Abstract
Activation of phospholipase C in nonexcitable cells causes the release of calcium (Ca2+) from intracellular stores and activation of Ca2+ influx by means of Ca2+ release-activated channels (ICRAC) in the plasma membrane. The molecular identity and the mechanism of ICRAC channel activation are poorly understood. Using the patch–clamp technique, here we describe the plasma membrane Ca2+ channels in human carcinoma A431 cells, which can be activated by extracellular UTP, by depletion of intracellular Ca2+ stores after exposure to the Ca2+-pump inhibitor thapsigargin, or by loading the cells with Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate. The observed channels display the same conductance and gating properties as previously described Imin channels, but have significantly lower conductance for monovalent cations than the ICRAC channels. Thus, we concluded that the depletion-activated Ca2+ current in A431 cells is supported by ICRAC-like (ICRACL) channels, identical to Imin. We further demonstrated synergism in activation of ICRACL Ca2+ channels by extracellular UTP and intracellular inositol (1,4,5)-triphosphate (IP3), apparently because of reduction in phosphatidylinositol 4,5-bisphosphate (PIP2) levels in the patch. Prolonged exposure of patches to thapsigargin renders ICRACL Ca2+ channels unresponsive to IP3 but still available to activation by the combined action of IP3 and anti-PIP2 antibody. Based on these data, we concluded that phospholipase C-mediated and store-operated Ca2+ influx pathways in A431 cells converge on the same ICRACL Ca2+ channel, which can be modulated by PIP2.
Activation of phospholipase C (PLC)-mediated signaling pathways in nonexcitable cells causes the release of Ca2+ from intracellular Ca2+ stores and activation of Ca2+ influx across the plasma membrane by means of capacitative Ca2+ entry or store-operated Ca2+ entry processes (1–3). These processes are mediated by plasma membrane Ca2+ channels termed “Ca2+ release activated channels” (ICRAC) (4–7). The molecular identity of ICRAC remains unclear, with mammalian trp channels (mTrp) usually considered the most likely candidate for the role of ICRAC (1–3, 8, 9). When compared with ICRAC, mTrp channels display relatively low selectivity for divalent cations, higher single channel conductance, and different kinetic and pharmacological properties. In experiments with a human carcinoma A431 cell line, we previously described plasma membrane Ca2+ channels (Imin) that are activated by application of uridine triphosphate and bradykinin to cell-attached patches or by application of inositol (1,4,5)-trisphosphate (IP3) to excised inside-out (i/o) patches (10–12). IP3-gated channels that share some common properties with Imin have been also observed in experiments with human T cells (13), rat macrophages (12), and endothelial cells (14, 15). Major functional properties of Imin channels, such as small conductance (1 pS for divalent cations), high selectivity for divalent cations (PCa/K > 1,000), inward rectification, and sensitivity to block by SKF95365 are similar to ICRAC channels (12, 16). Thus, we previously suggested that Imin and ICRAC may in fact be the same channels (17).
The mechanism of ICRAC activation remains similarly controversial (1–3). When studied in a heterologous expression system, activation of mTrp channels by IP3 appear to be mediated by direct conformational coupling between the cytosolic carboxyl-terminal tail of mTrp and the amino-terminal ligand-binding domain of intracellular IP3 receptor (IP3R) (18–21). However, whether mTrp can serve as an appropriate model system for understanding ICRAC activation is unresolved (18, 21, 22). In previous studies, we demonstrated that activity of Imin in i/o patches is potentiated by addition of IP3R-enriched microsomes as predicted by an Imin-IP3R conformational coupling model (16). More recently, we discovered that anti-PIP2 antibody (PIP2Ab) sensitizes Imin to IP3 activation and proposed an Imin-IP3R-PIP2 functional coupling model based on these findings (17). In parallel with our results, a potential role of PIP2 in trp-like (trpl) channel activation has been recently demonstrated in Sf9 cells (23). The Imin-IP3R-PIP2 coupling model can adequately explain activation of Imin channels by direct action of PLC but not the activation of ICRAC channels resulting from Ca2+ store depletion (4–6).
A number of critical questions related to a depletion-activated Ca2+ influx pathway remain unanswered. Most importantly, do store-depletion and PLC-dependent pathways activate the same or a different channel type? To answer this question, we compare the effects of PLC-linked agonist UTP, Ca2+ pump inhibitor thapsigargin (Tg), and Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) on plasma membrane Ca2+ channel activity in patch–clamp experiments performed with human carcinoma A431 cells. We conclude that PLC activation and depletion of intracellular Ca2+ stores activate the same Ca2+ channel in A431 cells. We found that the conductance and selectivity properties of the store-operated channel in A431 cells are identical to the properties of Imin and somewhat different from the properties of ICRAC channels described in Jurkat T cells (5–7). Thus, we will refer to store-operated channels in A431 cells as ICRACL (“crac-like”). We also concluded that PIP2 plays a role in modulation of ICRACL activity.
Materials and Methods
Electrophysiological Recordings.
Human carcinoma A431 cells (Cell Culture Collection, Institute of Cytology, St. Petersburg, Russia) were kept in culture, as described elsewhere (12). For patch–clamp experiments, cells were seeded onto coverslips and maintained in culture for 1–3 days before use. Single-channel currents were recorded by using the cell-attached and i/o configuration of the patch–clamp technique (24). Currents filtered at 500 Hz were recorded with a PC-501A patch–clamp amplifier (Warner Instruments, Hamden, CT) with a conventional resistance feedback in the headstage (10 GΩ). The currents were digitized at 2.5 kHz. For data analysis and presentation, currents were additionally digitally filtered at 100 Hz.
NPo was determined by using the following equation: NPo = 〈I〉/i, where 〈I〉 and i are the mean channel current and unitary current amplitude, respectively. 〈I〉 was estimated from the time integral of the current above the baseline, and i was determined from current records and all-point amplitude histograms. Data were collected from current records after channel activity reached steady state. Because channel activity was transient and displayed significant fluctuations, we used NPo collected during 30 s of maximal activity (NPomax30) as a standard way to compare open channel probability among different experiments. Average NPomax30 values of channel activity from several experiments are presented in the text and on the figures as mean ± SEM. The pipette solution contained (in mM): 105 BaCl2, 105 CaCl2, or 140 NaCl as indicated, and 10 Tris/HCl (pH 7.4). In cell-attached experiments, the bath solution contained 140 mM KCl and 2 mM CaCl2 to nullify the cell's resting potential. For BAPTA-AM loading, 100 μM BAPTA-AM and 1 μM Tg were added to the bath solution containing (in mM): 140 KCl, 5 NaCl, 10 Hepes/KOH, and 2 EGTA (pH 7.4). For i/o experiments, patches were excised into the standard intracellular solution containing (in mM): 140 K glutamate, 5 NaCl, 1 MgCl2, 10 Hepes/KOH, 1.13 CaCl2, and 2 EGTA (pCa 7, pH 7.4), with or without IP3 as indicated. The cell-attached and i/o recordings were performed at −70 mV holding potential. All experiments were carried out at room temperature (22–24°C).
Materials.
Monoclonal anti-PIP2 antibody (PIP2Ab) (25) was from PerSeptive Biosystems (Framingham, MA), and monoclonal anti-PIP antibody (PIPAb) was from Assay Designs (Ann Arbor, MI). PIP2Ab and PIPAb were reconstituted in PBS (titer 1:1,500), diluted 1:100 by intracellular solution and used for chamber perfusion. Hepes, UTP, and Tg were from Sigma; EGTA was from Fluka Chemie AG (Buchs, Switzerland). IP3 and BAPTA-AM were from Calbiochem.
Results
Exposure to Extracellular UTP Sensitizes Imin to IP3 Activation.
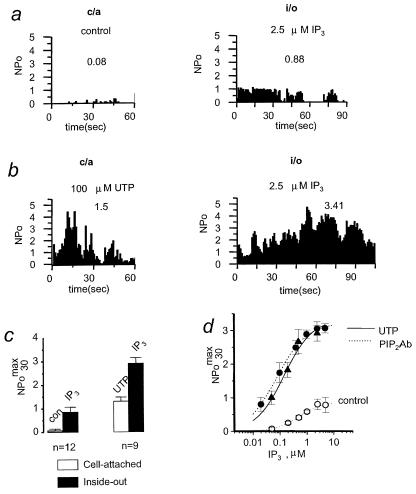
When cell-attached (c/a) recordings of Imin in A431 cells were performed in control recording conditions, the channel activity was very low with NPomax30 equal to 0.08 ± 0.06 (n = 12) (Fig. 1 a and c). After patch excision in bath solution containing 2.5 μM IP3, moderate activity of Imin in i/o patches was observed with NPomax30 equal to 0.86 ± 0.2 (n = 12) (Fig. 1 a and c). Similar behavior of Imin channels in c/a and i/o configurations has been described (10–12, 17). As we previously reported, addition of 100 μM UTP or 10 μM bradykinin to the solution bathing A431 cells leads to activation of PLC-coupled receptors and an increase in Imin activity in c/a patches to NPomax30 of 0.7–1.0 (12). When 100 μM UTP was included in the pipette solution, significantly higher Imin channel activity was observed with NPomax30 equal to 1.5 ± 0.17 (n = 33) (Fig. 1b). With either bath (12) or pipette (Fig. 1b) UTP application, activity of Imin was transient and resulted in channel inactivation within several minutes. After patch excision into intracellular solution containing 2.5 μM IP3, very high levels of Imin channel activity were observed (Fig. 1b). On an average, Imin channel NPomax30 increased from 1.31 + 0.17 (c/a) to 2.91 + 0.23 (i/o) in this series of experiments (n = 9) following patch excision (Fig. 1c).
Figure 1.
Sensitization of Imin to IP3 by extracellular UTP. (a) Plot of Imin open channel probability (NPo) in cell-attached (c/a) patch recorded in control conditions and in i/o patch from the same cell in the presence of 2.5 μM IP3. The NPo was averaged over 1-s intervals and plotted vs. time in the experiment. Mean NPo max 30 was 0.08 in c/a and 0.88 in i/o for the experiment shown. Data are representative of 12 experiments. (b) Same plot as in a for the experiment with 100 μM UTP in the pipette. Mean NPomax30 was 1.5 in c/a and 3.41 in i/o for the experiment shown. Data are representative of nine experiments. (c) The summary plot of Imin open channel probability in c/a (open bars) and i/o (closed bars) recordings performed in control conditions (n = 12, left) or in the presence of 100 μM UTP in the pipette (n = 9, right). Imin activity is represented as NPomax30 (mean ± SEM). (d) NPomax30 of Imin channels in i/o experiments at IP3 concentrations as indicated measured with 100 μM UTP in the pipette (▴), in the presence of PIP2Ab (●) and in control conditions (○). Average data at each IP3 concentration are shown as mean ± SEM (n ≥ 6). Smooth curve, best fit the data obtained with UTP in the pipette by using the equation NPomax30 = (NPomax30)max [IP3]nH/([IP3]nH + KappnH), the values of parameters are in the text. Dashed lines, fit to the similar data obtained in control conditions (curve on the right) and in the presence of PIP2Ab (curve on the left). The data for control conditions and in the presence of PIP2Ab are taken from ref. 17.
To gain insight into the mechanism responsible for the unusually high activity of Imin channels in i/o recordings observed in the experiments with UTP in the pipette (Fig. 1 b and c), we determined the sensitivity of Imin activation by IP3 when 100 μM of UTP was included in the pipette solution. In all experiments of this series, we waited until Imin activity in c/a patches subsided before the patch excision. A single IP3 concentration in the 0.05–2.5 μM range was tested in each experiment to avoid IP3-induced Imin desensitization (12). Fitting the Hill equation to the data (Fig. 1d, ▴) yielded an apparent affinity (Kapp) of 0.15 μM IP3, maximal NPomax30 (NPomax) of 3.33, and a Hill coefficient (nH) of 0.83 (Fig. 1d, curve). When similar experiments were performed in control recording conditions, sensitivity of Imin to IP3 activation was much lower (Kapp = 0.51 μM IP3, NPomax = 0.87, nH = of 1.05) (17) (Fig. 1d, ○, and dashed line on the right). The dramatic increase in Imin apparent affinity for IP3 and in NPomax induced by exposure to UTP in the pipette quantitatively matches with the effects exerted by PIP2Ab on Imin (Kapp = 0.08 μM IP3, NPomax = 3.21, nH = of 0.8) (17) (Fig. 1d, ●, and dashed line on the left) and on the IP3R (26). We reasoned that synergistic actions of extracellular UTP and intracellular IP3 in our experiments (Fig. 1) result from UTP receptor stimulation of PLC which decreases PIP2 levels in the patch. Reduction of PIP2 levels leads to an increase in the apparent affinity of IP3R for IP3 (26) and in the potency of IP3 to activate Imin.
Imin Is the ICRACL Channel Activated by Depletion of Intracellular Ca2+ Stores.
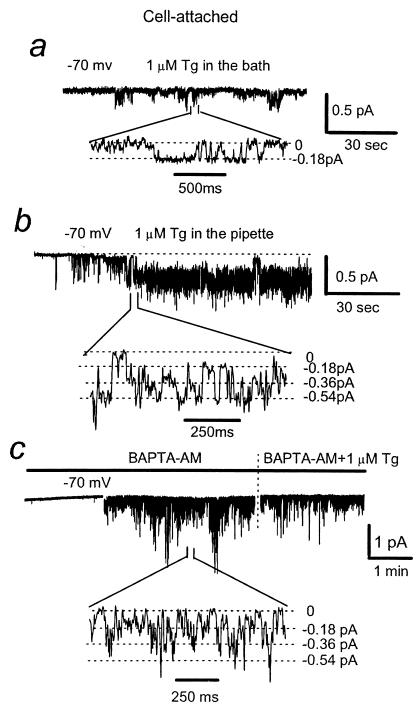
ICRAC currents can be activated in cells without PLC activation as a result of intracellular Ca2+ store depletion following exposure to Ca2+-ATPase inhibitor Tg or intracellular Ca2+ chelators BAPTA and EGTA (4, 5). The experiments described in the previous section support the Imin-IP3R-PIP2 coupling model (17). This model explains activation of Imin channels by direct action of PLC but not the activation of ICRAC channels by Ca2+ store depletion. Does depletion of Ca2+ stores activate the same channel as activation of PLC? To answer this question, we evaluated effects of Tg on Ca2+ channel activity in patch–clamp experiments. As in our previous studies (12), addition of 1 μM Tg to the bath had only minimal effect on Imin activity when compared with control conditions, with NPomax30 equal to 0.11 ± 0.03 (n = 9) (Fig. 2a; also see Fig. 4 a and h). In contrast to these results, if 1 μM Tg was included in the pipette, active Imin channels were observed following a short delay after patch formation, with NPomax30 equal to 1.7 ± 0.24 (n = 18) (Figs. 2b and 4 b and h). We interpret this delay as the time needed for depletion of submembrane Ca2+ stores by Tg entering the cell from the pipette.
Figure 2.
Activation of Imin channels by Tg. (a) Ca2+ channel current traces in c/a patches recorded in the presence of 1 μM Tg in the bath solution. The fragments of current records are shown on the bottom on expanded time scale. The unitary current amplitude in used recording conditions (−70 mV membrane resting potential) is −0.18 pA. (b) Same as in a with 1 μM Tg in the pipette. (c) Same as in a with 100 μM BAPTA-AM and 1 μM Tg in the bath.
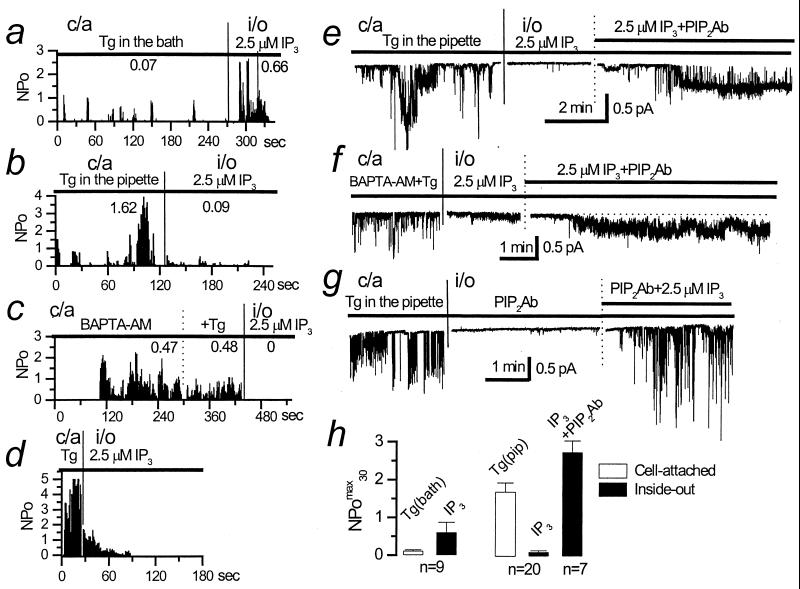
Figure 4.
Role of PIP2 in ICRACL modulation. (a) Plot of ICRACL open channel probability (NPo) in c/a patch recorded with 1 μM Tg in the bath and in i/o patch from the same cell in the presence of 2.5 μM IP3. The NPO was averaged over 1-s intervals and plotted vs. time in the experiment. Mean NPomax30 was 0.07 in c/a and 0.66 in i/o for the experiment shown. Data are representative of nine experiments. (b) Same plot as in a for the experiment with 1 μM Tg in the pipette. Mean NPomax30 was 1.62 in c/a and 0.09 in i/o for the experiment shown. Data are representative of 20 experiments. (c) Same plot as in a for the experiment with 100 μM BAPTA-AM and 1 μM Tg in the bath. Mean NPomax30 was 0.48 in c/a and 0 in i/o for the experiment shown. Data are representative of nine experiments. (d) Same plot as in b, but with patch excision within 30 s after ICRACL activation. Data are representative of four experiments. (e) ICRACL channel current traces in c/a patches recorded in the presence of 1 μM Tg in the pipette solution followed by i/o current recordings in the presence of 2.5 μM IP3 and PIP2Ab as shown. Data are representative of seven experiments. (f) Same as in e with 100 μM BAPTA-AM and 1 μM Tg in the bath. Data are representative of four experiments. (g) Same as in e with the order of PIP2Ab and IP3 additions to i/o patch reversed. Data are representative of five experiments. (h) The summary plot of ICRACL open channel probability in c/a (open bars) and i/o (closed bars) recordings performed in the presence of 1 μM Tg in the bath (n = 9, left) or in the presence of 1 μM Tg in the pipette (n = 20, right). ICRACL activity is represented as NPomax30 (mean ± SEM).
One potential explanation of different effects caused by bath and pipette applications of Tg is Ca2+-induced inactivation of Imin. From comparison of Imin rundown kinetic with Ca2+ or Ba2+ as a current carrier, we previously concluded that Imin is likely to undergo Ca2+-induced inactivation process (11). Massive Ca2+ release from the stores resulting from bath application of Tg may quickly inactivate Imin, but if Tg is included only in the pipette, Ca2+ leak is much slower, and Imin inactivation may be reduced or decelerated. To test this hypothesis, we clamped Ca2+ concentration in A431 cells by loading them with the membrane-permeable Ca2+ chelator BAPTA-AM. Bath application of 0.1 mM BAPTA-AM by itself resulted in Imin activity in 9 of 15 experiments. In six remaining experiments, application of Tg to BAPTA-loaded cells evoked Imin channel activity. To simplify experimental procedure, we combined application of Tg and BAPTA-AM to the bath, which resulted in Imin channel activity in 7 of 10 experiments (Figs. 2c and 4c). From these results, we concluded that the low potency of Tg in the bath to activate Imin in our previous experiments (Fig. 2a) (12) mostly likely results from Ca2+-dependent inactivation of Imin.
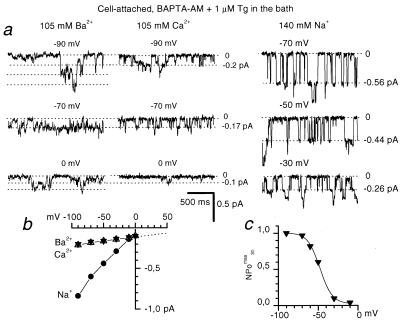
Activation of Imin by depletion of intracellular Ca2+ stores with Tg and BAPTA-AM (Fig. 2 b and c) reinforces the idea that Imin and ICRAC may in fact be the same channels (17). To test this idea further and in the absence of molecular information and specific blockers, we resorted to comparison of Imin and ICRAC single-channel properties. The divalent single-channel conductance of ICRAC channels in Jurkat T cells has been estimated to be 24 fS from the noise analysis (6), and the monovalent single-channel conductance has been measured at 40 pS with Na+ as a current carrier (7). It has also been demonstrated that the permeability of ICRAC to Ca2+ is higher than for Ba2+ (6, 27). With 105 mM divalent cations in the pipette, the store-operated channels in A431 cells were equally permeable to Ca2+ and Ba2+ (Fig. 3), displayed a single-channel current amplitude of −0.18 pA at −70 mV membrane potential (Figs. 2 and 3) and a single-channel conductance of about 1 pS (Fig. 3). Thus, conductance properties of these channels are identical to the properties of Imin channels activated by UTP (in c/a) or by IP3 (in i/o) (12). We also demonstrated that the open probability of store-operated channels in A431 cells is strongly dependent on the membrane potential (Fig. 3), in line with the properties of Imin (12). Using 140 mM Na+ as a current carrier, we determined that store-depletion activated channels in A431 cells displayed the main conductance level of −0.56 pA at −70 mV membrane potential and the corresponding single channel conductance of 6 pS (Fig. 3), which is several-fold smaller than conductance of ICRAC channels in Jurkat T cells in similar ionic conditions (7). From these results, we concluded that the store-depletion activated Ca2+ current in A431 is carried by ICRAC-like (ICRACL) channels, which are identical to the previously described Imin channels (12). In the remaining section of the paper, these channels will be referred to simply as ICRACL.
Figure 3.
Conductance properties of store-operated channels in A431 cells. (a) Store-operated channels in A431 cells, activated by the mixture of 100 μM BAPTA-AM and 1 μM Tg in the bath solution, were recorded in c/a mode with 105 mM Ba2+ (Left), 105 mM Ca2+ (Center), and 140 mM Na+ (Right) in the pipette solution at membrane potential as indicated. (b) Fit to the unitary current-voltage relationship of store-operated channels with Ba2+ (▾, n = 4–6), Ca2+ (▵, n = 4), Na+ (●, n = 3) yielded slope single-channel conductance of 1 pS for Ca2+ and Ba2+ and 6 pS for Na+. (c) Open channel probability of store-operated channels (NPomax30) expressed as a function of membrane potential. Data from six independent experiments in c/a mode with 105 mM Ba2+ as a current carrier were averaged at each membrane potential (▾). (b and c) The average values are shown as mean ± SEM, unless the size of the error bars is smaller than the size of the symbols.
PIP2 Is a Modulator of ICRACL.
When activated by UTP (Fig. 1b) or Tg (Fig. 4b), ICRACL channel activity was transient, with channels typically lasting between 2 and 5 min. Loading of A431 cells with BAPTA-AM dramatically extended the period of Tg-induced ICRACL activity, effectively preventing ICRACL inactivation (Fig. 4c). Thus, we concluded that the Ca2+-dependent mechanism plays a major role in ICRACL inactivation, similar to the previous studies of ICRAC (27, 28). To get additional insight into the mechanisms of ICRACL inactivation, we evaluated responses of ICRACL channels to IP3 in i/o patches. With 1 μM Tg in the bath, normal activation of ICRACL channels by 2.5 μM IP3 was observed in i/o patches (Fig. 4 a and h), similar to control experiments (Fig. 1a). However, exposure to 1 μM Tg in the pipette, which initially resulted in ICRACL activation, eventually led to channel inactivation and greatly diminished activity of IP3-gated ICRACL channels in i/o mode (Fig. 4b). On average, ICRACL channel activity in i/o patches with Tg in the pipette was reduced to NPomax30 equal to 0.11 ± 0.03 (n = 20) (Fig. 4h). Tg-induced loss of ICRACL channel sensitivity to activation by IP3 developed in time. Indeed, when patches were excised within 30 s from the initial channel activation, substantial ICRACL channel activity in i/o patches was initially observed in the presence of 2.5 μM IP3 in 1 of 4 experiments (Fig. 4d). Although loading the cells with BAPTA-AM almost completely removed ICRACL inactivation in c/a mode (Fig. 4c), the channels in these experiments were also unresponsive to IP3 in i/o mode (Fig. 4c). Thus, following exposure to Tg, patch excision lead to a loss of ICRACL responsiveness to IP3, even in the absence of Ca2+-dependent inactivation.
Inclusion of UTP in the pipette resulted in sensitization of ICRACL channels to IP3 (Fig. 1 c and d), which we concluded was related to PLC-dependent reduction in PIP2 levels in the patch (see above). What if depletion of Ca2+ stores, which leads to a loss of ICRACL sensitivity to IP3 in i/o patches (Fig. 4 b–d and h), increases the fraction of PIP2-tethered IP3R-ICRACL complexes? To test this hypothesis, we analyzed the effect of PIP2Ab on ICRACL in i/o patches taken from cells exposed to Tg in the pipette or to the BAPTA-AM/Tg mixture in the bath. Although ICRACL was rendered sensitive to IP3 as a result of prolonged patch exposure to Tg, addition of PIP2Ab restored ICRACL channel activity (Fig. 4e), with NPomax30 = 2.73 ± 0.3 (n = 7) (Fig. 4h). Similar results were obtained in the experiments (n = 4) where ICRACL channels were initially activated by a BAPTA-AM/Tg mixture in the bath (Fig. 4f). The observed effect was specific for PIP2Ab, as addition of PIPAb had no effect on ICRACL channel activity in control experiments (n = 5). Similar to our previous results (17), PIP2Ab alone did not induce channel activity in these conditions, but instead greatly potentiated the ability of IP3 to activate the ICRACL (Fig. 4g). The experiments with PIP2Ab support the hypothesis that, following exposure to Tg and store-depletion, all ICRACL-IP3R complexes in the patch are shifted to the PIP2-tethered state. In the absence of Ca2+-induced inactivation, ICRACL channels in ICRACL-IP3R-PIP2 complexes remain active as long as store is depleted but do not respond to IP3.
Discussion
PLC-Dependent and Store-Operated Pathways of ICRACL Activation.
Our results lead us to conclude that both PLC-linked and Ca2+ store-operated Ca2+ entry pathways in A431 cells are in fact supported by the same Ca2+ channel, with single-channel properties identical to the properties of the previously described Imin channel (12). Similar to Imin, the store-operated channels in A431 cells are equally permeable to Ca2+ and Ba2+ and display a divalent single channel conductance of 1 pS. Monovalent single-channel conductance of these channels is 5.5–6 pS with 140 mM Na+ as a current carrier, which is several-fold smaller than single-channel conductance of ICRAC channels in Jurkat T cells measured in similar ionic conditions (40 pS) (7). To account for the observed differences in conductance and selectivity properties, we called the store-operated channel in A431 cells ICRACL (ICRAC-like). Ca2+ channels activated by depletion of intracellular stores in A431 cells were previously described (29). However, these channels are clearly distinct from ICRACL as they display higher permeability to Ba2+ than to Ca2+ (16 pS at 160 mM Ba2+ and 2 pS at 200 mM Ca2+), not permeable to Na+, not voltage-dependent, and do not respond to IP3 in i/o patches (29). Therefore, these channels constitute an alternative depletion-activated Ca2+ influx pathway in A431 cells. We have not observed channels described by Luckhoff and Clapham (29) in our experiments, most likely because of variability between different A431 clones or effects of culture conditions on channel expression. In some patches on A431 cells, we observed nonselective cation permeable channels with large conductance, which were clearly distinct from the ICRACL. These channels did not respond to IP3 or Tg, and the patches containing these channels were discarded.
What is a mechanism of ICRACL activation? From the present results and from our previous work on Imin, we conclude that ICRACL channels in A431 cells are conformationally coupled to intracellular IP3R and can be activated: (i) by changes in the IP3R receptor conformation on IP3 binding (16); (ii) by direct cleavage of ICRACL-IP3R-tethered PIP2 by PLC (17); and (iii) by the store-operated mechanism as in the conformational coupling mechanism originally proposed by Irvine (ref. 30) (present results). Gating of ICRACL-IP3R complexes by IP3 probably accounts for the low background channel activity in resting cells (Fig. 1a) (endogenous IP3 level is estimated at 40–100 nM in unstimulated cells; ref. 31), and for the substantial activity of ICRACL channels in excised patches in the presence of 2.5 μM IP3 (Fig. 1a). Cleavage of IP3R-tethered PIP2 by PLC is likely to be responsible for activation of ICRACL channels by UTP in the pipette in our experiments (Fig. 1b). The activation of ICRACL channels by Tg in the pipette (Fig. 2b) and by BAPTA-AM/Tg in the bath (Fig. 2c) results from the IP3R conformational changes on intracellular Ca2+ store depletion. In physiological conditions, stimulation of cells by agonist leads to PLC activation, increase in IP3 levels, and depletion of Ca2+ stores. Therefore, an additive or even synergistic action of three different pathways of ICRACL activation in cells is expected in response to application of agonist in situ. Similar to ICRAC (27, 28), ICRACL channels are under strong negative inhibitory control by cytosolic Ca2+, which normally leads to a transient nature of ICRACL activity (Figs. 1b and 4b). Loading A431 cells with BAPTA removes Ca2+-dependent inactivation and dramatically increases the duration of ICRACL activity (Fig. 4c).
Role of PIP2 as a Modulator of ICRACL Channels.
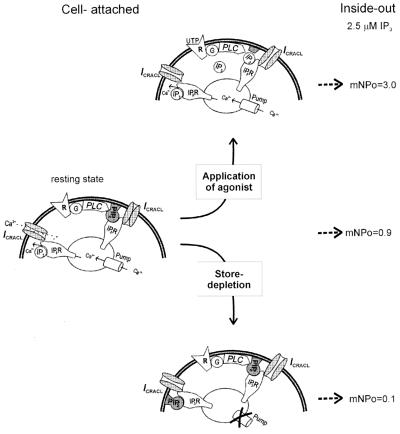
Our data also suggest that PIP2 may play a role of ICRACL modulator by regulating a dynamic equilibrium between ICRACL-IP3R and ICRACL-IP3R-PIP2 complexes (Fig. 5 Left). Following exposure to UTP, activation of PLC and cleavage of PIP2 in the patch, the majority of ICRACL channels are shifted to PIP2-free ICRACL-IP3R state (Fig. 5 Top), as manifested by NPomax30 = 3 in i/o patches with 2.5 μM IP3 in these experiments (Fig. 5 Top Right) compared with NPomax30 = 0.86 in control patches (Fig. 5 Right). Depletion of the stores with Tg or BAPTA appears to shift the equilibrium in the opposite direction, with all of ICRACL channels driven to ICRACL-IP3R-PIP2 complexes (Fig. 5 Bottom). ICRACL channels in these experiments were unresponsive to IP3 in i/o patches with NPomax30 = 0.1 (Fig. 5 Bottom Right) but responded essentially at the maximal level (NPomax30 = 2.7) to a combination of 2.5 μM IP3 and PIP2Ab (Fig. 4d). Despite loss of sensitivity to activation by IP3, ICRACL channels in ICRACL-IP3R-PIP2 complexes remain active in c/a mode (but not in i/o mode, for reasons that need to be further investigated) as long as stores are depleted and ICRACL inactivation is prevented by chelating Ca2+ (Fig. 4c). Possible mechanisms responsible for the store-dependent shift toward a PIP2-occupied state of the IP3R may include physical rearrangement of mobile Ca2+ stores (32), changes in local PIP2 levels in the patch (33), or an increase in IP3R affinity for PIP2 following Ca2+ stores depletion. Additional experiments will be needed to discriminate between these possibilities.
Figure 5.
Model of ICRACL conformational coupling to IP3R and modulation by PIP2. ICRACL-IP3R and ICRACL-IP3R-PIP2 complexes exist in equilibrium in resting cells (Left). Background ICRACL channel activity in cell-attached patches in resting cells results from endogenous IP3 (40–100 nM) (31) activating ICRACL-IP3R complexes. Exposure of patches excised from the resting cells to 2.5 μM IP3 leads to elevated ICRACL channel activity in i/o configuration (Right). Exposure to UTP in the pipette triggers cleavage of IP3R-tethered PIP2 and direct activation of ICRACL-IP3R-PIP2 complexes in cell-attached patches as previously proposed (17). The shift from ICRACL-IP3R-PIP2 to ICRACL-IP3R complexes (Top) explains high activity of ICRACL channels in i/o patches in the presence of 2.5 μM IP3 (Top Right). Exposure to Tg in the pipette or to BAPTA-AM/Tg in the bath causes depletion of local Ca2+ stores and activation of ICRACL channels by means of conformational coupling mechanism (30). Depletion of Ca2+ stores by some unknown mechanism promotes formation of ICRACL-IP3R-PIP2 complexes (Bottom), which leads to the loss of ICRACL sensitivity to activation by 2.5 μM IP3 in excised patches (Bottom Right). Despite loss of sensitivity to activation by IP3, ICRACL channels in ICRACL-IP3R-PIP2 complexes remain active as long as stores are depleted and ICRACL inactivation is prevented by chelating Ca2+ with BAPTA. The model drawing is adapted from ref. 47.
Conformational Coupling Model of ICRACL Activation.
ICRACL-IP3R association is likely to involve direct binding of the IP3R amino-terminal region to the ICRACL protein, similar to mTrp-IP3R association (21). Interestingly, the same amino-terminal region of IP3R also includes specific IP3 (34, 35) and PIP2 (36) binding sites. Thus, ligand-induced conformational changes of the IP3R amino-terminal region can be transmitted directly to the ICRACL channel. The store-operated ICRACL activation is likely to involve an IP3R-associatied endoplasmic reticulum resident Ca2+-binding protein, such as calreticulin (37–39), which serves as a sensor of intraluminal Ca2+. Additional signaling components are likely to be recruited to the ICRACL-IP3R complex via actions of a modular adaptor protein, such as mGluR1/IP3R-binding protein Homer in neuronal cells (40), the Syk/Btk/Grb2/PLCγ-binding protein BLNK in B lymphocytes (41), or the trp/PKC/PLC-binding protein inaD in Drosophila photoreceptors (42). The actin cytoskeleton may also play an important role in correct spatial arrangement of required signaling components (43–45). In chicken B lymphocytes, removal of all three IP3R isoforms by genetic means had no effect on Tg-induced Ca2+ influx (46), in apparent conflict with the conformational coupling model of ICRACL activation in A431 cells (Fig. 5). From these results, we conclude that the B lymphocytes must have an additional or alternative Ca2+ influx pathway, coupled to Ca2+ store depletion by means of IP3R-independent mechanism that may involve a global “diffusible messenger.” Additional functional studies with B lymphocytes will be required for its detailed characterization.
Acknowledgments
We thank V. A. Alexeenko for technical assistance, Phyllis Foley for help with preparation of the manuscript, and Dr. Bertil Hille for comments on the paper. This work is supported by the Russian Basic Research Foundation 98-04-49512 (to G.N.M), the National Institutes of Health NS38082 (to I.B.), and the Civilian Research and Development Foundation RB1-2018 (G.N.M. and I.B.).
Abbreviations
- PLC
phospholipase C
- ICRAC
Ca2+ release activated channel
- ICRACL
ICRAC-like
- IP3
inositol 1,4,5-triphosphate
- IP3R
IP3 receptor
- PIP2
phosphatidylinositol (1,4,5)-bisphosphate
- c/a
cell-activated
- i/o
inside-out
- Tg
thapsigargin
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Berridge M J. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parekh A B, Penner R. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 3.Putney J W, Jr, McKay R R. BioEssays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Hoth M, Penner R. Nature (London) 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 5.Premack B A, McDonald T V, Gardner P. J Immunol. 1994;152:5226–5240. [PubMed] [Google Scholar]
- 6.Zweifach A, Lewis R S. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerschbaum H H, Cahalan M D. Science. 1999;283:836–839. doi: 10.1126/science.283.5403.836. [DOI] [PubMed] [Google Scholar]
- 8.Clapham D E. Neuron. 1996;16:1069–1072. doi: 10.1016/s0896-6273(00)80132-4. [DOI] [PubMed] [Google Scholar]
- 9.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. Proc Natl Acad Sci USA. 1996;93:15195–15202. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozhayeva G N, Naumov A P, Kuryshev Y A. FEBS Lett. 1990;277:233–234. doi: 10.1016/0014-5793(90)80853-b. [DOI] [PubMed] [Google Scholar]
- 11.Kiselyov K I, Mamin A G, Semyonova S B, Mozhayeva G N. FEBS Lett. 1997;407:309–312. doi: 10.1016/s0014-5793(97)00366-9. [DOI] [PubMed] [Google Scholar]
- 12.Kiselyov K I, Semyonova S B, Mamin A G, Mozhayeva G N. Pflugers Arch. 1999;437:305–314. doi: 10.1007/s004240050784. [DOI] [PubMed] [Google Scholar]
- 13.Kuno M, Gardner P. Nature (London) 1987;326:301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- 14.Luckhoff A, Clapham D E. Nature (London) 1992;355:356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- 15.Vaca L, Kunze D L. Am J Physiol. 1995;269:C733–C738. doi: 10.1152/ajpcell.1995.269.3.C733. [DOI] [PubMed] [Google Scholar]
- 16.Zubov A I, Kaznacheeva E V, Nikolaev A V, Alexeenko V A, Kiselyov K, Muallem S, Mozhayeva G N. J Biol Chem. 1999;274:25983–25985. doi: 10.1074/jbc.274.37.25983. [DOI] [PubMed] [Google Scholar]
- 17.Kaznacheyeva E, Zubov A N, Nikolaev A, Alexeenko V, Bezprozvanny I, Mozhayeva G N. J Biol Chem. 2000;275:4561–4564. doi: 10.1074/jbc.275.7.4561. [DOI] [PubMed] [Google Scholar]
- 18.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Nature (London) 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 19.Kiselyov K, Mignery G A, Zhu M X, Muallem S. Mol Cell. 1999;4:423–429. doi: 10.1016/s1097-2765(00)80344-5. [DOI] [PubMed] [Google Scholar]
- 20.Vannier B, Peyton M, Boulay G, Brown D, Qin N, Jiang M, Zhu X, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:2060–2064. doi: 10.1073/pnas.96.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boulay G, Brown D M, Qin N, Jiang M, Dietrich A, Zhu M X, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma H-T, Patterson R L, Rossum D B, Birnbaumer L, Mikoshiba K, Gill D L. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- 23.Estacion, M., Sinkins, W. G. & Schilling, W. P. (2000) J. Physiol. (London), in press.
- 24.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pfluegers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 25.Fukami K, Matsuoka K, Nakanishi O, Yamakawa A, Kawai S, Takenawa T. Proc Natl Acad Sci USA. 1988;85:9057–9061. doi: 10.1073/pnas.85.23.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lupu V D, Kaznacheyeva E, Krishna U M, Falck J R, Bezprozvanny I. J Biol Chem. 1998;273:14067–14070. doi: 10.1074/jbc.273.23.14067. [DOI] [PubMed] [Google Scholar]
- 27.Hoth M, Penner R. J Physiol (London) 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zweifach A, Lewis R S. J Gen Physiol. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luckhoff A, Clapham D E. Biophys J. 1994;67:177–182. doi: 10.1016/S0006-3495(94)80467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irvine R F. FEBS Lett. 1990;263:5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- 31.Luzzi V, Sims C E, Soughayer J S, Allbritton N L. J Biol Chem. 1998;273:28657–28662. doi: 10.1074/jbc.273.44.28657. [DOI] [PubMed] [Google Scholar]
- 32.Yao Y, Ferrer-Montiel A V, Montal M, Tsien R Y. Cell. 1999;98:475–485. doi: 10.1016/s0092-8674(00)81976-5. [DOI] [PubMed] [Google Scholar]
- 33.Raucher D, Stauffer T, Chen W, Shen K, Guo S, York J D, Sheetz M P, Meyer T. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 34.Mignery G A, Sudhof T C. EMBO J. 1990;9:3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyawaki A, Furuichi T, Ryou Y, Yoshikawa S, Nakagawa T, Saitoh T, Mikoshiba K. Proc Natl Acad Sci USA. 1991;88:4911–4915. doi: 10.1073/pnas.88.11.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glouchankova L, Krishna U M, Potter B V L, Falck J R, Bezprozvanny I. Mol Cell Biol Res Comm. 2000;3:153–158. doi: 10.1006/mcbr.2000.0208. [DOI] [PubMed] [Google Scholar]
- 37.Camacho P, Lechleiter J D. Cell. 1995;82:765–771. doi: 10.1016/0092-8674(95)90473-5. [DOI] [PubMed] [Google Scholar]
- 38.Fadel M P, Dziak E, Lo C M, Ferrier J, Mesaeli N, Michalak M, Opas M. J Biol Chem. 1999;274:15085–15094. doi: 10.1074/jbc.274.21.15085. [DOI] [PubMed] [Google Scholar]
- 39.Michalak M, Corbett E F, Mesaeli N, Nakamura K, Opas M. Biochem J. 1999;344:281–292. [PMC free article] [PubMed] [Google Scholar]
- 40.Tu J C, Xiao B, Yuan J P, Lanahan A A, Leoffert K, Li M, Linden D J, Worley P F. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 41.Kurosaki T, Tsukada S. Immunity. 2000;12:1–5. doi: 10.1016/s1074-7613(00)80153-3. [DOI] [PubMed] [Google Scholar]
- 42.Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker C S. Nature (London) 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- 43.Rossier M F, Bird G S J, Putney J W J. Biochem J. 1991;274:643–650. doi: 10.1042/bj2740643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holda J R, Blatter L A. FEBS Lett. 1997;403:191–196. doi: 10.1016/s0014-5793(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro C M, Reece J, Putney J W., Jr J Biol Chem. 1997;272:26555–26561. doi: 10.1074/jbc.272.42.26555. [DOI] [PubMed] [Google Scholar]
- 46.Sugawara H, Kurosaki M, Takata M, Kurosaki T. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Putney J W., Jr Proc Natl Acad Sci USA. 1999;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]