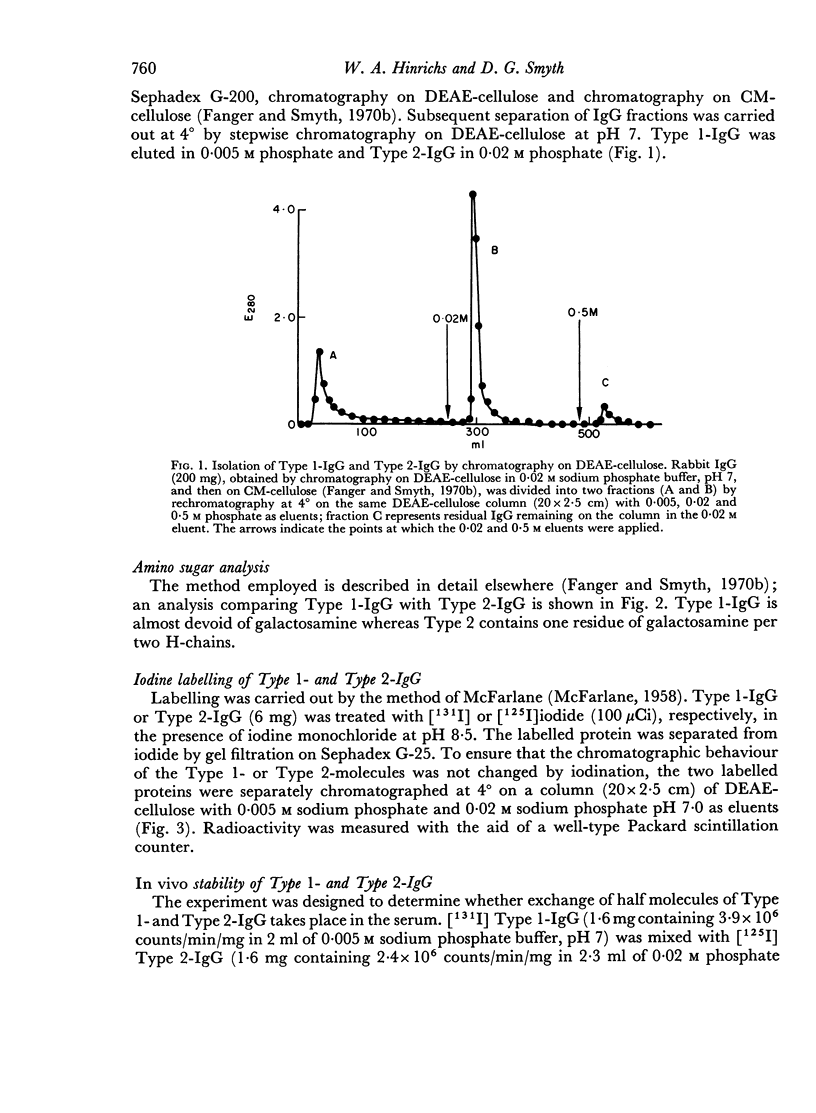
Abstract
Two populations of IgG molecules have been isolated from rabbit serum: Type 1, which is devoid of C2-oligosaccharide, and Type II, which has the C2-oligosaccharide attached to only one of the H-chains (Fanger & Smyth, 1970b). By labelling with different isotopes, it was possible to study the stability of the two IgG Types in vitro and in vivo; conclusions could then be drawn concerning biosynthesis of the carbohydrate moiety.
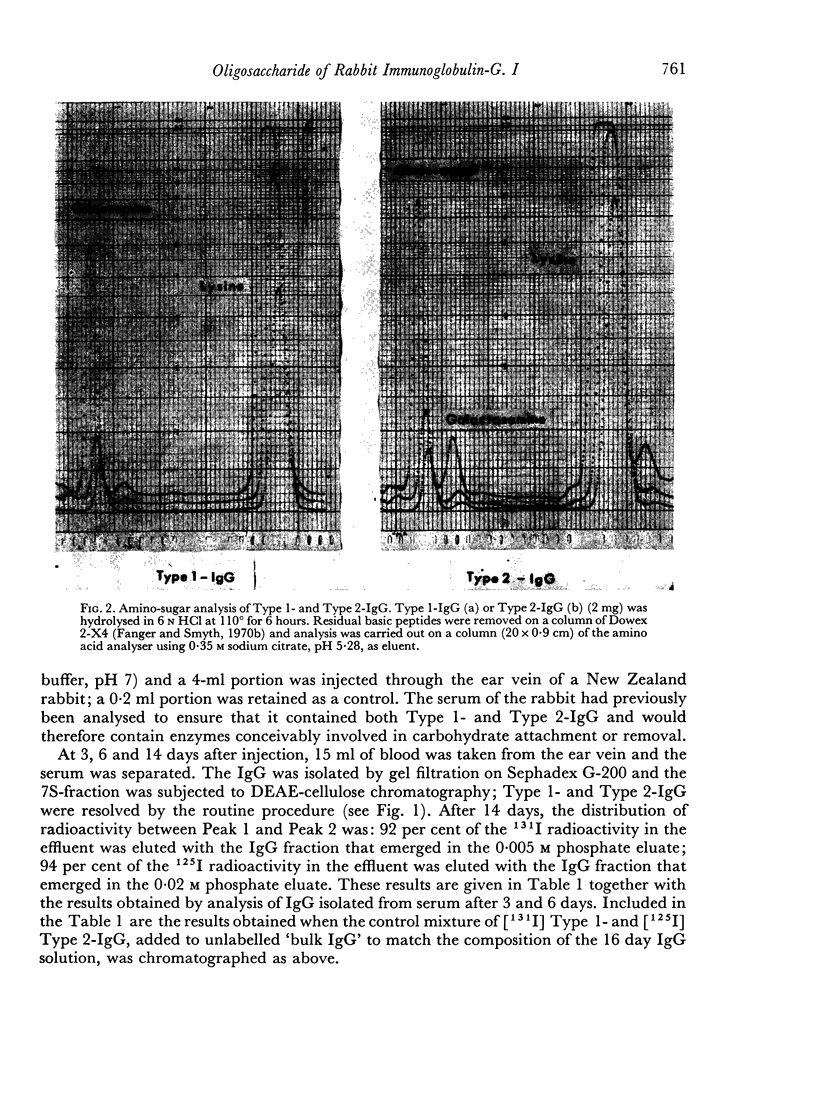
The C2-oligosaccharide is not affixed to or deleted from the IgG molecule during its life span in the circulation; carbohydrate attachment takes place exclusively within the biosynthetic cell. Furthermore, a symmetrical molecule with the C2-oligosaccharide on both H-chains does not appear to be formed in vivo, although this molecule could easily be formed in vitro. It follows that the order of events in biosynthesis of IgG is that first the polypeptide chains are assembled and then the carbohydrate moiety is added to complete the molecule.
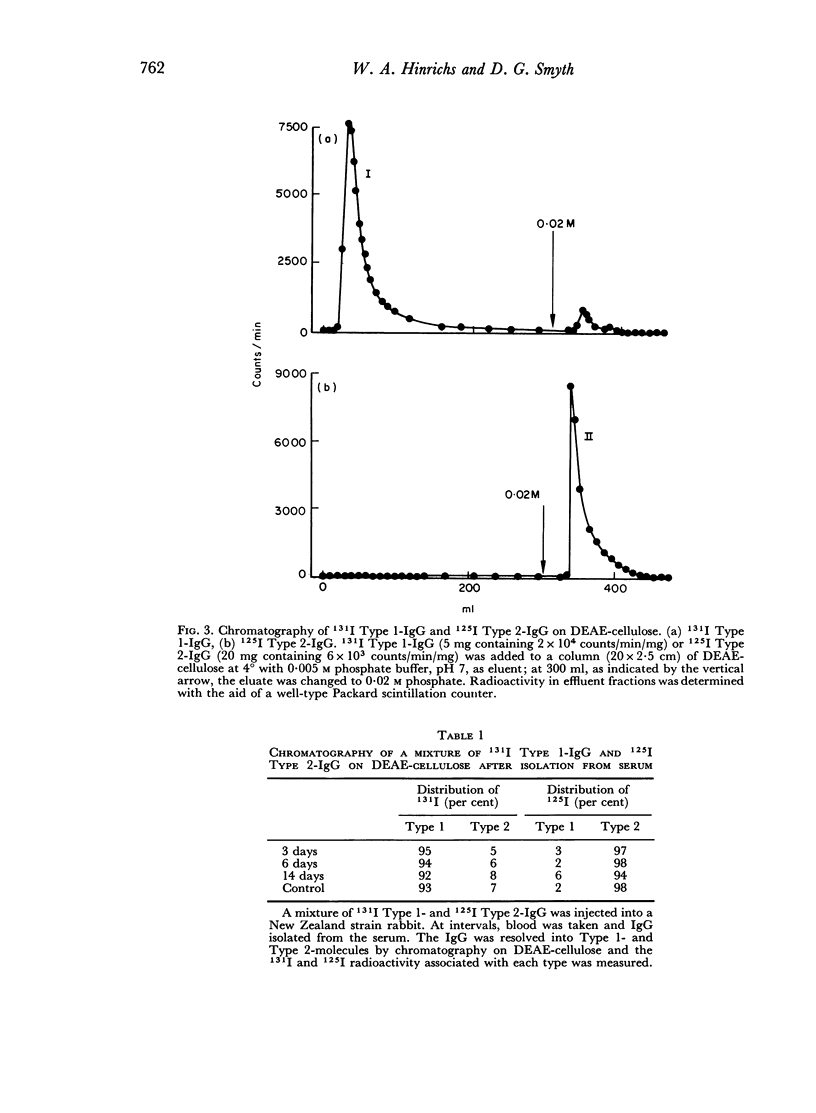
No exchange of half molecules takes place between different IgG molecules in the serum but in vitro this exchange could be induced under mild conditions. Thus after secretion from the cell the IgG molecule retains its structural integrity, both with regard to its constituent polypeptide chains and to its characteristic complement of oligosaccharides.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Williamson A. R. Interchain disulphide-bond formation in the assembly of immunoglobulin G. Heavy-chain dimer as an intermediate. Biochem J. 1968 Oct;109(4):637–643. doi: 10.1042/bj1090637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Steiner L. A., Porter R. R. The partial sequence of two large peptides from the N-terminal half of heavy chains from normal rabbit immunoglobulin G. Biochem J. 1968 Mar;107(1):79–88. doi: 10.1042/bj1070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givol D., De Lorenzo F. The position of various cleavages of rabbit immunoglobulin G. J Biol Chem. 1968 Apr 25;243(8):1886–1891. [PubMed] [Google Scholar]
- Hong R., Nisonoff A. Relative labilities of the two types of interchain disulfide bond of rabbit gamma G-immunoglobulin. J Biol Chem. 1965 Oct;240(10):3883–3891. [PubMed] [Google Scholar]
- Smyth D. S., Utsumi S. Structure at the hinge region in rabbit immunoglobulin-G. Nature. 1967 Oct 28;216(5113):332–335. doi: 10.1038/216332a0. [DOI] [PubMed] [Google Scholar]



