Abstract
PC-cell-derived growth factor (PCDGF) is an 88-kDa glycoprotein corresponding to the granulin precursor. We have reported that PCDGF was expressed in human breast cancer cells. In estrogen-receptor positive cells, 17-β-estradiol (E2) transcriptionally stimulated PCDGF expression in a dose- and time-dependent fashion. We demonstrate here that PCDGF mediates the mitogenic effect of E2 in MCF-7 cells. PCDGF substituted for E2 to stimulate DNA synthesis. The E2 mitogenic effect was inhibited in a dose-dependent fashion by anti-PCDGF neutralizing antibody. Inhibition of PCDGF expression by antisense transfection also inhibited the E2 mitogenic effect. In contrast, overexpression of PCDGF in MCF-7 cells resulted in cells that were able to proliferate in the absence of estrogen and were tamoxifen resistant. The PCDGF signaling pathway was examined. Like E2, PCDGF stimulated mitogen-activated protein kinase activity. PCDGF could substitute for E2 in stimulating cyclin D1 expression. The cyclin D1 stimulation by E2 was 50% inhibited by anti-PCDGF antibody. In contrast, PCDGF did not stimulate c-myc expression, another molecular target of E2. We conclude that autocrine PCDGF mediates the E2 mitogenic effect via stimulation of cyclin D1. These studies provide information on estrogen action and identify an autocrine molecular target in human breast cancer cells.
Breast cancer is a major worldwide cause of morbidity and mortality among women. Estrogen is known to be the main stimulator for estrogen receptor-positive (ER+) human breast cancer cell growth in vivo and in vitro (1, 2). Although breast tumors initially require estrogen for establishment and proliferation, the development of estrogen-independent tumors, resulting in poor prognosis, is observed during the course of the disease (1, 2). It has been postulated that the mitogenic effect of estrogen in breast cancer cells is mediated, at least partially, by autocrine growth factors regulated or not by estrogen (3, 4). Thus, the identification in human breast cancer cells of estrogen-responsive genes, particularly the ones encoding growth factors, has been very important because their characterization can contribute to the understanding of estrogen action in these cells.
PC-cell-derived growth factor (PCDGF) is an 88-kDa glycoprotein purified from the highly tumorigenic mouse teratoma-derived cell line PC (5, 6). Sequencing indicated that PCDGF corresponded to the precursor for a novel family of double cysteine-rich 6-kDa polypeptides called epithelins or granulins shown to either promote or inhibit cell growth, depending on the cell type tested (7–9). The precursor originally was reported to be biologically inactive unless it was processed into the 6-kDa forms by a yet-unknown mechanism (7, 8). Our laboratory demonstrated the biological activity of the precursor for mesenchymal cells (5, 6). Others later demonstrated the growth-promoting activity of the precursor for other mesenchymal and epithelial cells as well as for preimplantation embryos (10–12).
Screening of human tumor cell lines for PCDGF indicated that it was highly expressed in estrogen receptor-negative (ER−) human breast carcinomas (13). Inhibition of PCDGF expression in these cells by antisense PCDGF cDNA transfection resulted in a 90% inhibition of tumor incidence and tumor size when injected into nude mice (13). Although these data implicated PCDGF in the maintenance of the tumor phenotype, they did not provide any clue to whether PCDGF was involved in the 17-β estradiol (E2) mitogenic effect in ER+ cells. In ER+ cells such as MCF-7 and T47D, PCDGF expression was transcriptionally stimulated by E2 and inhibited by the antiestrogen tamoxifen (14). Because E2 is an important factor for the growth of ER+ breast cancer cells (3), we attempted to determine here whether PCDGF played a role in mediating the E2 mitogenic effect on ER+ breast cancer cell lines.
Materials and Methods
17β-Estradiol (E2) and 4-OH-tamoxifen were obtained from Sigma. Anti-Erk2 antibody was purchased from Santa Cruz Biotechnology. Anti-human pRb monoclonal antibody was from PharMingen. Anti-ER and anti-cyclin D1 mouse monoclonal antibodies and anti-c-Myc polyclonal antibody were from Upstate Biotechnology (Lake Placid, NY). PD98095 was from New England Biolabs. Culture media and FBS were purchased from Life Technologies (Grand Island, NY).
Cell Culture.
Human breast cancer MCF-7 cells, obtained from the American Type Culture Collection, were cultivated in a 1:1 mixture of DMEM and Ham's F-12 medium supplemented with 5% (vol/vol) FBS.
Cell Growth Assays.
Two types of assays (thymidine incorporation and increase in cell number) were used to investigate the effect of PCDGF on the proliferation of MCF-7 cells in estrogen-depleted medium. Both assays have been described (15).
Proliferation assays were carried out with human recombinant PCDGF produced in Chinese hamster ovary cells and purified as described (16). Neutralization experiments of PCDGF activity were performed by using rabbit anti-human PCDGF IgG that had been affinity purified as described (13).
Antisense and Overexpressing PCDGF cDNA Vector Constructs and Stable Transfection.
The antisense PCDGF cDNA expression vector used here has been described (13). PCDGF overexpression vector was prepared by ligating the coding region of human PCDGF cDNA into PcDNA3 mammalian expression vector. Plasmid DNA corresponding to each construct was transfected into MCF-7 cells with Lipofectamine according to the manufacturer's specifications. MCF-7 cells transfected with empty PCDNA3 vector were used as a control. Transfected MCF-7 cells were first selected for their ability to grow with G418 (800 μg/ml). Stable clones were selected after 3 weeks. Antisense and overexpressing clones were analyzed for either inhibition of PCDGF expression or an increase in PCDGF expression by Western blot analyses as described (11). In the case of overexpressing clones, all of the selected clones (10 total) similarly overexpressed PCDGF. Studies presented here were done with one representative clone (O4). Similar data were obtained with other randomly picked clones.
Mitogen-Activated Protein (MAP) Kinase Assay.
MCF-7 cells were cultured in a 1:1 mixture of DMEM and Ham's F-12 medium supplemented with 5% (vol/vol) FBS. One day before the start of the experiment, the medium was changed to E2-depleted PFMEM (phenol red-free α-MEM) supplemented with 5% charcoal-extracted FBS as described (15). Twenty-four hours later, the medium was replaced with PFMEM. PCDGF or various factors to be tested were added for 10 min to measure MAP kinase activity, using myelin basic protein as the substrate. The intensity of phosphorylated myelin basic protein bands was determined by densitometric scanning. The intensities of the Erk1 and Erk2 protein signals determined by Western blot analysis of duplicate samples with anti-Erk antibody was used as the internal control to normalize the MAP kinase assays.
Western Blot Detection of Cyclin D1, c-myc, and Rb Expression.
MCF-7 cells were cultivated in PFMEM and synchronized by treatment with 1 μM tamoxifen for 48 h as described (17). The cells were then treated with 10−9 M E2 or 200 ng/ml human PCDGF for the indicated times. The cells were then lysed as described (14). Sixty micrograms of cell lysate (100 μg for Rb detection) were separated by SDS/PAGE on a 10% polyacrylamide gel. Western blot analysis was done either with anti-human cyclin D1 monoclonal antibody or c-myc polyclonal antibody at 1 μg/ml or anti-human Rb monoclonal antibody at 2 μg/ml. Horseradish peroxidase-conjugated goat anti-rabbit IgG or goat-anti-mouse IgG was used as the secondary antibody. Protein detection was done by enhanced chemoluminescence.
Inhibition of Cyclin D1 Expression by Treatment with Anti-PCDGF Antibody.
The culture conditions of the MCF-7 cells were similar to those described above, except that the cells were treated with 10−9 M E2 in the presence of 300 μg/ml of nonimmune IgG or with 300 μg/ml anti-PCDGF antibody. The cells were lysed 3, 5, and 12 h later, and Western blot detection of cyclin D1 expression was performed by using anti-cyclin D1 antibody followed by incubation with 125I-labeled goat anti-mouse IgG (2 μCi/ml). The signals were quantified with a PhosphoImager and expressed as a percentage inhibition of the cyclin D1 signals in cells treated with E2 and nonimmune IgG, taken as controls at each time point.
Statistics.
All experiments were performed by using triplicate dishes and were repeated three times. The data were expressed as mean ± SD. A two-tailed Student's t test was used for statistical analysis of the data. P < 0.05 was taken as the level of significance.
Results
PCDGF Stimulates DNA Synthesis of MCF-7 Cells in the Absence of Estrogen.
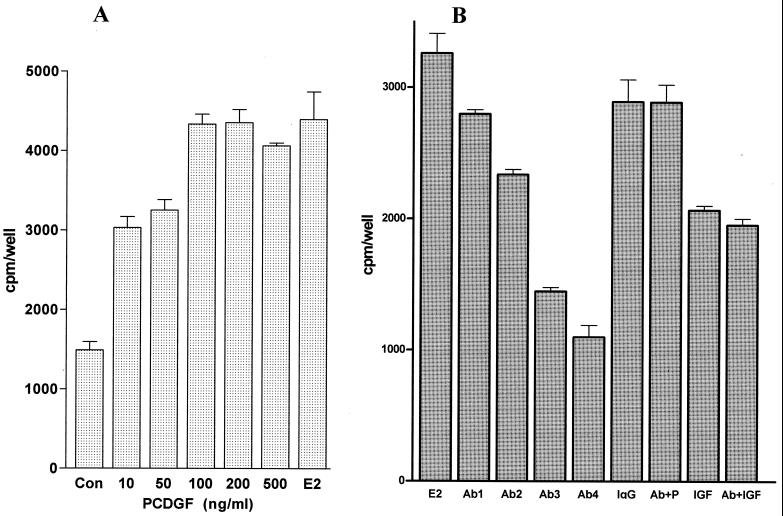
We first examined whether the addition of PCDGF stimulated DNA synthesis of MCF-7 cells maintained in the absence of E2. In these conditions, endogenous production of PCDGF is very low (14). A 2-fold increase in [3H]thymidine incorporation over the control (cells maintained in the absence of E2 and PCDGF; P < 0.01) was observed at 10 ng/ml of PCDGF (Fig. 1A). A 4-fold maximal stimulation was observed with 100 ng/ml PCDGF (P < 0.001), similar to the one observed with 10−9 M E2. PCDGF stimulated DNA synthesis of another ER+ cell line, T47D. A 2.9 ± 0.3-fold stimulation of DNA synthesis was observed with 100 ng/ml PCDGF.
Figure 1.
Effect of PCDGF on the proliferation of MCF-7 cells. (A) PCDGF stimulates DNA synthesis of MCF-7 cells in the absence of E2. MCF-7 cells (105 cells per well) were plated in a 1:1 mixture of DMEM and Ham's F-12 medium plus 5% FBS. After 48 h, the medium was replaced with PFMEM, followed 24 h later by serum-free α-MEM. Increasing concentrations of human PCDGF were added in triplicate to the medium. EtOH only (CON) and 10−9 M E2 (E2) were used as negative and positive controls, respectively. [3H]Thymidine (1 μCi/ml) was added 24 h later for 5 h. The results are expressed as means ± SD of triplicate determinations. (B) Anti-PCDGF antibody specifically inhibits the E2 mitogenic effect. MCF-7 cells were treated with 10−9 M E2 (E2) alone or with increasing concentrations of affinity-purified anti-PCDGF antibody: 50 μg/ml (Ab1), 100 μg/ml (Ab2), 200 μg/ml (Ab3), and 300 μg/ml (Ab4). Cells treated with 10−9 M E2 and 300 μg/ml preimmune IgG (preIgG) and cells treated with 10 ng/ml of IGF-II (IGF) alone or in the presence of 300 μg/ml anti-PCDGF antibody (Ab + IGF) were used as controls. The results are expressed as means ± SD of triplicate determinations.
E2 Mitogenic Effect Is Inhibited by Treatment of the Cells with Anti-PCDGF Antibody.
Based on these results and given that E2 stimulates PCDGF expression in MCF-7 cells (14), we determined whether endogenous PCDGF could mediate the E2 mitogenic effect via an autocrine loop. For this purpose, we first examined whether treatment with anti-PCDGF antibody that blocks PCDGF produced by the MCF-7 cells would inhibit the E2 mitogenic activity. The addition of affinity-purified anti-human PCDGF antibody inhibited in a dose-dependent fashion the growth of the cells stimulated by E2 (Fig. 1B). A 74% inhibition of E2 mitogenic effect was observed with 300 μg/ml of anti-PCDGF antibody (P < 0.003), whereas a similar concentration of nonimmune IgG had no effect. This antibody concentration displayed no cellular toxicity. Addition of PCDGF (200 ng/ml) restored the proliferation of the cells treated with anti-PCDGF antibody. The specificity of the PCDGF antibody effect also was demonstrated because it could not inhibit the stimulatory effect of insulin-like growth factor-II (IGF-II), a known growth stimulator of MCF-7 cells (18). These results suggest that PCDGF acts as an autocrine growth factor to mediate the mitogenic effect of E2 for MCF-7 cells.
Inhibition of PCDGF Expression Inhibits the Growth of MCF-7 Cells in the Presence of E2.
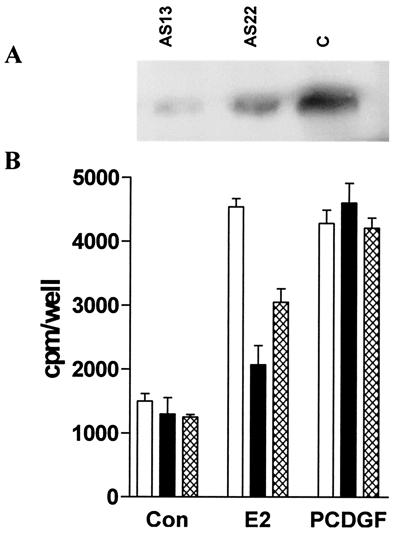
We next attempted to determine whether inhibiting PCDGF expression in MCF-7 cells would prevent the growth-stimulatory effect of E2. For this purpose, we examined the E2 mitogenic effect in MCF-7 cells, where PCDGF expression had been inhibited by antisense PCDGF cDNA transfection. The PCDGF levels in two representative antisense clones, As13 and As22, and empty vector control transfected MCF-7 (MCF-7C) cells were determined by Western blot analysis of samples prepared by using identical cell numbers (Fig. 2A). As13 cells displayed an 80% inhibition of PCDGF expression, whereas As22 showed a 50% inhibition when compared with MCF-7C cells.
Figure 2.
Effect of inhibiting PCDGF expression on the E2 mitogenic effect. (A) Inhibition of PCDGF expression by antisense PCDGF cDNA transfection in MCF-7 cells. PCDGF expression in antisense (As13 and As22) and empty vector control transfected cells (MCF7-C) was examined by Western blot analysis. AS13 (lane 1), AS22 (lane 2), and MCF-7C cells (lane 3) were treated with 10−9 M E2 in PFMEM. The conditioned media were collected after 24 h and normalized to the same cell number (4 × 106 cells) for the measurement of PCDGF expression. (B) Inhibition of PCDGF expression in MCF-7 cells inhibits E2 mitogenic activity. The E2 mitogenic effect was examined in antisense and control MCF-7C cells. AS13 (filled bars), AS22 (hatched bars), and MCF-7C cells (open bars) were treated with 10−9 M E2 (E2), 100 ng/ml recombinant PCDGF (PCDGF), or 0.1% EtOH only (Con). [3H]Thymidine incorporation was measured as described above. Results are expressed as means ± SD.
The effect of E2 on DNA synthesis then was examined in these antisense and control MCF-7C cells. A [3H]thymidine incorporation assay showed that the E2 stimulatory effect in MCF-7C cells had been reduced in AS13 and AS22 in correlation with the degree of inhibition of PCDGF expression (Fig. 2B). AS13 showed the highest inhibition of the E2 effect. DNA synthesis mediated by E2 was only 25% (P < 0.001) in AS13 and 60% (P < 0.001) in AS22, respectively, of that observed in MCF-7C cells. The addition of PCDGF restored the proliferation of the antisense clones to the level found in the control MCF-7 cells cultivated in the presence of E2.
Overexpression of PCDGF Leads to Estrogen Independence and Tamoxifen Resistance.
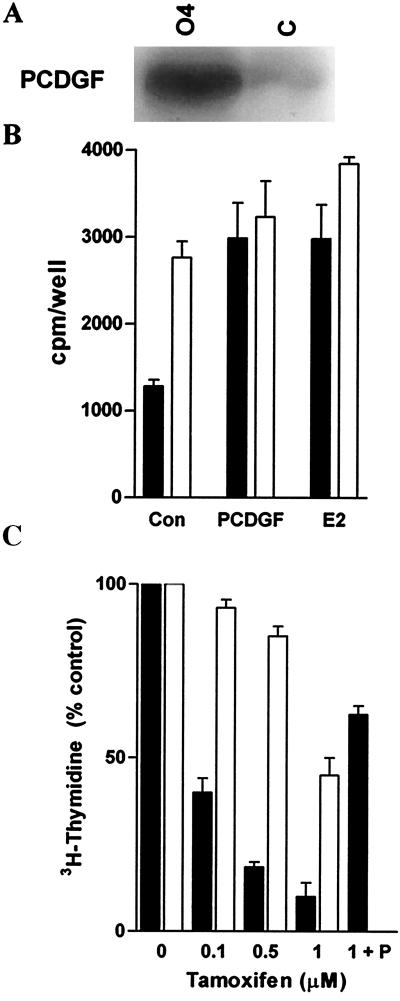
Based on the above results, we examined the effect of the constitutive overexpression of PCDGF in ER+ MCF-7 cells on their estrogen and tamoxifen responsiveness. This was achieved by transfecting MCF-7 cells with a pcDNA3 expression vector containing PCDGF cDNA under the control of the cytomegalovirus promoter. Several PCDGF overexpressing clones were obtained. One representative clone called O4 was further studied. Fig. 3A shows that O4 cells produced elevated levels of PCDGF when compared with control MCF-7C cells in E2-depleted medium. The ability of O4 cells to proliferate in the absence of E2 was clearly demonstrated (Fig. 3B), inasmuch as the thymidine incorporation in O4 cells was 2.2-fold higher than in MCF-7C cells (P < 0.01). Moreover, there was no significant difference between the thymidine incorporation level for O4 cells in E2-depleted medium and for MCF-7C cells treated with either PCDGF or E2 (P > 0.05). The addition of E2 or PCDGF to O4 cells did not have any significant additional effect in contrast to MCF-7C cells. In long-term growth assays, whereas MCF-7C cells could not grow in the absence of E2, O4 cells proliferated with a doubling time of 42 h, close to the 36-h doubling time of MCF-7C cells in the presence of E2. These data suggest that PCDGF overexpression provided a growth advantage in the absence of E2.
Figure 3.
Overexpression of PCDGF in MCF-7 cells results in cells that are able to proliferate in the absence of E2 and are resistant to tamoxifen. (A) PCDGF expression in O4 cells and control MCF-7C cells. O4 and empty vector control MCF-7 cells were cultivated in PFMEM. The conditioned media were collected after 24 h to determine PCDGF expression. (B) Proliferation of O4 and control MCF-7 cells in estrogen-depleted PFMEM. Cells were plated in triplicate in estrogen-depleted PFMEM. Cells were either maintained in the absence of E2 (controls: Con) or were treated for 24 h with either 10−9 M E2 or with 200 ng/ml PCDGF. [3H]Thymidine then was added to measure DNA synthesis. The results are expressed as means ± SD. (C) Comparison of the response of MCF-7 and O4 cells to tamoxifen. MCF-7C cells (filled bars) and O4 cells (open bars) received 10−9 M E2 only or in the presence of increasing concentrations of tamoxifen. MCF-7C cells treated with 200 ng/ml of human PCDGF and 1 μM tamoxifen (T + P) were also examined. DNA synthesis was measured as described in Fig. 1. The results were expressed as the percentage of the DNA synthesis values in cells treated with E2 only.
Based on these results, we then examined the tamoxifen responsiveness of O4 and MCF-7C cells. Tamoxifen inhibited the proliferation of MCF-7C cells in a dose-dependent fashion with a 60% inhibition of E2 effect at 100 nM, an 80% inhibition at 500 nM, and a maximal 90% inhibition at 1 μM tamoxifen (Fig. 3C). In contrast, O4 cells displayed a markedly decreased responsiveness to tamoxifen, inasmuch as their proliferation was not affected by 100 nM tamoxifen, whereas only a 20% and a 55% inhibition of the E2 effect was observed at 500 nM and 1 μM tamoxifen, respectively (P < 0.001). Interestingly, the addition of PCDGF to MCF-7 cells reduced the tamoxifen inhibition from 90% to 38% (P < 0.0001). These data suggest that PCDGF can overcome the tamoxifen inhibition and that increased PCDGF expression in MCF-7 cells leads to tamoxifen resistance.
Experiments were then carried out in all transfected cells to check the status of other parameters associated with estrogen responsiveness, such as estrogen receptor expression, activation of an estrogen response element by E2, and stimulation of progesterone receptor mRNA expression by E2. No difference was observed in these assays between antisense, control, and PCDGF overexpressing cells (data not shown). These data indicate that the changes in E2 proliferative response observed in the antisense or overexpressing cells were not due to an overall alteration in ER number or function but rather correlated with changes of PCDGF expression.
PCDGF Stimulates DNA Synthesis in MCF-7 Cells via Activation of the MAP Kinase Pathway.
We next examined the signal transduction pathways stimulated by PCDGF in MCF-7 cells, in comparison with E2. It has been shown that E2 stimulates progression through the G1 phase of the cell cycle (17, 19). Some of the underlying molecular targets have now been elucidated. It is known that E2 stimulates MAP kinase activity in MCF-7 cells (20, 21). Downstream, E2 activates the c-myc pathway and D-type cyclin/cdk complexes (22–25). Recent studies have postulated that the c-myc and cyclin D1 pathways were independently activated by E2 in breast cancer cells (26). Thus, we attempted to determine whether PCDGF could replace E2 to stimulate MAP kinase, cyclin D1, and/or c-myc pathways in MCF-7 cells.
Two approaches showed the involvement of MAP kinase in mediating PCDGF action in MCF-7 cells. First, the stimulatory effect of PCDGF on DNA synthesis was inhibited in a dose-dependent fashion by the MAP kinase kinase (MEK) inhibitor PD098059 (Fig. 4A). The PCDGF effect was almost completely abolished by 10 μM PD098059. Complete inhibition was observed with 30 μM PD098059, a concentration known to completely inhibit MAP kinase activity in other systems (27). Second, by using an in vitro MAP kinase assay, we showed that PCDGF (200 ng/ml) caused a 3-fold increase in MAP kinase activity in MCF-7 cells (Fig. 4B). This effect was abolished by PD098059 (30 μM).
Figure 4.
Determination of the PCDGF signaling pathway in MCF-7 cells. (A) Effect of MAP kinase inhibitor on the mitogenic effect of PCDGF. MCF-7 cells were cultivated as described in Fig. 1. Cells were pretreated for 60 min with 10 μM (PD10) or 30 μM (PD30) PD098059 before the addition of vehicle only (Con) or 100 ng/ml of recombinant human PCDGF alone (P). Thymidine incorporation data are presented as means ± SD. (B) Activation of MAP kinase by PCDGF. Cells were cultivated in PFMEM for 24 h followed by serum-free α-MEM. The samples examined were MCF-7 cells treated for 10 min with 0, 50 ng/ml, and 200 ng/ml of human PCDGF (lanes 1, 2, and 3, respectively) and MCF-7 cells treated for 10 min with 200 ng/ml of PCDGF and 30 μM PD098059 (4). The kinase assay was conducted as described in Materials and Methods. The intensity of the phosphorylated myelin basic protein bands was analyzed by densitometric scanning. An equal amount of original supernatant was used to check the expression of MAP kinase (Erk1 and Erk2) as an internal control to normalize MAP kinase activity.
PCDGF Stimulates Cyclin D1 but Not c-myc Expression in MCF-7 Cells.
Experiments were performed to determine the effect of PCDGF on cyclin D1 expression. PCDGF in a time-dependent fashion stimulated cyclin D1 expression in MCF-7 cells, reaching a maximum of 4-fold over untreated controls after 5 h (Fig. 5A). This effect was abolished by PD98095, in agreement with its action on the PCDGF mitogenic effect.
Figure 5.
Effect of PCDGF on cyclinD1 and c-myc expression in MCF-7 cells. (A) Stimulation of cyclin D1 expression by PCDGF. MCF-7 cells were cultivated in triplicate in PFMEM and synchronized by treatment with 1 μM tamoxifen (22). Medium was replaced by fresh medium supplemented with 200 ng/ml PCDGF alone or with 30 μM PD98095 (Upper) or with 10−9 M E2 (Lower). Cells were lysed at the indicated times to determine cyclin D1 expression by Western blot analysis with 60 μg of whole-cell lysates, using anti-cyclin D1 antibody. Samples were MCF-7 cells treated with 200 ng/ml PCDGF at time 0 (lane 1), 3 h (lane 2), 5 h (lane 3), and 12 h (lane 4); untreated MCF-7 cells at 5 h (lane 5); MCF-7 cells treated with 200 ng/ml PCDGF and PD08950 at 5 h (lane 6); and MCF-7 cells treated with 10−9 M E2 at 0 h, 3 h, and 5 h (lanes 7, 8, and 9, respectively). (B) Effect of E2 and PCDGF on c-myc expression. MCF-7 cells were synchronized by treatment with 1 μM tamoxifen (22). Cells then were treated with 10−9 M E2 (Upper) or 200 ng/ml PCDGF (Lower) for the indicated periods of time. Western blot detection of c-myc expression was performed by using 60 μg of whole-cell lysates and anti-myc polyclonal antibody.
The stimulation of cyclin D1 expression by PCDGF was accompanied by the increase in the phosphorylation and expression of pRb, strictly required for G1 phase progression (28, 29). After 6 h of PCDGF treatment, pRb became hyperphosphorylated. After 24 h, all of the pRb was in the hyperphosphorylated form, with a 5-fold increase in protein expression when compared with untreated cells. This effect also was blocked by PD98095 (data not shown).
We next compared the abilities of E2 and PCDGF to stimulate c-myc expression, another target for estrogen action in MCF-7 cells (22, 30). As shown in Fig. 5B, E2 (10−9 M) stimulated a rapid increase in c-myc expression in steroid-deprived MCF-7 cells with a maximal 4-fold induction within 3 h. The level of c-myc induction by E2 was sustained for more than 10 h. In contrast, PCDGF had no effect on c-myc protein expression during the same period (Fig. 5B).
Because PCDGF induced cyclin D1 but not c-myc expression, we determined whether the increase of endogenous PCDGF was involved in the ability of E2 to stimulate cyclin D1 expression. The treatment of MCF-7 cells with anti-PCDGF neutralizing antibody (300 μg/ml) resulted in a 52 ± 8% inhibition of the stimulation of cyclin D1 by E2, 5 h after the addition of the antibody. This degree of inhibition was sustained 12 h after antibody addition. These data suggest that the ability of E2 to stimulate cyclin D1 in MCF-7 cells is mediated, at least partially, by the endogenously produced PCDGF.
Discussion
We have reported previously that ER+ human mammary carcinoma cells express PCDGF and that E2 stimulates PCDGF expression in a dose- and time-dependent fashion (14). We demonstrate here that PCDGF mediates the growth stimulatory effect of E2. We show that PCDGF stimulates the proliferation of the human breast cancer cells MCF-7 and T47D maintained in the absence of E2. We next show that blocking the PCDGF autocrine pathway with anti-PCDGF antibody inhibits the mitogenic effect of E2. This effect was specific to PCDGF because the antibody was unable to inhibit the IGF-II mitogenic effect. The involvement of PCDGF in the mitogenic effect of E2 was further demonstrated by showing that the inhibition of PCDGF expression by antisense cDNA transfection reduced the E2 mitogenic effect on MCF-7 cells in correlation with the degree of inhibition of PCDGF expression. The addition of exogenous PCDGF restored the proliferation of these antisense cells. In addition, increased expression of PCDGF in MCF-7 cells led to cells able to proliferate in the absence of E2 and that were tamoxifen resistant. The fact that there was no change in other parameters of E2 responsiveness, such as ER expression, activation of estrogen response element–luciferase reporter gene, or stimulation of progesterone receptor expression, indicated that alteration of the E2 mitogenic effect in transfected cells was not due to a change in E2 receptors but specifically to a change in PCDGF expression. The present studies point to the possible role of increased PCDGF expression in the transition from estrogen dependence to estrogen independence and tamoxifen resistance, which is a critical stage for the development of breast cancer with poor prognosis (31).
In support of this hypothesis, preliminary studies of human breast cancer biopsies by immunocytochemistry indicate that PCDGF expression is elevated in epithelial cells from ductal invasive carcinoma (G.S. and O. Ioffe, unpublished observations).
Even though the results presented here demonstrate the direct involvement of autocrine PCDGF in the mitogenic effect of E2, they also suggest that PCDGF alone cannot account for all of the E2 effects. Inhibition of PCDGF action by neutralization antibody or PCDGF expression by antisense transfection resulted in a 75% reduction of the E2 mitogenic effect. Although this lack of complete inhibition could be due to limitations of the methodologies, it also is possible that E2 activates more than one pathway to regulate MCF-7 cell growth.
This later possibility was supported by the presence of similarities and differences between PCDGF and E2 at the level of the signaling pathways they stimulate. Similar to E2 and several growth factors, PCDGF activated MAP kinase activity to stimulate the proliferation of MCF-7 cells. These results are in agreement with the report that granulin precursor activated MAP kinase in mouse embryo fibroblasts from IGF-I receptor null mice (10). MAP kinase has been reported to be a critical element in the initiation and metastasis processes of human breast cancer formation, and its expression and activity are elevated in human breast cancer tissues (21). Moreover, c-myc and cyclin D1 have been shown to be overexpressed in breast cancer cells (30, 32). The inducible overexpression of cyclin D1 in breast cancer cells has been reported to reverse the growth-inhibitory effect of antiestrogens (25). We show here that like E2, PCDGF stimulated cyclin D1 protein expression in a time-dependent fashion, followed by stimulation of pRb expression and hyperphosphorylation. Both were inhibited in the presence of PD98095, indicating a MAP kinase requirement. Interestingly and in contrast to E2, PCDGF failed to stimulate c-myc expression, another target of E2 in breast cancer cells (22, 30). Recently, Prall et al. (26) have postulated that c-myc and cyclin D1 were the two key regulators of separate pathways mediating the mitogenic effects of E2. In support of this hypothesis, they demonstrated, using an inducible system in MCF-7 cells, that c-myc expression was not accompanied by increased cyclin D1 expression or Cdk4 activation, nor was cyclin D1 induction accompanied by an increase in c-myc. However, coexpression of inducible c-myc and cyclin D1 in MCF-7 cells mimicked estrogen effects on cyclin E-Cdk2 activation and cell cycle reentry (26). Their results suggested that E2 up-regulated c-myc and cyclin D1 pathways separately. Transcriptional activation of c-myc is rapid and is thought to be directly mediated via interaction with the ER (22), whereas estrogen stimulation of cyclin D1 expression requires de novo synthesis (28), indicating an indirect mechanism, possibly involving autocrine growth factors. Our results suggest that cyclin D1 is the target that PCDGF up-regulates to mediate the mitogenic effect of E2 in human breast cancer cells. In support of this hypothesis, the stimulation of cyclin D1 expression by E2 was inhibited by 52% by treatment of MCF-7 cells within 5–12 h of treatment with anti-PCDGF neutralizing antibody, in agreement with the time of induction of PCDGF expression by E2 (14). However, the fact that the inhibition was not complete would suggest that PCDGF alone cannot account for all of the E2 effect on cyclin D1. E2 has been shown to stimulate the expression of other autocrine growth factors such as transforming growth factor-α and IGF-II in MCF-7 cells (33–37) that may be involved in growth regulation mediated by E2, providing an optimal control of proliferation. In rat uterine epithelial cells, Zhang et al. (38) have reported that the mitogenic effect of E2 was mediated by heparin-binding epidermal growth factor via possible stimulation of cyclin D1. In conclusion, the present study identifies PCDGF as a mediator, at least in part, of the mitogenic effect of E2 on the human breast cancer cell line MCF-7. This finding not only expands our understanding regarding mechanisms of the E2 effect in human breast cancer cells; it offers a possible molecular target in the treatment of breast cancer.
Acknowledgments
We thank Drs. Daniel Sussman and Jun Hayashi for critically reading the manuscript and Dr. Jun You for his help in preparing PCDGF. This work was supported by Grants DAMD 17-96-1-6072 from the U. S. Army Medical Research and Material Command and Grant 9857 from the Susan G. Komen Breast Cancer Foundation. R.L. is a recipient of the Dr. Frank J. Slama Endowed Predoctoral Fellowship.
Abbreviations
- PCDGF
PC-cell-derived growth factor
- E2
17-β-estradiol
- MAP
mitogen-activated protein
- ER
estrogen receptor
- PFMEM
phenol red-free α-MEM
- IGF
insulin-like growth factor
- MCF-7C cells
empty vector control transfected MCF-7 cells
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.011525198.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.011525198
References
- 1.Jensen E V. Curr Top Pathol. 1991;83:365–431. doi: 10.1007/978-3-642-75515-6_11. [DOI] [PubMed] [Google Scholar]
- 2.Hoskins K, Weber B L. Curr Opin Oncol. 1994;6:554–559. doi: 10.1097/00001622-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Dickson R B, Lippman M E. Endocr Rev. 1987;16:29–43. doi: 10.1210/edrv-8-1-29. [DOI] [PubMed] [Google Scholar]
- 4.Arteaga C L, Osborne C K. Cancer Treat Res. 1991;53:289–304. doi: 10.1007/978-1-4615-3940-7_14. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Gao G, Crabb J W, Serrero G. J Biol Chem. 1993;268:10863–10869. [PubMed] [Google Scholar]
- 6.Zhang H, Serrero G. Proc Natl Acad Sci USA. 1998;95:14202–14207. doi: 10.1073/pnas.95.24.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plowman G D, Green J M, Neubauer M G, Buckley S D, McDonald V L, Todaro G J, Shoyab M. J Biol Chem. 1992;267:13073–13078. [PubMed] [Google Scholar]
- 8.Bhandari V, Palfree R G E, Bateman A. Proc Natl Acad Sci USA. 1992;89:1715–1719. doi: 10.1073/pnas.89.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoyab M, McDonald V L, Byles C, Todaro G J, Plowman G D. Proc Natl Acad Sci USA. 1990;87:7912–7916. doi: 10.1073/pnas.87.20.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu S Q, Tang D, Chamberlain S, Pronk G, Marsiaz F, Kaur S, Prisco M, Zanocco-Marani T, Baserga R. J Biol Chem. 1998;273:20078–20083. doi: 10.1074/jbc.273.32.20078. [DOI] [PubMed] [Google Scholar]
- 11.He Z, Bateman A. Cancer Res. 1999;59:3222–3229. [PubMed] [Google Scholar]
- 12.Diaz-Cueto L, Stein P, Jacobs A, Schultz R M, Gerton G L. Dev Biol. 2000;217:406–418. doi: 10.1006/dbio.1999.9564. [DOI] [PubMed] [Google Scholar]
- 13.Lu R, Serrero G. Proc Natl Acad Sci USA. 2000;97:3993–3998. doi: 10.1073/pnas.97.8.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu R, Serrero G. Biochem Biophys Res Commun. 1999;256:204–207. doi: 10.1006/bbrc.1999.0253. [DOI] [PubMed] [Google Scholar]
- 15.Lu R, Serrero G. J Cell Physiol. 1999;179:297–304. doi: 10.1002/(SICI)1097-4652(199906)179:3<297::AID-JCP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 16.Xia X, Serrero G. Biochem Biophys Res Commun. 1998;245:539–543. doi: 10.1006/bbrc.1998.8498. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland R L, Watts C K, Musgrove E A. J Steroid Biochem Mol Biol. 1993;47:99–106. doi: 10.1016/0960-0760(93)90062-2. [DOI] [PubMed] [Google Scholar]
- 18.Osborne C K, Clemmons D R, Arteaga C L. J Steroid Biochem Mol Biol. 1990;37:805–809. doi: 10.1016/0960-0760(90)90423-i. [DOI] [PubMed] [Google Scholar]
- 19.Prall O W, Hogan E M, Sutherland R L. J Steroid Biochem Mol Biol. 1998;65:169–174. doi: 10.1016/s0960-0760(98)00021-1. [DOI] [PubMed] [Google Scholar]
- 20.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 21.Sivaraman V S, Wang H G, Nuovo J, Malbon C C. J Clin Invest. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubik D, Shiu R P. Oncogene. 1992;7:1587–1594. [PubMed] [Google Scholar]
- 23.Prall O W J, Sarcevic B, Musgrove E A, Watts C F K, Sutherland R L. J Biol Chem. 1997;272:10882–10894. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 24.Dubik D, Dembinski T C, Shiu R P C. Cancer Res. 1987;47:6517–6529. [PubMed] [Google Scholar]
- 25.Wilcken N R, Prall O W, Musgrove E A, Sutherland R L. Clin Cancer Res. 1997;3:849–854. [PubMed] [Google Scholar]
- 26.Prall O W, Rogan E M, Musgrove E A, Watts C K, Sutherland R L. Mol Cell Biol. 1998;18:4499–4508. doi: 10.1128/mcb.18.8.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alessi D R, Cuenda A, Cohen P, Dudley P T, Saltiel A R. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg R A. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 29.Watts C K, Brady A, Sarcevic B, deFazio A, Musgrove E A, Sutherland R L. Mol Endocrinol. 1995;9:1804–1813. doi: 10.1210/mend.9.12.8614416. [DOI] [PubMed] [Google Scholar]
- 30.Watson P H, Pon R T, Shiu R P. Cancer Res. 1991;51:3996–4000. [PubMed] [Google Scholar]
- 31.Wolf D M, Jordan V C. Breast Cancer Res Treat. 1993;27:27–40. doi: 10.1007/BF00683191. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein I B. J Cell Biochem. 1996;,Suppl.25:23–28. [PubMed] [Google Scholar]
- 33.Westley B R, May F E. J Steroid Biochem Mol Biol. 1994;51:1–9. doi: 10.1016/0960-0760(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 34.Sciacca L, Constantino A, Pandini G, Mineo R, Frasca F, Scalia B, Sbraccia P, Goldfine I D, Vigneri R, Belfiore A. Oncogene. 1999;18:2471–2479. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- 35.Daly R J, Harris W H, Wang D Y, Darbre P D. Cell Growth Differ. 1991;2:457–464. [PubMed] [Google Scholar]
- 36.Reddy K B, Yee D, Hilsenbeck S G, Coffey R J, Osborne C K. Cell Growth Differ. 1994;5:1275–1282. [PubMed] [Google Scholar]
- 37.Leung B S, Stout L, Zhou L, Ji H J, Zhang Q Q, Leung H T. J Cell Biochem. 1991;46:125–133. doi: 10.1002/jcb.240460206. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Laping J, Glasser S, Day P, Mulholland J. Endocrinology. 1998;139:961–966. doi: 10.1210/endo.139.3.5794. [DOI] [PubMed] [Google Scholar]