Abstract
Mice homozygous for the mutation nude (nu), which are born without a thymus, accept allogeneic skin grafts. Prior intraperitoneal injection of a thymic cell suspension, or implantation of a thymus on the first day after birth, enables the `nude' mice to reject skin grafts.
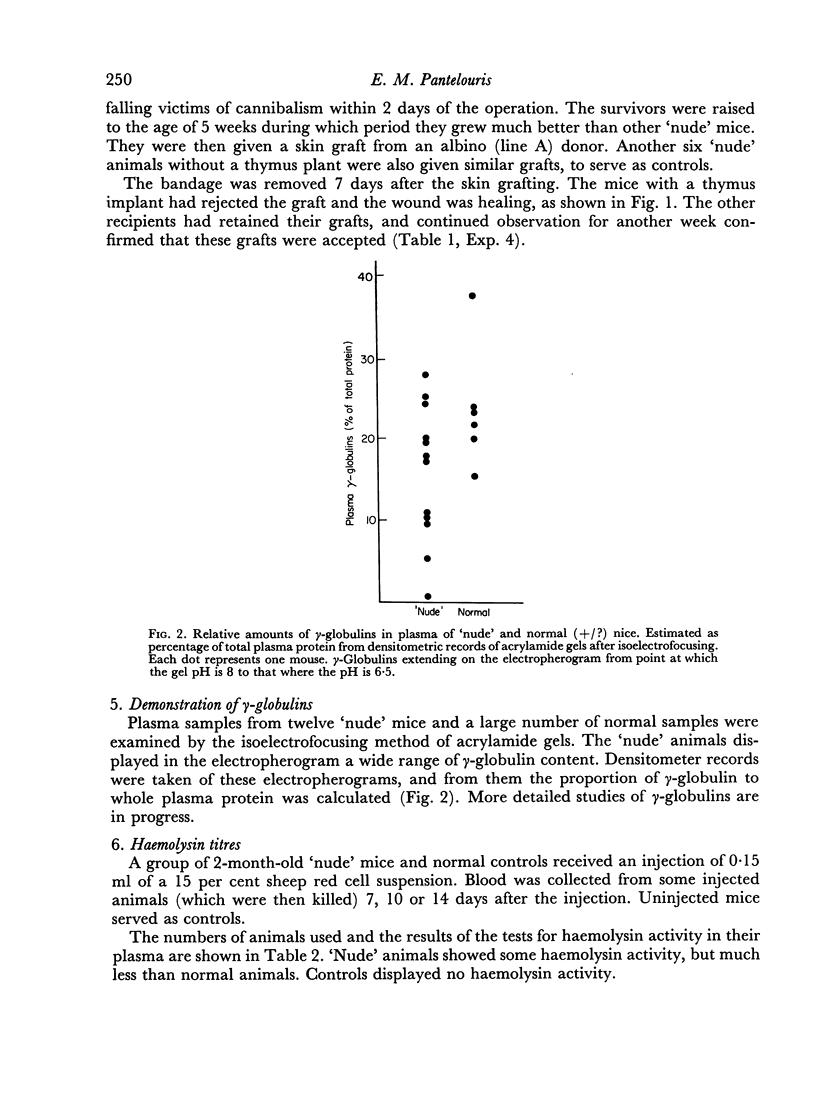
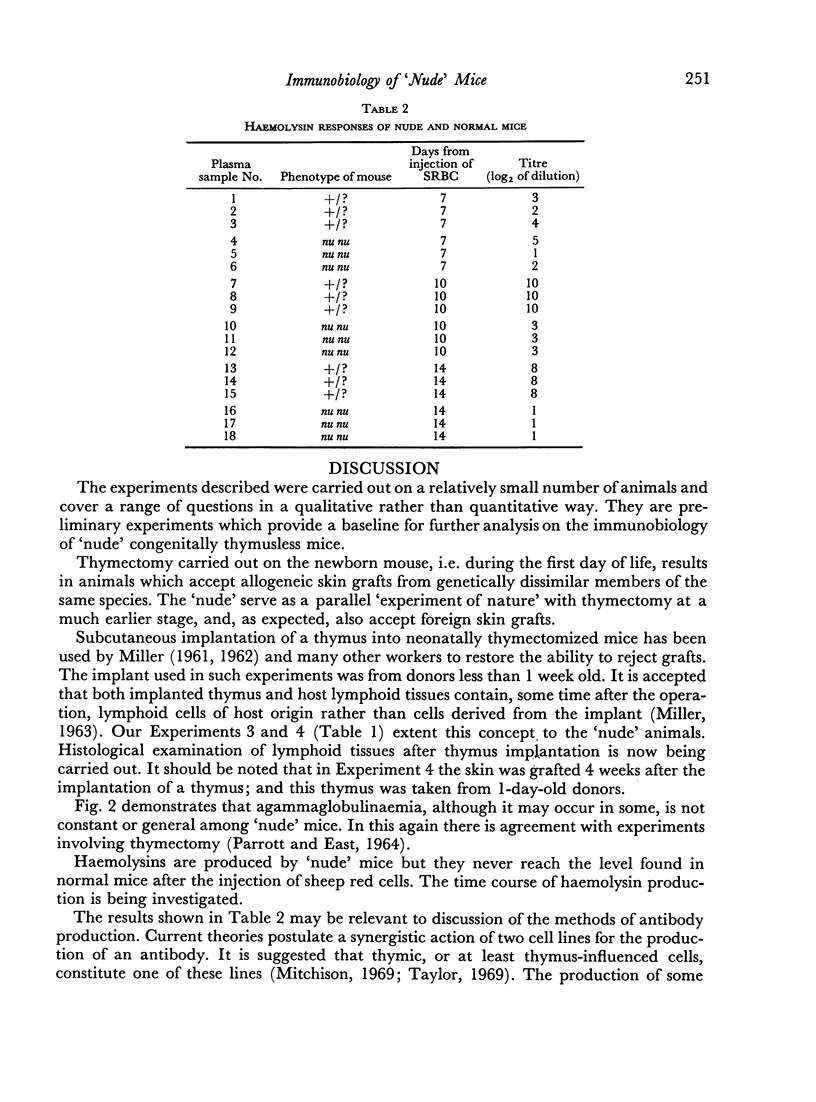
γ-Globulins are generally present in `nude' mice. Haemolysin activity in response to sheep red cell injection is detectable but much lower than in normal animals.
The similarity of the `nude' syndrome to certain human primary immunological deficiencies is briefly considered in the light of these experiments and of embryological evidence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awdeh Z. L., Williamson A. R., Askonas B. A. Isoelectric focusing in polyacrylamide gel and its application to immunoglobulins. Nature. 1968 Jul 6;219(5149):66–67. doi: 10.1038/219066a0. [DOI] [PubMed] [Google Scholar]
- De Sousa M. A., Parrott D. M., Pantelouris E. M. The lymphoid tissues in mice with congenital aplasia of the thymus. Clin Exp Immunol. 1969 Jun;4(6):637–644. [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Slater F. D., White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. 1966 Sep;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. F. Etiology and pathogenesis of mouse leukemia. Adv Cancer Res. 1961;6:291–368. doi: 10.1016/s0065-230x(08)60623-5. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The immunogenic capacity of antigen taken up by peritoneal exudate cells. Immunology. 1969 Jan;16(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- Pantelouris E. M. Absence of thymus in a mouse mutant. Nature. 1968 Jan 27;217(5126):370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- Seligmann M., Fudenberg H. H., Good R. A. A proposed classification of primary immunologic deficiencies. Am J Med. 1968 Dec;45(6):817–825. doi: 10.1016/0002-9343(68)90180-0. [DOI] [PubMed] [Google Scholar]
- Sinclair N. R., Millican D. Delayed development of immunological responsiveness in neonatally thymectomized mice: a time-course study. Clin Exp Immunol. 1967 May;2(3):269–274. [PMC free article] [PubMed] [Google Scholar]
- Taylor R. B. Cellular cooperation in the antibody response of mice to two serum albumins: specific function of thymus cells. Transplant Rev. 1969;1:114–149. doi: 10.1111/j.1600-065x.1969.tb00138.x. [DOI] [PubMed] [Google Scholar]



