Abstract
Isolated epithelial cells from intestinal mucosae are a suitable object for the study of the regulation of ion transport in the gut. This regulation possesses a great importance for human and veterinary medicine, as diarrheal diseases, which often are caused by an inadequate activation of intestinal anion secretion, are one of the major lethal diseases of children or young animals. The aim of this paper is to describe a method for the isolation of intact colonic crypts, e.g. for the subsequent investigation of the regulation of anion secretion by the intracellular second messenger, Ca2+ using electrophysiological and imaging techniques.
Keywords: Electrophysiology, Epithelial Cells, Ion channels
Introduction
Colonic ion transport is controlled by the intracellular concentration of second messengers such as Ca2+, cAMP and cGMP (1). An increase in the intracellular concentration of Ca2+, e.g. caused by neurotransmitters such as acetylcholine (2) or bacterial toxins such as the toxin from Vibrio parahaemolyticus (3), causes the opening of basolateral Ca2+-dependent K+ channels. This in turn, hyperpolarizes the cell (4,5) and thereby indirectly stimulates Cl- secretion via an increase in the driving force for Cl- exit across spontaneously open Cl- channels in the apical membrane (7). In order to study the mechanisms involved in this process, e.g. for the characterization of the Ca2+-permeable channels in the plasma membrane or for the study of the origin of the Ca2+ responsible for activation of anion secretion, experiments can be performed on isolated intact intestinal crypts (15), a preparation, in which the epithelial cells are accessible for electrophysiological or imaging measurements.
Materials and Methods
This section describes a method to isolate intact crypts from rat distal colon, a protocol to obtain whole-cell patch clamp recordings on these crypts, and a technique to measure changes in the intracellular Ca2+ concentration with the aid of the fluorescent dye, fura-2. Details of the methods can be found in the protocols at the end of this article.
Crypt isolation
We use Wistar rats with a weight of 120 - 220 g since older animals with a higher body weight give a poor yield of intact crypts. The rats have free access to water and food until the day of the experiment. The animals are stunned by a blow on the head and killed by exsanguination. Then the abdomen is opened with a midline incision. The colon (from the pelvic ring until the caecum) is visualized by dislocating the small intestinal loops to the left side. With the aid of fine scissors the colon is removed from the animal starting at its distal end until the beginning of the proximal colon. In the rat, the appearance of palm-like striae can be used to define the beginning of the proximal colon (10). In our hands it is only possible to obtain viable crypts from the distal, not from the proximal colon.
The interior of the distal colon is then washed using ice-cold Parsons solution (11) containing (mmol·l-1): NaCl 107, KCl 4.5, NaHCO3 25, Na2HPO4 1.8, NaH2PO4 0.2, CaCl2 1.25, MgSO4 1, and glucose 12, equilibrated with carbogen (5% CO2 in 95% O2) with a pH of 7.4. To this purpose, a 20 ml syringe is connected with a small tube ending in a thin pipette tip in order to introduce the washing fluid into the colon. Then a small plastic rod (diameter about 5 mm) is introduced into the colonic lumen. A circular dissection is made with the blunt side of a scalpel at the distal end of the organ, which only cuts through the outer muscle layer, but not through the submucosa or the mucosa. Then the serosa and muscularis propria are gently stripped away by hand to obtain a mucosa-submucosa preparation. During all steps the gut is often rinsed with the ice cold buffer solution in order to prevent drying. This stripping is necessary to remove diffusion barriers, which otherwise might prevent the Ca2+chelator used to isolate the crypts from its action site.
When the muscle layer is removed, the prepared tube (with the plastic rode still in the colonic lumen) is cut open along its longitudinal axis with a scalpel. The resulting square of mucosa-submucosa is glued onto a plastic holder using a cyanacrylate glue (every household cyanacrylate glue works for this purpose). The holders (Fig. 1) are made from Lucite glass by our mechanical workshop.
Fig. 1. Lucite glass holder to incubate the mucosa-submucosa preparation in the isolation buffer.
The total length of the holder is about 8 cm. The oval indicates the opening of the holder, over which the tissue is glued.
The holder with the adjacent mucosa-submucosa preparation is then transferred into an isolation Parsons solution with the following composition (mmol·l-1): NaCl 107, KCl 4.5, NaH2PO4 0.2, Na2HPO4 1.8, NaHCO3 25, EDTA (ethylenediamino-tetraacetic acid) 10, glucose 12, with 1 g·l-1 bovine serum albumin. The solution is gassed with carbogen (5% CO2 / 95% O2) and kept at 37°C. After 6 to 8 min (the shorter times for mucosae from younger, the longer times for those from older animals) the holder is vibrated (Chemap Vibromixer, A1-Biotech, Martinsried, FRG) for 30 s in order to isolate intact crypts (Fig. 2).
Fig. 2. Microscopic view of an isolated crypt from rat distal colon.
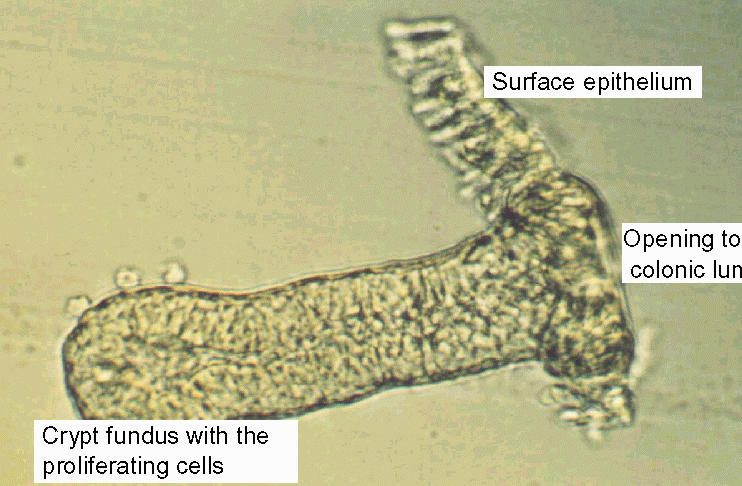
The crypts are collected in an intracellular-like high K+ Tyrode buffer (4) of the following composition (mmol·l-1): K+ gluconate 100, KCl 30, NaCl 20, CaCl2 1.25, MgCl2 1, HEPES (N-(2-hydroxyethyl)piperazine-N'-2-ethansulfonic acid) 10, glucose 12, Na+ pyruvate 5, and 1 g·l-1 of bovine serum albumin; pH 7.4. The storage in such an intracellular-like high K+, low Cl- buffer strongly prolongs the survival of the enterocytes (6).
Patch clamp experiments
The crypts are pipetted into the experimental chamber consisting of a Lucite ring glued to a glass slide. The volume of the chamber is about 0.5 ml with an inner diameter of 1.5 cm. The crypts are fixed to the glass bottom of the chamber with the aid of poly-L-lysine (molecular weight > 300 kDa; 0.1 g · l-1). Between 50 - 70 µl of the poly-L-lysine solution is distributed over the glass slide lying on a heating block (~40 °C) until the fluid has evaporated and the protein uniformly covers the glass slide. The chamber is mounted on the stage of an inverted microscope (Olympus IX-70). All patch clamp experiments are carried out at room temperature.
The preparation is superfused hydrostatically throughout the experiment (perfusion rate about 1 ml · min-1). The standard solution for the superfusion is a Tyrode solution containing (mmol · l-1): NaCl 140, KCl 5.4, HEPES 10, CaCl2 1.25, MgCl2 1 and glucose 12; pH 7.4.
Patch pipettes are pulled from thick-walled borosilicate glass capillaries (Jencons Scientific Ltd., Bedfordshire, UK; outer diameter 1.7 mm, inner diameter 1.16 mm) on a vertical two-stage puller (H. Ochotzki, Homburg/Saar, FRG). The front tips of the pipettes are fire-polished, i.e. they are approached very close to a red-heated wire using a mechanical micromanipulator (Märzhäuser, Wetzlar, Germany) under microscopical control. Also, the back ends of the pipettes are polished in a flame in order to avoid damaging of the rubber rings within the pipette holder (necessary to apply pressure or suction to the interior of the patch pipette) by the sharp edges of the upper end of the pipette. The fire-polished pipettes have electrical resistances of 5 to 10 MΩ when filled with the usual pipette solution. The standard pipette solution contains: K+ gluconate 100, KCl 30, NaCl 10, MgCl2 2, EGTA (ethyleneglycol bis-(ß-aminoethylether) N,N,N',N'-tetraacetic acid) 0.1, Tris 10, ATP (adenosine 5'-triphosphate disodium salt) 5; pH 7.2. Before the patch pipette is moved into the superfusion solution, a positive pressure is applied in order to prevent dust from contaminating the pipette tip when passing the water-air interface.
The patch clamp electrode is gently put onto the surface of a crypt cell using a hydraulic micromanipulator (Narishige MHW-103). The pipette is mounted at an angle of 30° onto the head of this manipulator. Under optical control (600 x amplification using 10x oculars, 40x objective with ultra-long working distance, and tubus magnification factor of 1.5) the pipette is gently placed onto the crypt, which is pressed in by about 1 µm. Then a small negative pressure is applied by suction to the pipette. During this process, pipette resistance is continuously registered using a test pulse (50 mV, duration 30 ms, applied every s) and measuring the resulting current across the pipette at a digital storage oscilloscope (5020A, AI Tektron, Meerbusch, Germany).
When a seal is established, the resistance of the connection membrane - glass tip of the pipette ("seal") continuously increases to values up to 5 GΩ for these cells. Seal formation is supported by transient increase of the Ca2+ concentration in the superfusing Tyrode solution to 10 mmol·l-1 and by hyperpolarizing the pipette tip. Care has to be taken so that the currents used to hyperpolarize the pipette do not exceed 100 pA, otherwise the seal may be lost.
To obtain a whole-cell recording, the membrane patch under the tip of the pipette is broken by a short, strong suction pulse after formation of the seal. Opening of the patch is indicated by an increase of the capacitance, a decrease of the resistance and a stable membrane potential under current-clamp conditions.
An alternative method to obtain a whole-cell recording is to use the permeabilized patch technique (Fig. 3). The tip of the pipette is filled via capillary forces by dipping it into a standard pipette solution for about 6 - 8 s. This filling time depends strongly on the shape of the pipette tip. Thus, the indicated time range has to be adjusted to the pipettes used. Then the back part of the pipette is filled with a pipette solution containing nystatin (300 µg.ml-1 from Sigma, Deisenhofen, FRG). If this ionophore reaches the surface of the patched cell by diffusion, it is incorporated into the membrane and allows an electrical access to the interior of the cell. When using this technique, no positive pressure can be applied to the pipette when approaching the cell, otherwise the nystatin would reach the whole crypt and permeabilize it. The advantage of the latter method is the prevention of a possible dilution of soluble cytosolic compounds.
Fig. 3. Methods to establish a whole-cell patch clamp recording.
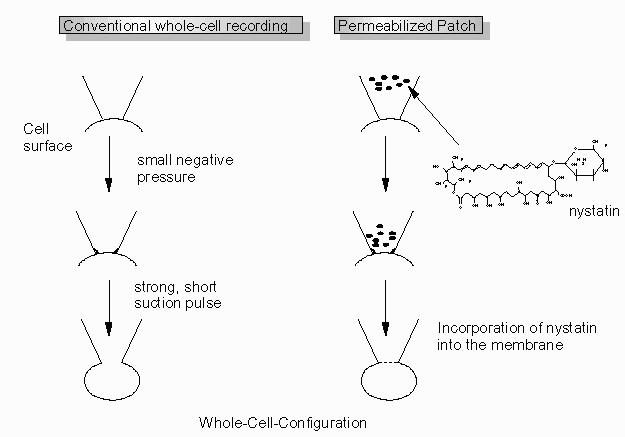
After establishment of the whole-cell configuration, the membrane capacitance can be corrected for by cancellation of the capacitance transient (subtraction) using the described 50 mV pulse.
In our laboratory, patch clamp currents are recorded on a RK-400 amplifier (Biologics, Meylan, France). Current and voltage signals are digitized at 48 kHz and stored on a modified digital audio recorder (DTR-1200, Biologics, Meylan, France). The reference point for the patch potentials is the extracellular side of the membrane, which is assumed to have zero potential.
Fura-2 experiments
Relative changes in intracellular Ca2+ concentration can be measured using the Ca2+-sensitive fluorescent dye, fura-2 (13). For this purpose, the crypts are pipetted into small chambers. They are constructed using thin glass slides (covered with poly-L-lysine; see above) mounted with silicon fat (Baysilone-Paste, mean viscosity; Bayer, Leverkusen, FRG) at the bottom side of a conventional 35 mm dish in which a hole was drilled. Total volume of the chamber is about 3 ml.
The crypts are fixed to the glass bottom of the chamber with the aid of poly-L-lysine (0.1 g·l-1). They are loaded for 60 min with 2.5 µmol·l-1 fura-2/AM (Molecular Probes, Leiden, The Netherlands) in the presence of 0.05 g·l-1 Pluronic® (BASF, Weyandotte, USA) in the dark at room temperature (12). Then, the fura-2 is washed away. The preparation is superfused hydrostatically throughout the experiment with the same standard Tyrode solution as used for the patch clamp experiments (see above) at a perfusion rate of about 1 ml·min-1.
Experiments are carried out on an inverted microscope (Olympus IX-50) equipped with an epifluorescence set-up and an image analysis system (Till Photonics, Martinsried, Germany). The emission above 470 nm is measured from several regions of interest, each with the size of about one cell. The cells are excited alternatively at 340 and 380 nm and the ratio of the emission signal at both excitation wavelengths is calculated. The duration of each light pulse is 20 ms. Data are sampled at 0.2 Hz.
To calculate intracellular Ca2+ concentrations, an extracellular calibration of the fura-2 ratio signal is used with commercial solutions (Molecular Probes, Leiden, The Netherlands) containing free fura-2 and 9 different concentrations of free Ca2+ ranging from 0 to 39.8 µmol·l-1. These data are then inserted into the Grynkiewicz equation (13) in order to estimate the intracellular Ca2+ concentration from the measured ratio signal.
Fig. 4. The Grynkiewicz equation for calibration of the fura-2 ratio signal.
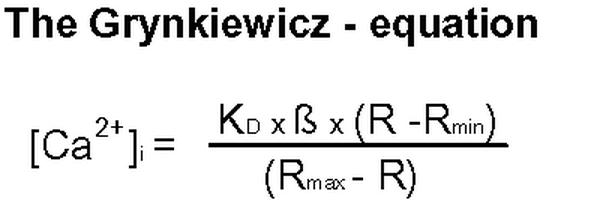
[Ca2+]I = intracellular Ca2+ concentration. KD = dissociation constant of fura-2 (224 nmol.l-1 under standard conditions). ß = fluorescence signal at 340 nm at 0 µmol.l-1 Ca2+ / fluorescence signal at 380 nm at 39.8 µmol.l-1 Ca2+. R = measured ratio. Rmin = ratio at 0 mmol.l-1 Ca2+. Rmax = ratio at 39.8 µmol.l-1 Ca2+.
Results and Discussion
In this section several problems are considered when performing imaging or electrophysiological experiments on isolated crypts.
The crypts
A combination of Ca2+ chelation together with mechanical perturbation has the big advantage that any enzymatic treatment, e.g. with collagenase or hyaluronidase, which have already been successfully used to isolate enterocytes (14), can be avoided. Consequently, there is no enzymatic digestion of the membrane, e.g. by tryptic activity of the used enzymes, and even more importantly, the procedure is independent of the specific activity of the individual enzyme batches used.
However, one has to take care about the integrity of the crypts used. If they are incubated too long in the EDTA isolation solution or if they are used longer than a maximum of about 6 h, the epithelial integrity may be lost. This can initially be recognized as a rounding of the basal pole of the crypt cells, which precedes the rounding of the completely isolated cell (15). This process, which is initiated after destruction of the tight junctions normally separating apical and basolateral membranes, leads to a mixing of apical and basolateral membrane components (16,17). Therefore, these crypts should no longer be used for experiments. The cell swelling will also lead to the activation of volume sensitive K+ and Cl- channels (18) and thus, alter the basal electrical properties of the cells. Because this rounding is much more accelerated at 37 °C, we perform all our patch clamp and imaging experiments at room temperature.
Patch clamping
When trying to perform patch clamp experiments on isolated colonic crypts, there is one major problem: the production of mucus by goblet cells in the intestinal mucosa. Establishment of a tight connection between the glass of the pipette and the membrane, i.e. the formation of a seal, requires an absolutely clean cell surface. If mucus covers the crypt cells, then the surface is no longer smooth and rough structures can be seen. Furthermore, during sealing no continuous increase of the resistance can be observed. Instead, the resistance remains at a lower level or changes stepwise without forming a real tight seal. The continuous production of mucus is also the reason that sealing of the membrane can only be well performed at the basolateral side. In order to reduce mucus production or to remove pre-existing mucus, several procedures may be used. One is to clean the mucosal surface with a soft filter paper just before the holder with the mucosa-submucosa preparation (Fig. 1) is incubated in the isolation solution. Another approach is to add atropine (10-6 mol·l-1) and indomethacin (10-6 mol·l-1) to the isolation solution in order to reduce stimulation of mucus production during the isolation procedure. Routinely, we keep the isolated crypts at 4°C just until use, which also diminishes the mucus production. Finally, a short (3 - 5 min) washing of the isolated crypts with the sulfhydryl reagent dithiothreitol (3 mmol·l-1, Sigma, Deisenhofen, BRD) may be helpful (15).
Another point, which has to be considered when working with isolated crypts, is that in contrast to conventional intestinal cell cultures, the crypt cells are not homogenous. Beside the existence of several cell types, e.g. enteroendocrine cells, columnar epithelial cells or goblet cells, there are even gradients in the cells in which we are mostly interested, i.e. the columnar epithelial cells. Here, the young proliferating cells are located at the fundus region of the crypt. They migrate during several days to the colonic lumen and thereby differentiate into mature surface cells, which are finally lost by apoptosis (19). During this procedure, the cells also change their electrical properties, e.g. they undergo a continuous decrease in basal membrane potential (4). Thus, for all electrical recordings the location of the patched cell along the crypt axis has to be considered. We routinely divide the crypt into 3 parts in order to classify the location of a cell in the fundus region, the middle third and the upper third.
Fura-2
In contrast to patch clamp recording, measurements with fluorescence dyes are technically easier to perform with colonic crypts. The crypt cells usually load quite well with the Ca2+ sensitive fluorescent dye fura-2, applied as acetoxymethylester (fura-2/AM). The protocol used can also be easily transferred e.g. for a pH sensitive dye such as BCECF (20). Usual technical problems can be an inadequate fixation of the crypt, e.g. if the poly-L-lysine is too old. We therefore only use an opened batch of poly-L-lysine for up to 1 month.
Acknowledgments
Supported by Deutsche Forschungsgemeinschaft, grant Di 388/6-1, and Graduiertenkolleg Molekulare Veterinärmedizin.
Appendix
Protocols
A. Isolation of intact colonic crypts
Equipment
Plastic rod (diameter 3 - 5 mm) for stripping the muscle layer.
Vibration mixer (Chemap Vibromixer, A1-Biotech, Martinsried, FRG).
Solutions
-
Parsons solution :
Substance Final concentration
(mmol.l-1)For 1 litre solution NaCl 107 6.253 g NaHCO3 25 2.100 g NaH2PO4 x H2O 0.2 0.028 g Na2HPO4 x 12H2O 1.8 0.645 g KCl 4.5 0.336 g Glucose 12.2 2.198 g CaCl2 1.25 1 ml from a 1.25 M stock solution (9.19 g CaCl2 x 2H2O in 50 ml aqua dest.) MgSO4 1.0 1 ml from a 1 M stock solution (12.325 g MgSO4 x 7H2O in 50 ml aqua dest.) The salts listed in line 1 to 5 can alternatively be mixed in a 10x stock solution. The pH is adjusted to 7.4 by adding HCl (1 M) or NaHCO3 (1 M) during gassing with carbogen (5% CO2 / 95% O2). Take care not to add the CaCl2 before the pH has fallen to a value below 7.8 during gassing, otherwise CaCO3 will precipitate.
-
Isolation solution:
Substance Final concentration
(mmol.l-1)For 1 litre solution NaCl 107 6.253 g NaHCO3 25 2.100 g NaH2PO4 x H2O 0.2 0.028 g Na2HPO4 x 12H2O 1.8 0.645 g KCl 4.5 0.336 g Glucose 12.2 2.198 g EDTA 10 0.372 g The salts listed in line 1 to 5 can alternatively be mixed in a 10fold concentrated stock solution. The pH is adjusted to 7.4 by adding HCl (1 M) or Tris-base (1 M) during gassing with carbogen (5% CO2 / 95% O2). In general, 100 ml are sufficient for one experiment.
-
Storage solution for the crypts:
Substance Final concentration
(mmol.l-1)For 1 litre solution K gluconate 100 23.425 g KCl 30 2.237 g HEPES 10 2.381 g NaCl 20 1.169 g CaCl2 1.25 1 ml from a 1.25 M stock solution (9.19 g CaCl2 x 2H2O in 50 ml aqua dest.) MgCl2 1 1 ml from a 1 M stock solution (10.166 g MgCl2 x 6H2O in 50 ml aqua dest.) Glucose 12.2 2.198 g Na pyruvate 5 0.553 g Bovine serum albumine (BSA) 1 g/l 1 g The pH is adjusted with KOH/HCl to pH 7.4. The BSA is added after the pH calibration in order to protect the pH electrode. In general, 100 ml is sufficient for one experiment.
-
Standard Tyrode superfusion solution for patch clamp and fura-2 experiments:
Substance Final concentration
(mmol.l-1)For 1 litre solution NaCl 140 8.182 g KCl 5.4 0.403 g HEPES 10 2.383 g Glucose 12.2 2.198 g CaCl2 1.25 1 ml from a 1.25 M stock solution (9.19 g CaCl2 x 2H2O in 50 ml aqua dest.) MgCl2 1 1 ml from a 1 M stock solution (10.166 g MgCl2 x 6H2O in 50 ml aqua dest.) The pH is adjusted with NaOH/HCl to pH 7.4.
-
Standard pipette solution for whole-cell patch clamp experiments:
Substance Final concentration
(mmol.l-1)For 1 litre solution K Gluc 100 23.425 g KCl 30 2.237 g NaCl 10 0.584 g EGTA 0.1 0.038 g Tris-base 10 1.211 g ATP (disodium salt) 5 2.756 g MgCl2 2 2 ml from a 1 M stock solution (10.166 g MgCl2 x 6H2O in 50 ml aqua dest.) The pH is adjusted with KOH/HCl to pH 7.2. In general, about 10 ml is sufficient for one experiment. The solution can be kept frozen (at -20 °C) for several weeks.
Isolation procedure
Excise the distal colon, wash it with ice-cold Parsons solution.
Insert a plastic rod into the lumen and strip away by hand the serosa and muscle layer after a blunt dissection of the muscle at the distal end of the colon.
Open the resulting mucosa-submucosa tube by a longitudinal cut and fix the sheet with cyanacrylate adhesive to an isolation holder.
Incubate the holder for 6 (younger animals) to 8 (older animals) min in isolation buffer at 37° C.
Vibrate the holder for 30 s and collect the crypts in high K+ / low Cl- storage buffer.
Pick up the crypts with a syringe (1 ml) and fix them to a glass slide covered with poly-L-lysine (molecular weight > 300 kDa; 0.1 gl-1; Biochrom, Berlin, FRG).
B. Patch clamp experiments
Equipment
Invert microscope IX-70 (Olympus, Hamburg, FRG)
Micromanipulator MHW-103 (Narishige, London, UK)
Pipette puller (Ochotzki Feinmechanik - Labortechnik, Homburg/Saar, FRG)
Patch clamp amplifier RK-400 (Biologics, Meylan, France)
DAAD-converter CED 1401 (Cambridge Electronic Design, Cambridge, UK)
Modified digital audio recorder DTR-1200 for storage of voltage and current data (Biologics, Meylan, France)
Digital storage oscilloscope 5020A (AI Tektron, Meerbusch, Germany)
Patch clamp software (Cambridge Electronic Design, Cambridge, UK) and a pentium III personal computer
Experimental protocol
Pull the pipette controlling the shape of the tip.
Fill the tip of the pipette by dipping it into the filling solution. Then fill the back using a syringe with a fine needle (0.40 x 42 mm). Remove air bubbles by gently tapping on the glass.
Mount the pipette in the pipette holder. Apply positive pressure, if you are not using nystatin. Approach the pipette to the crypt under optical control with the micromanipulator. When the pipette is in the bath, measure the offset potential in the current-clamp mode of the amplifier and compensate the offset if necessary with the amplifier.
When the crypt is reached and is gently pressed down, apply negative pressure. Measure the pipette resistance by applying a 50 mV pulse in the voltage-clamp mode of the amplifier. When the seal resistance has increased above 1 GΩ, release the negative pressure. If the seal does not form in this way, increase the Ca2+ concentration in the superfusion solution to 10 mmol.l-1. Alternatively, control the cellular surface if there is some mucus present. Select a different cell for trying with a new patch pipette.
Apply a negative suction for a few seconds to break into the cell. When using nystatin, simply wait (for maximal 15 min) until the ionophore permeabilizes the cell. A successful whole-cell recording is indicated by an increase in the membrane capacitance, a decrease in resistance, and a stable membrane potential in the current-clamp mode of the amplifier.
Permeabilization of patches
Nystatin (Sigma, Deisenhofen, BRD) stock solution 50 g.l-1 kept under light protection. 6 μl of this stock solution is added to 1 ml pipette solution kept under light protection. Just prior the beginning of the experiment, the solution is ultrasonicated.
Fura-2 experiments
Equipment
Invert microscope IX-50 with epifluorescence set-up (Olympus, Hamburg, FRG)
Image analysis system (Till Photonics, Martinsried, Germany)
Experimental protocol
Add 2.5 μmol.l-1 fura-2/AM together with 0.05 g.l-1 Pluronic® to the crypts. Incubate in the dark for 1 h.
Wash away the fura-2/AM with standard Tyrode solution.
Control the loading by exciting shortly the crypts with one wavelength, e.g. 340 nm.
Select several regions of a crypt with the image analysis system, each with the size of about one cell.
During the experiment, the crypt is alternatively exposed to light of 340 nm and 380 nm. We use light pulse of 20 ms duration applied every 5 s.
Drugs used for imaging
Fura-2-acetoxymethylester (fura-2/AM; Molecular Probes, Leiden, The Netherlands) dissolved as stock solution (1 mg/1 ml dimethylsulfoxide [DMSO]), kept at -20° C in small aliquots. For loading of the crypts, 2.5 μl is added to 0.5 ml of crypt suspension.
Pluronic® (BASF, Weyandotte, USA) is dissolved in DMSO as a 200 g.l-1 stock solution, kept at room temperature). Add 1 μl to 0.5 ml of crypt suspension.
References
- Binder HJ, Sandle GJ. Electrolyte transport in the mammalian colon. In: Johnson LR (ed) Physiology of the gastrointestinal tract. 3rd ed. New York: Raven Press; 1994. p. 2133-2171.
- Diener M, Eglème C, Rummel W. Phospholipase C-induced anion secretion and its interaction with carbachol in the rat colonic mucosa. Eur J Pharmacol. 1991;200:267–276. doi: 10.1016/0014-2999(91)90581-a. [DOI] [PubMed] [Google Scholar]
- Raimondi F, Kao JPY, Kaper JB, Guandalini S, Fasano A. Calcium-dependent intestinal chloride secretion by vibrio parahaemolyticus thermostable direct hemolysin in a rabbit model. Gastroenterology. 1995;109:381–386. doi: 10.1016/0016-5085(95)90324-0. [DOI] [PubMed] [Google Scholar]
- Böhme M, Diener M, Rummel W. Calcium- and cyclic-AMP-mediated secretory responses in isolated colonic crypts. Pflügers Arch. 1991;419:144–151. doi: 10.1007/BF00373000. [DOI] [PubMed] [Google Scholar]
- Bleich M, Riedemann N, Warth R, Kerstan D, Leipziger J, Hör M, Van Driessche W, Greger R. Ca2+ regulated K+ and nonselective cation channels in the basolateral membrane of rat colonic crypt base cells. PflüSgers Arch. 1996;432:1011–1022. doi: 10.1007/s004240050229. [DOI] [PubMed] [Google Scholar]
- Del Castillo JR. The use of hyperosmolar intracellular-like solutions for the isolation of epithelial cells from guinea-pig small intestine. Biochim Biophys Acta. 1987;901:201–208. doi: 10.1016/0005-2736(87)90116-7. [DOI] [PubMed] [Google Scholar]
- Strabel D, Diener M. Evidence against direct activation of chloride secretion by carbachol in the rat distal colon. Eur J Pharmacol. 1995;274:181–191. doi: 10.1016/0014-2999(94)00728-p. [DOI] [PubMed] [Google Scholar]
- Siemer C, Gögelein H. Activation of nonselective basolateral cation channels in the basolateral membrane of rat distal colon crypt cells. Pflügers Arch. 1992;420:319–328. doi: 10.1007/BF00374465. [DOI] [PubMed] [Google Scholar]
- Warth R, Hamm K, Bleich M, Kunzelmann K, von Hahn T, Schreiber R, Ullrich E, Mengel M, Trautmann N, Kindle P, Schwab A, Greger R. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflügers Arch. 1999;438:437–444. doi: 10.1007/s004249900059. [DOI] [PubMed] [Google Scholar]
- Lindström CG, Rosengren JE. Colon of the rat. An anatomic histologic and radiographic investigation. Acta Radiol Diagn. 1979;20:523–536. doi: 10.1177/028418517902000314. [DOI] [PubMed] [Google Scholar]
- Parsons DS, Paterson CR. Fluid and solute transport across rat colonic mucosa. Q J Exp Physiol. 1965;50:220–231. doi: 10.1113/expphysiol.1965.sp001784. [DOI] [PubMed] [Google Scholar]
- Frings M, Schultheiss G, Diener M. Electrogenic Ca2+ entry in the rat colonic epithelium. Pflügers Arch. 1999;439:39–48. doi: 10.1007/s004249900159. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hartmann F, Owen R, Bissell DM. Characterization of isolated epithelial cells from rat small intestine. Am J Physiol. 1985;242:G147–G155. doi: 10.1152/ajpgi.1982.242.2.G147. [DOI] [PubMed] [Google Scholar]
- Diener M, Rummel W, Mestres P, Lindemann B. Single chloride channels in colon mucosa and isolated colonic enterocytes of the rat. J Membr Biol. 1989;108:21–30. doi: 10.1007/BF01870422. [DOI] [PubMed] [Google Scholar]
- Ziomek CA, Schulman S, Edidin M. Redistribution of membrane proteins in isolated intestinal epithelial cells. J Cell Biol. 1980;86:849–857. doi: 10.1083/jcb.86.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman DJ, Allan CH, Hagen SJ, Trier J. Structural features of absorptive cell and microvillus preparations from rat small intestine. Gastroenterology. 1986;91:1401–1414. doi: 10.1016/0016-5085(86)90194-0. [DOI] [PubMed] [Google Scholar]
- Diener M, Nobles M, Rummel W. Activation of basolateral Cl- channels in the rat colonic epithelium during regulatory volume decrease. Pflügers Arch. 1992;421:530–538. doi: 10.1007/BF00375048. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Proliferation and differentiation of normal and diseased gastrointestinal cells. In: Johnson LR (ed) Physiology of the gastrointestinal tract. 2nd ed. New York: Raven press; 1994. p. 255-284.
- Schultheiss G, Ribeiro R, Diener M. Fatty acids inhibit anion secretion in rat colon: apical and basolateral action sites. Pflügers Arch. 2001;442:603–613. doi: 10.1007/s004240100574. [DOI] [PubMed] [Google Scholar]


