Abstract
HOLLAND, P.C. AND G.D. PETROVICH. A neural systems analysis of the potentiation of feeding by conditioned stimuli. PHYSIOL BEHAV xx(x) 000-000, 200x. Associative learning processes play many important roles in the control of food consumption. Although these processes can complement regulatory mechanisms in the control of eating by providing opportunities for the anticipation of upcoming needs, they may also contribute to inappropriate or pathological consumption patterns by overriding internal regulatory signals. In this article, we first review some of the ways in which associative learning can contribute to the control of feeding, and then describe a neural systems analysis of a simple animal model of the control of feeding by Pavlovian conditioned stimuli (CSs). Food-sated rats increase their food consumption after presentation of CSs that were previously paired with food while the rats were food-deprived. This cue-potentiated feeding is independent of conditioned approach responses, and is at least somewhat specific to the foods associated with those CSs. A series of studies that used neuroanatomical tract tracing, immediate early gene expression, and neurotoxic disconnection lesion techniques implicated circuitry that includes the basolateral complex of the amygdala, the lateral hypothalamus, and the medial prefrontal cortex, but not the amygdala central nucleus, nucleus accumbens, or lateral orbitofrontal cortex, in cue-potentiated feeding. These studies also showed dissociations between cue-potentiated feeding and other learned motivational phenomena that are known to depend on function of amygdala systems. The data suggest that cue-potentiated feeding is uniquely mediated by cortical and amygdalar neurons that directly target the lateral hypothalamus, and thus gain access to hypothalamic neuropeptide and other systems involved in the promotion and suppression of eating.
Keywords: potentiation of feeding, amygdala, hypothalamus, prefrontal cortex, nucleus accumbens, Arc, homer 1a
1. Introduction
Evidence presented in many articles from this symposium attest to the role of “non-regulatory” controls over feeding, including a variety of preabsorptive signals provided by food itself, and features of the food environment. These cues may lie at the heart of the recent rapid growth in overweight and obesity, as through learning, they may come to induce and maintain eating even in the face of cues normally linked to satiety and the cessation of eating. In this article, we discuss roles for associative learning, especially Pavlovian conditioning, in the control of eating under conditions of satiety. We first outline some of the many ways in which associative learning may influence feeding in general. Next, we describe a neural systems analysis of a simple animal model of the control of feeding by Pavlovian conditioned stimuli (CSs) under conditions of satiety. Finally, we relate this analysis to broader conceptualizations of motivation and the control of behavior.
2. Influences of associative learning on feeding
Psychologists distinguish between two models of associative learning, instrumental or operant conditioning, which involves response-outcome contingencies, and Pavlovian or classical conditioning, which involves stimulus-outcome contingencies. Although it is often difficult to determine the extent to which each of these contingencies contributes in any particular behavioral context, analyses of the role of associative learning in feeding typically apply one or the other model, but not both.
Most analyses of foraging and food procurement are based on instrumental conditioning models, in which current behavior is based on past experience with the consequences of particular responses [1]. For example, optimal foraging theory and related approaches [2, 3, 4] have been quite successful in describing both global and local (real-time) response-outcome contingencies that govern many aspects of foraging and food procurement. Decisions about when to initiate and terminate foraging and consumption, and choices between different food-containing patches and between different food items, are thought to be based on the differential reinforcement history of making these choices in the past. Likewise, as Balleine ([5] this issue) describes, studies using instrumental conditioning procedures have been very informative about how food-procuring behavior is guided by representations of the outcomes of that behavior, representations that may include both affective and detailed sensory properties of the foods procured.
In this article we focus instead on roles for Pavlovian learning in feeding. In discussing these roles it is useful to consider two questions: what are the critical events for Pavlovian food-based conditioning, and what are the products of that Pavlovian learning?
2.1. Critical events in Pavlovian learning
In the simplest Pavlovian conditioning procedures, a relation is arranged between two events, without respect to the subject’s behavior. In the prototypical case, an initially neutral stimulus, a metronome (the conditioned stimulus, or CS) was sounded immediately before the delivery of food (the unconditioned stimulus, or US) to a dog subject. Pavlov [6] found that these sound-food pairings resulted in the dog’s eventual display of various digestive responses such as salivary and gastric secretions in the presence of the metronome alone, before food was delivered.
Although in this example, the metronome and food delivery were identified as the nominal CS and US, respectively, specifying the events that truly serve these roles in any particular experimental or real-life feeding situation is often difficult. In our example, what aspects of the “food delivery” US are critical to its ability to support the acquisition of CRs? The food’s smell? Oral, but preingestive properties, such as taste or texture? Post-ingestive, but pre-absorptive, such as gastric, duodenal, or intestinal stimulation? Metabolic consequences of food consumption? The regulatory responses to those metabolic consequences? The identification of which aspects of “food delivery” are critical for learning various CRs and other products of learning (described below) is often a key part of understanding the contribution of Pavlovian learning to the control of feeding and food-related behaviors. For example, many investigators have attempted to parcel out the roles of oral and post-ingestive features of food in learning by using non-nutritive but highly palatable foods as reinforcers, sham feeding procedures in which food is consumed but does not reach various stages of digestion, or procedures in which food is infused directly into components of the digestive system without active consummatory responses [7, 8, 9].
Identifying the CS can be equally difficult. Although the experimenter may easily identify the nominal CS in a laboratory study, natural feeding situations encompass many potential signals, including spatial locations, more discrete external signals, and internal state cues, each of which may come to predict food and control CRs [10, 11, 12]. Furthermore, in both laboratory and field settings, some aspects of food delivery itself occur in signaling relations with other aspects of food delivery, and thus may themselves serve as CSs. For example, sensory features of food may serve as signals for later events in the feeding sequence, including post-ingestive stimulation of the digestive system and the initiation of metabolic processes. Indeed, the ability of some sensory features of food to serve as reinforcers may be related to learning of the relations between those features and other properties of food. For example, Pavlov noted that the ability of food odor alone to elicit salivation and to support CRs depended on explicit developmental experience of the odor in concert with other properties of the food. Several investigators at Purdue’s Ingestive Behavior Research Center, including Mattes, Powley, and Swithers, consider this point in their contributions to this issue.
2.2. Products of Pavlovian learning
Pavlovian conditioning has many consequences that are important for feeding. Perhaps most obvious are the overt autonomic and skeletal CRs that occur when CSs are presented. However, other products of Pavlovian conditioning may be at least as important in modulating feeding. These include changes in one’s evaluation of the CS, learned stimulus control over motivational states, alterations in attention to particular internal and external events, the establishment of outcome expectancies that encode sensory and affective information about the US, and the acquisition of modulatory power over the action of other learned associations. In this section, we briefly outline each of these functions.
2.2.1. Overt CRs
Early reviews of Pavlovian conditioning emphasized CRs mediated by the autonomic nervous system. Clearly, these CRs are important to adaptive function in feeding, both in terms of increasing the efficiency of feeding and digestion and protecting the body from deleterious side effects of large meals [13]. For example, the conditioned secretion of saliva and stomach acids in response to reliable signals for food may facilitate early digestion of that food. Likewise, as Woods and others point out [10, 14 this issue], the conditioned release of insulin in response to food-related cues protects individuals from massive hyperglycemia, which would normally be expected with digestion of large meals of carbohydrates. In general, regulatory activity is more effective and efficient if it can occur proactively, rather than in response to homeostatic perturbations.
However, organized skeletal responses are subject to Pavlovian conditioning as well. For example, cues paired with food come to evoke approach behavior, which brings the animal into contact with that food. In the natural world, these cues include a variety of stimuli including spatial location cues and more discrete visual, auditory or olfactory cues that serve as “beacons” in directing approach from a distance. Indeed, in some circumstances this conditioned tendency to approach stimuli paired with food can be so strong as to be maladaptive, such that animals approach and contact the signal rather than the food itself [15]. Timberlake and others [16, 17] have described how a number of detailed aspects of organized behavior systems, ranging from activity related to the general search for food, to focal foraging or predatory responses, to consummatory responses such as chewing, can come under the control of cues for food.
2.2.2. Changes in CS evaluation
CSs may acquire value as a result of Pavlovian learning. The most common example is that of conditioned reinforcement, in which a CS paired with food acquires the ability to serve as a reinforcer for new learning, as if it acquired its own motivational significance. Conditioned reinforcement is observed in both Pavlovian and instrumental settings. In Pavlovian “second-order conditioning”, learning about a new stimulus occurs when it is paired with a previously trained CS, but in the absence of the original US. For example, after a light CS is paired with food, subsequent tone-light pairings result in the acquisition of CRs to the tone, even though it is never paired with food [18]. Various control procedures are used to show that this second-order conditioning of the tone depends on both tone-light pairings and prior conditioning of the light. Similarly, in instrumental “secondary reinforcement”, subjects will learn to perform a new response that earns only presentations of a previously conditioned stimulus, in the absence of any primary reinforcer such as food [19]. For example, rats will learn to press a lever that earns presentations of the light CS from our last example.
Observations that initially neutral stimuli such as lights and tones can acquire the ability to serve as reinforcers as a result of Pavlovian learning are consistent with the claim that such learning may involve changes in the motivational significance of initially neutral stimuli. These learned changes in motivational significance are important to feeding for two reasons. First, the occurrence of second-order conditioning and secondary reinforcement extends the domain of Pavlovian conditioning to situations in which food is not present, but in which food cues are present. Thus, new stimuli paired with food cues established by previous Pavlovian learning may themselves come to influence feeding and food-related behavior. Second, as noted briefly in section 2.1, the ability of certain aspects of food itself (e.g., its sensory properties) to serve as reinforcers may in some circumstances depend on prior learning of associations between those stimulus properties and other aspects of food (e.g., post-ingestive consequences).
According to many investigators [20, 21], the frequently-observed conditioned approach responses to localizable cues for food (described in section 2.2.1) may reflect acquisition of value by those cues. From this perspective, as a result of learning, animals approach food-related cues just as they would approach more intrinsically-valued objects, such as food itself. A particularly interesting, but contentious, case of acquired value of a CS has been termed “alliesthesia” [22, 23], in which the value invested in the CS as a result of learning represents a “hedonic shift” [24] in the individual’s response to the stimulus in its own right. For example, evidence from analysis of the microstructure of rats’ licking suggests that initially neutral (or even preferred) flavors paired with illness come to be unpalatable, to taste “bad”, apart from the rats’ learning to suppress consumption of those flavors [25]. Likewise, pairing an initially neutral flavor with either intraoral [26] or intragastric [9] infusion of sugars can lead to increased palatability of that flavor.
2.2.3. Conditioned emotional states
Many descriptions of Pavlovian conditioning, especially those of two-process theories [27], emphasize the conditioning of emotional or motivational states. Although the largest application of these ideas has been in the case of aversive, “fear” conditioning, when CSs are paired with noxious stimuli such as electric shock, a variety of motivational states have also been ascribed to CSs paired with the presentation (or omission) of food, including appetite, hunger, incentive, wanting, craving, hope, satiety, disappointment, and frustration [28, 29, 30, 31, 32, 33, 34]. Interestingly, none of these terms has won wide acceptance in describing the emotional/motivational consequences of CSs paired with food, whereas the array of consequences of aversive conditioning is almost universally described as reflecting conditioned fear. Nevertheless, several behavioral outcomes are widely viewed as indicating conditioned appetitive motivational states. Two of these outcomes are notable because of their relevance to the feeding studies described in section 3 of this article. First, CSs previously paired with food may enhance the rate of food-reinforced instrumental responding (e.g., lever-pressing) by food-deprived rats, a phenomenon that is now termed “Pavlovian-instrumental transfer”. Second, CSs paired with food while rats are food-deprived can increase the amount of food consumed by those rats later, when they are food-sated [35]. Thus, Pavlovian CSs paired with food can modulate the performance of both appetitive responses leading up to food and consummatory responses to food itself.
2.2.4. Changes in attention
Pavlovian relations arranged between CSs and food can alter subsequent processing of those CSs in new learning tasks. In the simplest case, arranging relations between a particular property of an event and reinforcement may make animals more likely to use that stimulus property in solving subsequent problems, even when those problems involve very different events. For example, faced with a choice of novel stimuli that differ in both odor and texture, rats are more likely to choose on the basis of texture if texture has been predictive of reinforcement in other tasks [36, 37]. Thus, prior experience in feeding situations may make animals more likely to use particular stimulus features (e.g., social cues, external signals, oral cues, or internal, state cues) in new learning situations involving food. Somewhat paradoxical is the observation in other circumstances, that the omission of an event predicted on the basis of prior Pavlovian learning (e.g., omission of S2 after S1→S2 learning) can make the stimulus that normally predicts the omitted event (S1 in this case) acquire new learning more rapidly [38, 39]. Given that various sensory properties of food itself may often play the role of CS, with post-ingestive consequences of food serving as the US ([40]; see section 2.1), changes in predictive relations among these aspects of food may encourage especially rapid new learning about the sensory features of food.
2.2.5. Outcome expectancies
In many conditioning situations, detailed sensory and affective information about the US is encoded with the CS. This information can be used in advance of US delivery in a variety of ways that allow for flexibility of behavior in changing conditions. For example, consider a “devaluation” experiment in which rats first receive pairings of one auditory CS with one food and another auditory CS with another, differently-flavored food. Next, the value of one of those foods is reduced by pairing it with an illness-inducing toxin, in the rats’ home cages, in the absence of the auditory CSs. Finally, responding to the two auditory CSs is assessed, in the absence of the foods themselves. Typically, responding to the to CS whose food partner had been devalued is substantially reduced relative to responding to the CS whose food partner maintained its value [41, 42].
This observation of reduced CRs after devaluation of the food US is important because it shows that Pavlovian CSs can code detailed information (in this case, flavor) about upcoming food reinforcers. As a result, the influence of Pavlovian CSs on food-related behavior may be very food-specific. For example, Pavlovian-instrumental transfer (described in section 2.2.3) is typically quite reinforcer-specific, that is, Pavlovian CSs previously paired with particular reinforcers are more likely to facilitate operant responding that had been reinforced by that same reinforcer [5: Balleine this issue, 43]. Likewise, the ability of a CS to enhance food consumption in sated rats is largely specific to the particular food that the CS originally signaled (see section 4.2.)
Specific Pavlovian outcome expectancies may influence food-related behavior in other ways as well. For example, they may serve as discriminative stimuli in the control of operant responses, as shown in “differential outcome expectancy” studies [5: Balleine this issue, 44, 45]. Thus, animals can use these Pavlovian outcome expectancies to facilitate learning to perform one response when they expect one food and another response when they expect a different food. Finally, in a variety of circumstances, a CS for food may substitute for that food itself in new learning. For example, Holland [42, 46, 47] established aversions to a particular flavored food by making rats ill in the presence of a CS that had been previously paired with that food, as if illness while “thinking about” a particular food was sufficient to establish an aversion to that food. The specificity of the aversion to that CS’s food partner in those experiments showed that the CS coded particular sensory features of the food.
2.2.6. Occasion-setting
In each of the previous examples, Pavlovian learning occurred with the arrangement of simple CS-US relations, such as pairing, between two stimuli. Slightly more complex Pavlovian relations may endow a stimulus with a modulatory function, occasion-setting, by which it signals the validity of a relation between other stimuli [48, 49]. Consider a discrimination in which a tone is paired with food only when it is preceded by a light: presentations of a light→tone sequence are followed by food but presentations of the tone alone are not reinforced. Although the logically simplest solution to this task is to acquire Pavlovian light-food associations, rats instead typically form tone-food associations, and the light acquires the ability to modulate the action of those associations. This modulatory power is in most cases independent of the light’s own Pavlovian associations with the US; for example, establishment or extinction of direct light-food associations neither interferes with nor enhances the light’s ability to set the occasion for the reinforcement of the tone. Importantly, the occasion-setting powers of CSs follow somewhat different rules from those of simple CS-US associations [48]. Thus, specifying the roles of Pavlovian learning processes in any particular feeding context requires distinguishing between the contributions of simple conditioning and occasion-setting.
Of special interest for the case of feeding is the observation that contextual cues [50] and internal state cues, such as those provided by drugs [51] or deprivation conditions [10, 11, 52: Davidson this issue], often seem to function as occasion-setters rather than simple Pavlovian CSs. For example, Davidson [10, 11] suggested that deprivation state cues may set the occasion for the action of associations between food cues and positive postingestive consequences of food. Within this perspective, hunger itself is not directly associated with these postingestive events, and thus does not elicit responses based on them, but instead signals when food cues will be followed by these positive consequences of food. Thus, through Pavlovian occasion-setting, hunger modulates feeding based on the learned relation between food cues and positive postingestive events.
This list is not meant to be exhaustive. Instead, our point is that simple Pavlovian event relations inherent in feeding situations provide opportunities for a variety of learning products, which can influence feeding in many ways. In the rest of this article, we focus on one of those sequellae of Pavlovian learning, the cue-potentiated feeding of food-satiated rats.
3. A neural systems analysis of cue-potentiated feeding
Presentation of meal-associated cues will encourage otherwise food-sated individuals to eat in many circumstances. For example, humans eat beyond satiety if presented with a food cue, such as the brief sight, smell and taste of a particular food prior to eating [53]. This effect of enhanced eating after pre-exposure to food cues is further exaggerated in restrained eaters [54, 55: Herman this issue). Eating is also augmented by CSs previously paired with food in rats, both under conditions of food deprivation [56] and satiation [57]. This augmentation is consistent with the common view of Pavlovian conditioning by which CSs gain control over motivational processes, such as craving, hunger, or incentive, which orchestrate food-related behavior (section 2.2.3). In collaboration with Michela Gallagher, we began exploiting this observation in the context of a broader research project concerned with the role of different subregions of the amygdala in the various aspects of associative learning described in section 2. The amygdala has long been identified with emotion and emotional behavior [58, 59]; our work was designed to illuminate its roles in other aspects of learning, ranging from cognitive control to the modification of species-specific behavior [38].
3.1. Amygdala subsystems and cue-potentiated feeding
Initially, we viewed the cue-potentiated feeding phenomenon as a simple assessment of motivational deficits consequent to amygdala damage. In our first experiments, sated rats’ consumption of food pellets was examined in the presence or absence of visual, auditory or visual+auditory compound CSs that had been paired with food while rats were food-deprived. Rats with sham lesions and rats with neurotoxic lesions of the central nucleus of the amygdala (CEA) consumed 15–20 more 45-mg food pellets in 10-min test sessions that included CSs than in similar test sessions that did not [60]. However, rats with neurotoxic lesions of the basolateral area of the amydala (BLA, including basolateral [“basal”], basomedial [“accessory basal”], and lateral nuclei) failed to eat additional pellets in the presence of the CSs [61, 62]. At the same time, those BLA-lesioned rats showed no deficits in the performance of a variety of overt CRs to the CSs, including conditioned ORs and food-cup approach responses. These results were consistent with other data that suggested a role for BLA but not CEA in conditioned motivational functions. For example, earlier we [63] found that BLA lesions, but not CEA lesions, interfered with the acquisition of second-order conditioning (section 2.2.2) and the sensitivity of CRs to post-training devaluation of the food US by taste aversion (section 2.2.5).
Subsequent studies in our laboratory have examined the neural systems underlying this phenomenon. We describe the first of these studies in some detail because its procedures are common to most of the experiments described here. In separate experiments, we [64] first made bilateral neurotoxic or sham lesions of BLA or CEA. After two-weeks recovery, the rats were food-deprived (85% ad lib weights) and trained on an auditory discrimination, in which one 10-s auditory cue (CS+) was paired with delivery of 2 45-mg food pellets to a recessed food cup and another (CS−) was presented without food reinforcement. Next, the rats were food-sated for 7 days, their weights reaching 110–115% of their original ad lib weights. Finally, each rat’s consumption of food pellets was tested in four conditions, on separate days. In each of these tests, the rats were first placed in their experimental chambers for 10 min with food pellets freely available. The purpose of these pretest periods was to give the rats opportunity to consume the food pellets used in training and testing, increasing the likelihood that the rats were satiated in the tests proper. In both rats and humans, nominally sated individuals often eat more when palatable foods are made available [53]. The rats were then removed, pellets quickly removed for later counting, and new pellets and the rats replaced for the test itself. In two of the tests, the food pellets were placed in the food cup used in training and in the other two tests the food pellets were placed in a bowl on the opposite side of the chamber. For each food location, in one test the CS+ was presented 10 times in 10 min and in the other test the CS− was presented 10 times in 10 min.
While food-deprived, amygdala- and sham-lesioned rats learned the auditory discrimination at the same rate, responding to the CS+ with a rapid approach to the food cup followed by maintaining their heads within the food cup. Similarly, they gained the same amounts of weight and entered the post-satiation tests with similar body weights. Figure 1 shows food pellet consumption in the test sessions. Both sham-lesioned and CEA-lesioned rats showed significantly more food consumption in the presence of CS+ than in the presence of CS−, regardless of whether the food pellets were found in the usual container and location (cup test) or a new location and container (bowl test). However, BLA-lesioned rats failed to show any potentiated feeding in CS+ test sessions.
Figure 1.
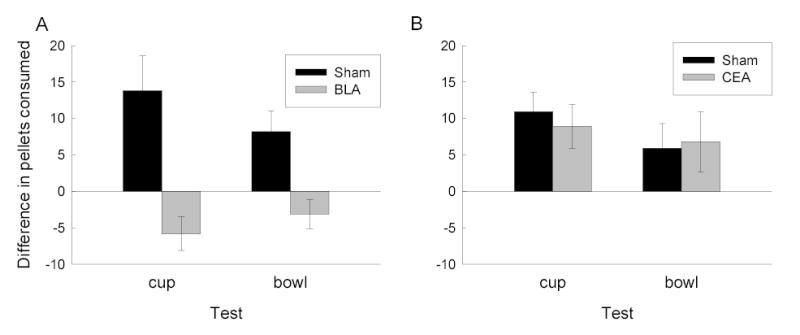
Cue-potentiated feeding in rats with bilateral neurotoxic or sham lesions of (A) basolateral amgydala (BLA) or (B) central nucleus of the amygdala (CEA). The bars show the mean ± sem differences in the number of 45-mg food pellets consumed in tests with a previously reinforced conditioned stimulus or a previously nonreinforced stimulus. In separate tests, food pellets were presented in either the original food cups used in conditioning or in bowls placed elsewhere in the conditioning chambers.
Several results of this study are important for understanding the nature of cue-potentiated feeding and the role of amygdala systems in that phenomenon. First, the observation that the effects of the CSs were discriminative (more consumption during CS+ than CS− sessions) showed that the associative history of the CSs was critical to potentiated feeding; the CSs were not just waking the rats, for example. Second, the observation of potentiated feeding when the food was presented in a different container and location showed that conditioned food-cup approach responses learned in the initial discrimination phase, which might increase the opportunity to eat, were not critical to the enhanced eating. Indeed, conditioned approach to the food cup might be expected to reduce consumption of food placed in the bowl. Third, regardless of food location in the consumption tests, food-sated rats spent relatively little time with their heads in the food cup during CS presentations themselves, and approached it only with long latencies. Instead, observations from video tapes suggested that the rats primarily consumed food many seconds after the termination of the CSs themselves. Indeed, measures of the rats’ latencies to approach the food cup after CS onset in the tests showed little evidence for discriminative performance of that response in CeA- and sham-lesioned rats, which nevertheless showed substantially more food consumption during CS+ sessions than during CS− sessions. Thus, the potentiated feeding effect seems more interpretable in terms of some learned motivational function than overt CRs acquired in the initial training.
Interestingly, unlike these rats, rats with BLA lesions, which failed to show greater food consumption during CS+ sessions, showed shorter latencies to enter the food cup on CS+ trials than on CS− trials. Furthermore, the latencies to enter the food cup on CS+ trials were shorter for BLA-lesioned rats than for rats in the other lesion conditions. This observation is likely interpretable in the context of devaluation studies, described in section 2.2.5. Post-conditioning food satiation is often used as a method of devaluing a food US after CS-food pairings (5: Balleine this issue, 18). The lack of a rapid overt CR to the CS+ after satiation in intact rats may in part reflect such devaluation effects: if food is of little value to food-satiated rats, then the rats might be expected to respond less to the CS+. In previous studies [63] we observed such devaluation effects in rats with sham or CEA lesions, but not in rats with BLA lesions. If the BLA-lesioned rats in this study were less susceptible to US-devaluation effects on the response to CS+, then they might be expected to respond more rapidly to that cue than sham-lesioned rats. Regardless of the viability of this devaluation interpretation of the food cup response latency data, it is notable that when the CS+ was presented, the BLA-lesioned rats entered the food cup faster than sham-lesioned rats, but they ate less food. This dissociation provides further evidence that potentiated feeding is not derived from simple approach CRs. Finally, the observation that appetitive and consummatory behavior was differentially affected by these lesions warns us that when we study the effects of brain lesions or other manipulations on feeding, we should be careful to measure feeding itself, and not just the latency to approach a food cup or the amount of time spent there.
3.2. BLA-lateral hypothalamus communication and cue-potentiated feeding
Our next study [65] showed that the occurrence of cue-potentiated feeding depends on communication between the BLA and the lateral hypothalamus (LH), an important part of hypothalamic circuitry involved in feeding, and which has historically been linked to the initiation of feeding [66]. Rats received unilateral neurotoxic lesions of the BLA and LH, either in the same or opposite hemispheres. Because the BLA-LH connections are almost exclusively ipsilateral, rats with contralateral lesions lack communication between BLA and LH in either hemisphere. Thus, any function that depends on BLA-LH communication would be disrupted in the contralateral lesion preparation. At the same time, because each structure is intact in one hemisphere, other circuitry and functions of BLA and LH, which do not require their communication, would be less impaired, if at all. By contrast, although rats with ipsilateral lesions of BLA and LH would have the same amount of damage to each structure as rats with contralateral lesions, they would retain intact communication between those structures in one hemisphere, and hence should display less impairment of potentiated feeding than rats with contralateral lesions. Rats with contralateral, ipsilateral, or sham lesions of BLA and LH were trained and tested as in the previous study. Lesioned rats were unimpaired in their acquisition of discriminated food cup approach CRs to the auditory CSs. However, only the sham-lesioned and ipsilaterally lesioned rats showed potentiated feeding (Figure 2A). Thus, BLA’s communication with LH is critical to the display of this phenomenon.
Figure 2.
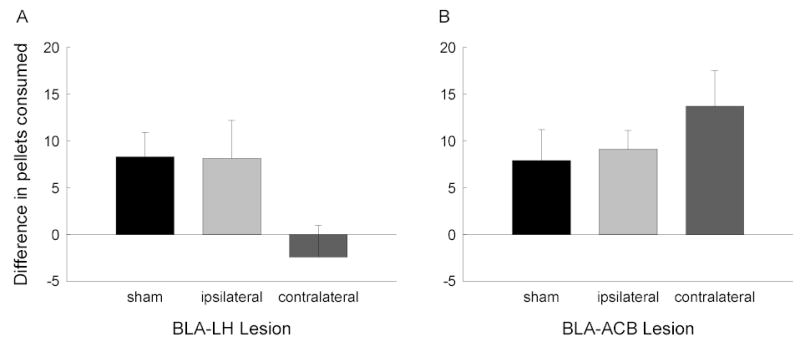
Cue-potentiated feeding in rats with unilateral neurotoxic or sham lesions of the basolateral amgydala (BLA) and (A) the lateral hypothalamus (LH) or (B) the nucleus accumbens (ACB). In rats with contralateral lesions of BLA and LH or ACB, these regions were functionally disconnected from each other in both hemispheres, whereas in rats with ipsilateral lesions, communication was intact in one hemisphere. The bars show the mean ± sem differences in the number of 45-mg food pellets consumed in tests with a previously reinforced conditioned stimulus or a previously nonreinforced stimulus.
However, this study does not identify how information necessary for cue-potentiated feeding reaches LH from BLA. Although BLA projects directly to LH [67], BLA also projects to several other brain regions that in turn project to LH, including the CEA, the nucleus accumbens (ACB), the prefrontal cortex (PFC), the hippocampal formation, the bed nuclei of the stria terminalis, the substantia innominata, and the ventromedial hypothalamus [67, 68, 69, 70, 71]. The remaining studies that we describe here were designed to uncover the particular amygdala-hypothalamic circuitry that mediates cue-enhanced eating.
3.3. A functional anatomical and immediate-early gene analysis of cue-potentiated feeding
This study [72] combined anatomical tract-tracing and immediate-early gene (IEG) methods to map circuitry engaged in cue-potentiated feeding. The first experiment focused on direct projections to LH from BLA, CEA, ACB, and PFC. Notably, each of these regions also receive direct projections from BLA. Rats first received injections of the retrograde tracer, FluoroGold (FG), into the LH. After recovery, they were trained on an auditory discrimination while food-deprived, and then placed on ad lib food for a week, as in the previous studies. Next they received testing of food consumption in the presence of CS+ or CS−, in two consecutive tests designed to permit the detection of CS-induced expression of the IEGs Arc ([73]; also known as Arg3.1 [74]), and Homer 1a (H1a) [75], which have a time-limited appearance in the nucleus of activated neurons [76]. Rats were first placed in their chambers with food available in food bowls for 5 min, removed for 25 min and then replaced in the chambers for 5 min with food again available. For half of the rats, the first test included CS+ presentations and the second test included CS− presentations, and for the other half of the rats the test order was reversed. Immediately after the second test, the rats were euthanized, perfused and their brains prepared for IEG and tracer analysis.
Arc mRNA appears in the nucleus about 2–10 min after activation, but is no longer visible there after 20 min, whereas H1a mRNA first appears in the nucleus 25–35 min after activation. Thus, neurons activated in the first test would express nuclear mRNA for H1a, and those activated in the second test would express nuclear mRNA for Arc. Because the test order was counterbalanced, each IEG equally often indexed activity induced by CS+ or CS−. Thus, neurons that were activated by aspects common to both test sessions, for example, placement in the chamber, the presence of food or auditory stimuli, or eating itself, would express both IEGs. By contrast, neurons that responded selectively to CS+ in the potentiated feeding situation would express only the IEG appropriate to the test that included CS+ presentations.
Importantly, recall that the rats were first injected with a retrograde tracer into the LH. This label permitted us to identify individual neurons in each of the regions of interest (BLA, CEA, ACB, and PFC) that projected directly to LH. Thus, the brain tissue was processed with combined double-label fluorescence in situ hybridization and immunohistochemistry to visualize the markers for the IEGs (nuclear mRNA) and retrograde tracer (FG), respectively. In each target region, neurons that both projected to LH and were activated by CS+ would label for both FG and the IEG specific to CS+, but not the IEG specific to CS−. Neurons displaying each of the labels were counted using fluorescent confocal microscopy.
The rats in this experiment acquired the auditory discrimination as in previous studies. Furthermore, in the test procedure, which was modified from previous experiments to accommodate measurement of the IEGs, they displayed significant potentiated feeding. That is, they consumed 6.0 ±1.1 more pellets in the CS+ session than during the CS− session. Thus, the CS-potentiatied feeding effect was robust even when consumption during the two CSs was assessed in tests only briefly separated in time.
Consistent with previous anatomical studies [70, 77, 78, 79, 80], substantial numbers of retrogradely-labeled neurons were found in each of these regions. In BLA, most FG-labeled neurons were concentrated in the posterior basomedial nucleus and a small strip within the adjacent posterior basolateral nucleus. In the CEA, FG-labeled neurons were concentrated in a discrete zone of the medial CEA, and in the ACB, labeled neurons were found primarily in the caudal shell. In the PFC, labeled neurons were found primarily along the medial wall of the prefrontal cortex within the prelimbic and infralimbic areas, extending into the medial and ventrolateral orbitofrontal cortical areas.
The primary purpose of this experiment was to determine which inputs to LH were selectively activated by CS+ in the feeding tests. In both BLA and PFC, significantly more of the FG-labeled neurons also labeled specifically for the IEG appropriate to CS+ tests (19.8 ± 2.9% and 19.0 ± 1.7%, respectively), than labeled specifically for the IEG appropriate to the CS− test (8.0 ±2.2% and 7.3 ±0.5%). By contrast, there was no such selectivity for either ACB (9.2 ± 2.6% vs 8.2 ±3.4%) or CEA (5.7 ±2.2% vs 13.3 ±3.4%). Thus, these results indicate that direct inputs to LH from BLA and PFC, but not those from ACB or CEA, contribute to the cue-potentiated feeding effect.
The role for BLA but not CEA inputs to LH is consistent with the lesion data described in section 3.1 [64], in which BLA but not CEA lesions prevented cue-potentiated feeding. But at first glance it is surprising that we found no selective activation of ACB neurons by CS+ neurons in this study, given that many researchers have found substantial involvement of this region in feeding and reward [81: Kelley this issue). However, note that the double-labeling results suggest only that ACB plays no specific role in cue-potentiated feeding. A high proportion of FG-labeled neurons in all of the regions of interest, including ACB, labeled for both IEGs. In the absence of no-stimulus, no-food, and no-test controls it is difficult to ascertain the origin of this nonselective activation, but one possibility is that these regions were nonselectively activated by the feeding experience. Our next two experiments sought to examine the roles of ACB and PFC further by using lesion techniques.
3.4. Role of BLA-ACB communication in cue-potentiated feeding
Communication between BLA and ACB is crucial to other learned motivational consequences of Pavlovian conditioning. Rats with contralateral lesions that disconnected BLA and ACB fail to acquire conditioned reinforcement value to a visual stimulus paired with food [82]. Although they acquired normal levels of overt CRs to that cue, they did not acquire second-order conditioning of a tone paired with that light (see section 2.2.2). Thus, it seemed reasonable to suspect that communication between BLA and ACB might be critical to the ability of a Pavlovian CS to enhance food consumption. Together with Barry Setlow, we recently examined the effects of BLA-ACB disconnection on potentiated feeding. Rats received either contralateral or ipsilateral lesions of BLA and ACB prior to auditory discrimination training and cue-potentiated feeding training as described in section 3.2 [65]. Figure 2B shows the results of the consumption tests. Rats with contralateral disconnection lesions and ipsilateral lesions showed equivalent levels of potentiated feeding. Thus, the BLA-LH interaction in potentiated feeding found by Petrovich et al., ([65]; section 3.2), was clearly not mediated by BLA’s projections to the ACB. Although the results of this experiment do not preclude a direct role of ACB-LH connections in cue-potentiated feeding, the lack of selective activation of ACB inputs to LH by CS+ in our labeling study described in section 3.3 makes that possibility seem less likely.
3.5. Role of prefrontal cortex in cue-potentiated feeding
Our labeling study (section 3.3) revealed highly selective activation of PFC inputs to LH by CS+. Recently, we examined the effects of lesions of this region on potentiated feeding. In this experiment, we used a different training procedure, in which the signal for food was a context (experimental chamber) rather than a discrete auditory cue. Rats received either neurotoxic or sham lesions of the medial PFC (mPFC) bilaterally. The lesions encompassed the prelimbic, infralimbic, and medial orbitofrontal areas, and closely matched the region of the PFC that was functionally activated in our labeling study. Half of the rats in each lesion condition were given food pellets in a distinct context (paired), while the other half of rats were placed in the context without the food (unpaired). All rats were food-deprived prior to training sessions. After training, the rats were sated, and then tested for food consumption in the training context. As shown in the left bars of Figure 3, sham-lesioned rats showed conditioned potentiation of eating: during the tests, rats in the paired group ate significantly more food pellets than rats in the unpaired group. In contrast, in rats with mPFC lesions, there was no difference in the number of pellets consumed during consumption tests by paired and unpaired groups. These results show that, like BLA, an intact mPFC is a critical part of the circuitry that mediates conditioned potentiation of feeding.
Figure 3.
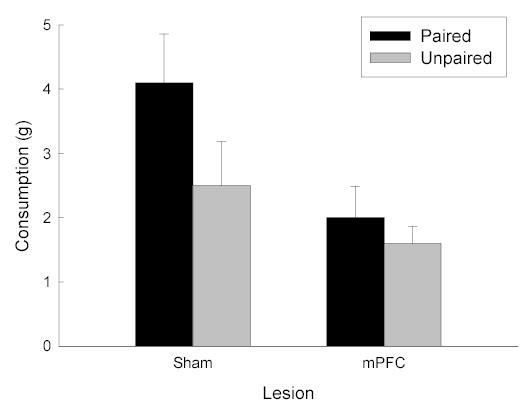
Cue-potentiated feeding in rats with bilateral neurotoxic or sham lesions of the medial prefrontal cortex (mPFC). Consumption of food pellets (in grams, mean ± sem) was measured in a context in which rats had previously been fed (paired) or not fed (unpaired) in the past.
It is notable that this region of the prefrontal cortex has substantial reciprocal connections with BLA [68, 71, 77, 78, 83]. Thus, BLA’s influence on LH might occur both via its direct connections and by modulating the activity of mPFC inputs to LH. In another functional labeling study, similar to the one described previously, we injected retrograde tracer (FG) into the region of mPFC in which we saw CS-activated neurons that projected to LH in the previous study. Substantial numbers neurons that project to the mPFC were found within BLA, primarily within the posterior basolateral nucleus itself. These neurons, like the neurons that project to LH, were selectively activated by CS+. Thus, in cue-potentiated feeding, BLA may influence hypothalamic systems for eating both via its direct projections to LH and indirectly via its projections to mPFC, which in turn also innervates the LH. It would be of interest to determine if the mPFC targets of BLA neurons activated by CS+ include neurons that project to LH.
Finally, a recent study from our laboratory [44] shows that another prefrontal region that also has substantial reciprocal connections with BLA, an area of the orbitofrontal cortex (OFC) that is both posterior and lateral to the PFC region we studied earlier, is not critical to cue-potentiated feeding. Using the discriminated auditory cue procedures described previously, we found no effect of bilateral lesions of this region on cue-potentiated feeding. Although this region was not a focal area in Petrovich et al.’s [72] study, a cursory examination of tissue from that study found no FG labeling there, which indicates that there are no direct projections from this region of the PFC to the LH.
3.6. A neural system for cue-potentiated feeding
Evidence presented in this article, from studies that combined behavioral and functional anatomical methods, highlight an amygdalo-prefrontal-hypothalamic network that is critical for the control of feeding by learned cues. The BLA and mPFC share extensive bidirectional connections, which permit a variety of distinct functional circuitry within the network. For example, direct BLA-LH connections and pathways via relays in the mPFC (BLA-mPFC-LH) might both be needed to mediate cue-potentiated eating. On the other hand it is possible that either of the two pathways is sufficient to mediate cue-potentiated eating.
In our experiments, lesions of the bilateral BLA, and disconnections of the BLA and LH system, which abolished potentiation of eating by CSs, were made prior to training. Thus, the results of these studies did not provide insights to whether these structures are critical during learning, memory maintenance, or expression of cue-potentiated eating. The roles of the BLA, and its communication with the LH, directly and via mPFC, as well as the roles of the mPFC and its pathways to the LH, might differ in the various phases of the cue-potentiated eating task. Interestingly, in two other tasks in which CS value acquired via associative appetitive learning also guides subsequent behavior, second-order conditioning (section 2.2.2) and reinforcer devaluation (section 2.2.5), an intact BLA is critical only during the learning phase [82, 84]. For example, in the reinforcer devaluation procedure (section 2.2.5), lesions of either BLA or OFC, or BLA-OFC disconnection lesions, when made prior to training, impair performance [63, 85, 86]. That is, unlike normal rats, lesioned rats fail to show spontaneous reductions in responding to the CS alone after devaluation of the food US. However, in the devaluation task, BLA and OFC serve different roles. Intact BLA function is necessary to acquire normal outcome expectancies during the initial CS-food training, but once those expectancies are formed, BLA function is no longer necessary for rats to use them to guide task performance when the food is later devalued. By contrast, OFC function is critical for that flexible use of previously-acquired information. Thus, post-training OFC lesions impair performance in this task, but post-training BLA lesions do not [84]. We are currently examining the role of the BLA and mPFC at different stages of the cue-potentiated eating paradigm.
In this article we showed that neurons within the BLA and mPFC that are selectively activated by the tests with CS+ presentations send direct projections to the LH. This evidence, together with the evidence from our BLA-LH disconnection study (section 3.2) suggests that the amygdalar and prefrontal pathways, independently or concurrently, influence feeding systems via LH. Unfortunately, nothing is known at this time about the mechanisms that allow such modulation of feeding. The exact peptide-phenotype, or hodology of LH neurons that receive BLA inputs directly or via the mPFC, or the exact neurotransmitters used by these pathways, are not yet known. However, evidence suggests that these pathways use glutamate [71], which is interesting because glutamatergic mechanisms within the LH have been shown to promote feeding in sated rats [87]. Thus, it is plausible that mechanisms mediating potentiation of feeding, at least in part, involve direct glutamatergic projections from the BLA and/or mPFC.
Interestingly, the BLA and mPFC pathways reach topographically distinct regions within the LH. The topography of these pathways suggest that the mPFC, but not BLA, reaches the region of the LH with neurons that express two recently discovered neuropeptides, melanin concentrating hormone and orexin [67, 80], which are regulated by hunger-satiety state and are linked to the initiation of feeding [14: Woods this issue, 66, 88: Levin this issue]. On the other hand, direct BLA-LH pathways might reach other parts of the feeding circuitry, as shown in a recent study using a viral labeling technique [89]. In that study, a pseudorabies virus constructed to infect either neuropeptide Y-expressing neurons or neurons expressing the leptin receptor was injected into the arcuate nucleus of the hypothalamus, and its retrograde transport across one or more synapses was mapped. The time course of retrograde labeling observed within the BLA suggested transynaptic transport, possibly via the LH and/or ventromedial hypothalamic nucleus. Thus, the BLA, which does not send direct projections to the arcuate nucleus, might reach it via its pathways to the LH. In turn, this evidence raises the possibility that the BLA and its circuitry could modulate feeding, including cue-potentiated feeding, via its influence on the NPY- and/or leptin-mediated mechanisms [14: Woods this issue, 88: Levin this issue, 90: Grove this issue]. Clearly, additional work is needed to illuminate the exact neurochemistry, as well as the precise function, of the BLA-LH and BLA-mPFC-LH circuitries.
As discussed in sections 3.2 and 3.3, the amygdala is connected with the hypothalamus via a highly organized network that includes direct pathways as well as pathways via relays in a number of telencephalic areas. Most notably, we discussed the role of the mPFC in cue-potentiated feeding. We also showed that the ACB, and CEA are not likely to participate in the amygdalo-hypothalamic circuitry that mediates cue-potentiated feeding. However, this observation does not imply that the ACB and CEA play no role in the control of feeding. Indeed, a variety of evidence has implicated the ACB in aspects of motivational control connected with feeding [20, 91, 92, 93] including opioid-mediated consumption and food preferences [81: Kelley this issue]. The CEA, which is critical in aversive Pavlovian conditioning [94, 95, 96, 97] is likely to mediate control of feeding by aversive states. Indeed, our unpublished observations suggest that the CEA is critical for allowing learned cues that signal danger to inhibit eating in hungry rats. Furthermore, distinct functional subsystems within the amygdalo-hypothalamic network might be recruited as needed under different circumstances for the control of feeding. Thus, it is tempting to speculate about the circumstances under which the ACB, and perhaps even CEA, which are not critical for cue-potentiated eating, would be recruited by the circuitry that mediates that phenomenon. For example, cue-potentiated consumption of highly palatable foods is likely to involve the ACB opioid system, and competition between an appetitive CS that enhances feeding and another CS that signals danger should involve the CEA and possibly ACB as well.
In addition to the systems discussed so far, pathways that involve the hippocampal formation represent a large portion of the amygdalo-hypothalamic network. The BLA sends a substantial, topographically organized output to the hippocampal formation, which in turn reaches hypothalamic behavioral systems directly, via the lateral septum, and via the mPFC. The extensive network of such an amygdalo-hippocampo-hypothalamic system is particularly interesting to consider in the light of evidence for the role of the hippocampal formation in some forms of inhibitory learning and the inhibition of eating [52: Davidson this issue, 98]. Thus, it would be interesting to examine a possible role of the hippocampal formation and its interactions with BLA and LH in cue-potentiated feeding. For example, cues for food might control food consumption in part by disinhibiting the normally suppressive action of the hippocampus.
In conclusion, the functional circuitry that mediates cue-potentiated eating delineated here could be taken within a broader view as a model for control of eating by learned cues. The anatomical blueprint of a complex and extensive amygdalo-hypothalamic network, which involves connections between the amygdalar subsystems important for processing emotion (including emotional learning), and forebrain systems critical for decision making (mPFC, [99]), learning and memory (hippocampal formation, [100]), and reward (ACB; 81: Kelley this issue) allows for control of appetite and food consumption based on a number of cognitive and motivational factors.
4. Cue-potentiated feeding and motivated behavior
As noted in section 3, our studies of neural systems in cue-potentiated feeding were initiated in the context of a broader program of investigation of amygdala systems of food-motivated behavior. A variety of learned, putatively motivational functions, including those tapped in conditioned reinforcement, reinforcer devaluation, Pavlovian-instrumental transfer, and cue-potentiated feeding procedures, were impaired by amygdala lesions. Interestingly, our research suggests that these functions are mediated by different amygdala projections. Studies of the effects of lesions of various amygdala projection regions and disconnections of those regions from the amygdala have yielded a number of double dissociations. After describing some of these dissociations, we will discuss their implications for theories of food-motivated behavior.
4.1. Neural systems dissociations of cue-potentiated feeding and other learned motivational functions
Although both cue-potentiated feeding and second-order conditioning, the ability of a CS for food to serve as a reinforcer for Pavlovian learning about a new cue (section 2.2.2), are impaired by BLA lesions [63, 64], those two phenomena are mediated by different BLA projections. Cue-potentiated feeding depends on BLA-LH projections whereas second-order conditioning requires intact BLA-ACB connectivity. In Petrovich et al.’s [65] study, the same rats (with BLA-LH disconnection lesions) that failed to show cue-potentiated feeding showed unimpaired second-order conditioning of a new cue. By contrast, although Petrovich and Setlow (section 3.4) showed that disconnection of BLA and ACB had no effect on cue-potentiated feeding, Setlow et al. [82] found that rats with these same disconnection lesions failed to show second-order conditioning. Likewise, although McDannald et al. [44] found no effects of lateral OFC lesions on cue-potentiated feeding, other investigators have found that these lesions impair a cue’s acquisition of conditioned reinforcement value in an instrumental secondary reinforcement procedure, akin to second-order conditioning. [101]. Furthermore, both these OFC lesions [85] and lesions that disconnect BLA from OFC [86] impair animals’ sensitivity to reinforcer devaluation procedures (section 2.2.5).
Perhaps most surprisingly, the ability of a food-predicting CS to enhance consumption of that food and its ability to enhance instrumental responding that yields that food also show dissociations. In two experiments [35], rats with BLA, CEA or sham lesions first received auditory discrimination training. Then they were tested in both cue-potentiated feeding and Pavlovian-instrumental transfer (section 2.2.3) tasks, in counterbalanced order. In sham-lesioned rats, the previously reinforced CS both enhanced consumption of the food while the rats were sated, and the rate of instrumental lever-pressing for that food under conditions of food deprivation. By contrast, rats with BLA lesions showed no evidence for cue-potentiated feeding, but normal Pavlovian-instrumental transfer, whereas rats with CEA lesions showed normal cue-potentiated feeding but no evidence of Pavlovian-instrumental transfer.
These dissociations show that each of these putatively motivational consequences of Pavlovian learning can be distinguished at the level of neural systems. Because the operating characteristics of these systems are likely to vary considerably, observations that the controls of reward, approach, and consumption often dissociate as well should hardly be surprising. Whether it is reasonable then to distinguish among several distinct sources of incentive motivation [5: Balleine this issue, 35], or revisit Hinde’s [102] critique of concepts of motivation remains to be seen. Identifying anatomically and functionally defined neural circuits that influence various aspects of feeding behavior in a variety of circumstances may prove useful to circumvent many of these arguments [99].
4.2. The motivational basis of cue-potentiated feeding
A question basic to all of this research is, why do rats eat more in the presence of a CS that predicts food? Our initial research (section 3.1) made less plausible some simple accounts couched in terms of enhanced opportunity to eat, mediated by learned food-cup approach responses. Another possibility is that food cues induce an internal incentive state comparable to that induced by food deprivation, and thus increased food consumption would be seen as a normal regulatory response to that state. More mechanistically, a CS might, for example, induce conditioned insulin secretion CRs [13, 14: Woods this issue], altering glycolosis so as to encourage feeding. Although the observations that lesions of the CEA interfere with the insulin CR [104] but do not affect cue-potentiated feeding (section 3.1) make this particular mechanism unlikely, incentive states may nevertheless be conditionable. For example, a CS might induce conditioned NPY release, as this neuropeptide was shown recently to play a role in conditioned food-anticipatory responses [105]. Alternately, the CS may affect behavior more directly, perhaps by eliciting “habitual eating” responses [106] or by potentiating the power of food itself to activate downstream motor control systems for feeding responses.
Recent evidence concerning the food-specificity of the cue-potentiated feeding makes these latter accounts less plausible as well. First, in our study of the effects of mPFC lesions (Figure 3), we also tested the ability of the contextual CS to enhance consumption of a different, novel food (grain pellets rather than sucrose pellets), as well as a different, familiar food (standard chow). Only the consumption of the original, training food was enhanced in the paired rats, relative to unpaired rats. It might be argued however, that the rats had never eaten either of these alternative foods in the experimental context and were thus displaying some sort of context-specific neophobia. A recent experiment conducted in our laboratory by Ezequiel Galarce provides stronger evidence for the food-specificity of cue-potentiated feeding. In his experiment, food-deprived rats were trained with two auditory CSs and two food USs, isopreferred solutions of sucrose and the polysaccharide maltodextrin. In six separate tests conducted while the rats were chow-sated, consumption of each of the two reinforcers was measured in the presence of the each of the two CSs and in the absence of any CS. Consumption of each solution was significantly greater when it was accompanied by the CS with which it was originally paired than when it was accompanied by the other CS or no CS. Thus, even when the rats had identical exposure to each food, enhanced consumption in the presence of a CS was US-specific. This specificity, although consistent with a large literature on sensory-specific satiety [107], is inconsistent with notions that food cues induce some general hunger or amplify feeding responses in general. By contrast, it supports the view that CSs code sensory properties of their US associates, consistent with previous studies in our lab, which used US-specific devaluation [42] and differential-outcome expectancy [44] procedures. Finally, it makes contact with studies that show rats are sensitive to micronutrient contents of their diets, and can associate those nutrients or their consequences with relatively arbitrary cues [108, 109, 110].
How might the cue-activation of sensory properties of its food associate produce specific potentiation of feeding? Among several potential accounts for potentiated feeding, Holland et al. [64] suggested that CSs might provoke incentive processes in the consumption tests by activating a representation of the food as it was perceived in training, when the rats were food-deprived. Many investigators have suggested that food deprivation may act in part by enhancing the value of food [18, 111]. Thus, presentation of the CS, last experienced in conjunction with the food when it was highly valued, might enhance the value of food in the consumption tests conducted under conditions of satiation. Because rats code taste information about the US, the CS might enhance the value of its original target only. This account is consistent with data from humans and nonhuman primates [112], as well as our observations that both cue-potentiated eating and reinforcer devaluation effects are eliminated by BLA lesions: if those lesions prevent CS-encoding of such information, then both phenomena would be disrupted. By contrast, at least at first glance, our observations that lesions of OFC [85] disrupt devaluation effects but not potentiated feeding [44] are inconsistent with this view. However, it should be noted that in both of those studies, lesions were made prior to all training. It is possible that BLA is required for encoding reinforcer information during learning, but that information is conveyed to other systems for use in specialized output functions. Thus, OFC may inform systems that generate the appetitive responses assessed in devaluation experiments, but not those involved in the regulation of feeding responses.
A particular version of this approach is that CSs enhance orosensory palatability of their specific associates. A number of labs have suggested that hedonic reactions, palatability, or “liking” can be indexed by certain features of the microstructure of eating responses. For example, Grill & Norgen [113] and Berridge [29] have identified a set of qualitatively identifiable taste reactivity behaviors that assess these sensory-hedonic features, and Davis [114] identified somewhat more prosaic aspects of licking behavior with putatively similar properties. Several investigators [42, 115, 116] found that CSs previously paired with foods of various current palatabilities could elicit such behaviors even in the presence of plain water. Likewise, pharamacological enhancement and suppression of ACB opioid activity produces corresponding changes in food consumption patterns, as if increasing and decreasing their palatability, respectively [117, 118]. Although the lack of CS-specific ACB activation in our labeling study (section 3.3) is inconsistent with such an origin of cue-potentiated feeding in our particular preparation, it does not rule out contributions of this system in other circumstances.
Berridge [119] has claimed that such changes in “liking” foods can be dissociated from “wanting” them, a primarily dopamine-mediated function, which may also be readily conditionable through associative learning. That is, CSs may activate cravings for foods without necessarily enhancing their palatability or value, in the same way a long-time drug addict may crave drug without “liking” its effects any more when administered. Food cravings are frequently highly specific, and the craver may pass over equally palatable foods for the craved food, without necessarily “liking” it more. Furthermore these specific cravings may engage striatal systems that are apparently immune to devaluation effects [5: Balleine this issue] and which thus may survive OFC lesions.
In our laboratory, Ezequiel Galarce is examining more mechanistic accounts for cue-potentiated feeding that embrace his observations of food-specificity of these effects, by examining the microstructure of licking. Davis [114, 120] asserted that different aspects of licking related to different aspects of control over drinking. For example, the initial lick rate on contact with a solution and aspects of lick burst and cluster size indicated palatability or potency of the gustatory stimulation, whereas the decay of lick rate and aspects of lick burst and cluster number early in a consumption interval indicated a learned negative feedback relation between drinking and post-ingestive consequences.
In Galarce’s experiments, fluids were delivered to transparent wells fitted with color TV cameras. In consumption tests, the fluids were replenished as they were consumed. Slow-motion analysis of the video tapes permitted quantifying both qualitative and quantitative aspects of drinking, including consumption itself. Preliminary results from these studies suggest that, in the context of Davis’s assertions, CSs in the cue-potentiated eating procedure both enhance the palatability of their associated fluid USs (but not other USs) and reduce negative feedback control of oral cues over drinking, resulting in a larger meal size. Alternately, the CSs may sensitize initial responding to their taste associates, and reduce the normal habituation of oral cues’ ability to elicit consummatory responding [121]. Regardless, it is this apparent overriding of normally effective cues for satiation that makes cue-potentiated eating relevant to our understanding of overweight and obesity. Powley [122: this issue] notes that these normal satiation cues are largely anticipatory and preabsorptive, and so themselves may involve associative learning. Thus, the overriding of such cues by external Pavlovian cues may involve interference processes studied extensively by learning theorists [123, 124].
Finally, it may be worth noting that in Galarce’s studies there were substantial individual differences in the in rats’ display of these microstructural patterns of drinking in potentiated feeding tests. It is tempting to speculate that these differences may reflect individual differences in susceptibility to weight gain [125: Blundell this issue]. Whether Galarce’s analyses provide any additional insight into the origins of environmental controls over eating remains to be seen.
4.3. Conditioning and obesity
The “food environment” [125: Blundell this issue] is exceedingly rich in both image and substance. We are bombarded with cues for food; signboards, radio and television advertisements, and catchy jingles and product names that are difficult to get out of our heads. From a perspective of associative learning, these cues can influence eating in many ways, from serving as conditioned reinforcers for instrumental foraging behavior, to inducing internal states normally linked to eating, to overriding normal cues for satiety, and hence increasing meal size. Woods [14: this issue) notes that the initiation of meals is based more on habit and convenience than acute energy needs. The multiplicity of learning functions involved in eating may contribute to the difficulty of breaking bad eating habits, because those behaviors may be controlled by a number of aspects of the feeding situation. Learning processes may enhance hunger and the pleasurable sensations of food and eating, and reduce our sensitivity to the more delayed, unpleasant aspects. And the impact of these learned changes is likely exacerbated by the widespread availability of prepared and nearly-prepared foods and trends toward larger portion and meal sizes [126: Popkin this issue].
Nevertheless, it is probably a mistake to view the induction of overweight and obesity by associative learning processes as inherently pathological. Each of the examples of cue learning we described may have an important functional role in normal feeding. That is, overweight and obesity may be less the result of pathology in some specific system or the result of some learning or regulatory process gone drastically awry, and more the result of minor changes in the gain or influence of these systems. Throughout most of our history, mammalian species probably lived in environments in which the costs of food were high and its caloric density low. In such an environment, methods for selecting high-caloric density foods, facilitating digestion and overcoming the deleterious effects of large meals, make adaptive sense. Liking sweets and fats, getting hungry in the presence of food and its harbingers, and stuffing oneself past repletion are good things in that context. It is probably a testament to the resilience of regulatory systems that the problem of obesity is not worse than it is in today’s environment.
We agree with Levitsky [127: this issue) that solutions to obesity require identifying and modifying environmental cues that lead many of us to overweight. Even putting aside the social and economic pressures that may make modifying these cues difficult, the notion that disorders of eating and weight control may stem in part from minor alterations in function of multiple learned control systems suggests that even the task of identifying them may be more complex than it seems at first glance. These cues and learning systems do not operate in a vacuum, but have acted in a coordinated fashion with a number of homeostatic mechanisms in solving problems of food getting throughout our evolutionary history. Understanding the mechanisms by which environmental cues exert control over eating and weight control may require identifying multiple critical internal events with which they are associated, and multiple processes that they instigate. Even after we have identified these mechanisms, we must recognize that the effects of altering one or more of them may not be so straightforward. Changes in the hedonics, caloric density, meal size or other properties of feeding experiences may have unintended consequences. For example, Davidson and Swithers [40, 128: this issue) argued that the adoption of high-hedonic, low calorie food and beverages may exacerbate rather than ameliorate the problem of overweight and obesity. Better understanding of what constitutes the food environment may help inform epidemiological analyses of recent population changes in body weight. These analyses, especially among groups that have experienced these changes at different times, or are yet to experience them, may provide information most relevant to environmental modulation of eating and weight gain.
Footnotes
Supported by NIMH grants MH53667, MH60179, and MH67252.
References
- 1.Staddon JER, Cerutti DT. Operant conditioning. Annu Rev Psychol. 2003;54:115–144. doi: 10.1146/annurev.psych.54.101601.145124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrnstein RJ. On the law of effect. J Exp Anal Behav. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houston, A.; McNamara, J. Models of adaptive behavior. Cambridge: Cambridge Univ. Press; 1999.
- 4.Kacelink, A.; Krebs, J.R. Yanomamo dreams and starling payloads: The logic of optimality. In: Betzig, L., ed., Human nature. New York: Oxford University Press; 1997, pp. 21–35.
- 5.Balleine, B.W. Affect, arousal and reward in limbic-striatal circuits. Physiol. Behav. this issue. [DOI] [PubMed]
- 6.Pavlov, I.P. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex (G.V. Anrep, Trans.). London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed]
- 7.Capaldi, E.D. Conditioned food preferences. In: Medin D, ed., The psychology of learning and motivation, vol. 28. New York: Academic Press, 1992, pp. 1–33.
- 8.Elizalde G, Sclafani A. Flavor preferences conditioned by intragrstric Polycose: a detailed analysis using an electronic esophagus preparation. Physiol Behav. 1990;47:63–77. doi: 10.1016/0031-9384(90)90043-4. [DOI] [PubMed] [Google Scholar]
- 9.Myers KP, Sclafani A. Conditioned enhancement of flavor evaluation reinforced by intragastric glucose. Physiol Behav. 2001;74:495–505. doi: 10.1016/s0031-9384(01)00596-0. [DOI] [PubMed] [Google Scholar]
- 10.Davidson TL. The nature and function of interoceptive signals to feed: Toward an integration of physiological and learning perspectives. Psychol Rev. 1993;100:640–657. doi: 10.1037/0033-295x.100.4.640. [DOI] [PubMed] [Google Scholar]
- 11.Davidson, T.L. Hunger cues as modulatory stimuli. In: Schmajuk, N.A.; Holland, P.C., eds., Occasion setting: Associative learning and cognition in animals. Washington, D.C.: American Psychological Association; 1998, pp. 223–249.
- 12.Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J Neurosci. 2004;204:6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods SC, Ramsay DS. Pavlovian influence over food and drug intake. Behav Brain Res. 2000;110:175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- 14.Woods, S.C. Signals that control food intake and body weight. Physiol. Behav. this issue [DOI] [PubMed]
- 15.Hearst, E. Pavlovian conditioning and directed movements. In: Bower, G., ed., The Psychology of Learning and Motivation, Vol. 9. New York: Academic Press; 1975, pp. 215–262.
- 16.Silva KM, Timberlake W. The organization and temporal properties of appetitive behavior in rats. Anim Learn Behav. 1988;26:182–195. [Google Scholar]
- 17.Timberlake, W.; Lucas, G.R. Behavior systems and learning: From misbehavior to general principles. In: Klein, S.B.; Mowrer, R.R., eds., Contemporary learning theories. Hillsdale, NJ: Lawrence Erlbaum; 1987, pp. 1–46.
- 18.Holland PC, Rescorla RA. The effect of two ways of devaluing the unconditioned stimulus after first and second order appetitive conditioning. J Exp Psychol Anim Behav Proc. 1975;1:355–363. doi: 10.1037//0097-7403.1.4.355. [DOI] [PubMed] [Google Scholar]
- 19.Fantino, E. Conditioned reinforcement: Choice and information. In: Honig, W.K.; Staddon, J.E.R., eds., Handbook of operant behavior. Englewood Cliffs, NJ: Prentice-Hall; 1977, pp. 313–339.
- 20.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 21.Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol Anim Behav Proc. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- 22.Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991;16:103–120. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- 23.Cabanac M, Lafrance L. Postingestive alliesthesia: The rat tells the same story. Physiol Behav. 1990;47:539–543. doi: 10.1016/0031-9384(90)90123-l. [DOI] [PubMed] [Google Scholar]
- 24.Garcia J, Kovner R, Green KS. Cue properties versus palatability of flavors in avoidance learning. Psychon Sci. 1970;20:313–314. [Google Scholar]
- 25.Zellner DA, Berridge KC, Grill HJ, Ternes JW. Rats learn to like the taste of morphine. Behav Neurosci. 1985;99:290–300. doi: 10.1037//0735-7044.99.2.290. [DOI] [PubMed] [Google Scholar]
- 26.Breslin PAS, Davidson TL, Grill HJ. Conditioned reversal of reactions to normally avoided tastes. Physiol Behav. 1990;47:535–538. doi: 10.1016/0031-9384(90)90122-k. [DOI] [PubMed] [Google Scholar]
- 27.Rescorla RA, Solomon RL. Two process learning theory: Relationships between classical conditioning and instrumental learning. Psychol Rev. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- 28.Amsel A. The role of frustrative nonreward in noncontinuous reward situations. Psychol Bull. 1958;55:102–119. doi: 10.1037/h0043125. [DOI] [PubMed] [Google Scholar]
- 29.Berridge, K.C. Reward learning: Reinforcement, incentives, and expectations. In: Medin, D.L., ed., The psychology of learning and motivation, Vol. 40. New York: Academic Press; 2001, pp. 223–279.
- 30.Bindra DA. A motivational view of learning, performance, and behavior modification. Physiol Rev. 1974;81:199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- 31.Booth DA. Food-conditioned eating preferences and aversions with interoceptive elements: conditioned appetites and satieties. Ann NY Acad Sci. 1985;443:22–37. doi: 10.1111/j.1749-6632.1985.tb27061.x. [DOI] [PubMed] [Google Scholar]
- 32.Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 33.Mowrer, O.H. Learning theory and behavior. Wiley, New York, 1960.
- 34.Siegel, S. The role of conditioning in drug tolerance and addiction. In: Keehn, J.D., ed., Psychopathology in animals. New York: Academic Press, New York; 1979, pp. 143–168.
- 35.Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on CS-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- 36.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox MT, Barense MD, Baxter MG. Perceptual attentional set-shifting is impaired in rats with neurotoxic lesions of posterior parietal cortex. J Neurosci. 2003;23:676–681. doi: 10.1523/JNEUROSCI.23-02-00676.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in CS processing. Behav Neurosci. 1993;107:246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- 39.Holland P, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cog Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 40.Davidson TL, Swithers SE. A Pavlovian approach to the problem of obesity. Int J Obes Relat Metab Disord. 2004;28:933–935. doi: 10.1038/sj.ijo.0802660. [DOI] [PubMed] [Google Scholar]
- 41.Colwill RM, Motzkin DK. Encoding of the unconditioned stimulus in Pavlovian conditioning. Anim Learn Behav. 1994;22:384–394. [Google Scholar]
- 42.Holland PC. Event representation in Pavlovian conditioning: Image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- 43.Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. J Exp Psychol Anim Behav Proc. 2004;30:104–117. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- 44.McDannald MA, Saddoris MP, Gallagher M, Holland PC. Lesions of orbitofrontal cortex impair rats’ differential outcome expectancy learning but not CS-potentiated feeding. J Neurosci. 2005;25:4626–2632. doi: 10.1523/JNEUROSCI.5301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trapold, M.A.; Overmier, J.B. The second learning process in instrumental training. In: Black, A.; Prokasy, W.F., eds., Classical Conditioning II. New York: Appleton-Century-Crofts; 1972, pp. 427–452.
- 46.Holland PC. Acquisition of representation mediated conditioned food aversions. Learn Motiv. 1981;12:1–18. doi: 10.1016/j.lmot.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holland PC. Amount of training affects associatively-activated event representation. Neuropharmacology. 1998;37:461–469. doi: 10.1016/s0028-3908(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 48.Holland, P.C. Occasion setting in Pavlovian feature positive discriminations. In: Commons, M.L.; Herrnstein, R.J.; Wagner, A.R., eds., Quantitative analyses of behavior: Discrimination processes, Vol. 4. New York: Ballinger; 1983, pp. 183–206.
- 49.Holland, P.C. Occasion setting in Pavlovian conditioning. In: Medin, D., ed., The psychology of learning and motivation, Vol. 28. San Diego: Academic Press; 1992, pp. 69–125.
- 50.Bouton ME, Swartzentruber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. J Exp Psychol Anim Behav Proc. 1986;12:333–350. [Google Scholar]
- 51.Skinner, D.M.; Goddard, M.J.; Holland, P.C. What can nontraditional features tell us about conditioning and occasion setting? In: Schmajuk, N.A.; Holland, P.C., eds., Occasion setting: Associative learning and cognition in animals. Washington, D.C.: American Psychological Association; 1998, pp. 113–144.
- 52.Davidson, T.L. Hypermnesia and hyperphagia: A vicious circle? Physiol. Behav. this issue
- 53.Cornell CE, Rodin J, Weingarten H. Stimulus-induced eating when satiated. Physiol Behav. 1989;45:695–704. doi: 10.1016/0031-9384(89)90281-3. [DOI] [PubMed] [Google Scholar]
- 54.Fedoroff I, Polivy J, Herman CP. The specificity of restrained versus unrestrained eaters' responses to food cues: general desire to eat, or craving for the cued food? Appetite. 2003;41:7–13. doi: 10.1016/s0195-6663(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 55.Herman, C.P. Normative influences on food intake. Physiol. Behav., this issue [DOI] [PubMed]
- 56.Zamble E. Augmentation of eating following a signal for feeding in rats. Learn Motiv. 1973;4:138–147. [Google Scholar]
- 57.Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 58.Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatr. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- 59.Gallagher M, Chiba AA. The amygdala and emotion. Curr Opin Neurobiol. 1996;6:221–227. doi: 10.1016/s0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- 60.Gallagher, M.; Holland, P.C. Understanding the function of the central nucleus: Is simple conditioning enough? In: Aggleton, J.P., ed., The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992, pp. 307–321.
- 61.Gallagher, M. The amygdala and associative learning. In Aggleton, J.P., ed., The Amygdala: A functional analysis. New York: Oxford University Press; 2000, pp. 311–329.
- 62.Holland PC, Hatfield T, Gallagher M. Rats with basolateral amygdala lesions show normal increases in conditioned stimulus processing but reduced conditioned potentiation of eating. Behav Neurosci. 2001;115:945–950. [PubMed] [Google Scholar]
- 63.Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- 65.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: Hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 67.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 68.Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- 69.Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- 70.Kita H, Oomura Y. An HRP study of the afferent connections to rat lateral hypothalamic region. Brain Res Bull. 1982;8:63–71. doi: 10.1016/0361-9230(82)90028-4. [DOI] [PubMed] [Google Scholar]
- 71.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 72.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DI, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 74.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Nat Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- 76.Vazdarjanova A, McNaughton BL, Barnes CA, Worley PA, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes Arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris Leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 78.Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- 79.Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- 80.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- 81.Kelley, A.E. Corticostriatal-hypothalamic circuitry and food motivation: integration of reward, cognition, and energy balance. Physiol. Behav. this issue [DOI] [PubMed]
- 82.Setlow B, Holland PC, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive Pavlovian second-order conditioned response. Behav Neurosci. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- 83.Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol. 1990;298:40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- 84.Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duva MA, Tomkins EM, Moranda LM, Kaplan R, Suskhaseum A, Jimenez A, Stanley BG. Reverse microdialysis of N-methyl-D-aspartic acid into the lateral hypothalamus of rats: effects on feeding and other behaviors. Brain Res. 2001;921:122–132. doi: 10.1016/s0006-8993(01)03108-0. [DOI] [PubMed] [Google Scholar]
- 88.Levin, B.E. Factors promoting the development and amelioration of obesity. Physiol. Behav. this issue [DOI] [PubMed]
- 89.DeFalco J, Tomishima M, Liu H, Zhao C, Cai XL, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 90.Grove, K.L. Development of metabolic systems. Physiol. Behav. this issue. [DOI] [PubMed]
- 91.Berridge KC. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 92.Phillips AG, Ahn S, Howland JG. Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev. 2003;27:543–554. doi: 10.1016/j.neubiorev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 93.Kelley AE. Ventral striatal control of appetitive motivation: role of ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 94.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 95.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 96.Davis M, Walker DL, Myers KM. Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci. 2003;985:218–232. doi: 10.1111/j.1749-6632.2003.tb07084.x. [DOI] [PubMed] [Google Scholar]
- 97.Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning, Annu. Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 98.Chan KH, Morell JR, Jarrard LE, Davidson TL. Reconsideration of the role of the hippocampus in learned inhibition. Behav Brain Res. 2001;119:111–130. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- 99.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 100.Squire LR, Stark CE, Clark RE. The medial temporal lobe, Annu. Rev Neurosci. 2004;27:79–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 101.Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. J Neurosci. 2003;23:11189–11201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hinde RA. Ethological models and the concept of drive. Brit J Phil Sci. 1956;6:321. [Google Scholar]
- 103.Smith GP. The controls of eating: A shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–820. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 104.Roozendaal B, Oldenburger WP, Strubbe JH, Koolhaas JM, Bohus B. The central amygdala is involved in the conditioned but not in the meal-induced cephalic insulin response in the rat. Neurosci Lett. 1990;116:210–215. doi: 10.1016/0304-3940(90)90412-3. [DOI] [PubMed] [Google Scholar]
- 105.Drazen DL, Wortman MD, Seeley RJ, Woods SC. Neuropeptide Y prepares rats for scheduled feeding. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1606–11. doi: 10.1152/ajpregu.00817.2004. [DOI] [PubMed] [Google Scholar]
- 106.Morgan MJ. Resistance to satiation. Animal Behav. 1974;22:449–466. [Google Scholar]
- 107.Rolls ET, Rolls JH. Olfactory sensory-specific satiety in humans. Physiol Behav. 1997;61:461–473. doi: 10.1016/s0031-9384(96)00464-7. [DOI] [PubMed] [Google Scholar]
- 108.Rozin P, Kalat JW. Specific hungers and poison avoidance as adaptive specializations of learning. Psychol Rev. 1971;78:459–486. doi: 10.1037/h0031878. [DOI] [PubMed] [Google Scholar]
- 109.Coldwell SE, Tordoff MG. Learned preferences for the flavor of salted food. Physiol Behav. 1993;54:999–1004. doi: 10.1016/0031-9384(93)90314-6. [DOI] [PubMed] [Google Scholar]
- 110.Benoit SC, Morell JR, Davidson TL. Lesions of the amygdala central nucleus abolish lipoprivic-enhanced responding during oil-predicting conditioned stimuli. Behav Neurosci. 1999;113:1233–1241. doi: 10.1037//0735-7044.113.6.1233. [DOI] [PubMed] [Google Scholar]
- 111.Tolman, E.C. Purposive behavior in animals and men. New York: Appleton Century; 1932.
- 112.Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85:45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 113.Grill HJ, Norgren R. The taste reactivity test. I Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 114.Davis, J.D. A model for the control of ingestion: 20 years later. In: Fluharty, S.J.; Morrison, A.R., eds., Progress in psychobiology and physiological psychology, Vol. 17. New York: Academic Press; 1998, pp. 123–173.
- 115.Kerfoot, E.C.; Lee, H.J.; Agarwal, I.; Holland, P.C. Conditioned auditory stimuli control appetitive and aversive taste-reactivity responses in a devaluation task. Society for Neuroscience Abstract, 2004, 206.10.
- 116.Delameter AR, LoLordo VM, Berridge KC. Control of fluid palatability by exteroceptive Pavlovian signals. J Exp Psychol: Anim Behav Proc. 1986;12:143–152. [PubMed] [Google Scholar]
- 117.Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278:1499–1507. [PubMed] [Google Scholar]
- 118.Zhang M, Kelley AE. Intake of saccharine, salt, and ethanol solutions is increased by infusion of a t opioid agonist into the nucleus accumbens. Psychopharmacology. 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- 119.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 120.Davis JD. The effectiveness of some sugars in stimulating licking behavior in the rat. Physiol Behav. 1973;12:377–384. doi: 10.1016/0031-9384(73)90120-0. [DOI] [PubMed] [Google Scholar]
- 121.Swithers SE, Hall WG. Does oral experience terminate ingestion? Appetite 1994, 23:113–138. doi: 10.1006/appe.1994.1041. [DOI] [PubMed] [Google Scholar]
- 122.Powley, T.L.; Chi, M.M.; Phillips, R.J. Nutrient signals beyond the tongue and before metabolism: visceral afferents. Physiol. Behav. this issue
- 123.Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 124.Wasserman EA, Miller RR. What's elementary about associative learning? Annu Rev Psychol. 1997;48:573–607. doi: 10.1146/annurev.psych.48.1.573. [DOI] [PubMed] [Google Scholar]
- 125.Blundell, J. Resistance and susceptibility to weight gain: Individual variability in response to diet. Physiol. Behav. this issue [DOI] [PubMed]
- 126.Popkin, B.; Duffey, K.; Gordon-Larsen, P. Environmental influences on food choice, physical activity and energy balance. Physiol. Behav. this issue [DOI] [PubMed]
- 127.Levitsky, D.A. The un-regulation of food intake in humans: Hope for reversing the epidemic of obesity. Physiol. Behav. this issue. [DOI] [PubMed]
- 128.Swithers, S.E. Influence of early dietary experience on energy regulation. Physiol. Behav. this issue [DOI] [PubMed]


