Abstract
Adenoviral (Ad) vectors can efficiently transduce a broad range of cell types and have been used extensively in preclinical and clinical studies for gene delivery applications. The presence of preexisting Ad immunity in the majority of human population and a rapid development of immune response against the Ad vector backbone following the first inoculation with the vector have impeded clinical use of these vectors. In addition, a number of animal inoculation studies have demonstrated that high systemic doses of Ad vectors invariably lead to initiation of acute inflammatory responses. This is mainly due to activation of innate immunity by vector particles. In general, vector and innate immune responses drastically limit the vector transduction efficiency and the duration of transgene expression. In order to have a predictable response with Ad vectors for gene therapy applications, the above limitations must be overcome. Strategies that are being examined to circumvent these drawbacks of Ad vectors include immunosuppression, immunomodulation, serotype switching, use of targeted Ad vectors, microencapsulation of Ad vectors, use of helper-dependent (HD) Ad vectors, and development of nonhuman Ad vectors. Here we review the current understanding of immune responses to Ad vectors, and recent advances in the strategies for immune evasion to improve the vector transduction efficiency and the duration of transgene expression. Development of novel strategies for targeting specific cell types would further boost the utility of Ad vectors by enhancing the safety, efficacy and duration of transgene expression.
1. ADVANTAGES OF ADENOVIRAL VECTORS FOR GENE THERAPY
Adenoviral (Ad) vectors have been the focus of considerable interest in the last few years for their potential applications as delivery vehicles for human gene therapy [Alemany et al. 2000; Bramson et al. 1995; Chuah et al. 2003; Curiel 2000; Hitt et al. 2000; Liu et al. 2002; Sadeghi et al. 2005; St George 2003]. Results of animal studies and clinical trials in humans for cancer therapy and other metabolic disorders using human Ad (HAd) vectors are encouraging [Akbulut et al. 2003; Ambar et al. 1999; Emtage et al. 1998; Parks et al. 1999; Trudel et al. 2001; Wen et al. 2001]. Some of the important reasons for the choice of Ad vectors for gene therapy are: (i) many human and animal adenoviruses are non-pathogenic for their natural hosts, (ii) a variety of both proliferating and quiescent cell types, such as epithelial cells, fibroblasts, hepatocytes, endothelial cells and stromal cells, can be infected with Ad vectors, (iii) Ad vectors can be grown to very high titers that offer a means to infect a large number of target cells, and (iv) replication-competent (e.g., early region (E) 3 (E3) -deleted vectors), conditional replication-competent (e.g., vectors in which E1A is under the control of a tissue- or cancer antigen-specific promoter), replication-defective (e.g., E1, E1 & E3, E2, E4, E2 & E4, or E1, E2 & E4-deleted vectors) and helper-dependent (e.g., vectors in which the majority of Ad genome is deleted) Ad vectors can easily be generated. Moreover, the absence of germ-line transmission of Ad vectors in mice highlights one of the safety aspects of HAd vectors [Paielli et al. 2000]. The E1- or E1 and E3-deleted vectors are routinely known as the first generation Ad vectors, and vectors having the deletion of E2 and/or E4, in addition to E1 or E1 and E3 deletion are called the second generation Ad vectors. Helper-dependent (HD)-Ad (HD-Ad) vectors are also referred as gutless, gutted, or high-capacity vectors and can be classified as the third generation Ad vectors.
2. SIGNIFICANCE OF VECTOR IMMUNITY IN GENE THERAPY
More than 50 different serotypes of HAd are known to infect humans. Due to ubiquitous nature of HAd, a majority of the human population is exposed to adenoviruses leading to the development of an HAd-specific immune response [Harvey et al. 1999]. The preexisting vector immunity is serotype-dependent and some HAd serotypes are less prevalence than others. Preexisting immunity against a particular serotype will significantly reduce the uptake of the homologous HAd vector. Currently, most HAd vectors are based on HAd serotype 5 (HAd5). The E1-deleted replication-defective HAd vectors are capable of expressing viral early and late genes at a magnitude sufficient to stimulate cellular and humoral immune responses [Dai et al. 1995; Elkon et al. 1997; Kafri et al. 1998; Yang et al. 1994; Yang et al. 1995]. HAd-specific neutralizing antibodies are directed against the viral capsid components [Toogood et al. 1992], and significantly inhibit the virus uptake following readministration of the same vector [Dong et al. 1996; Moffatt et al. 2000; Sailaja et al. 2002; Walter et al. 1996]. The cellular immune response, mediated through HAd5-specific CD8+ T cells, eliminates the target cells expressing viral and transgene products. This causes rapid loss of transgene expression in Ad vector-inoculated experimental animals [Crystal 1995].
It has been shown that in addition to the E1 deletion, E2A, E2B and E4 deletions resulted in minimal Ad late gene expression but without a drastic improvement in the duration of transgene expression [Engelhardt et al. 1994; Kafri et al. 1998; Yang et al. 1995]. Subsequently, HD-Ad vectors that have a large portion of the genome deleted were developed [Fisher et al. 1996; Kochanek et al. 1996; Morsy et al. 1998; Parks et al. 1996]. Inoculation of animals with an HAd vector having all viral genes deleted resulted in improved safety and prolonged expression of the transgene [Kochanek et al. 1996; Morsy et al. 1998; Parks et al. 1999]. However, significant levels of humoral and cellular immunity were elicited in HD-Ad inoculated animals indicating that expression of viral proteins was not essential for the induction of immune responses [Kafri et al. 1998]. Therefore, the first inoculation with any type of Ad vector would result in varying degrees of vector-specific immune response [Hackett et al. 2000; Kass-Eisler et al. 1996]. Since a number of inoculations with the vector containing the transgene may be needed for most gene therapy applications (Fig. 1), it is important to develop strategies that could effectively elude immunity to the vector.
Fig. (1).
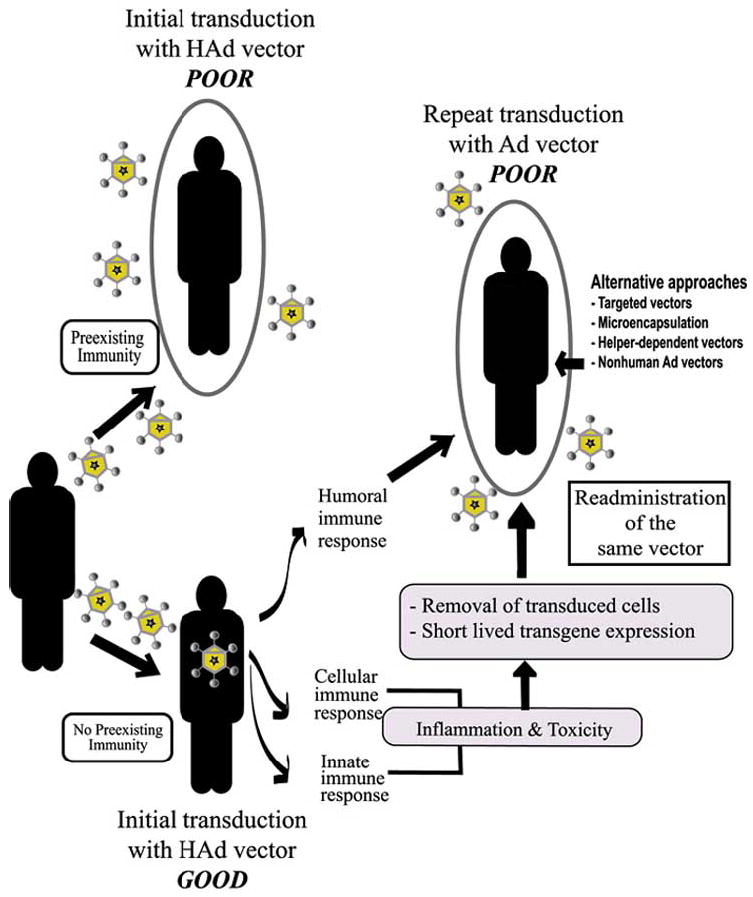
Preexisting immunity as a barrier to adenoviral (Ad) gene therapy. The presence of preexisting immunity in the majority of human population interferes with initial transduction with HAd vectors. In case of individuals with no preexisting immunity, the first inoculation with an HAd vector may be successful but subsequent development of strong cellular and humoral immunity renders repeat administration of the same vector less effective. Ad, adenoviral vector; HAd, human Ad vector
3. INDUCTION OF INNATE IMMUNE RESPONSE AND TOXICITY BY AD VECTORS
Scientists working on Ad vectors for gene therapy learnt a bitter lesson on September 17, 1999, when 18-year-old Jesse Gelsinger died after receiving a very high dose (3.8 x 1013 particles) of HAd vector containing the ornithine transcarbamylase (OTC) gene. This tragedy and a number of subsequent animal inoculation studies demonstrated that higher vector doses invariably lead to hepatotoxicity and acute inflammatory response mainly due to activation of innate immunity. Innate immune response is activated following recognition of molecular patterns on Ad capsid by pattern recognition receptors on macrophages (mφ) and dendritic cells (DC), resulting in activation of multiple signaling pathways such as mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB pathways that augment expression of several proinflammatory cytokines and chemokines [Bruder et al. 1997; Lieber et al. 1997; Muruve et al. 1999; Shifrin et al. 2005] (Fig. 2). Multiple inflammatory cytokines and chemokines including interleukin (IL)-6, IL-8, IL-12, tumor necrosis factor (TNF)-α, interferon-&;ambda;, RANTES (Regulated upon Activation, Normal T-cell Expressed and Secreted), interferon-inducible protein 10 (IP-10), macrophage inflammatory protein (MIP)-1β, and MIP-2 are expressed following Ad vector administration in a dose-dependent manner [Elkon et al. 1997; Zaiss et al. 2002].
Fig. (2).
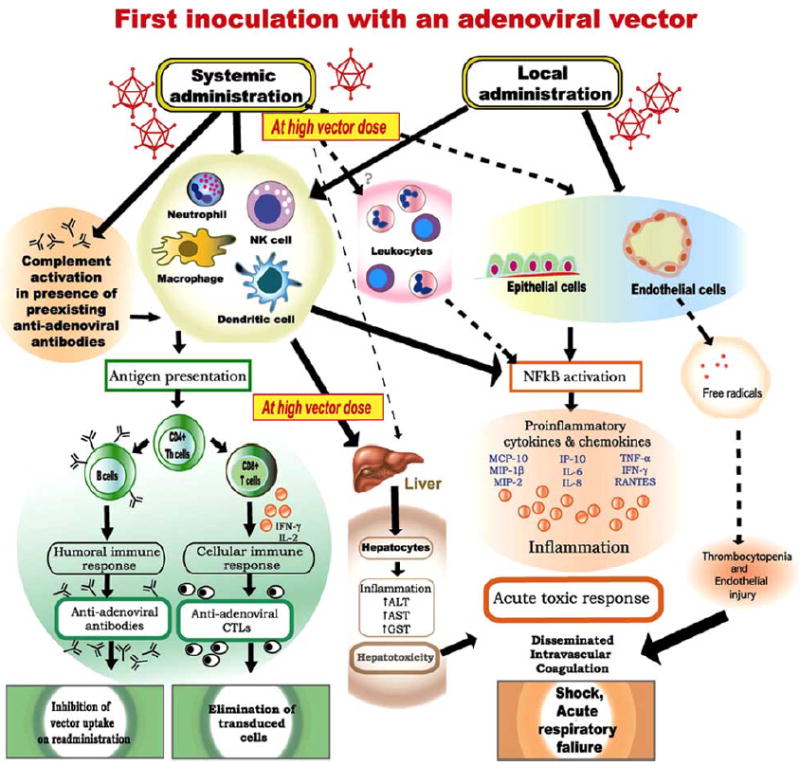
Development of adenoviral (Ad) vector immunity. The first use of an Ad vector leads to a strong innate as well as adaptive immune responses resulting in development of neutralizing antibodies and elimination of transduced cells. In response to high amount of vector administration, a strong innate immune is initiated, which is characterized by production of a variety of proinflammatory cytokines and chemokines leading to an acute toxic response and hepatotoxicity.
3.1. Implication of Route of Inoculation on Innate Immunity
Following intravenous (i.v.) inoculation, HAd vectors are in general taken up by the reticuloendothelial cells in the liver, leading to a rapid induction of an innate immune response. Intraportal infusion of an E1-deleted HAd5 vector (7.5 × 1012 particles/kg) in nonhuman primates resulted in acute activation of mφ and DC followed by considerable apoptosis of splenocytes and hepatocytes due to activation of innate immunity by viral capsid proteins [Schnell et al. 2001]. However, vector doses up to 5 × 1012 particles/kg usually lead to only limited hepatitis suggesting that vector toxicity could be diminished by lowering the vector dose per inoculation. In another study, i.v. inoculation of mice with a HAd5 vector (2 × 1011 genomes/mouse) led to acute inflammatory response characterized by high levels of IL-6 and IL-12 expression due to preferential activation of mφ and DC [Zhang et al. 2001].
The i.v. delivery of Ad vectors also lead to activation of endothelial cells as detected by expression of phosphorylated Akt/PKB kinase, activated endothelial nitric oxide synthase (eNOS), and nitrotyrosine due to interaction of viral particles with Kupffer cells [Schiedner et al. 2003a]. Conserved arg-gly-asp (RGD) motifs of the adenovirus capsid appeared to be important for efficient vector transduction and endothelial cell activation [Liu et al. 2003]. In rhesus monkeys, i.v. inoculation of HAd vector induced thrombocytopenia by enhancing in vivo platelet clearance [Wolins et al. 2003]. Similarly, a baboon inoculated with an E1-deleted HAd vector developed acute symptoms, decreased platelet counts, increased liver enzymes, injury to the vascular endothelium, and became moribund at 48 h post-inoculation [Morral et al. 2002]. These studies underscore the importance of induction of a strong innate immune response following Ad vector administration in mediating an acute inflammatory reaction.
In a subcutaneous mouse mammary tumor model, pre-immunization with an HAd5 vector resulted in significantly reduced transgene expression in the tumor and normal tissues, however, the inhibition was more in the liver than in the mammary tumor [Bramson et al. 1997; Vlachaki et al. 2002]. Increasing the vector dose by 10- to 100-fold restored the level of transgene expression in preimmunized mice, but higher vector doses (2 × 1011 virus particles or more per inoculation) also led to significantly higher hepatotoxicity compared to naïve animals. Readministration of a second vector dose was associated with the same degree of toxicity as the first vector, but prompted a much more vigorous neutralizing antibody response [Nagao et al. 2001; Nunes et al. 1999]. Increased mortality was observed when pre-immunized mice were inoculated systemically with a high dose of Ad vector [Varanvski et al. 2005]. Pre-exposure failed to inhibit induction of pro-inflammatory cytokines but tissue toxicity was reduced. In cirrhotic rats, the biodistribution of HAd vectors shifted from the liver to the lungs due to the presence of pulmonary intravascular mφ [Smith et al. 2004b]. High doses of HAd vectors in cirrhotic rats not only upregulated TNF-α and IL-6 expression, but also led to markedly prolonged coagulation times, and resulted in fatal pulmonary hemorrhagic edema [Smith et al. 2004a]. Cellular gene expression in response to wild type Ad, Ad vectors, or empty Ad particles was similar [Stilwell et al. 2003], suggesting the importance of the viral capsid proteins in mediating vector toxicity without viral gene expression. Additionally, it has been shown that intramuscular (i.m.) inoculation but not i.v. inoculation resulted in prolonged and sustained transgene expression and effective evasion of preexisting Ad immunity [Maione et al. 2001].
4. STRATEGIES FOR CIRCUMVENTION OF VECTOR IMMUNITY
In order to improve the clinical application of Ad vectors, it is most important to reduce or evade the vector immune response and enhance target cell transduction. Several approaches have been developed to meet these contradictory requirements for improving the efficacy of Ad vector-based gene transfer.
4.1. Immunosupression or Immunomodulation
It has been shown that the use of immunosuppressive agents, such as cyclosporin, cyclophosphamide [Smith et al. 1996], deoxyspergualin [Kaplan et al. 1997], FK506, [Ilan et al. 1997] and CTLA4Ig [Guerette et al. 1996; Jooss et al. 1998], or transient depletion of CD4 lymphocytes using an anti-CD4 monoclonal antibody [Ye et al. 2000], use of anti-CD40 ligand antibody to block CD40-CD40 ligand interactions [Chirmule et al. 2000], and oral tolerization to Ad proteins [Ilan et al. 1998] enhance the duration of transgene expression following systemic delivery of Ad vectors. These approaches help in inhibiting humoral, cell-mediated, or both responses to Ad. Since mφ play an important role in the induction of innate immune response following vector inoculation, depletion of mφ and DC in the liver and spleen following administration of liposome-encapsulated dichloro-methylene-biphosphonate resulted in reduced cytokine production [Zhang et al. 2001]. Short-term depletion of hepatic mφ resulted in increased hepatic transgene expression and reduced transgene-specific humoral immune response following Ad vector inoculation in mice [Schiedner et al. 2003b]. Similarly, depletion of alveolar mφ prior to intratracheal (i.t.) administration of an Ad vector improved vector transduction and persistence in both immunocompetent and immunodeficient mice [Worgall et al. 1997]. The use of immunosuppressive agents or depletion of mφ will not be preferred in clinical cases due to the inherent toxicity of such strategies. Nevertheless, these studies have demonstrated the feasibility of manipulation of the host innate immune response against Ad vectors to allow increased vector survival and prolonged transgene expression.
4.2. Covalent Modification of Ad Capsid
Alteration of the immunodominant epitopes of the Ad capsid was also helpful in evading Ad immunity [Roy et al. 1998]. Covalent attachment of polymers such as polyethylene glycol (PEG) [Croyle et al. 2000; Croyle et al. 2002; Lanciotti et al. 2003; O'Riordan et al. 1999] or N-(2-hydroxypropyl) methacrylamide (HPMA) [Fisher et al. 2001] to Ad capsid has been shown to curtail antibody-mediated virus neutralization. Such modifications are also expected to elude innate immunity since they will potentially mask the molecular patterns on the viral capsid with little or no effect on virus infectivity. Consistent with this, mono-methoxypolyethylene glycol conjugation of Ad vector lead to reduced innate immunity and improved therapeutic index in mice when compared to unmodified Ad vectors [Geest et al. 2005].
PEGylation of vectors substantially lowered innate Il-6 responses by HD-Ad as well as first-generation Ad vectors without significantly affecting transduction efficiency [Mok et al. 2005]. These reduced innate responses paralleled reductions in vector uptake by mφ in vitro and Kupffer cells in vivo. In addition to demonstrating the possibility of evading vector immunity by covalent modification of HD-Ad vectors, these studies also highlighted the role of nonspecific vector uptake by mφ in inducing innate immunity against Ad [Mok et al. 2005]. PEGylation of HD-Ad vectors did not adversely affect in vitro and in vivo transduction efficiencies but lowered peak serum IL-6, IL-12 and TNF-α levels compared to normal HD-Ad vectors [Croyle et al. 2005] suggesting that innate immune response elicited by Ad capsid components is critical in mediating vector toxicity.
4.3. Altering Native Ad Vector and Cell Surface Receptor Interactions
HAd5 attachment to a susceptible cell occurs via the interaction between the Ad fiber knob and cosackievirus adenovirus receptor (CAR) on the host cell membrane [Bergelson et al. 1997; Tomko et al. 1997]. CAR is a member of the immunoglobulin superfamily and serves as a high-affinity receptor for HAd in families A, C, D, E, and F but not B [Bergelson et al. 1997; Roelvink et al. 1998; Tomko et al. 1997]. In addition, major histocompatibility (MHC) class I α2 domain [Hong et al. 1997], heparin sulfate glycosaminoglycan [Smith et al. 2003] or sialic acid saccharide [Arnberg et al. 2000] may also serve as the primary receptor for HAd.
In addition to these primary receptors, host cell integrins serve as co-receptors for Ad entry [Wickham et al. 1993]. The HAd penton base protein interacts with vitronectin-binding integrins, specifically αvβ3 and αvβ5, for virus uptake [Wickham et al. 1993]. This process is facilitated by the arginine-glycine-asparagine (RGD) motif of the penton base. Interestingly, the RGD motif is also found in a number of adhesion molecules that are known to interact with integrins [Bai et al. 1993]. The interaction of HAd penton and αvβ1 integrins promotes actin cytoskeletal reorganization via activation of several signaling molecules [Li et al. 2001]. Binding of the HAd5 fiber knob to CAR receptor could be effectively prevented with a knob-specific antibody. For targeting HAd5 vectors to receptors other than CAR, knob-specific neutralizing antibody could be complexed either to a specific ligand or a receptor-specific antibody [Bilbao et al. 1998] (Fig. 3). This complex molecule will efficiently bind Ad knob on one side and a specific receptor on the other side. With this technology, a wide variety of HAd5 vectors have been successfully targeted to a number of receptors including folate, epidermal growth factor, fibroblast growth factor, epithelial cell adhesion molecule (EpCAM), tumor-associated glycoprotein (TAG)-67, and CD40 [Bilbao et al. 1998; Curiel 1999; Douglas et al. 1996; Gu et al. 1999; Krasnykh et al. 1998]. These modified vectors should be preferentially taken up by the specific cells.
Fig. (3).
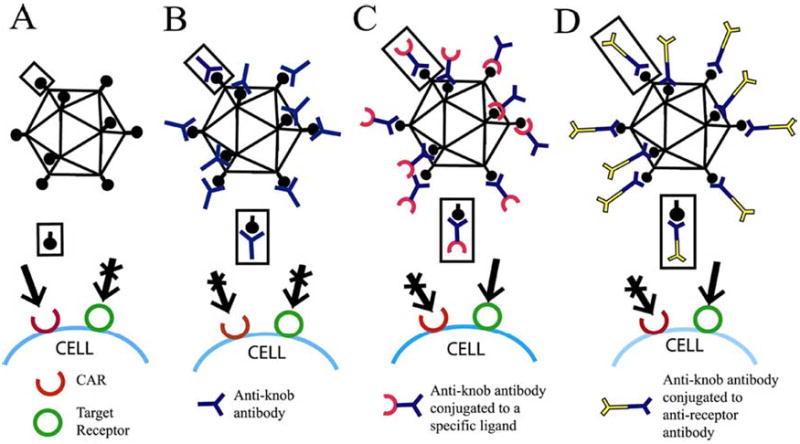
Some of the strategies for designing targeted adenoviral (Ad) vectors. A) Binding of adenovirus to cells via the knob domain of the fiber to CAR. B) Adenovirus complexed with an anti-knob antibody fails to bind to CAR. C) Conjugation of a specific ligand to the anti-knob antibody would allow virus binding to the targeted receptor on the cell surface. D) Conjugation of anti-receptor antibody to anti-knob antibody would target the Ad vector to the specific receptor on the cell surface.
HAd5 fiber knob has been shown to induce DC activation and maturation [Molinier-Frenkel et al. 2003]. Virus-induced maturation of DC was significantly reduced when knobless Ad particles were incubated with immature DC. Therefore, fiber knob modifications to incorporate cellular ligands with novel cell-binding capacity might confer targeting and decrease vector immunogenicity. Ad fiber and CAR interactions are considered important for preferential hepatic sequestration of Ad vectors following intravenous delivery. Uptake of Ad vectors by hepatocytes and Kupffer cells lead to an increase in cytokine and chemokine mRNA expression, and subsequently an enhanced innate immune response [Schoggins et al. 2005]. Fiber-pseudotyped Ad vectors were found to induce significantly lower innate immune response following systemic delivery, highlighting the importance of fiber-modification in Ad gene delivery. Similarly, immunogenicity of a chimeric vector containing HAd35 capsid and HAd5 fiber knob was enhanced indicating a potential role of the fiber knob in the immunogenicity of HAd5 vectors [Nanda et al. 2005]. It is very important to mention here that, despite a lower innate immune response, adaptive cellular and humoral responses were not affected by fiber modification. Since virus neutralizing antibodies are primarily directed to Ad hexon [Ostapchuk et al. 2001; Sumida et al. 2005], it is anticipated that modification of hexon will evade vector immunity.
Targeting of Ad vectors could also be achieved by fusing the extracellular domain of CAR to peptide-targeting ligands [Kim et al. 2002]. Genetic targeting of Ad vectors by engineering small peptides into the HAd fiber [Aoki et al. 2001; Belousova et al. 2002; Biermann et al. 2001; Douglas et al. 1999; Mizuguchi et al. 2001; Nicklin et al. 2001], protein IX [Dmitriev et al. 2002; Zakhartchouk et al. 2004] or by replacing the fiber protein with the phage T4 fibritin [Krasnykh et al. 2001] has been also demonstrated, but the size of the peptide appears to be a limitation. Similarly, the use of bifunctional polyethylene glycol molecules is useful in ablating the vector tropism by CAR-mediated interaction and providing specific vector targeting by incorporating a ligand for a particular receptor [Lanciotti et al. 2003].
4.4. Vector Microencapsulation
The use of polyethylene glycol-cationic lipid to coat HAd vectors [Chillon et al. 1998] and poly (lactic-glycolic) acid (PLGA) copolymer encapsulation [Beer et al. 1998] has also been shown to elude virus-neutralizing antibodies. Sodium alginate-based biodegradable microparticles have been shown to encapsulate purified protein, bacteria, DNA or viruses and can be delivered to the animals by various routes of inoculation [Aggarwal et al. 1999; Bowerstock T.L. et al. 1999; Hilbert et al. 1999; Mittal et al. 2000; Periwal et al. 1997]. Since alginate microspheres are biodegradable and no harsh treatments or organic solvents are used in the process of their synthesis, the viability of Ad vectors in these microparticles is usually very high. Encapsulation of a HAd5 vector into alginate microparticles could effectively evade the vector-specific immune response [Sailaja et al. 2002]. More than 70% of alginate microspheres are approximately 5–10 μm in size, and therefore, it is expected that majority of them will be taken up by mφ and DC [Lomotan et al. 1997]. It appears that alginate microspheres may be an attractive delivery system to target mφ and DC, but there is a need to study the role of these microparticles in modulating the immune response through mφ and DC. Use of bilamellar cationic liposomes to encapsulate HAd vectors also provided protection from preexisting humoral immune responses [Yotnda et al. 2002]. Similarly, microsphere-liposome complexes guard HAd vectors from neutralizing antibody responses and are capable of effectively transducing cells leading to successful transgene expression [Steel et al. 2004]. It seems that transgene expression levels by encapsulated vectors are usually lower (approximately 50–70%) than those of unencapsulated vectors both in the naïve and vector-primed animals [Sailaja et al. 2002]. It may be due to slow release of the vector from microparticles over time that will also prolong the duration of transgene expression.
4.5. Use of Alternate HAd Serotypes (Serotype Switching)
Since more than 50 HAd serotypes exist, and the neutralizing humoral immune response to Ad is serotype-specific, another strategy to overcome Ad vector immune response could be serotype switching in vector construction [Kass-Eisler et al. 1996; Mack et al. 1997; Mastrangeli et al. 1996; Parks et al. 1999]. Subgroup B Ad, such as HAd3, HAd11, and HAd35, have been shown to utilize the membrane cofactor protein CD46 as an attachment receptor [Gaggar et al. 2003; Segerman et al. 2003; Sirena et al. 2004]. This particular feature makes these viruses attractive for targeting cell types that are refractory to HAd5 vectors that are primarily dependent on CAR-mediated internalization. Low seroprevalence of HAd11 and HAd35 makes them promising vectors for in vivo applications. Replication-defective HAd35 vectors efficiently transduced human cells and eluded preexisting HAd immunity [Gao et al. 2003; Reddy et al. 2003; Sakurai et al. 2003; Vogels et al. 2003]. Similarly, HAd11 based replication-defective vectors have shown expanded tropism [Holterman et al. 2004; Stone et al. 2005]. HAd35 based replication-defective vector vaccines evaded preexisting HAd5 immunity in mice [Barouch et al. 2004] as well as in rhesus monkeys [Shiver et al. 2004].
4.6. Use of Helper-Dependent Ad (HD-Ad) Vectors
HD-Ad vectors are constructed by removing all coding sequences of the Ad genome except the packaging sequence and inverted terminal repeats, thereby eliminating the problem of residual viral gene expression associated with E1/E3-deleted Ad vectors [Mitani et al. 1995; Parks et al. 1996]. Initial studies showed that HD-Ad vectors elicited limited cell-mediated immune response, had high cloning capacity, and produced long-term gene expression in both naïve small laboratory animals [Morsy et al. 1998; Schiedner et al. 1998], and nonhuman primates [Morral et al. 1998; Morral et al. 1999; Morsy et al. 1998] without causing significant liver damage and toxicity. Systemic delivery of HD-Ad vectors has been shown to provide strong transgene expression for prolonged period with minimal toxicity in the baboon, mouse, or canine model [Brown et al. 2004; Kim et al. 2001; Morral et al. 1999].
HD-Ad vectors also induce vector-specific immune response similar to that generated by E1-deleted HAd [Roth et al. 2002]. Systemic administration of HD-Ad vectors in baboons also leads to acute toxicity accompanied by activation of the innate response in a dose-dependent manner [Brunetti-Pierri et al. 2004] indicating that vector-mediated acute toxicity is independent of viral gene expression. Sequential delivery of different HD-Ad vector serotypes circumvented the humoral response to the virus [Morral et al. 1999] suggesting that long-term transgene expression was possible by sequential delivery of HD-Ad vectors of different serotypes. However, acute toxicity due to vector is not prevented or reduced [Brunetti-Pierri et al. 2004; Stilwell et al. 2003], implying the importance of the viral capsid components in vector toxicity. The generation and potential applications of HD-Ad vectors have been reviewed [Ng et al. 2002].
It has been demonstrated that HD-Ad vectors could be used for in utero gene delivery for long-term transgene expression for genetic disorders such as Duchenne muscular dystrophy (DMD) [Bilbao et al. 2005]. Like first-generation HAd vectors, HD-Ad vectors do not integrate into the host cell genome; therefore, vector DNA will be gradually diluted out in dividing cells. In situations where long-term gene expression is desired, such as DMD, vector integration into the host genome will further improve longevity of transgene expression. The hybrid HAd-adeno-associated virus (AAV) vectors could provide nonrandom integration of double-stranded DNA by ex vivo or in vivo gene delivery [Recchia et al. 2004], thereby serving as a continuous source of trans-gene expression without potential systemic toxicity. Alternatively, long-term gene expression can be achieved by using a novel binary HD-Ad-Epstein-Barr virus (HDAd-EBV) hybrid system for stable transfection of mammalian cells [Dorigo et al. 2004]. This system consists of a cre-recombinase expressing HD-Ad, and a HD-Ad carrying EBV episome and a transgene flanked by loxP sites.
HD-Ad vectors have also been investigated for their application in long-term neurological gene therapy. While, transgene expression from a first-generation Ad vector was completely eliminated following peripheral immune priming, HD-Ad vectors produced sustained transgene expression in the rat brain [Thomas et al. 2000]. Even in the presence of anti-HAd immunity, an HD-Ad system resulted in sustained and regulatable transgene expression in the brain [Xiong et al. 2006]. It may obviate the need to screen patients for pre-existing vector immunity especially for gene delivery to the brain.
Following i.v. inoculation of HD-Ad vectors, an early expression of inflammatory cytokine and chemokine genes, including IP-10, MIP-2, and TNFα, was induced in the liver in a pattern similar to that induced by first generation HAd vectors [Muruve et al. 2004]. HD-Ad vectors also induced the recruitment of CD11b-positive leukocytes to the transduced liver cells within hours of administration [Muruve et al. 2004]. While first-generation HAd vectors induced a second phase of liver inflammation, consisting of inflammatory gene expression and CD3-positive lymphocytic infiltrate at 7 days post-transduction, these changes were not detected in the livers of mice receiving HD-Ad beyond 24 h post-transduction [Muruve et al. 2004]. In addition, adaptive immune responses generated by HD-Ad vectors was also attenuated in comparison to that of first-generation HAd vectors [Muruve et al. 2004].
4.7. Use of Nonhuman Ad Vectors
Since Ad viruses are species-specific, nonhuman Ad are expected to be nonprevalent in humans, and therefore, they evade preexisting HAd immunity. In order to extend the range of Ad vectors that could be used to evade HAd neutralizing immune response, a number of nonhuman Ad such as bovine Ad type 3 (BAd3) [Mittal et al. 1995; Reddy et al. 1999b; van Olphen et al. 2002], canine Ad type 2 [Hemminki et al. 2003; Klonjkowski et al. 1997], ovine Ad [Hofmann et al. 1999], chimpanzee Ad [Farina et al. 2001; Xiang et al. 2002], and porcine Ad type 3 [Bangari et al. 2004; Reddy et al. 1999a] were exploited for vector construction. It has been shown that nonhuman Ad vectors infect human cells in culture leading to expression of the transgene [Bangari et al. 2004; Bangari et al. 2005; Farina et al. 2001; Klonjkowski et al. 1997; Mittal et al. 1995; Rasmussen et al. 1999]. Since HAd5-, BAd3- and PAd3-specific neutralizing antibodies do not cross-neutralize [Moffatt et al. 2000], it is expected that sequential administration of HAd5, BAd3 and PAd3 would effectively evade the vector-specific neutralizing immune response. The sera of mice immunized with HAd serotypes 2, 4, 5, 7, and 12 did not neutralize chimpanzee Ad [Farina et al. 2001] indicating the utility of such vectors in evading HAd preexisting immunity. Following the decline in transgene expression to background levels, readministration of the vector is necessary to maintain therapeutic levels of transgene expression; it seems that sequential administration of nonhuman Ad vectors could provide that opportunity. The progress in design and construction of various nonhuman Ad vectors has been reviewed recently [Bangari et al. 2006].
5. CONCLUDING REMARKS
Various Ad vectors seem to have considerable potential for preventive or therapeutic applications where transgene expression for a short duration may be enough for the desired effects, e.g., for developing recombinant vaccines for human and veterinary use and for cancer gene therapy. The use of Ad vectors for gene therapy of genetic disorders will be more challenging since therapeutic gene expression will be required for extended period of time. It should be noted that induction of proinflammatory cytokines and chemokines by Ad vectors might not be a limitation in every situation. On the contrary, it may be advantageous in some situations such as cancer immunotherapy and preventive vaccination.
In addition to vector immunity, the immune response could also be induced against the transgene product in situations where it is recognized as a foreign antigen by the host. This would also adversely affect the persistence of transgene expression. It is known that the E3 gene products are involved in modulating host immune responses to the virus; therefore, inclusion or deletion of one or more E3 genes will have implications in vector immunity. All novel modifications in vector design are required to be tested extensively in experimental animal models to evaluate their usefulness in evading vector immunity and toxicity. The use of transgenic mice will be useful in further evaluating the strategies for evading vector-induced innate and adaptive immune responses and toxicity. For the purpose of expanding the tropism and modifying vector immunity and toxicity, development of nonhuman adenoviral vectors and human-nonhuman chimeric vectors hold considerable potential. Further investigations on various human and nonhuman Ad surface proteins involved in receptor-mediated internalization will also help to develop better vectors with the ability to target specific receptors. The cross-reactivity of cellular immune responses among different Ad needs to be evaluated to develop strategies for eluding vector cellular immunity by sequential administration of human and/or nonhuman Ad vectors. Adaptation of the information gleaned from other viral vector systems, micro- or nano-particle technology, and mechanism/s of induction of innate and adaptive immune responses will certainly facilitate further improvement in Ad vector design and delivery.
Acknowledgments
We wish to thank Harm HogenEsch for his critical review and Jane Kovach for her secretarial assistance in the preparation of this manuscript. This work was supported by Public Health Service grant CA110176 from the National Cancer Institute.
Since the area of Ad vector research is rapidly expending, it was impossible to cite all related references in this review. Our apologies are to all investigators whose work could not be cited.
References
- Aggarwal N, HogenEsch H, Guo P, North A, Suckow M, Mittal SK. Biodegradable alginate microspheres as a delivery system for naked DNA. Can J Vet Res. 1999;63:148–152. [PMC free article] [PubMed] [Google Scholar]
- Akbulut H, Zhang L, Tang Y, Deisseroth A. Cytotoxic effect of replication-competent adenoviral vectors carrying L-plastin promoter regulated E1A and cytosine deaminase genes in cancers of the breast, ovary and colon. Cancer Gene Ther. 2003;10:388–395. doi: 10.1038/sj.cgt.7700579. [DOI] [PubMed] [Google Scholar]
- Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- Ambar BB, Frei K, Malipiero U, Morelli AE, Castro MG, Lowenstein PR, Fontana A. Treatment of experimental glioma by administration of adenoviral vectors expressing Fas ligand. Hum Gene Ther. 1999;10:1641–1648. doi: 10.1089/10430349950017644. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Hosaka S, Kawa S, Kiyosawa K. Potential tumor-targeting peptide vector of histidylated oligolysine conjugated to a tumor-homing RGD motif. Cancer Gene Ther. 2001;8:783–787. doi: 10.1038/sj.cgt.7700362. [DOI] [PubMed] [Google Scholar]
- Arnberg N, Edlund K, Kidd AH, Wadell G. Adenovirus type 37 uses sialic acid as a cellular receptor. J Virol. 2000;74:42–48. [PMC free article] [PubMed] [Google Scholar]
- Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangari DS, Mittal SK. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004;105:127–136. doi: 10.1016/j.virusres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24:849–862. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem Biophys Res Commun. 2005;327:960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- Beer SJ, Matthews CB, Stein CS, Ross BD, Hilfinger JM, Davidson BL. Poly (lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 1998;5:740–746. doi: 10.1038/sj.gt.3300647. [DOI] [PubMed] [Google Scholar]
- Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J Virol. 2002;76:8621–8631. doi: 10.1128/JVI.76.17.8621-8631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Biermann V, Volpers C, Hussmann S, Stock A, Kewes H, Schiedner G, Herrmann A, Kochanek S. Targeting of high-capacity adenoviral vectors. Hum Gene Ther. 2001;12:1757–1769. doi: 10.1089/104303401750476258. [DOI] [PubMed] [Google Scholar]
- Bilbao G, Gomez-Navarro J, Curiel DT. Targeted adenoviral vectors for cancer gene therapy. Adv Exp Med Biol. 1998;451:365–374. doi: 10.1007/978-1-4615-5357-1_57. [DOI] [PubMed] [Google Scholar]
- Bilbao R, Reay DP, Wu E, Zheng H, Biermann V, Kochanek S, Clemens PR. Comparison of high-capacity and first-generation adenoviral vector gene delivery to murine muscle in utero. Gene Ther. 2005;12:39–47. doi: 10.1038/sj.gt.3302392. [DOI] [PubMed] [Google Scholar]
- Bowerstock TL, HogenEsch H, Suckow M, Guimond P, Martin S, Borie D, Torregrosa S, Park H, Park K. Oral vaccination of animals with antigens encapsulated in alginate microspheres. Vaccine. 1999;17:1804–1811. doi: 10.1016/s0264-410x(98)00437-x. [DOI] [PubMed] [Google Scholar]
- Bramson JL, Graham FL, Gauldie J. The use of adenoviral vectors for gene therapy and gene transfer in vivo. Curr Opin Biotechnol. 1995;6:590–595. doi: 10.1016/0958-1669(95)80097-2. [DOI] [PubMed] [Google Scholar]
- Bramson JL, Hitt M, Gauldie J, Graham FL. Pre-existing immunity to adenovirus does not prevent tumor regression following intratumoral administration of a vector expressing IL-12 but inhibits virus dissemination. Gene Ther. 1997;4:1069–1076. doi: 10.1038/sj.gt.3300508. [DOI] [PubMed] [Google Scholar]
- Brown BD, Shi CX, Powell S, Hurlbut D, Graham FL, Lillicrap D. Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood. 2004;103:804–810. doi: 10.1182/blood-2003-05-1426. [DOI] [PubMed] [Google Scholar]
- Bruder JT, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Chillon M, Lee JH, Fasbender A, Welsh MJ. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther. 1998;5:995–1002. doi: 10.1038/sj.gt.3300665. [DOI] [PubMed] [Google Scholar]
- Chirmule N, Raper SE, Burkly L, Thomas D, Tazelaar J, Hughes JV, Wilson JM. Readministration of adenovirus vector in nonhuman primate lungs by blockade of CD40-CD40 ligand interactions. J Virol. 2000;74:3345–3352. doi: 10.1128/jvi.74.7.3345-3352.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah MK, Collen D, VandenDriessche T. Biosafety of adenoviral vectors. Curr Gene Ther. 2003;3:527–543. doi: 10.2174/1566523034578140. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y, Wilson JM. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther. 2002;13:1887–1900. doi: 10.1089/104303402760372972. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Le HT, Linse KD, Cerullo V, Toietta G, Beaudet A, Pastore L. PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther. 2005;12:579–587. doi: 10.1038/sj.gt.3302441. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Yu QC, Wilson JM. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum Gene Ther. 2000;11:1713–1722. doi: 10.1089/10430340050111368. [DOI] [PubMed] [Google Scholar]
- Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- Curiel DT. Strategies to adapt adenoviral vectors for targeted delivery. Ann N Y Acad Sci. 1999;886:158–171. doi: 10.1111/j.1749-6632.1999.tb09409.x. [DOI] [PubMed] [Google Scholar]
- Curiel DT. The development of conditionally replicative adenoviruses for cancer therapy. Clin Cancer Res. 2000;6:3395–3399. [PubMed] [Google Scholar]
- Dai Y, Schwarz EM, Gu D, Zhang WW, Sarvetnick N, Verma IM. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev IP, Kashentseva EA, Curiel DT. Engineering of adenovirus vectors containing heterologous peptide sequences in the C terminus of capsid protein IX. J Virol. 2002;76:6893–6899. doi: 10.1128/JVI.76.14.6893-6899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JY, Wang D, Van Ginkel FW, Pascual DW, Frizzell RA. Systematic analysis of repeated gene delivery into animal lungs with a recombinant adenovirus vector. Hum Gene Ther. 1996;7:319–331. doi: 10.1089/hum.1996.7.3-319. [DOI] [PubMed] [Google Scholar]
- Dorigo O, Gil JS, Gallaher SD, Tan BT, Castro MG, Lowenstein PR, Calos MP, Berk AJ. Development of a novel helper-dependent adenovirus-Epstein-Barr virus hybrid system for the stable transformation of mammalian cells. J Virol. 2004;78:6556–6566. doi: 10.1128/JVI.78.12.6556-6566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JT, Miller CR, Kim M, Dmitriev I, Mikheeva G, Krasnykh V, Curiel DT. A system for the propagation of adenoviral vectors with genetically modified receptor specificities. Nat Biotechnol. 1999;17:470–475. doi: 10.1038/8647. [DOI] [PubMed] [Google Scholar]
- Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M, Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- Elkon KB, Liu CC, Gall JG, Trevejo J, Marino MW, Abrahamsen KA, Song X, Zhou JL, Old LJ, Crystal RG, Falck-Pedersen E. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc Natl Acad Sci USA. 1997;94:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtage PC, Wan Y, Muller W, Graham FL, Gauldie J. Enhanced interleukin-2 gene transfer immunotherapy of breast cancer by coexpression of B7-1 and B7-2. J Interferon Cytokine Res. 1998;18:927–937. doi: 10.1089/jir.1998.18.927. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Ye X, Doranz B, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina SF, Gao GP, Xiang ZQ, Rux JJ, Burnett RM, Alvira MR, Marsh J, Ertl HC, Wilson JM. Replication-defective vector based on a chimpanzee adenovirus. J Virol. 2001;75:11603–11613. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V, Seymour LW. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- Fisher KJ, Choi H, Burda J, Chen SJ, Wilson JM. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology. 1996;217:11–22. doi: 10.1006/viro.1996.0088. [DOI] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Gao W, Tamin A, Soloff A, D'Aiuto L, Nwanegbo E, Robbins PD, Bellini WJ, Barratt-Boyes S, Gambotto A. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geest BD, Snoeys J, Linthout SV, Lievens J, Collen D. Elimination of innate immune responses and liver inflammation by PEGylation of adenoviral vectors and methylprednisolone. Hum Gene Ther. 2005;16:1439–1451. doi: 10.1089/hum.2005.16.1439. [DOI] [PubMed] [Google Scholar]
- Gu DL, Gonzalez AM, Printz MA, Doukas J, Ying W, D'Andrea M, Hoganson DK, Curiel DT, Douglas JT, Sosnowski BA, Baird A, Aukerman SL, Pierce GF. Fibroblast growth factor 2 retargeted adenovirus has redirected cellular tropism: evidence for reduced toxicity and enhanced antitumor activity in mice. Cancer Res. 1999;59:2608–2614. [PubMed] [Google Scholar]
- Guerette B, Vilquin JT, Gingras M, Gravel C, Wood KJ, Tremblay JP. Prevention of immune reactions triggered by first-generation adenoviral vectors by monoclonal antibodies and CTLA4Ig. Hum Gene Ther. 1996;7:1455–1463. doi: 10.1089/hum.1996.7.12-1455. [DOI] [PubMed] [Google Scholar]
- Hackett NR, Kaminsky SM, Sondhi D, Crystal RG. Antivector and antitransgene host responses in gene therapy. Curr Opin Mol Ther. 2000;2:376–382. [PubMed] [Google Scholar]
- Harvey BG, Worgall S, Ely S, Leopold PL, Crystal RG. Cellular immune responses of healthy individuals to intradermal administration of an E1-E3- adenovirus gene transfer vector. Hum Gene Ther. 1999;10:2823–2837. doi: 10.1089/10430349950016555. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Kanerva A, Kremer EJ, Bauerschmitz GJ, Smith BF, Liu B, Wang M, Desmond RA, Keriel A, Barnett B, Baker HJ, Siegal GP, Curiel DT. A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther. 2003;7:163–173. doi: 10.1016/s1525-0016(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Hilbert AK, Fritzsche U, Kissel T. Biodegradable microspheres containing influenza A vaccine: immune response in mice. Vaccine. 1999;17:1065–1073. doi: 10.1016/s0264-410x(98)00323-5. [DOI] [PubMed] [Google Scholar]
- Hitt MM, Graham FL. Adenovirus vectors for human gene therapy. Adv Virus Res. 2000;55:479–505. doi: 10.1016/s0065-3527(00)55014-3. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Loser P, Cichon G, Arnold W, Both GW, Strauss M. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J Virol. 1999;73:6930–6936. doi: 10.1128/jvi.73.8.6930-6936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holterman L, Vogels R, van der V, Sieuwerts M, Grimbergen J, Kaspers J, Geelen E, van der Helm E, Lemckert A, Gillissen G, Verhaagh S, Custers J, Zuijdgeest D, Berkhout B, Bakker M, Quax P, Goudsmit J, Havenga M. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J Virol. 2004;78:13207–13215. doi: 10.1128/JVI.78.23.13207-13215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SS, Karayan L, Tournier J, Curiel DT, Boulanger PA. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan Y, Jona VK, Sengupta K, Davidson A, Horwitz MS, Roy-Chowdhury N, Roy-Chowdhury J. Transient immunosuppression with FK506 permits long-term expression of therapeutic genes introduced into the liver using recombinant adenoviruses in the rat. Hepatology. 1997;26:949–956. doi: 10.1002/hep.510260422. [DOI] [PubMed] [Google Scholar]
- Ilan Y, Sauter B, Chowdhury NR, Reddy BV, Thummala NR, Droguett G, Davidson A, Ott M, Horwitz MS, Chowdhury JR. Oral tolerization to adenoviral proteins permits repeated adenovirus-mediated gene therapy in rats with pre-existing immunity to adenoviruses. Hepatology. 1998;27:1368–1376. doi: 10.1002/hep.510270525. [DOI] [PubMed] [Google Scholar]
- Jooss K, Turka LA, Wilson JM. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 1998;5:309–319. doi: 10.1038/sj.gt.3300595. [DOI] [PubMed] [Google Scholar]
- Kafri T, Morgan D, Krahl T, Sarvetnick N, Sherman L, Verma I. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, Smith AE. Transient immunosuppression with deoxyspergualin improves longevity of transgene expression and ability to readminister adenoviral vector to the mouse lung. Hum Gene Ther. 1997;8:1095–1104. doi: 10.1089/hum.1997.8.9-1095. [DOI] [PubMed] [Google Scholar]
- Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 1996;3:154–162. [PubMed] [Google Scholar]
- Kim IH, Jozkowicz A, Piedra PA, Oka K, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Smith T, Idamakanti N, Mulgrew K, Kaloss M, Kylefjord H, Ryan PC, Kaleko M, Stevenson SC. Targeting adenoviral vectors by using the extracellular domain of the coxsackie-adenovirus receptor: improved potency via trimerization. J Virol. 2002;76:1892–1903. doi: 10.1128/JVI.76.4.1892-1903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonjkowski B, Gilardi-Hebenstreit P, Hadchouel J, Randrianarison V, Boutin S, Yeh P, Perricaudet M, Kremer EJ. A recombinant E1-deleted canine adenoviral vector capable of transduction and expression of a transgene in human-derived cells and in vivo. Hum Gene Ther. 1997;8:2103–2115. doi: 10.1089/hum.1997.8.17-2103. [DOI] [PubMed] [Google Scholar]
- Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S, Caskey CT. A new adenoviral vector: Replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnykh V, Belousova N, Korokhov N, Mikheeva G, Curiel DT. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J Virol. 2001;75:4176–4183. doi: 10.1128/JVI.75.9.4176-4183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti J, Song A, Doukas J, Sosnowski B, Pierce G, Gregory R, Wadsworth S, O'Riordan C. Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol Ther. 2003;8:99–107. doi: 10.1016/s1525-0016(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Li E, Brown SL, Stupack DG, Puente XS, Cheresh DA, Nemerow GR. Integrin alpha(v)beta1 is an adenovirus coreceptor. J Virol. 2001;75:5405–5409. doi: 10.1128/JVI.75.11.5405-5409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, Kay MA. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zaiss AK, Colarusso P, Patel K, Haljan G, Wickham TJ, Muruve DA. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum Gene Ther. 2003;14:627–643. doi: 10.1089/104303403321618146. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, Saxena A, Xiang J. Intratumoral coinjection of two adenoviral vectors expressing functional interleukin-18 and inducible protein-10: respectively, synergizes to facilitate regression of established tumors. Cancer Gene Ther. 2002;9:533–542. doi: 10.1038/sj.cgt.7700466. [DOI] [PubMed] [Google Scholar]
- Lomotan EA, Brown KA, Speaker TJ, Offita PA. Aqueous-based microcapsules are detected primarily in gut-associated dendritic cells after oral inoculation of mice. Vaccine. 1997;15:1959–1962. doi: 10.1016/s0264-410x(97)00108-4. [DOI] [PubMed] [Google Scholar]
- Mack CA, Song WR, Carpenter H, Wickham TJ, Kovesdi I, Harvey BG, Magovern CJ, Isom OW, Rosengart T, Falck-Pedersen E, Hackett NR, Crystal RG, Mastrangeli A. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum Gene Ther. 1997;8:99–109. doi: 10.1089/hum.1997.8.1-99. [DOI] [PubMed] [Google Scholar]
- Maione D, Della Rocca C, Giannetti P, D'Arrigo R, Liberatoscioli L, Franlin LL, Sandig V, Ciliberto G, La Monica N, Savino R. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc Natl Acad Sci USA. 2001;98:5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangeli A, Harvey BG, Yao J, Wolff G, Kovesdi I, Crystal RG, Falck-Pedersen E. "Sero-switch" adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum Gene Ther. 1996;7:79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- Mitani K, Wakamiya M, Hasty P, Graham FL, Bradley A, Caskey CT. Gene targeting in mouse embryonic stem cells with an adenoviral vector. Somat Cell Mol Genet. 1995;21:221–231. doi: 10.1007/BF02255777. [DOI] [PubMed] [Google Scholar]
- Mittal SK, Aggarwal N, Sailaja G, van Olphen A, HogenEsch H, North A, Hays J, Moffatt S. Immunization with DNA, adenovirus or both in biodegradable alginate microspheres: effect of route of inoculation on immune response. Vaccine. 2000;19:253–263. doi: 10.1016/s0264-410x(00)00170-5. [DOI] [PubMed] [Google Scholar]
- Mittal SK, Prevec L, Graham FL, Babiuk LA. Development of a bovine adenovirus type 3-based expression vector. J Gen Virol. 1995;76:93–102. doi: 10.1099/0022-1317-76-1-93. [DOI] [PubMed] [Google Scholar]
- Mizuguchi H, Koizumi N, Hosono T, Utoguchi N, Watanabe Y, Kay MA, Hayakawa T. A simplified system for constructing recombinant adenoviral vectors containing heterologous peptides in the HI loop of their fiber knob. Gene Ther. 2001;8:730–735. doi: 10.1038/sj.gt.3301453. [DOI] [PubMed] [Google Scholar]
- Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000;272:159–167. doi: 10.1006/viro.2000.0350. [DOI] [PubMed] [Google Scholar]
- Mok H, Palmer Donna J, Ng Philip, Barry Michael A. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther. 2005;11:66–79. doi: 10.1016/j.ymthe.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Molinier-Frenkel V, Prevost-Blondel A, Hong SS, Lengagne R, Boudaly S, Magnusson MK, Boulanger P, Guillet JG. The maturation of murine dendritic cells induced by human adenovirus is mediated by the fiber knob domain. J Biol Chem. 2003;278:37175–37182. doi: 10.1074/jbc.M303496200. [DOI] [PubMed] [Google Scholar]
- Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra PA, Zhou H, Parks RJ, Velji R, Aguilar-Cordova E, Wadsworth S, Graham FL, Kochanek S, Carey KD, Beaudet AL. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morral N, O'Neal WK, Rice K, Leland MM, Piedra PA, Aguilar-Cordova E, Carey KD, Beaudet AL, Langston C. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum Gene Ther. 2002;13:143–154. doi: 10.1089/10430340152712692. [DOI] [PubMed] [Google Scholar]
- Morral N, Parks RJ, Zhou H, Langston C, Schiedner G, Quinones J, Graham FL, Kochanek S, Beaudet AL. High doses of a helper-dependent adenoviral vector yield supraphysiological levels of alpha1-antitrypsin with negligible toxicity. Hum Gene Ther. 1998;9:2709–2716. doi: 10.1089/hum.1998.9.18-2709. [DOI] [PubMed] [Google Scholar]
- Morsy MA, Gu M, Motzel S, Zhao J, Lin J, Su Q, Allen H, Franlin L, Parks RJ, Graham FL, Kochanek S, Bett AJ, Caskey CT. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci USA. 1998;95:7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Cotter MJ, Zaiss AK, White LR, Liu Q, Chan T, Clark SA, Ross PJ, Meulenbroek RA, Maelandsmo GM, Parks RJ. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao S, Kuriyama S, Okuda H, Tominaga K, Nakatani T, Tsujinoue H, Yoshiji H, Fukui H. Adenovirus-mediated gene transfer into tumors: evaluation of direct readministration of an adenoviral vector into subcutaneous tumors of immunocompetent mice. Int J Oncol. 2001;18:57–65. [PubMed] [Google Scholar]
- Nanda A, Lynch DM, Goudsmit J, Lemckert AA, Ewald BA, Sumida SM, Truitt DM, Abbink P, Kishko MG, Gorgone DA, Lifton MA, Shen L, Carville A, Mansfield KG, Havenga MJ, Barouch DH. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J Virol. 2005;79:14161–14168. doi: 10.1128/JVI.79.22.14161-14168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng P, Parks RJ, Graham FL. Preparation of helper-dependent adenoviral vectors. Methods Mol Med. 2002;69:371–388. doi: 10.1385/1-59259-141-8:371. [DOI] [PubMed] [Google Scholar]
- Nicklin SA, Von Seggern DJ, Work LM, Pek DC, Dominiczak AF, Nemerow GR, Baker AH. Ablating adenovirus type 5 fiber-CAR binding and HI loop insertion of the SIGYPLP peptide generate an endothelial cell-selective adenovirus. Mol Ther. 2001;4:534–542. doi: 10.1006/mthe.2001.0489. [DOI] [PubMed] [Google Scholar]
- Nunes FA, Furth EE, Wilson JM, Raper SE. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum Gene Ther. 1999;10:2515–2526. doi: 10.1089/10430349950016852. [DOI] [PubMed] [Google Scholar]
- O'Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, Francis GE. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- Ostapchuk P, Hearing P. Pseudopackaging of adenovirus type 5 genomes into capsids containing the hexon proteins of adenovirus serotypes B, D, or E. J Virol. 2001;75:45–51. doi: 10.1128/JVI.75.1.45-51.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paielli DL, Wing MS, Rogulski KR, Gilbert JD, Kolozsvary A, Kim JH, Hughes J, Schnell M, Thompson T, Freytag SO. Evaluation of the biodistribution, persistence, toxicity, and potential of germ-line transmission of a replication-competent human adenovirus following intraprostatic administration in the mouse. Mol Ther. 2000;1:263–274. doi: 10.1006/mthe.2000.0037. [DOI] [PubMed] [Google Scholar]
- Parks R, Chen L, Anton M, Sankar U, Rudnicki M, Graham F. A new helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks R, Evelegh C, Graham F. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999;6:1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- Periwal SB, Speaker TJ, Cebra JJ. Orally administered microencapsulated reovirus can bypass suckled, neutralizing maternal antibody that inhibits active immunization of neonates. J Virol. 1997;71:2844–2850. doi: 10.1128/jvi.71.4.2844-2850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen UB, Benchaibi M, Meyer V, Schlesinger Y, Schughart K. Novel human gene transfer vectors: evaluation of wild-type and recombinant animal adenoviruses in human-derived cells. Hum Gene Ther. 1999;10:2587–2599. doi: 10.1089/10430349950016636. [DOI] [PubMed] [Google Scholar]
- Recchia A, Perani L, Sartori D, Olgiati C, Mavilio F. Site-specific integration of functional transgenes into the human genome by adeno/AAV hybrid vectors. Mol Ther. 2004;10:660–670. doi: 10.1016/j.ymthe.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Reddy PS, Ganesh S, Limbach MP, Brann T, Pinkstaff A, Kaloss M, Kaleko M, Connelly S. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- Reddy PS, Idamakanti N, Chen Y, Whale T, Babiuk LA, Mehtali M, Tikoo SK. Replication-defective bovine adenovirus type 3 as an expression vector. J Virol. 1999a;73:9137–9144. doi: 10.1128/jvi.73.11.9137-9144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PS, Idamakanti N, Hyun BH, Tikoo SK, Babiuk LA. Development of porcine adenovirus-3 as an expression vector. J Gen Virol. 1999b;80:563–570. doi: 10.1099/0022-1317-80-3-563. [DOI] [PubMed] [Google Scholar]
- Roelvink PW, Lizonova A, Lee JG, Li Y, Bergelson JM, Finberg RW, Brough DE, Kovesdi I, Wickham TJ. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MD, Cheng Q, Harui A, Basak SK, Mitani K, Low TA, Kiertscher SM. Helper-dependent adenoviral vectors efficiently express transgenes in human dendritic cells but still stimulate antiviral immune responses. J Immunol. 2002;169:4651–4656. doi: 10.4049/jimmunol.169.8.4651. [DOI] [PubMed] [Google Scholar]
- Roy S, Shirley PS, McClelland A, Kaleko M. Circumvention of immunity to the adenovirus major coat protein hexon. J Virol. 1998;72:6875–6879. doi: 10.1128/jvi.72.8.6875-6879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi H, Hitt MM. Transcriptionally targeted adenovirus vectors. Curr Gene Ther. 2005;5:411–427. doi: 10.2174/1566523054546189. [DOI] [PubMed] [Google Scholar]
- Sailaja G, HogenEsch H, North A, Hays J, Mittal SK. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9:1722–1729. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai F, Mizuguchi H, Hayakawa T. Efficient gene transfer into human CD34+ cells by an adenovirus type 35 vector. Gene Ther. 2003;10:1041–1048. doi: 10.1038/sj.gt.3301959. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Bloch W, Hertel S, Johnston M, Molojavyi A, Dries V, Varga G, van Rooijen N, Kochanek S. A hemodynamic response to intravenous adenovirus vector particles is caused by systemic Kupffer cell-mediated activation of endothelial cells. Hum Gene Ther. 2003a;14:1631–1641. doi: 10.1089/104303403322542275. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Hertel S, Johnston M, Dries V, van Rooijen N, Kochanek S. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol Ther. 2003b;7:35–43. doi: 10.1016/s1525-0016(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Morral N, Parks RJ, Wu Y, Koopmans SC, Langston C, Graham FL, Beaudet AL, Kochanek S. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat Genet. 1998;18:180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, Chen SJ, Varnavski AN, LeClair C, Raper SE, Wilson JM. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Schoggins JW, Nociari M, Philpott N, Falck-Pedersen E. Influence of fiber detargeting on adenovirus-mediated innate and adaptive immune activation. J Virol. 2005;79:11627–11637. doi: 10.1128/JVI.79.18.11627-11637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G, Arnberg N. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol. 2003;77:9183–9191. doi: 10.1128/JVI.77.17.9183-9191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifrin AL, Chirmule N, Gao GP, Wilson JM, Raper SE. Innate immune responses to adenoviral vector-mediated acute pancreatitis. Pancreas. 2005;30:122–129. doi: 10.1097/01.mpa.0000151578.99413.88. [DOI] [PubMed] [Google Scholar]
- Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- Sirena D, Lilienfeld B, Eisenhut M, Kalin S, Boucke K, Beerli RR, Vogt L, Ruedl C, Bachmann MF, Greber UF, Hemmi S. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004;78:4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Tian J, Lozier JN, Byrnes AP. Severe pulmonary pathology after intravenous administration of vectors in cirrhotic rats. Mol Ther. 2004a;9:932–941. doi: 10.1016/j.ymthe.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Smith JS, Tian J, Muller J, Byrnes AP. Unexpected pulmonary uptake of adenovirus vectors in animals with chronic liver disease. Gene Ther. 2004b;11:431–438. doi: 10.1038/sj.gt.3302149. [DOI] [PubMed] [Google Scholar]
- Smith TA, Idamakanti N, Rollence ML, Marshall-Neff J, Kim J, Mulgrew K, Nemerow GR, Kaleko M, Stevenson SC. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum Gene Ther. 2003;14:777–787. doi: 10.1089/104303403765255165. [DOI] [PubMed] [Google Scholar]
- Smith TA, White BD, Gardner JM, Kaleko M, McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- St George JA. Gene therapy progress and prospects: adenoviral vectors. Gene Ther. 2003;10:1135–1141. doi: 10.1038/sj.gt.3302071. [DOI] [PubMed] [Google Scholar]
- Steel JC, Cavanagh HM, Burton MA, Kalle WH. Microsphere-liposome complexes protect adenoviral vectors from neutralising antibody without losses in transfection efficiency, in-vitro. J Pharm Pharmacol. 2004;56:1371–1378. doi: 10.1211/0022357044643. [DOI] [PubMed] [Google Scholar]
- Stilwell JL, McCarty DM, Negishi A, Superfine R, Samulski RJ. Development and characterization of novel empty adenovirus capsids and their impact on cellular gene expression. J Virol. 2003;77:12881–12885. doi: 10.1128/JVI.77.23.12881-12885.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D, Ni S, Li ZY, Gaggar A, DiPaolo N, Feng Q, Sandig V, Lieber A. Development and assessment of human adenovirus type 11 as a gene transfer vector. J Virol. 2005;79:5090–5104. doi: 10.1128/JVI.79.8.5090-5104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, Lockman S, Peter T, Peyerl FW, Kishko MG, Jackson SS, Gorgone DA, Lifton MA, Essex M, Walker BD, Goudsmit J, Havenga MJ, Barouch DH. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toogood CI, Crompton J, Hay RT. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J Gen Virol. 1992;73:1429–1435. doi: 10.1099/0022-1317-73-6-1429. [DOI] [PubMed] [Google Scholar]
- Trudel S, Li Z, Dodgson C, Nanji S, Wan Y, Voralia M, Hitt M, Gauldie J, Graham FL, Stewart AK. Adenovector engineered interleukin-2 expressing autologous plasma cell vaccination after high-dose chemotherapy for multiple myeloma--a phase 1 study. Leukemia. 2001;15:846–854. doi: 10.1038/sj.leu.2402077. [DOI] [PubMed] [Google Scholar]
- van Olphen AL, Tikoo SK, Mittal SK. Characterization of bovine adenovirus type 3 E1 proteins and isolation of E1-expressing cell lines. Virology. 2002;295:108–118. doi: 10.1006/viro.2002.1389. [DOI] [PubMed] [Google Scholar]
- Varnavski AN, Calcedo R, Bove M, Gao G, Wilson JM. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005;12:427–436. doi: 10.1038/sj.gt.3302347. [DOI] [PubMed] [Google Scholar]
- Vlachaki MT, Hernandez-Garcia A, Ittmann M, Chhikara M, Aguilar LK, Zhu X, Teh BS, Butler EB, Woo S, Thompson TC, Barrera-Saldana H, Aguilar-Cordova E, The BS. Impact of preimmunization on adenoviral vector expression and toxicity in a subcutaneous mouse cancer model. Mol Ther. 2002;6:342–348. doi: 10.1006/mthe.2002.0669. [DOI] [PubMed] [Google Scholar]
- Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, Cecchini M, Wetterwald A, Sprangers M, Lemckert A, Ophorst O, Koel B, van Meerendonk M, Quax P, Panitti L, Grimbergen J, Bout A, Goudsmit J, Havenga M. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, You Q, Hagstrom JN, Sands M, High KA. Successful expression of human factor IX following repeat administration of adenoviral vector in mice. Proc Natl Acad Sci USA. 1996;93:3056–3061. doi: 10.1073/pnas.93.7.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen XY, Mandelbaum S, Li ZH, Hitt M, Graham FL, Hawley TS, Hawley RG, Stewart AK. Tricistronic viral vectors co-expressing interleukin-12 (1L-12) and CD80 (B7-1) for the immunotherapy of cancer: preclinical studies in myeloma. Cancer Gene Ther. 2001;8:361–370. doi: 10.1038/sj.cgt.7700321. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Wolins N, Lozier J, Eggerman TL, Jones E, guilar-Cordova E, Vostal JG. Intravenous administration of replication-incompetent adenovirus to rhesus monkeys induces thrombocytopenia by increasing in vivo platelet clearance. Br J Haematol. 2003;123:903–905. doi: 10.1046/j.1365-2141.2003.04719.x. [DOI] [PubMed] [Google Scholar]
- Worgall S, Leopold PL, Wolff G, Ferris B, Van Roijen N, Crystal RG. Role of alveolar macrophages in rapid elimination of adenovirus vectors administered to the epithelial surface of the respiratory tract. Hum Gene Ther. 1997;8:1675–1684. doi: 10.1089/hum.1997.8.14-1675. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, Wilson JM, Ertl HC. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76:2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Goverdhana S, Sciascia SA, Candolfi M, Zirger JM, Barcia C, Curtin JF, King GD, Jaita G, Liu C, Kroeger K, Agadjanian H, Medina-Kauwe L, Palmer D, Ng P, Lowenstein PR, Castro MG. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Robinson MB, Pabin C, Batshaw ML, Wilson JM. Transient depletion of CD4 lymphocyte improves efficacy of repeated administration of recombinant adenovirus in the ornithine transcarbamylase deficient sparse fur mouse. Gene Ther. 2000;7:1761–1767. doi: 10.1038/sj.gt.3301299. [DOI] [PubMed] [Google Scholar]
- Yotnda P, Chen DH, Chiu W, Piedra PA, Davis A, Templeton NS, Brenner MK. Bilamellar cationic liposomes protect adenovectors from preexisting humoral immune responses. Mol Ther. 2002;5:233–241. doi: 10.1006/mthe.2002.0545. [DOI] [PubMed] [Google Scholar]
- Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhartchouk A, Connors W, van Kessel A, Tikoo SK. Bovine adenovirus type 3 containing heterologous protein in the C-terminus of minor capsid protein IX. Virology. 2004;320:291–300. doi: 10.1016/j.virol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, Tazelaar J, Wilson JM. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]


