/ml), gentamycin sulfate (50 µg/ml), amphotericin B (50 ng/ml) and 0.2% BSA. The cells were stimulated with 1 µg/ml VEGF-D or VEGF-C156S for 10 min at +37°C, washed twice with ice-cold PBS containing 100 µM sodium orthovanadate and lysed with RIPA buffer (10 mM Tris pH 7.4, 50 mM NaCl, 0.5% sodium deoxycholate, 0.5% NP-40, 0.1% SDS) containing 1 mM sodium orthovanadate, 1 mM PMSF and 0.1 U/ml aprotinin. The lysates were sonicated, clarified by centrifugation at +4°C, and immunoprecipitated with antiserum specific for VEGFR-2 (a kind gift from Lena Claesson-Welsh) or monoclonal antibodies against VEGFR-3 (Jussila et al., 1998). Western blotting analysis was carried out using PY20 phosphotyrosine-specific monoclonal antibodies (Transduction Laboratories) and the ECL method, followed by stripping and analysis using receptor-specific antibodies.
Generation of transgenic mice and northern blot analysis
The cDNAs encoding full-length human VEGF-D and VEGF-C156S were cloned into a human K14 promoter expression cassette (Vassar et al., 1989) and injected into fertilized mouse oocytes of the strain FVB/NIH. Several independent inbred lines of transgenic mice were generated and those lines with high levels of transgene mRNA expression were used for the study. In the transgenic mouse lines used there was only one site of transgene insertion in the genome, and the phenotype had 100% penetrance.
Total RNA from the back skin of wild-type, K14-VEGF-D and K14-VEGF-C156S mice was extracted using the RNeasy kit (Qiagen). RNA (10 µg) was electrophoresed in agarose gels containing formaldehyde, blotted and hybridized with human VEGF-D or VEGF-C156S, mouse VEGFR-2 or mouse VEGFR-3 cDNA probes.
Analysis of blood and lymphatic vessels
For immunohistochemistry, biopsies from the back, ear and internal organs of the transgenic mice were fixed in 4% paraformaldehyde (PFA), dehydrated and embedded in paraffin. After rehydration and microwave treatment for antigen retrieval, 5 µm sections were stained for VEGFR-3 and VEGFR-2 (Kubo et al., 2000), VEGF-C (Joukov et al., 1998), VEGF-D (R&D Systems), LYVE-1 (Banerji et al., 1999), PECAM-1 (Pharmingen), von Willebrand factor (DAKO), α-smooth muscle actin (Sigma) or collagen IV (Chemicon).
Lectin staining was used to visualize blood vessels in whole-mount tissue preparations (Thurston et al., 1999). One hundred microlitres of 1 mg/ml biotinylated L.esculentum lectin (Sigma) were injected intra venously via the femoral vein into anaesthesized mice and allowed to circulate for 2 min. The mouse was then sacrificed and the tissues were fixed by perfusion with 1% PFA/0.5% glutaraldehyde in PBS. The ears were dissected, washed with PBS, and the cartilage was removed. Bound lectin was visualized by the ABC-DAB peroxidase method, the ears were mounted onto slides and examined by light microscopy. In K14-VEGF-D × VEGFR-3+/LacZ and K14-VEGF-C156S × VEGFR-3+/LacZ compound heterozygous mice blood vessels were visualized as above. Lymphatic vessels were then visualized by incubating the fixed tissues in the β-galactosidase substrate X-Gal (Sigma) followed by dehydration and mounting.
Studies on lymphatic transport
Microlymphography was performed to visualize the lymphatic network in the ear and tail. TRITC-dextran (mol. wt 2000 kDa; Sigma) was injected into the tip of the tail using a 30 gauge needle, and the progressive staining of the lymphatic network was followed by fluorescence microscopy and photographed.
Ferritin (type I ferritin from horse spleen, mol. wt 480 kDa; Sigma) was injected into the ear. Forty-five minutes after injection the mouse was sacrificed and the ears were prepared for histology. The iron component of ferritin was visualized in histological sections by potassium ferrocyanide/HCl (Prussian Blue) followed by counterstaining.
A bolus of 5 mg/ml Evans Blue (Sigma) in PBS was injected intradermally into the hind footpads of mice. The skin from the lateral surface of the hind limb was removed to expose the ischiatic vein. Transport of the dye through the lymphatic vessels along the ischiatic vein was followed under direct microscopic observation and photographed.
The morphometric parameters of the transgenic and wild-type lymphatic capillaries were determined after visualization by an injection of fluorescent dextran into the tail. Lymphatic capillary diameter, as well as the horizontal and vertical mesh sizes of the lymphatic network, was measured from appropriate photomicrographs, and statistically significant variation from wild type was determined by the Mann–Whitney test.
ELISA
For determination of VEGF-D from serum samples, MaxiSorp plates (Nunc) were coated overnight at +4°C with 2.5 µg/ml monoclonal anti-human VEGF-D (R&D Systems) in PBS. The wells were blocked with 5% BSA, 0.05% Tween-20 in PBS for 30 min at room temperature (RT). Serum samples were diluted in 5 mg/ml BSA, 0.05% Tween-20 in PBS and incubated in the wells for 1 h at RT, the wells were washed three times with incubation buffer, and 2.5 µg/ml biotinylated rabbit polyclonal anti-VEGF-D antibody (R&D Systems) was added for 1 h at RT. After washes as above, the wells were incubated with ZyMax Streptavidin-Alkaline Phosphatase (Zymed) at 300 ng/ml for 30 min at RT, washed, and the 4-nitrophenyl phosphate (4-NPP) substrate (Roche) at 1 mg/ml in diethanolamine pH 10.3 was added. Optical density was read at 405 nm.
For quantification of VEGF-D in transgenic tissues, age-matched transgenic and wild-type mice were sacrificed and the hair of back skin was removed. Skin, lung and heart were snap-frozen in liquid nitrogen, pulverized using a dismembranator and lysed in 20 mM Tris pH 7.4, 1 mM EDTA, 50 mM NaCl, 50 mM NaF, 1% Triton X-100, 1 mM PMSF, 4 µg/ml leupeptin and 0.1 U/ml aprotinin. Equal amounts of total protein were used for the ELISA, which was performed as above.
The half-lives of the purified factors in blood circulation were estimated by giving anaesthesized mice an intravenous injection of 1 µg of VEGF-D, VEGF-C or VEGF165 in 100 µl of PBS via the femoral vein. Blood was drawn at different time points after the injection and VEGF-D levels were analysed by ELISA as above. For VEGF-C determination, MaxiSorp plates were coated overnight with 15 µg/ml monoclonal anti-human VEGF-D (clone VD4, cross-reacts with VEGF-C; Achen et al., 2000), and 8 µg/ml rabbit anti-human VEGF-C (Joukov et al., 1997) was used for detection. For VEGF determination, Quantikine human VEGF colorimetric sandwich ELISA (R&D Systems) was used.
Acknowledgments
Acknowledgements
We thank Eija Koivunen, Pipsa Ylikantola, Riikka Kivirikko, Tapio Tainola, Sanna Karttunen and Sirke Haaka-Lindgren for excellent technical assistance. The K14 expression vector was a kind gift from Dr Elaine Fuchs. Dr David G.Jackson provided the LYVE-1 antibody. This study was supported by grants from the Ida Montini Foundation, the Finnish Cultural Foundation, the Foundation of the Finnish Cancer Institute, the Paolo Fondation, the Finnish Academy of Sciences and the Novo Nordisk Foundation.
References
- Achen M.G., Jeltsch,M., Kukk,E., Makinen,T., Vitali,A., Wilks,A.F., Alitalo,K. and Stacker,S.A. (1998) Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl Acad. Sci. USA, 95, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achen M.G. et al. (2000) Monoclonal antibodies to vascular endothelial growth factor-D block its interactions with both VEGF receptor-2 and VEGF receptor-3. Eur. J. Biochem., 267, 2505–2515. [DOI] [PubMed] [Google Scholar]
- Aiello L.P., Pierce,E.A., Foley,E.D., Takagi,H., Chen,H., Riddle,L., Ferrara,N., King,G.L. and Smith,L.E. (1995) Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl Acad. Sci. USA, 92, 10457–10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S., Ni,J., Wang,S.-X., Clasper,S., Su,J., Tammi,R., Jones,M. and Jackson,D.G. (1999) LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol., 144, 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C., Tainsky,M. and Fuchs,E. (1994) Programming gene expression in developing epidermis. Development, 120, 2369–2383. [DOI] [PubMed] [Google Scholar]
- Cao Y., Linden,P., Farnebo,J., Cao,R., Eriksson,A., Kumar,V., Qi,J.-H., Claesson-Welsh,L. and Alitalo,K. (1998) Vascular endothelial growth factor C induces angiogenesis in vivo. Proc. Natl Acad. Sci. USA, 95, 14389–14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmar M. et al. (1998) Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J. Invest. Dermatol., 111, 1–6. [DOI] [PubMed] [Google Scholar]
- Dumont D., Jussila,L., Taipale,J., Mustonen,T., Pajusola,K., Breitman,M. and Alitalo,K. (1998) Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science, 282, 946–949. [DOI] [PubMed] [Google Scholar]
- Eriksson U. and Alitalo,K. (1999) Structure, expression and receptor-binding properties of novel vascular endothelial growth factors. Curr. Top. Microbiol. Immunol., 237, 41–57. [DOI] [PubMed] [Google Scholar]
- Ferrara N. (1999) Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int., 56, 794–814. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Chen,H., Davis-Smyth,T., Gerber,H.-P., Nguyen,T.-N., Peers,D., Chisholm,V., Hillan,K.J. and Schwall,R.H. (1998) Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nature Med., 4, 336–340. [DOI] [PubMed] [Google Scholar]
- Fong G.H., Rossant,J., Gertsenstein,M. and Breitman,M.L. (1995) Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature, 376, 66–70. [DOI] [PubMed] [Google Scholar]
- Goldman C.K. et al. (1998) Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastasis and mortality rate. Proc. Natl Acad. Sci. USA, 95, 8795–8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch M. et al. (1997) Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science, 276, 1423–1425. [DOI] [PubMed] [Google Scholar]
- Joukov V., Pajusola,K., Kaipainen,A., Chilov,D., Lahtinen,I., Kukk,E., Saksela,O., Kalkkinen,N. and Alitalo,K. (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J., 15, 290–298. [PMC free article] [PubMed] [Google Scholar]
- Joukov V., Sorsa,T., Kumar,V., Jeltsch,M., Claesson-Welsh,L., Cao,Y., Saksela,O., Kalkkinen,N. and Alitalo,K. (1997) Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J., 16, 3898–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V., Kumar,V., Sorsa,T., Arighi,E., Weich,H., Saksela,O. and Alitalo,K. (1998) A recombinant mutant vascular endothelial growth factor-C that has lost vascular endothelial growth factor receptor-2 binding, activation and vascular permeability activities. J. Biol. Chem., 273, 6599–6602. [DOI] [PubMed] [Google Scholar]
- Jussila L. et al. (1998) Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res., 58, 1599–1604. [PubMed] [Google Scholar]
- Kaipainen A., Korhonen,J., Mustonen,T., van Hinsbergh,V.M., Fang,G.-H., Dumont,D., Breitman,M. and Alitalo,K. (1995) Expression of the fms-like tyrosine kinase FLT4 gene becomes restricted to endothelium of lymphatic vessels during development. Proc. Natl Acad. Sci. USA, 92, 3566–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen M., Ferrell,R.E., Lawrence,E.C., Kimak,M.A., Levinson,K.L., McTigue,M.A., Alitalo,K. and Finegold,D.N. (2000) Missense mutations interfere with VEGFR-3 signaling in primary lymphoedema. Nature Genet., 25, 153–159. [DOI] [PubMed] [Google Scholar]
- Kong H.L., Hecht,D., Song,W., Kovesdi,I., Hackett,N.R., Yayon,A. and Crystal,R.G. (1998) Regional suppression of tumor growth by in vivo transfer of a cDNA encoding a secreted form of the extracellular domain of the flt-1 vascular endothelial growth factor receptor. Hum. Gene Ther., 9, 823–833. [DOI] [PubMed] [Google Scholar]
- Kubo H. et al. (2000) Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood, 96, 546–553. [PubMed] [Google Scholar]
- Lee J., Gray,A., Yuan,J., Louth,S.-M., Avraham,H. and Wood,W. (1996) Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc. Natl Acad. Sci. USA, 93, 1988–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu A.J., Berk,D.A., Lymboussaki,A., Alitalo,K. and Jain,R.J. (2000) Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res., 60, 4324–4327. [PubMed] [Google Scholar]
- Lin P., Sankar,S., Shan,S., Dewhirst,M.W., Polverini,P.J., Quinn,T.Q. and Peters,K.G. (1998) Inhibition of tumor growth by targeting tumor endothelium using a soluble vascular endothelial growth factor receptor. Cell Growth Differ., 9, 49–58. [PubMed] [Google Scholar]
- Lymboussaki A., Olofsson,B., Eriksson,U. and Alitalo,K. (1999) VEGF and VEGF-C show overlapping binding sites in embryonic endothelia and distinct sites in differentiated adult endothelia. Circ. Res., 85, 992–999. [DOI] [PubMed] [Google Scholar]
- Makinen T. et al. (2001) Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nature Med., 7, 199–205. [DOI] [PubMed] [Google Scholar]
- Mandriota S.J. et al. (2001) Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J., 20, 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconcini L., Marchio,S., Morbidelli,L., Cartocci,E., Albini,A., Ziche,M., Bussolino,F. and Oliviero,S. (1999) c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc. Natl Acad. Sci. USA, 96, 9671–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.J., Jeltsch,M.M., Birkenhager,R., McCarthy,J.E., Weich,H.A., Christ,B., Alitalo,K. and Wilting,J. (1997) VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol., 188, 96–109. [DOI] [PubMed] [Google Scholar]
- Orlandini M., Marconcini,L., Ferruzzi,R. and Oliviero,S. (1996) Identification of a c-fos-induced gene that is related to the platelet-derived growth factor/vascular endothelial growth factor family. Proc. Natl Acad. Sci. USA, 93, 11675–11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen K., Puolakkainen,P., Jussila,L., Jahkola,T. and Alitalo,K. (2000) Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am. J. Pathol., 156, 1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen T.A., Alitalo,K. and Miettinen,M. (1999) Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer, 86, 2406–2412. [PubMed] [Google Scholar]
- Shalaby F., Rossant,J., Yamaguchi,T.P., Gertsenstein,M., Wu,X.F., Breitman,M.L. and Schuh,A.C. (1995) Failure of blood island formation and vasculogenesis in Flk-1-deficient mice. Nature, 376, 62–66. [DOI] [PubMed] [Google Scholar]
- Skobe M. et al. (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature Med, 7, 192–198. [DOI] [PubMed] [Google Scholar]
- Stacker S.A. et al. (1999a) Biosynthesis of vascular endothelial growth factor-D (VEGF-D) involves proteolytic processing which generates non-covalent homodimers. J. Biol. Chem., 274, 32127–32136. [DOI] [PubMed] [Google Scholar]
- Stacker S.A., Vitali,A., Caesar,C., Domagala,T., Groenen,L.C., Nice,E., Achen,M.G. and Wilks,A.F. (1999b) A mutant form of vascular endothelial growth factor (VEGF) that lacks VEGF receptor-2 activation retains the ability to induce vascular permeability. J. Biol. Chem., 274, 34884–34892. [DOI] [PubMed] [Google Scholar]
- Stacker S.A. et al. (2001) VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nature Med., 7, 186–191. [DOI] [PubMed] [Google Scholar]
- Takayama K., Ueno,H., Nakanishi,Y., Sakamoto,T., Inoue,K., Shimizu,K., Oohashi,H. and Hara,N. (2000) Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res., 60, 2169–2177. [PubMed] [Google Scholar]
- Thurston G., Suri,C., Smith,K., McClain,J., Sato,T.N., Yancopoulos,G.D. and McDonald,D.M. (1999) Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science, 286, 2511–2514. [DOI] [PubMed] [Google Scholar]
- Valtola R. et al. (1999) VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am. J. Pathol., 154, 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R., Rosenberg,M., Ross,S., Tyner,A. and Fuchs,E. (1989) Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc. Natl Acad. Sci. USA, 86, 1563–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veikkola T., Karkkainen,M., Claesson-Welsh,L. and Alitalo,K. (2000) Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res., 60, 203–212. [PubMed] [Google Scholar]
- Witzenbichler B. et al. (1998) Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am. J. Pathol., 153, 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Nezu,J., Shimane,M. and Hirata,Y. (1997) Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics, 42, 483–488. [DOI] [PubMed] [Google Scholar]