Abstract
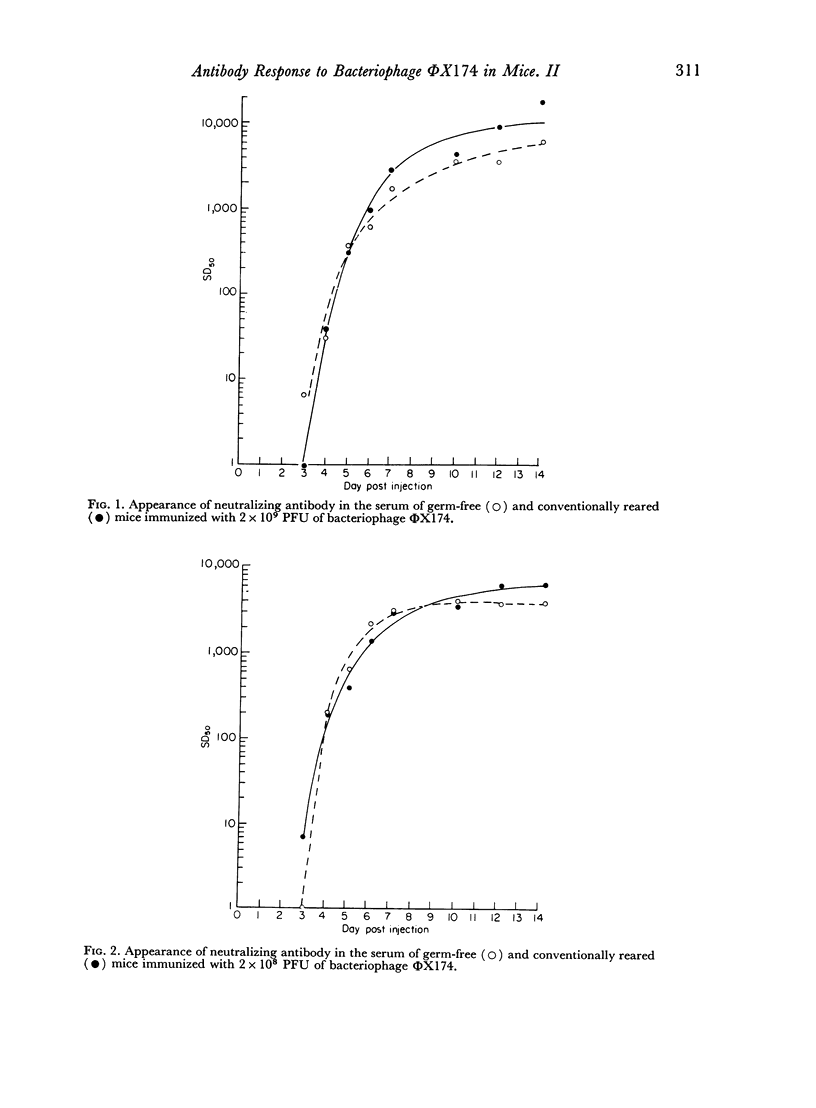
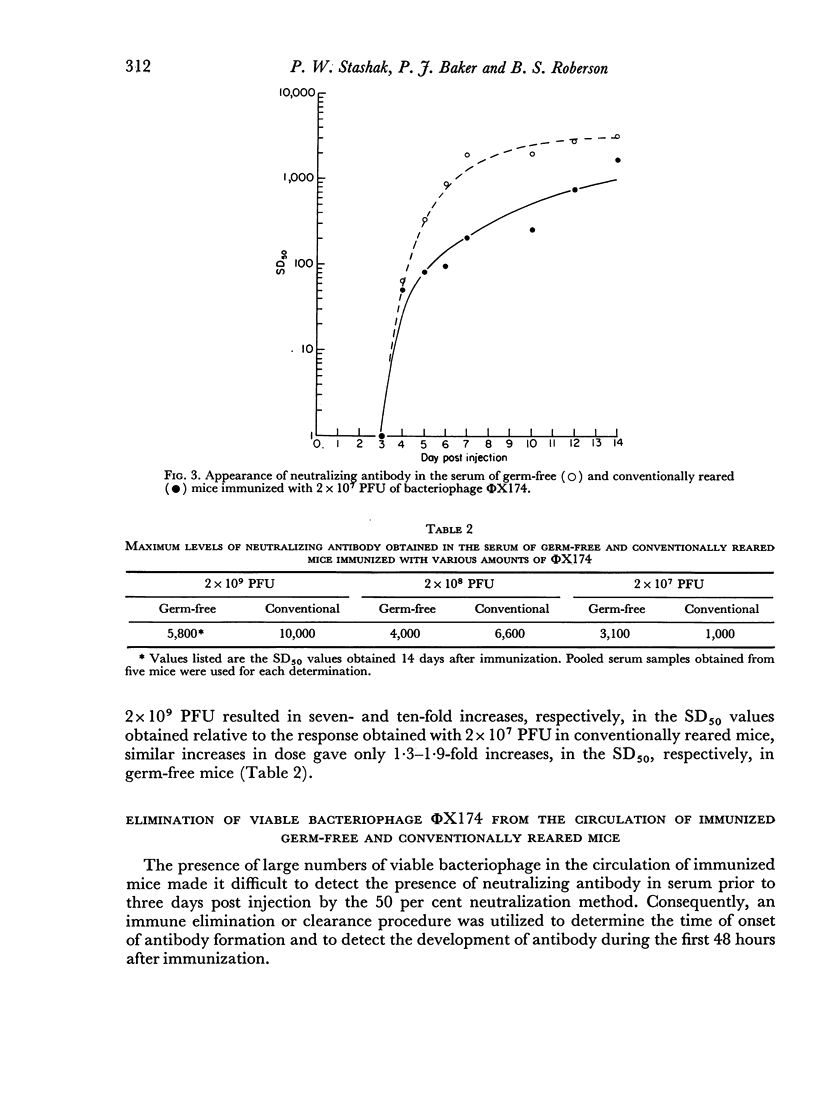
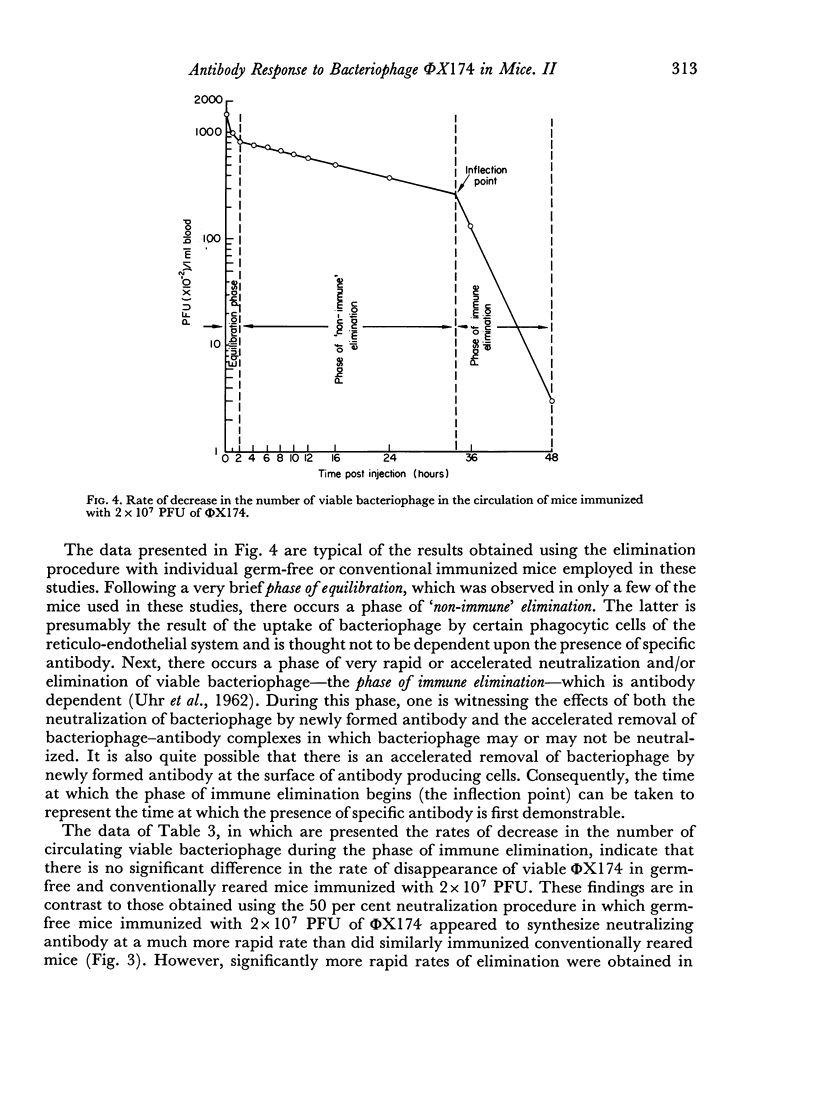
The kinetics of the serum antibody response to various amounts of bacteriophage ΦX174 were compared in germ-free and conventionally reared mice using a 50 per cent neutralization procedure for the assay of neutralizing antibody. Ten- and 100-fold increases in the amount of ΦX174 used to immunize resulted in seven and ten-fold increases, respectively, in the amount of antibody produced in conventionally reared mice; however, the same amounts of antigen produced only 1.3- and 1.9-fold increases in germ-free mice.
Greater SD50 values, i.e. the reciprocal of the highest dilution of serum which neutralized 50 per cent of the added bacteriophage, were obtained in conventionally reared than germ-free mice during the later stages of the antibody response; no other significant differences were noted in the kinetics of the response produced in both groups of animals immunized with high doses of antigen. However, neutralizing antibody was produced at a more rapid rate and in larger amounts in germ-free than conventionally reared mice immunized with a low dose of antigen.
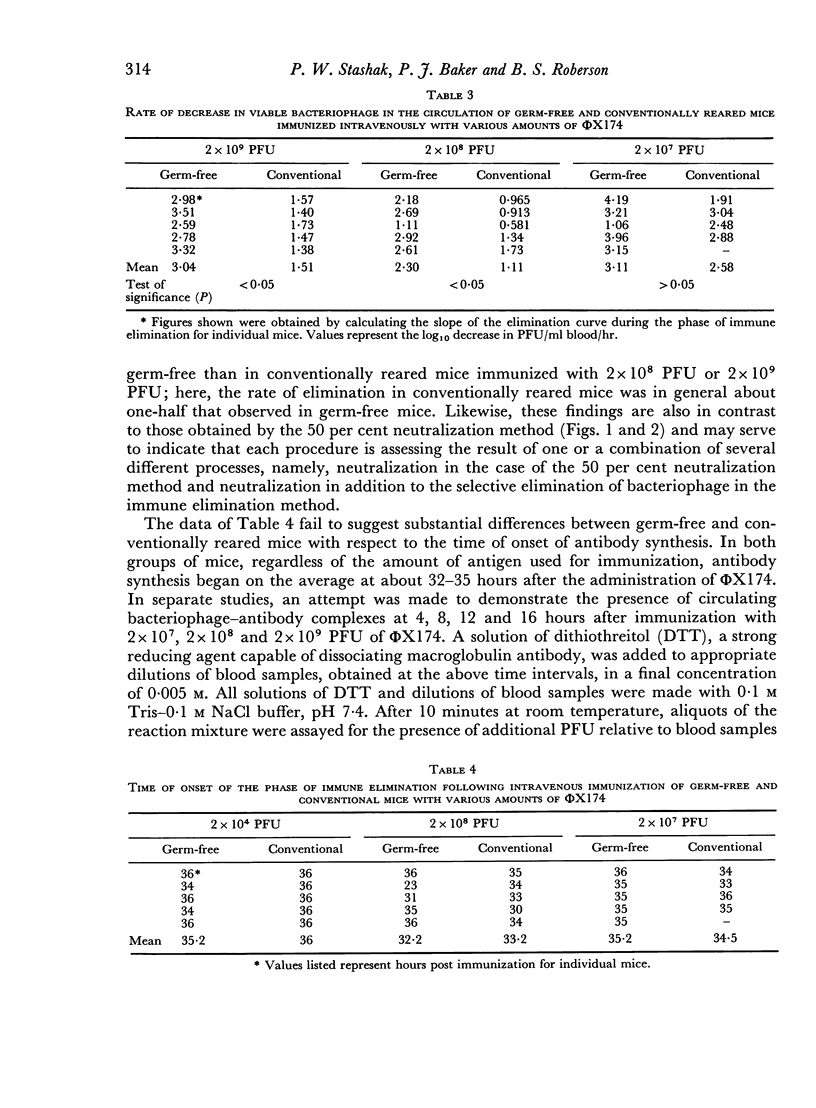
Regardless of the amount of antigen used to immunize, the time of onset of antibody formation was essentially the same (32–35 hours after injection) in both groups of mice. Conventionally reared mice eliminated antigen at half the rate observed in germ-free mice similarly immunized with high doses of ΦX174, but with low doses of antigen, no significant differences in elimination rate were noted.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERNATHY R. S., BRADLEY G. M., SPINK W. W. Increased susceptibility of mice with brucellosis to bacterial endotoxins. J Immunol. 1958 Oct;81(4):271–275. [PubMed] [Google Scholar]
- BAUER H., HOROWITZ R. E., WATKINS K. C., POPPER H. IMMUNOLOGIC COMPETENCE AND PHAGOCYTOSIS IN GERMFREE ANIMALS WITH AND WITHOUT STRESS. JAMA. 1964 Mar 7;187:715–718. doi: 10.1001/jama.1964.03060230043011. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Landy M. Brevity of the inductive phase in the immune response of mice to capsular polysaccharide antigens. J Immunol. 1967 Oct;99(4):687–694. [PubMed] [Google Scholar]
- Bauer H., Paronetto F., Burns W. A., Einheber A. The enhancing effect of the microbial flora on macrophage function and the immune response. A study in germfree mice. J Exp Med. 1966 Jun 1;123(6):1013–1024. doi: 10.1084/jem.123.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook W. A., Toussaint A. J., Simonton L. A., Muschel L. H. Appearance of natural antibodies in young rabbits. Nature. 1966 Apr 30;210(5035):543–544. doi: 10.1038/210543a0. [DOI] [PubMed] [Google Scholar]
- Hájek P. Properties of the neutralizing factors against T 2 and phi X 174 phages present in normal sera. Folia Microbiol (Praha) 1966;11(4):290–293. doi: 10.1007/BF02878899. [DOI] [PubMed] [Google Scholar]
- Kim Y. B., Bradley S. G., Watson D. W. Ontogeny of the immune response. I. Development of immunoglobulins in germfree and conventional colostrum-deprived piglets. J Immunol. 1966 Jul;97(1):52–63. [PubMed] [Google Scholar]
- Kim Y. B., Bradley S. G., Watson D. W. Ontogeny of the immune response. IV. The role of antigen elimination in the true primary immune response in germfree, colostrum-deprived piglets. J Immunol. 1967 Aug;99(2):320–326. [PubMed] [Google Scholar]
- Olson G. B., Wostmann B. S. Cellular and humoral immune response of germfree mice stimulated with 7S HGG or Salmonella typhimurium. J Immunol. 1966 Aug;97(2):275–286. [PubMed] [Google Scholar]
- Olson G. B., Wostmann B. S. Lymphocytopoiesis, plasmacytopoiesis and cellular proliferation in nonantigenically stimulated germfree mice. J Immunol. 1966 Aug;97(2):267–274. [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. The susceptibility of mice to bacterial endotoxins. J Exp Med. 1961 Mar 1;113:559–570. doi: 10.1084/jem.113.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashak P. W., Baker P. J., Roberson B. S. The serum antibody response to bacteriophage phi chi 174 in germ-free and conventionally reared mice. I. Assay of neutralizing antibody by a 50 per cent neutralization method. Immunology. 1970 Feb;18(2):295–305. [PMC free article] [PubMed] [Google Scholar]
- THORBECKE G. J. Some histological and functional aspects of lymphoid tissue in germfree animals. I. Morphological studies. Ann N Y Acad Sci. 1959 May 8;78:237–246. doi: 10.1111/j.1749-6632.1959.tb53106.x. [DOI] [PubMed] [Google Scholar]
- UHR J. W., FINKELSTEIN M. S., BAUMANN J. B. Antibody formation. III. The primary and secondary antibody response to bacteriophage phi X 174 in guinea pigs. J Exp Med. 1962 Mar 1;115:655–670. doi: 10.1084/jem.115.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]


