Abstract
A method for the simultaneous determination of several classes of aldehydes in exhaled breath condensate (EBC) was developed using liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry (LC/APCI-MS/MS). EBC is a biological matrix obtained by a relatively new, simple and noninvasive technique and provides an indirect assessment of pulmonary status. The measurement of aldehydes in EBC represents a biomarker of the effect of oxidative stress caused by smoke, disease, or strong oxidants like ozone. Malondialdehyde (MDA), acrolein, α,β-unsaturated hydroxylated aldehydes [namely 4-hydroxyhexenal (4-HHE) and 4-hydroxynonenal (4-HNE)], and saturated aldehydes (n-hexanal, n-heptanal and n-nonanal) were measured in EBC after derivatization with 2,4-dinitrophenylhydrazine (DNPH). Atmospheric pressure chemical ionization of the analytes was obtained in positiveion mode for MDA, and in negativeion mode for acrolein, 4-HHE, 4-HNE, and saturated aldehydes. DNPH derivatives were separated on a C18 column using variable proportions of 20 mM aqueous acetic acid and methanol. Linearity was established over 4–5 orders of magnitude and limits of detection were in the 0.3–1.0 nM range. Intra-day and inter-day precision were in the 1.3–9.9% range for all the compounds. MDA, acrolein and n-alkanals were detectable in all EBC samples, whereas the highly reactive 4-HHE and 4-HNE were found in only a few samples. Statistically significant higher concentrations of MDA, acrolein and n-hexanal were found in EBC from smokers.
Production of reactive oxygen species (ROS) contributes to oxidation of cell macromolecules, e.g. lipids, DNA and proteins, leading to a variety of products1 including alkanes, aldehydes, oxidated nucleotides and oxidated amino acids. Among the mechanisms of free radical damage, lipid peroxidation is probably the most extensively investigated process. ROS oxidation of cell membrane phospholipids produces chain reactions whose targets are the (poly)unsaturated fatty acids (P)UFA esterified in the sn-1 and sn-2 glyceride positions, and results in the formation of unstable lipid hydroperoxides and of secondary carbonyl compounds such as aldehydic products. Oxidative stress plays an important role in pathobiology of the lung due to its large exchange surface with oxygen and with environmental pollutants contaminating inhaled air. The main (P)UFAs in epithelial pulmonary cells are oleic acid (C18:1), linoleic acid (C18:2) and arachidonic acid (C20:4), distributed among phosphati-dylcholine (46%), phosphatidylethanolamine (32%) and phosphatidylinositol (39%).2
Among the products of lipid peroxidation, malondial-dehyde (MDA) is commonly used as a marker of oxidative stress.3 It has been determined in several biological matrices3 including plasma,4–6 serum,7 urine,5 bronchoalveolar lavage (BAL) fluid,7 expired breath condensate,8 and tissues,9 as well as in lipid-rich foods.10 Its colorimetric determination after reaction with 2-thiobarbituric acid (the so-called TBARS assay) has been criticized because of the nonspecifi-city of the TBARS reaction.11,12 Therefore, several analytical methods have proposed the use of chromatographic techniques coupled with sensitive detectors.13,14 Besides MDA, some α,β-unsaturated aldehydes, namely, 4-hydroxyhexenal (4-HHE) and 4-hydroxynonenal (4-HNE), are known to be formed by peroxidation of ω −3 and ω-6 PUFAs,3 respectively. Small amounts of acrolein can also be formed from both ω −3 and ω −6 PUFAs during lipid peroxidation.3 These highly reactive unsaturated aldehydes can covalently bind in a Michael-type reaction to protein SH groups and to glutathione (GSH),15 as well as to amino groups of proteins to form Schiff bases;3 the aldehyde group can also react with the NH2 group of deoxyguanosine.16 Although free 4-HNE hasbeen measured in cell extracts17 and in plasma,18 it is more frequently determined as an adduct to proteins19 or GSH.20 Saturated aldehydes, namely, n-hexanal (C6), n-heptanal (C7) and n-nonanal (C9), were found in BAL after induction of free radical damage in the lung of both rat21 and humans22 by ozone, and they may represent breakdown products of oxidized linoleic acid and arachidonic acid (C6), palmitoleic acid (C7) and oleic acid (C9), respectively.
Aldehydes have long been determined using gas chromatography/mass spectrometry (GC/MS), and liquid chromatography (LC) coupled with nonspecific detectors.23 More recently, several methods based on the use of liquid chromatography/mass spectrometry (LC/MS) have been proposed. Carbonyl compounds including saturated, unsaturated and aromatic aldehydes and ketones have been quantitated as 2,4 dinitrophenylhydrazone derivatives in ambient air samples24–26 by LC combined with atmospheric pressure chemical ionization (APCI) and ion trap multiple MS (MSn), and in disinfected water27 using LC and electro-spray (ESI) tandem MS (MS/MS). Formaldehyde, acetaldehyde, benzaldehyde, acrolein and C3–C6 n-alkanals were also determined as 2,4-dinitro-3,5,6-trideuterophenylhydra-zones in air samples using LC/APCI-MS,28 whereas the Hantzsch reaction was proposed to derivatize aliphatic aldehydes before LC/MS analysis.29 Very recently, a method has been proposed30 for the qualitative and quantitative analysis of aldehydes (alkanals, alkenals, 4-HHE and 4-HNE, but not MDA) as cyclohexanedione (CHD) derivatives in plasma by LC/MS/MS using electrospray ionization.
The aim of the present work was to develop and validate a new analytical method for the simultaneous determination of biologically relevant aldehydes arising from oxidative stress, including MDA, acrolein, α,β-unsaturated aldehydes and saturated aldehydes, present at trace levels in exhaled breath condensate. EBC, obtained by cooling exhaled air under conditions of spontaneous breathing, is a biological matrix which could provide an indirect and nonsystemic assessment of pulmonary status.31 EBC collection is simple to perform and noninvasive; it does not disrupt the respiratory mucosa and does not have a dilution factor as observed in samples obtained via bronchoscopy and BAL. During expiration the exhaled air flows through a condenser which is cooled to 0°C by melting ice or to −20°C by a refrigerated circuit, and breath condensate is then collected into a cooled collection vessel. A low temperature may be important for preserving labile markers during the collection period, which usually takes 10–20 min to obtain 1–3 mL of condensate.
Up to now, more than 100 peer-reviewed articles on this subject have appeared in the literature,32,33 most of them based on the use of colorimetric and immunochemical assays for the determination of inflammatory mediators and markers of oxidative stress in different lung pathologies. The lack of specificity of these tests, together with the need for standardization in sample collection, make still doubtful the clinical applicability of EBC. For these reasons, there are high expectations for the results of the application of reliable analytical techniques like LC/MS/MS to this relatively new but still unexplored biological matrix.
EXPERIMENTAL
Chemicals and standard preparation
Malondialdehyde (MDA) tetrabutylammonium salt (purity 98%), acrolein (95%), n-alkanal standards (n-hexanal, C6; n-heptanal, C7; n-nonanal, C9; >95%), 2,4-dinitrophenyl-hydrazine (DNPH, 97%), cyclohexanedione (CHD, 97%), thiobarbituric acid (TBA, 98%) and butylated hydroxyto-luene (BHT, 99%) were purchased from Sigma-Aldrich (Milan, Italy). All these standards were used without further purification. 4-Hydroxy-2-hexenal (4-HHE, >98%), 4-hydroxy-2-nonenal (4-HNE, 98%) dissolved in ethanol (87.6 and 64.0 mM) were obtained from Cayman Chemicals (Michigan, USA). These solutions are stated to be stable for 6 months at −80°C. HPLC-grade water, methanol and acetonitrile were from LabScan (Dublin, Ireland). Analytical-grade acetic acid (>99%), formic acid (>98%), hydrochloric acid (37%) and perchloric acid (>70%) were obtained from Sigma-Aldrich.
Standard stock solutions of other aldehydes (about 50 mM) were prepared in acetonitrile and stored at −20°C for up to 1 month. From these solutions a concentrated solution containing all the aldehydes (5 mM) was prepared weekly in acetonitrile. Calibration standards for the determination of the linear dynamic range were prepared by serial dilution with water of the 5 mM solution to the appropriate concentrations in the 1 nM to 500 μM range. Three aliquots of each calibration standard were individually derivatized and injected. For quantitative analysis of aldehydes in EBC, working standard solutions containing 1 μM, 500 nM, 250 nM and 100 nM of aldehydes were prepared daily by dilution of the 5 mM solution with water, and were used for standard addition to pooled EBC samples in order to obtain concentration increases of 100, 50, 25 and 10 nM. Each calibration sample was then individually derivatized and injected twice.
Exhaled breath condensate collection
EBC samples were collected using a commercially available EcoScreen®breath condenser (Jaeger, Würzburg, Germany). Twenty-five healthy subjects, 12 nonsmoking controls and 13 asymptomatic smokers, were asked to breath tidally for 15 min, without nose clip in place, through a two-way non-rebreathing valve by which inspiratory and expiratory air are separated and saliva is trapped. EBC thus produced during a 15-min period (2–3 mL) was collected in polypropylene tubes and stored at −80°C until analysis.
Preparation of the dinitrophenylhydrazone derivatives
To perform derivatization with DNPH, a procedure adapted from the literature was followed. Briefly, DNPH (50 mg) was dissolved in 20 mL of acetonitrile and acidified with 0.4 mL of formic acid. The DNPH solution (12 mM) was stable for 1 week when stored at 4°C. Derivatization of aldehydes was performed by mixing 100 μL of the standard solutions (or EBC samples) with 100 μL of the derivatizing agent and incubating at room temperature for 1 h. Then, 20 μL of derivatized samples were directly injected onto the LC/APCI-MS/MS system. Each sample was injected twice.
In the optimization of the derivatization procedure the use of formic and acetic acid was compared with that of perchloric and hydrochloric acid,27 and derivatization was conducted both at room temperature and at 70°C. These comparative experiments were done on both aqueous aldehyde standard solutions and on EBC samples spiked at nine concentration levels in the 25 nM to 10 μM range. Then, experiments using formic acid were performed at room temperature to establish the time (0–6 h) and the excess of DNPH needed to obtain a complete reaction. The use of BHT was also tested to evaluate artefact formation during storage of samples. For comparative purposes, derivatization with both TBA and CHD was performed following the procedures described in the literature.14,30
Liquid chromatography/mass spectrometry
The LC/MS/MS system consisted of a Perkin Elmer series 200 liquid chromatograph (Norwalk, CT, USA) coupled with a PE-Sciex API 365 triple-quadrupole mass spectrometer (Sciex, Concord, Canada) equipped with an Ionspray interface for pneumatically assisted electrospray or a heated nebulizer (HN) interface for atmospheric pressure chemical ionization (APCI). Injections were performed using an ASPEC XL autosampler (Gilson, Villiers-le Bel, France). A Power Macintosh G3 computer was used for instrument control, data acquisition and processing. Chromatography of DNPH derivatives was performed on a SupelcosilTM LC-18-DB column (75 −4.6 mm i.d., 3 μm; Supelco, Bellefonte, PA, USA) using 20 mM aqueous acetic acid and methanol under gradient elution conditions at a flow rate of 0.80 mL/min. Gradient program: from 43% to 98% methanol in 3.2 min, linear gradient, and then hold for 2.5 min. The analytes were ionized by both electrospray and APCI in positiveion mode (PI) for MDA, and in negativeion (NI) mode for acrolein, 4-HHE, 4-HNE, and all saturated aldehydes. ESI conditions: for positive ions (PI), ionspray voltage 5800 V, orifice voltage 50 V; and for negative ions (NI), ionspray voltage –4500 V, orifice voltage −30 V. The APCI temperature was 450°C, and the needle current +or −3 μA, in PI and NI, respectively. APCI production spectra were obtained by flow injections of standards of the DNPH derivatives (about 50 μM) dissolved in a 1:1 (v/v) acetonitrile/aqueous HCOOH (2%) mixture and acquired in the m/z range 70–350 (scan rate 0.67 scan/s; w1/2 0.6 Da). Detection for sample analyses was obtained in selected-reaction monitoring (SRM) mode, following the reactions m/z 235 →159 (collision energy 20 eV) for MDA, m/z 235 →163 (17 eV) for acrolein, m/z 293 →167 (18 eV) for 4-HHE, m/z 335 →167 (18 eV) for 4-HNE, m/z 279 →163 (14 eV) for C6, m/z 293 →163 (17 eV) for C7, and m/z 321 →163 (17 eV) for C9.
Statistics
Statistical analysis was performed by using the SPSS/PC+ software (version 10.0 for Windows, Chicago, IL, USA). Comparisons between different acid treatments were made by one-way ANOVA followed by Tukey’s test. Differences between two groups were assessed using the Mann–Whitney U-test. A p value of less than 0.05 indicated statistical significance. Linear regression analysis was performed using the least-squares method.
RESULTS AND DISCUSSION
Choice and optimization of the derivatization procedure
For EBC aldehyde analysis, DNPH as derivatizing agent was preferred over thiobarbituric acid (TBA) and cyclohexanedione (CHD), since it allows the simultaneous derivatization of all the classes of aldehydes investigated (Fig. 1). In fact, whereas TBA is known to be suitable for the derivatization of MDA,3,14 CHD is a derivatizing agent specific for most aldehydes but it was found to not react with MDA30 (confirmed by our findings). Hydrazine reagents are the most widely used derivatizing agents for carbonyl compounds in ambient air samples;34 the reaction is rapid and leads to stable products. Nevertheless, problems of chemical interferences are reported for the determination of DNPH derivatives of small aldehydes like acrolein,35 formaldehyde and acetaldehyde36 in air samples, mainly due to the presence of ozone and nitrogen dioxide. Similar interference problems in the analysis of acrolein were not observed by us in EBC.
Figure 1.
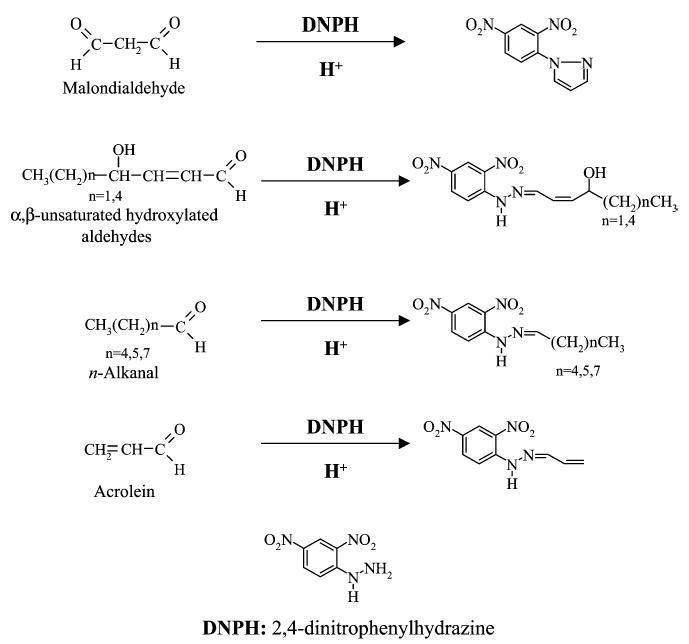
Structures of the DNPH derivatives representative of the several classes of the aldehydes investigated.
The present method makes use of a modified derivatization procedure. The use of formic or acetic acid instead of perchloric or hydrochloric acid was initially considered, due to their higher compatibility with MS detection. Experiments conducted with the aim of comparing the derivatization yield using hydrochloric, perchloric, formic or acetic acid demonstrated that the highest MS response and the highest reproducibilty for all aldehydes except MDA were obtained when derivatization was conducted with hydrochloric or formic acid at room temperature. Figure 2 shows the influence of the different derivatization conditions (different acids at room temperature) on the slope of the calibration curve obtained when EBC samples spiked at nine different concentrations were analyzed using LC/ESI-MS/MS. Similar behavior was observed for aqueous samples. The use of higher temperatures (70°C) was found not to significantly influence the derivatization yield (data not shown). As shown in Fig. 2, hydrochloric and formic acid gave comparable results for all aldehydes, whereas acetic acid gave a considerably lower response for MDA (p <0.001 vs. all other acids). Moreover, the use of perchloric acid was found to increase the response of MDA but had a detrimental effect on the yield of derivatization of acrolein (p <0.001 vs. all other acids), probably due to the highly oxidative properties of the acid; a similar effect was observed using hydrochloric or formic acid at 70°C and could be ascribed to the thermal lability of acrolein in the presence of water traces. By variation of the reaction time between 1 and 6 h, we demonstrated that the derivatization was complete in less than 1 h, even under the mild conditions applied. It has recently been reported for water samples that an excess of DNPH at a molar ratio of approximately 200 was sufficient for quantitative derivatization of carbonyl compounds.27 We have verified that such a large excess of DNPH ensures a high reproducibility of the derivatization process even at very low concentrations like those measured in EBC samples (%RSD <10% for all DNPH derivatives, six derivatized samples, each injected four times).
Figure 2.
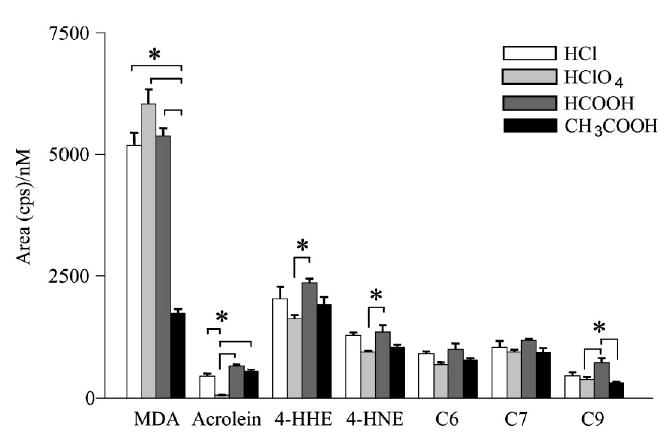
Comparison between the intensities of the MS response obtained using four different acids for the derivatization of aldehydes with DNPH at room temperature. EBC samples spiked with aldehyde mixtures at nine concentration levels between 25 nM and 10 μM were derivatized using a 12 mM DNPH solution acidified with hydrochloric, perchloric, formic or acetic acid, and then analyzed by LC/ESI-MS/MS (PI for MDA and NI for all other aldehydes). The histograms show the slopes of the calibration curves calculated in the 25 nM to 10 μM range for each DNPH hydrazone and for each treatment. Comparisons between different acid treatments were made by one-way ANOVA followed by Tukey’s test (*p<0.05).
The use of antioxidant is considered fundamental to prevent adventitious formation of aldehydes in complex and lipid-rich matrices like food or plasma during multi-step sample preparation. In contrast, we found that the addition of antioxidants like BHT did not further improve the stability of EBC samples, as already reported for the case of MDA determination.14 Moreover, it should be considered that EBC samples were collected using a special condenser which stores samples at low temperature in the dark, and that sample collection requires a relatively short time (10–20 min), thus limiting the risk of artifactual formation of aldehydes.
MS characterization of the DNPH derivatives
The NI mass spectrometric characterization of carbonyl compounds, including saturated and unsaturated aldehydes, was previously described using both APCI24–26 and ESI with a TurboIon Spray interface.27 None of these studies included MDA and α,β-unsaturated hydroxylated aldehydes. In our studies, ESI- and APCI-MS and production MS/MS mass spectra of derivatized aldehydes were similar in terms of both ionization and fragmentation, although a higher response was obtained using APCI. All aldehydes except MDA, for which only PI was satisfactory, were efficiently ionized in both PI and NI modes with generation of intense [M+H]+ and [M – H]− ions, respectively. The protonated molecule of MDA at m/z 235 is consistent with the structure of the cyclic mono-DNPH derivative and with its EI spectrum ([M]+, m/z 234) previously reported.13 NI spectra also showed the presence of the radical anion [M]− with a relative abundance of about 20% with respect to the [M–H]− ion. The formation of radical anions of DNPH derivatives has never been reported before, to our knowledge. It may result from the relatively high electron affinity of the nitro groups. On the other hand, radical anions are regularly observed for various compound classes in addition to deprotonated molecules in NI mode, especially in APCI. This is proposed to be related to electron capture of thermal electrons generated in one of the first steps of the ionization processes.37
Production APCI-MS/MS spectra of selected DNPH derivatives representative of the different aldehyde classes, namely MDA, acrolein, C9 and 4-HNE, obtained in PI mode, are shown in Fig. 3. The production spectrum of the [M+H]+ ion of MDA (precursor at m/z 235) is characterized by an intense fragment ion at m/z 189 due to the loss of a NO2 molecule, probably from the ortho position. Subsequent loss of a NO molecule leads to the formation of the ion at m/z 159. Production spectra of the [M+H]+ ions of acrolein, C9 and other saturated aldehydes are characterized by the loss of 17 Da (100%) due to an ortho-effect-driven release of OH, whereas, for α,β-unsaturated aldehydes 4-HHE (m/z 277) and 4-HNE (m/z 319), the loss of water was more abundant than the loss of OH. Other common peaks were observed at m/z 193, 184, 183, 167, 164, 153 and 138 for all n-alkanals, and at m/z 184, 138 and [M+H−182]+ for α,β-unsaturated aldehydes. Definitive spectral interpretation in this case, where both odd- and even-electron fragments are generated, would require accurate mass determination and was outside the scope of the present work.
Figure 3.
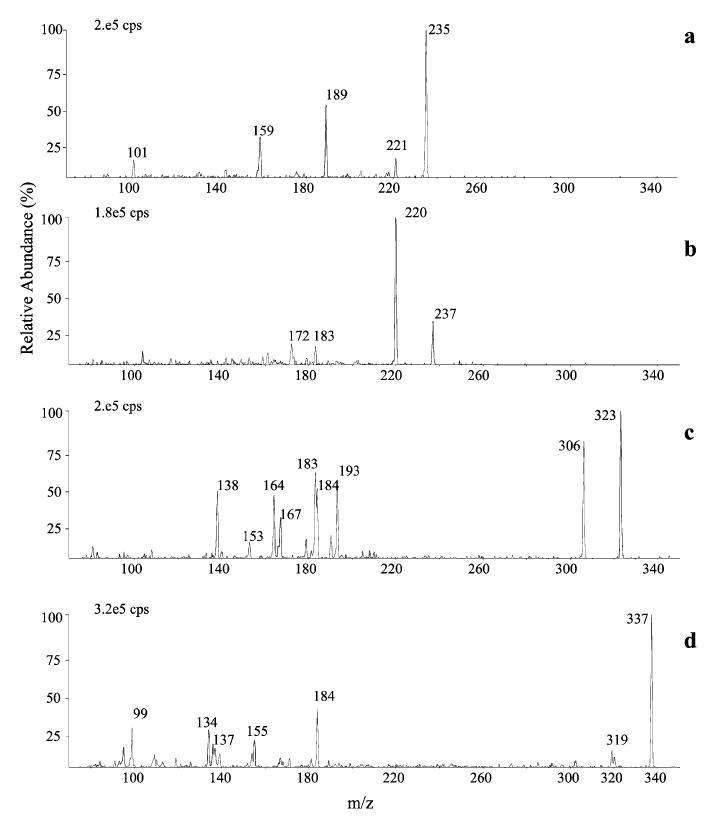
Production APCI mass spectra of the DNPH derivatives of MDA (a), acrolein (b), n-nonanal (c) and 4-HNE (d) obtained in positiveion mode.
Production spectra of DNPH aldehydes (acrolein, C9 and 4-HNE) in NI mode ([M–H]−) are shown in Fig. 4. The spectra show fragments at m/z 163, 153 and 152, common to all aldehydes, and others present only for subgroups of aldehydes, e.g. m/z 205, 191, 179 and 120 for saturated aldehydes and at m/z 182 and 167 for α,β-unsaturated compounds. The same fragment ions were previously observed by other authors using both APCI24 and ESI,27 and some of these peaks were attributed to the 2,4-dinitrophenylhydrazone residue by using isotopically labelled compounds.38 The presence of another characteristic ion, [M–H–164]−, probably related to the aliphatic chain, was observed at m/z 115, 129 and 157 for C6, C7 and C9, respectively. A characteristic pattern of three ions, [M–H]−, [M–H–NO]−. and [M–H–NO2]−., is shown byall saturated aldehydes, whereas the loss of HNO2 (47 Da),typical of unsaturated aldehydes, was observed for both 4-HHE (at m/z 246) and 4-HNE (at m/z 288).
Figure 4.
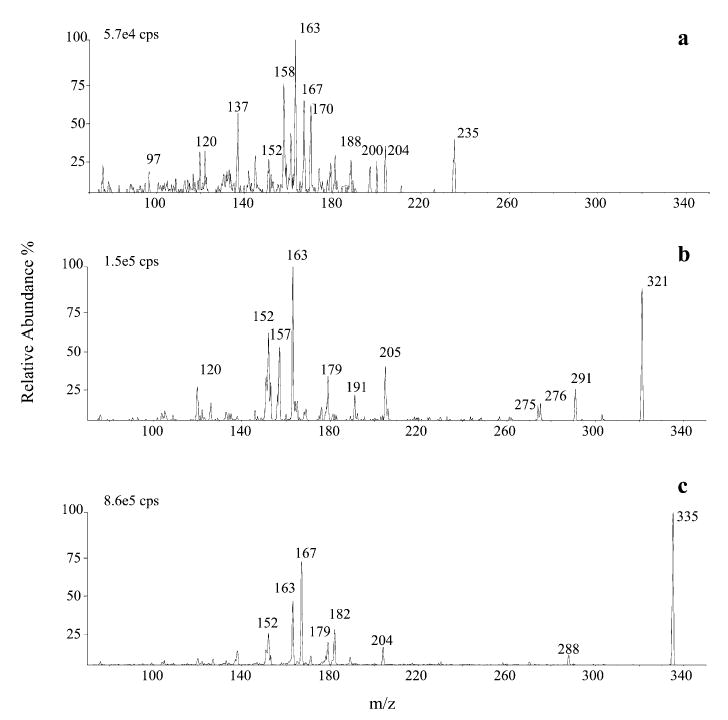
Product-ion APCI mass spectra of the DNPH derivatives of acrolein (a), n-nonanal (b) and 4-HNE (c) obtained in negativeion mode.
The occurrence of the odd-electron fragments is clearly related to the nitro character of the derivatives. Nitro compounds readily lose two stable neutral radicals, NO. and NO2. When the radical ions [M]−. instead of the deprotonated molecules [M–H]− were fragmented, a shift of 1 Th was observed for some fragment ions, e.g. at m/z 153, 164, 192 and 206 for saturated aldehydes and at m/z 153, 164, 168 and [M–47]− for unsaturated aldehydes.
The most abundant fragment ion in each spectrum, i.e.[C7H5N3O2]− at m/z 163 for acrolein and n-alkanals and [C6H5N3O3]−at m/z 167 for 4-HHE and 4-HNE, was selected for the SRM transition for quantitative analysis. However, in the case of MDA, the choice of the fragment at m/z 159, although less abundant, was found to be more favorable in terms of a better signal-to-noise (S/N) ratio. This means that for all compounds analyzed in NI mode, the fragment selected arises from the DNPH part of the derivative, while only the selected precursor ion is indicative of the aldehyde part of the molecule. For these derivatives the charge is retained by the dinitrophenyl group rather than by the aldehyde part, which is the reason underlying the need to perform derivatization in the first place.
Validation of the method for the determination of DNPH derivatives in EBC
A chromatogram of a pooled EBC sample, spiked with a 25 nM mixture of aldehydes and then analyzed by LC-APCI-SRM, is shown in Fig. 5, together with the chromatogram of an authentic nonspiked EBC sample obtained under the same conditions. All the investigated aldehydes could be examined in a 11 min chromatographic run, switching from positive (for the early-eluting MDA) to negative (for other aldehydes) ionization. A 43–98% methanol/water gradient containing 20 mM acetic acid was applied, providing a 10- to 100-fold higher response in NI than in PI mode for both saturated and α,β-unsaturated aldehydes. Owing to the competitive role played by methanol in the derivatization of the aldehyde function, acetonitrile was used as a solvent instead of methanol in the initial characterization of the DNPH derivatives by flow injection of standards. Surprisingly, similar responses of the DNPH derivatives were observed in PI and NI mode under the latter conditions [1:1 (v/v) acetonitrile/aqueous HCOOH (2%)]. The use of methanol instead of the more commonly used acetoni-trile24–27 was found to enhance both the chromatographic separation and the ionization efficiency of the DNPH derivatives.
Figure 5.
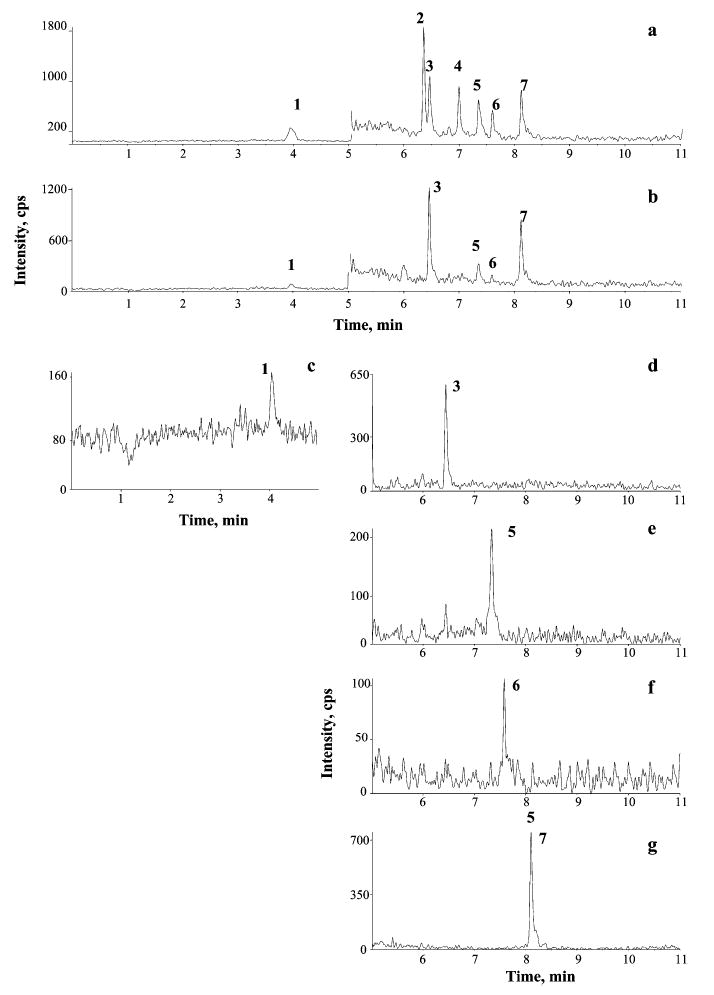
LC/APCI-MS/MS chromatograms obtained in SRM mode of a pooled EBC sample spiked with a 25 nM mixture of derivatized aldehyde standards (a) and of an authentic EBC sample (b), for which separate SRM traces are shown (c)–(g). Chromatographic conditions: see Experimental. Peak identification and SRM transitions:1. MDA (m/z 235 →159), 2 4-HHE (m/z 293 →167), 3 acrolein(m/z 235 →163), 4 4-HNE (→ 335 →167), 5 n-hexanal (→ 279 →163), 6 n-heptanal (→ 293 →163), 8 n-nonanal (→ 3215 →163).
Table 1 summarizes the linearity data and the limits of detection of the LC/APCI-MS/MS method for the determination of aldehydes as DNPH derivatives in aqueous solutions, obtained under SRM conditions. As compared with ESI, APCI ionization allowed a wider linear dynamic range, up to 5 orders of magnitude, and about 10-fold lower limits of detection (LODs). LODs, calculated on the basis of a S/N ratio >3, were comparable to or even lower than those reported in the literature for LC/MS/MS of 4-HNE17 and other aldehydes27,30 and for HPLC-FLD of MDA.14 Since EBC is mostly water with a salt and protein content much lower than other biological matrices,39 no differences due to matrix effects were observed between water and pooled EBC samples. Nevertheless, for sample analyses, addition of standards in a restricted concentration range (up to 100 nM) was done on pooled EBC samples. The results of the short-and long-term reproducibility of the LC/APCI-MS/MS method, determined for EBC samples, are reported in Table 2.
Table 1.
Linear dynamic range, correlation cofficients (r2) and limits of detections (LODs) of the LC/APCI-MS/MS method for the determination of aldehydes as DNPH derivativesa
| Compound | SRM (m/z) | Range (μM) | ab × 10−4 | r2 | n | LOD (nM)c |
|---|---|---|---|---|---|---|
| MDA | 235 → 159 | 0.004–400 | 2.96 ± 0.05 | 0.997 | 39 | 1.0 |
| Acrolein | 235 → 163 | 0.003–200 | 1.55 ± 0.03 | 0.995 | 36 | 1.0 |
| 4-HHE | 293 → 167 | 0.003–150 | 9.22 ± 0.20 | 0.995 | 36 | 0.3 |
| 4-HNE | 335 → 167 | 0.003–150 | 9.14 ± 0.20 | 0.994 | 36 | 0.6 |
| n-hexanal | 279 → 163 | 0.004–350 | 3.90 ± 0.07 | 0.997 | 39 | 1.0 |
| n-heptanal | 293 → 163 | 0.003–350 | 3.78 ± 0.08 | 0.995 | 39 | 1.0 |
| n-nonanal | 321 → 163 | 0.003–350 | 3.70 ± 0.09 | 0.998 | 39 | 1.0 |
Calibration curve: y = ax, forced through zero.
± Values are confidence intervals at 95% probability level.
Limit of detection (S/N ratio = 3) calculated under SRM conditions.
Table 2.
Repeatability and intra-day and inter-day precision of the LC/APCI-MS/MS method, calculated at 25 nM. Results are expressed as % relative standard deviation (% RSD)
| Compound | Repeatabilitya % | Intra-dayb % | Inter-day %c |
|---|---|---|---|
| MDA | 1.3 | 5.2 | 6.9 |
| Acrolein | 2.7 | 3.5 | 7.5 |
| 4-HHE | 2.6 | 4.4 | 6.9 |
| 4-HNE | 2.1 | 2.6 | 7.8 |
| n-hexanal | 2.2 | 4.7 | 7.4 |
| n-heptanal | 4.4 | 7.9 | 9.9 |
| n-nonanal | 3.6 | 6.2 | 8.5 |
n = 12.
n = 24; four derivatized samples, each injected twice, at the beginning, in the middle and at the end of the working session.
n = 24; solutions of aldehydes were prepared daily from the 5 mM solution in acetonitrile and derivatized.
Application: determination of aldehydes in EBC
EBC is a biological matrix currently encountering both much enthusiam due to its noninvasiveness, and also a lot of scepticism due to the problems related to difficulties concerning the standardization of its collection procedures and the absence of a gold standard.33 To date, only a few papers based on the use of specific analytical techniques have been published. Very recently, a method for the determination of MDA in EBC by HPLC with fluorescence detection (FLD) has been proposed,14 although use of this method revealed no statistically significant differences between patients with and without asthma.
The LC/APCI-MS/MS method we propose was applied to the quantitative analysis of aldehydes in EBC samples from healthy subjects, both asymptomatic smokers and nonsmo-kers. The results of EBC analyses are summarized in Table 3. MDA and n-alkanals were detectable in all EBC samples, whereas 4-HHE and 4-HNE were found in only a few samples. MDA concentrations were found to be consistent with those determined in EBC using an independent HPLC-FLD method,14 and about fourfold higher than those reported in the case of BAL.7 A similar dilution factor was found between the n-alkanal levels we found in EBC and the values reported by other authors for human BAL, measured as oximes of pentafluorobenzylhydroxylamine (PFBHA) by GC/ECD.22 The reason why α,β-unsaturated hydroxylated aldehydes were not detected in some EBC samples, despite the very low LODs, could be attributed to their well-known reactivity towards thiol groups of glutathione and proteins.15,19 These adducts have already been characterized,20 and methods aimed at assessing their presence in EBC are still under development. Despite the limited number of samples examined and the problems related to the standardization of sample collection, EBC from smokers showed statistically significant higher levels of MDA, acrolein, and n-hexanal (p <0.05). Preliminary data obtained for subjects with chronic obstructive pulmonary disease (COPD) suggest a statistically significant increase in MDA, n-hexanal and n-heptanal concentrations (data not shown).
Table 3.
Results of the LC/APCI-MS/MS analysis of aldehydes (nM) produced by lipid peroxidation of membranes in breath condensate of healthy nonsmoking subjects and asymptomatic smokers, and determined as DNPH derivatives
| Healthy (n = 12)
|
Smokers (n = 13)
|
|||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| MDA | 11.2 | 1.07–23.4 | 34.7* | 16.4–56.9 |
| Acrolein | 2.33 | 1.39–3.95 | 6.78* | 6.26–7.45 |
| 4-HHE | 0.94 | 0.85–12.6 | 4.72 | 2.56–33.9 |
| 4-HNE | 0.74 | 0.25–9.04 | 3.77 | 0.74–5.75 |
| n-hexanal | 41.9 | 8.34–87.3 | 68.6* | 38.4–75.2 |
| n-heptanal | 29.9 | 7.93–46.8 | 30.8 | 14.9–39.5 |
| n-nonanal | 17.8 | 6.68–27.7 | 12.9 | 4.72–24.7 |
p < 0.05, vs. nonsmoking subjects.
Oxidative stress is a general mechanism involved in several pathological processes, including degenerative diseases,40 COPD, and other lung diseases.41 Up to now, the degree of oxidative stress has been mainly evaluated by formation of MDA, which is produced starting from PUFAs with three or more unsaturations. The approach we propose in this study to monitor free radical damage is new, and is based on the simultaneous determination of patterns of aldehydes. These patterns can reflect either a different composition in membrane cell phospholipids occurring in lung diseases42 or different membrane cell targets and mechanisms involved in the ROS attack. Further studies will be published in due course.
CONCLUSIONS
The method proposed here has the potential to be applied to the monitoring of levels of EBC aldehydes, with the aim of evaluating the oxidative status of epithelial pulmonary cells both in healthy subjects and in patients suffering from different lung pathologies including COPD, asthma, and lung cancer. Since EBC collection is noninvasive, it can be repeated several times (e.g. during and after exacerbations) and can also be applied to children.43 The application of mass spectrometry to EBC analysis could play a fundamental role in the validation of EBC as a new accessible biological matrix and in the definition of standardized EBC collection procedures.44
Acknowledgments
This study was supported in part by the Italian Ministry of Health through a grant from I.S.P.E.S.L. (Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro, Rome, Italy) and Grant Number 1R01 HL72323-01 from NHLBI. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or NIH.
References
- 1.De Zwart LL, Meerman JHN, Commandeur JNM, Vermeulen NPE. Free Rad Biol Med. 1999;26:202. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 2.Holtzman MJ, Grunberger D, Hunter JA. Biochim Biophys Acta. 1986;877:459. doi: 10.1016/0005-2760(86)90212-2. [DOI] [PubMed] [Google Scholar]
- 3.Esterbauer H, Schaur RJ, Zollner H. Free Rad Biol Med. 1991;11:81. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Clin Chem. 1997;43:1209. [PubMed] [Google Scholar]
- 5.Stalikas CD, Konidari CN. Anal Biochem. 2001;290:108. doi: 10.1006/abio.2000.4951. [DOI] [PubMed] [Google Scholar]
- 6.Hong Y-L, Yeh S-L, Chang C-Y, Hu M-L. Clin Biochem. 2000;33:619. doi: 10.1016/s0009-9120(00)00177-6. [DOI] [PubMed] [Google Scholar]
- 7.Ozaras R, Tahan V, Turkmen S, Talay F, Besirli K, Aydin S, Uzun H, Cetinkaya A. Respirology. 2000;5:289. doi: 10.1046/j.1440-1843.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 8.Antczak A, Nowak D, Shariati B, Król M, Piasecka G, Kurmanowska Z. Eur Respir J. 1997;10:1235. doi: 10.1183/09031936.97.10061235. [DOI] [PubMed] [Google Scholar]
- 9.Csallany AS, Der Guan M, Manwaring JD, Addis PB. Anal Biochem. 1984;142:277. doi: 10.1016/0003-2697(84)90465-2. [DOI] [PubMed] [Google Scholar]
- 10.Hartman PE. Environ Mutagen. 1983;5:603. doi: 10.1002/em.2860050409. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Yeo HC, Doniger SJ, Ames BN. Anal Biochem. 1997;245:161. doi: 10.1006/abio.1996.9990. [DOI] [PubMed] [Google Scholar]
- 12.Scott MD, Eaton JW. In Free Radical Toxicology, Wallace KB (ed.). Taylor & Francis: Washington, 1997; 401.
- 13.Fenaille F, Mottier P, Turesky RJ, Ali S, Guy PA. J Chromatogr A. 2001;921:237. doi: 10.1016/s0021-9673(01)00883-4. [DOI] [PubMed] [Google Scholar]
- 14.Lärstad M, Ljungkvist G, Olin A-C, Torén K. J Chromatogr B. 2002;766:107. doi: 10.1016/s0378-4347(01)00437-6. [DOI] [PubMed] [Google Scholar]
- 15.Esterbauer H. Monatsh Chem. 1970;101:782. [Google Scholar]
- 16.Sodum RS, Chung FL. Carcinogenesis. 1987;28:98. [Google Scholar]
- 17.Gioacchini AM, Calonghi N, Boga C, Cappadone C, Masotti L, Roda A, Traldi P. Rapid Commun Mass Spectrom. 1999;13:1573. doi: 10.1002/(SICI)1097-0231(19990815)13:15<1573::AID-RCM675>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Yazdanpanah M, Luo X, Lau R, Greenberg M, Fisher LJ, Lehotay D. Free Rad Biol Med. 1997;23:870. doi: 10.1016/s0891-5849(97)00070-1. [DOI] [PubMed] [Google Scholar]
- 19.Rahman I, van Schadewijk AM, Crowther AJL, Hiemstra PS, Stolk J, MacNee W, De Beor WI. Am J Respir Crit Care Med. 2002;166:490. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 20.Boon PJ, Marinho HS, Oosting R, Mulder GJ. Toxicol Appl Pharmacol. 1999;159:214. doi: 10.1006/taap.1999.8742. [DOI] [PubMed] [Google Scholar]
- 21.Pryor WA, Bermúdez E, Cueto R, Squadrito GL. Fundam Appl Toxicol. 1996;34:148. doi: 10.1006/faat.1996.0185. [DOI] [PubMed] [Google Scholar]
- 22.Frampton MW, Pryor WA, Cueto R, Cox C, Morrow PE, Utell MJ. Am J Resp Crit Care Med. 1999;159:1134. doi: 10.1164/ajrccm.159.4.9807057. [DOI] [PubMed] [Google Scholar]
- 23.Esterbauer H, Zollner H. Free Rad Biol Med. 1989;7:197. doi: 10.1016/0891-5849(89)90015-4. [DOI] [PubMed] [Google Scholar]
- 24.Kölliker S, Oehme M, Dye C. Anal Chem. 1998;70:1979. doi: 10.1021/ac9709458. [DOI] [PubMed] [Google Scholar]
- 25.Brombacher S, Oehme M, Beukes JA. J Environ Monit. 2001;3:311. doi: 10.1039/b101204p. [DOI] [PubMed] [Google Scholar]
- 26.Brombacher S, Oehme M, Dye C. Anal Bioanal Chem. 2002;372:622. doi: 10.1007/s00216-001-1227-1. [DOI] [PubMed] [Google Scholar]
- 27.Zwiener C, Glauner T, Frimmel FH. Anal Bioanal Chem. 2002;372:615. doi: 10.1007/s00216-002-1233-y. [DOI] [PubMed] [Google Scholar]
- 28.Zurek G, Karst U. J Chromatogr A. 2000;869:251. doi: 10.1016/s0021-9673(99)01240-6. [DOI] [PubMed] [Google Scholar]
- 29.Zurek G, Karst U. J Chromatogr A. 1999;864:191. doi: 10.1016/s0021-9673(99)01041-9. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien-Coker I, Perkins G, Mallet AI. Rapid Commun Mass Spectrom. 2001;15:920. doi: 10.1002/rcm.324. [DOI] [PubMed] [Google Scholar]
- 31.Mutlu GM, Garey KW, Robbins RA, Danziger LH, Rubinstein I. Am J Respir Crit Care Med. 2001;164:731. doi: 10.1164/ajrccm.164.5.2101032. [DOI] [PubMed] [Google Scholar]
- 32.Kharitonov SA, Barnes PJ. Biomarkers. 2002;7:1. doi: 10.1080/13547500110104233. [DOI] [PubMed] [Google Scholar]
- 33.Hunt J. J Allergy Clin Immunol. 2002;110:28. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 34.Vogel M, Büldt A, Karst U. Fresenius J Anal Chem. 2000;366:781. doi: 10.1007/s002160051572. [DOI] [PubMed] [Google Scholar]
- 35.Schulte-Ladbeck R, Lindahl R, Levin J-O, Karst U. J Environ Monit. 2001;3:306. doi: 10.1039/b101354h. [DOI] [PubMed] [Google Scholar]
- 36.Zurek G, Luftmann H, Karst U. Analyst. 1999;124:1291. [Google Scholar]
- 37.Singh G, Gutierrez A, Xu K, Blair IA. Anal Chem. 2000;72:3007. doi: 10.1021/ac000374a. [DOI] [PubMed] [Google Scholar]
- 38.Kölliker S, Oehme M, Merz L. Rapid Commun Mass Spectrom. 2001;15:2117. doi: 10.1002/rcm.434. [DOI] [PubMed] [Google Scholar]
- 39.Effros RM, Hoagland KW, Bosbous M, Castillo D, Foss B, Dunning M, Gare M, Lin W, Sun F. Am J Respir Crit Care Med. 2002;165:663. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 40.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Eur J Biochem. 2002;267:4904. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 41.Rahman I, Morrison D, Donaldson K, MacNee W. Am J Respir Crit Care Med. 1996;154:1055. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 42.Honda Y, Tsunematsu K, Suzuki A, Akino T. Lung. 1988;166:293. doi: 10.1007/BF02714060. [DOI] [PubMed] [Google Scholar]
- 43.Corradi M, Folesani G, Andreoli R, Manini P, Bodini A, Piacentini G, Carraro S, Zanconato S, Baraldi E. Am J Respir Crit Care Med. 2003;167:395. doi: 10.1164/rccm.200206-507OC. [DOI] [PubMed] [Google Scholar]
- 44.Corradi M, Rubinstein I, Andreoli R, Manini P, Caglieri A, Poli D, Mutti A. Am. J. Respir. Crit. Care Med. 2003, published ahead of print on January 9, 2003. [DOI] [PubMed]


