Abstract
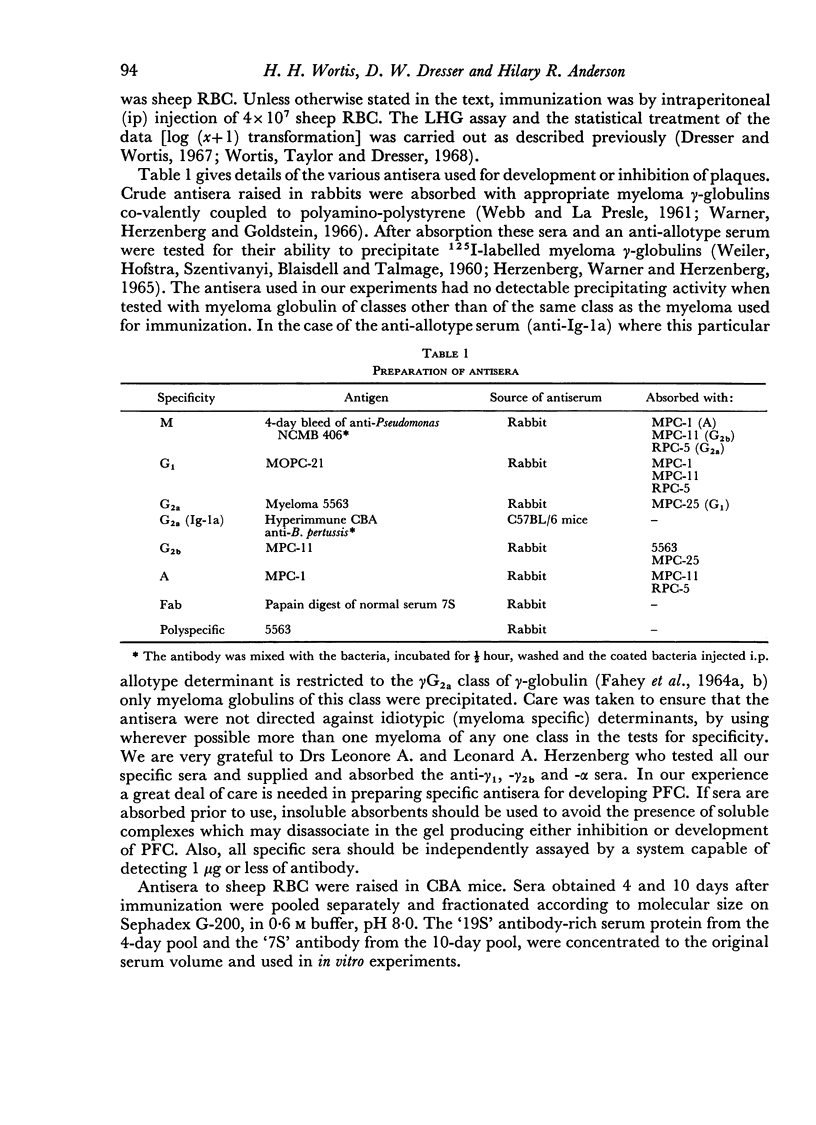
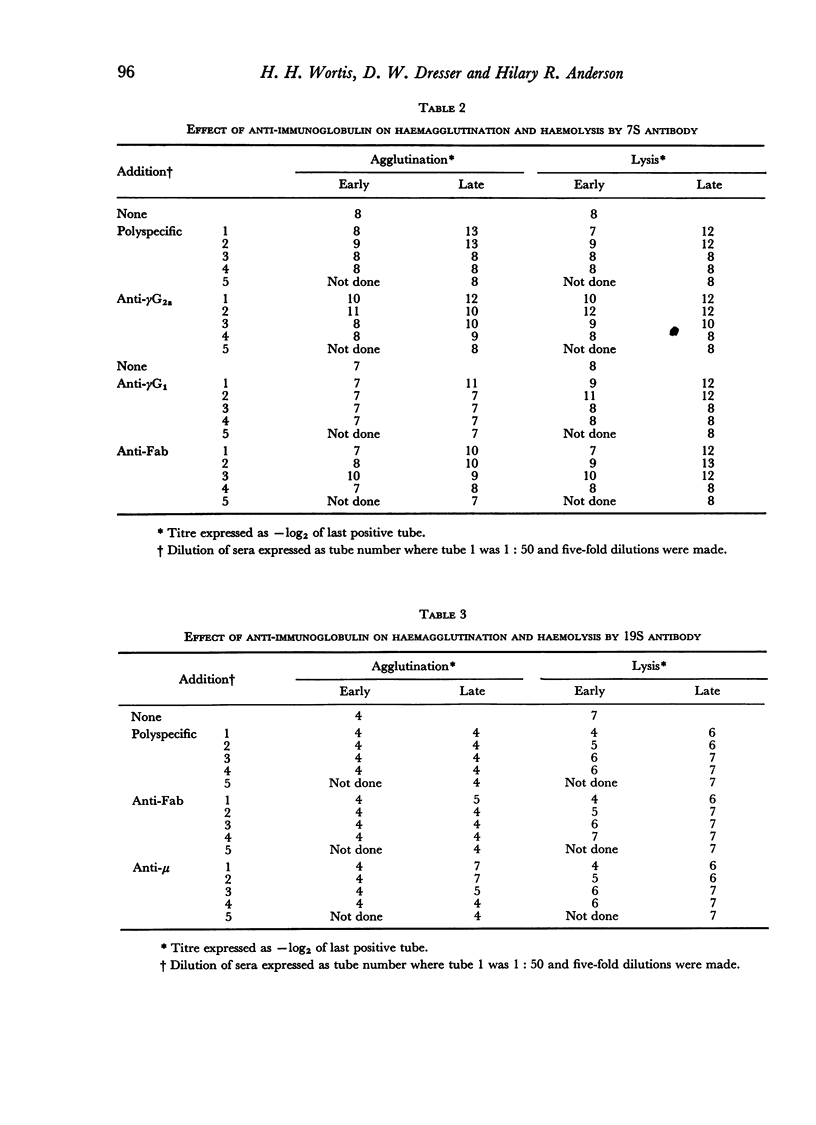
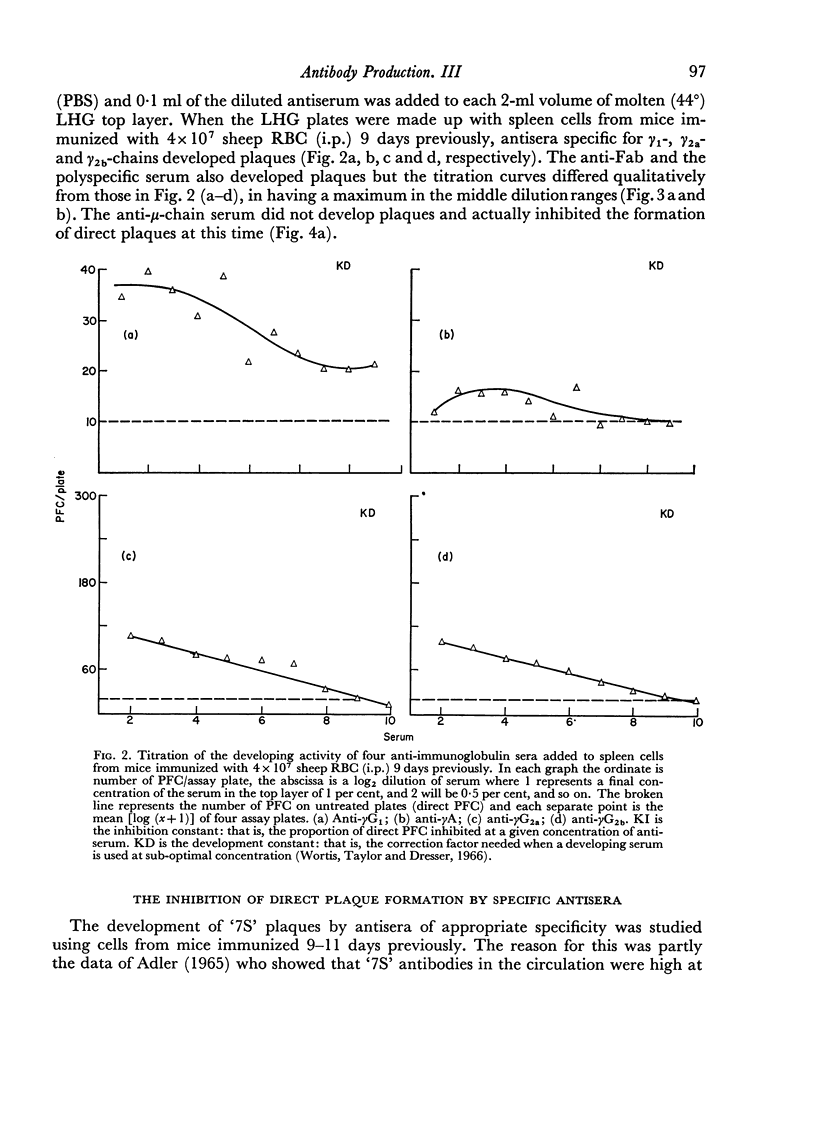
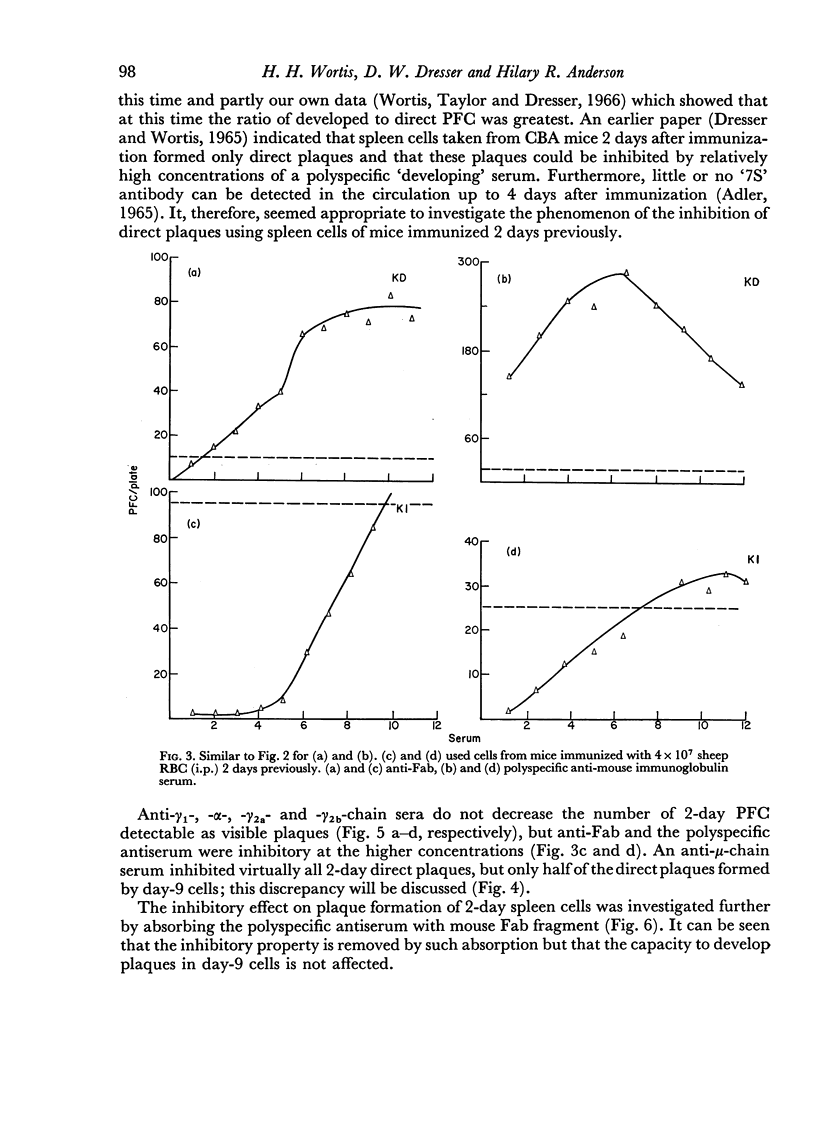
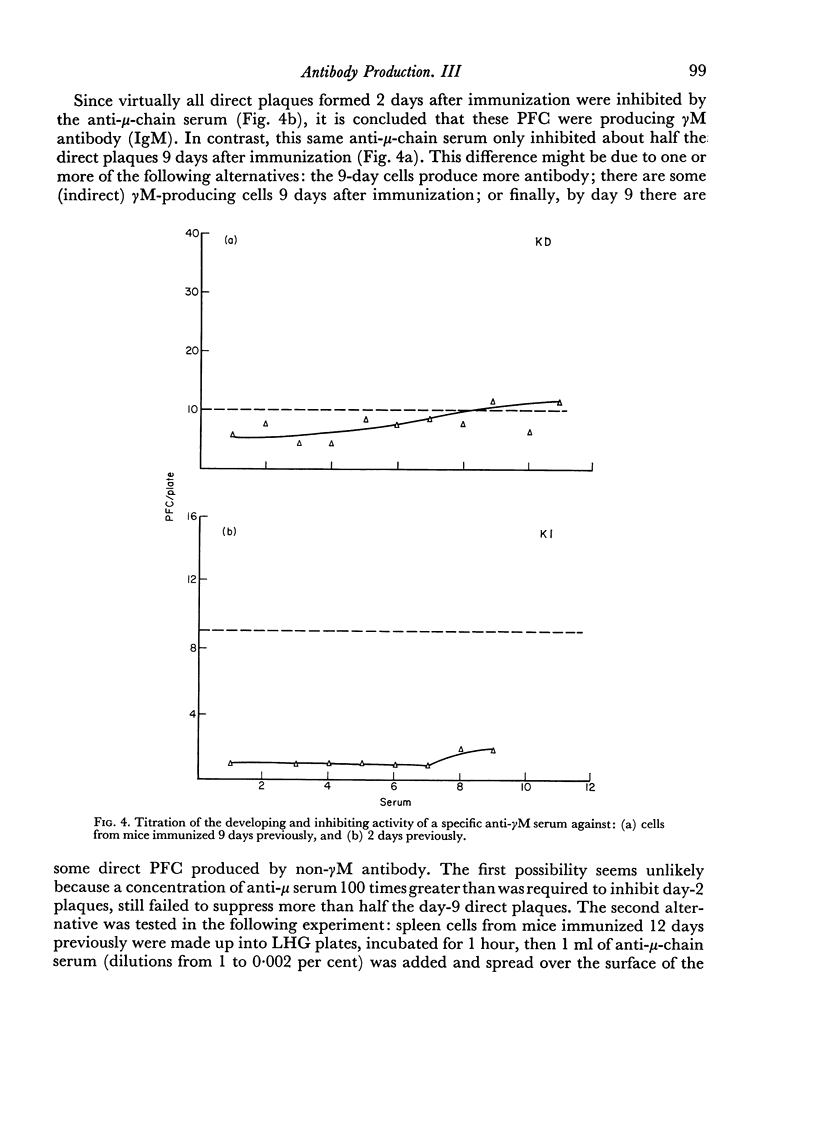
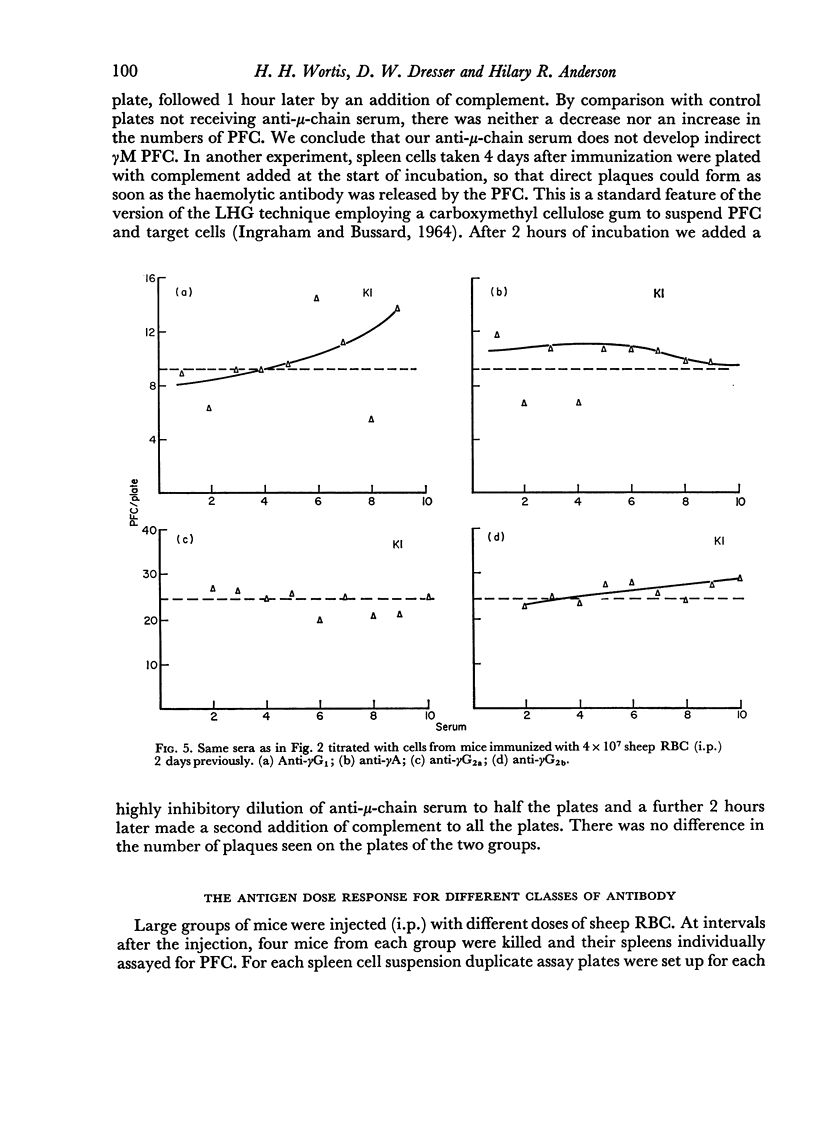
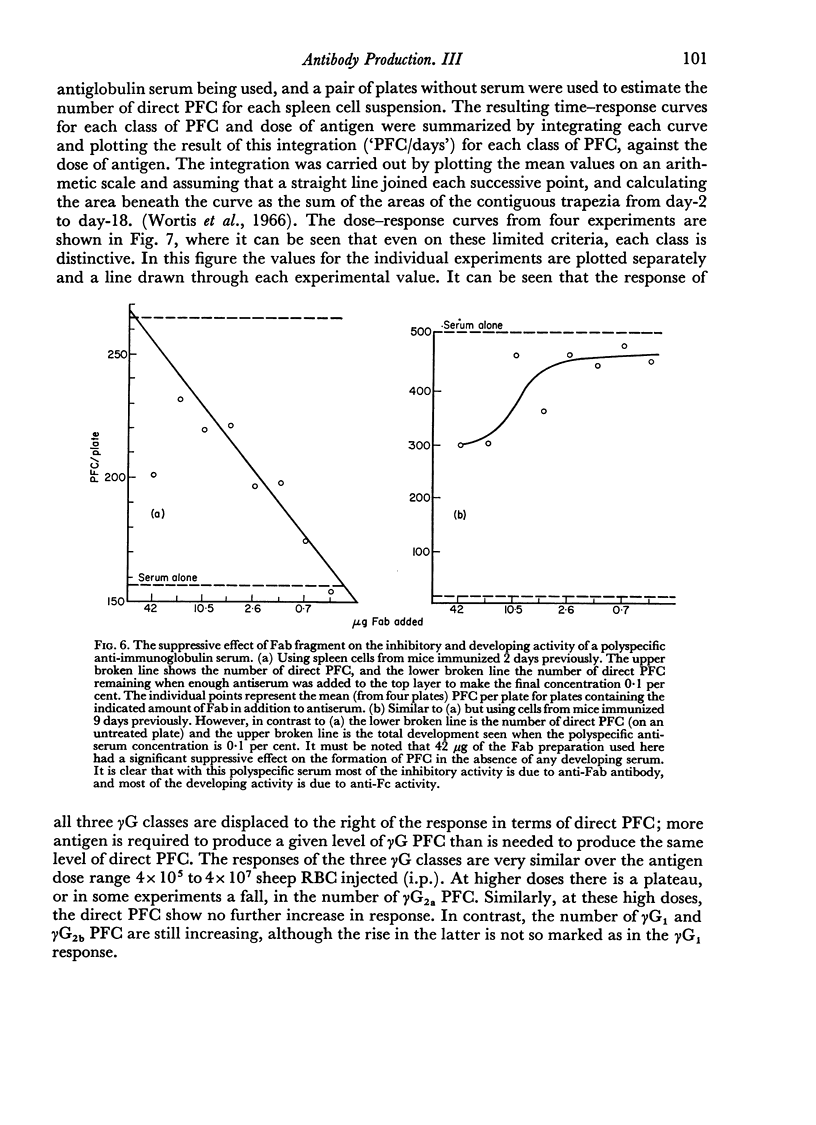
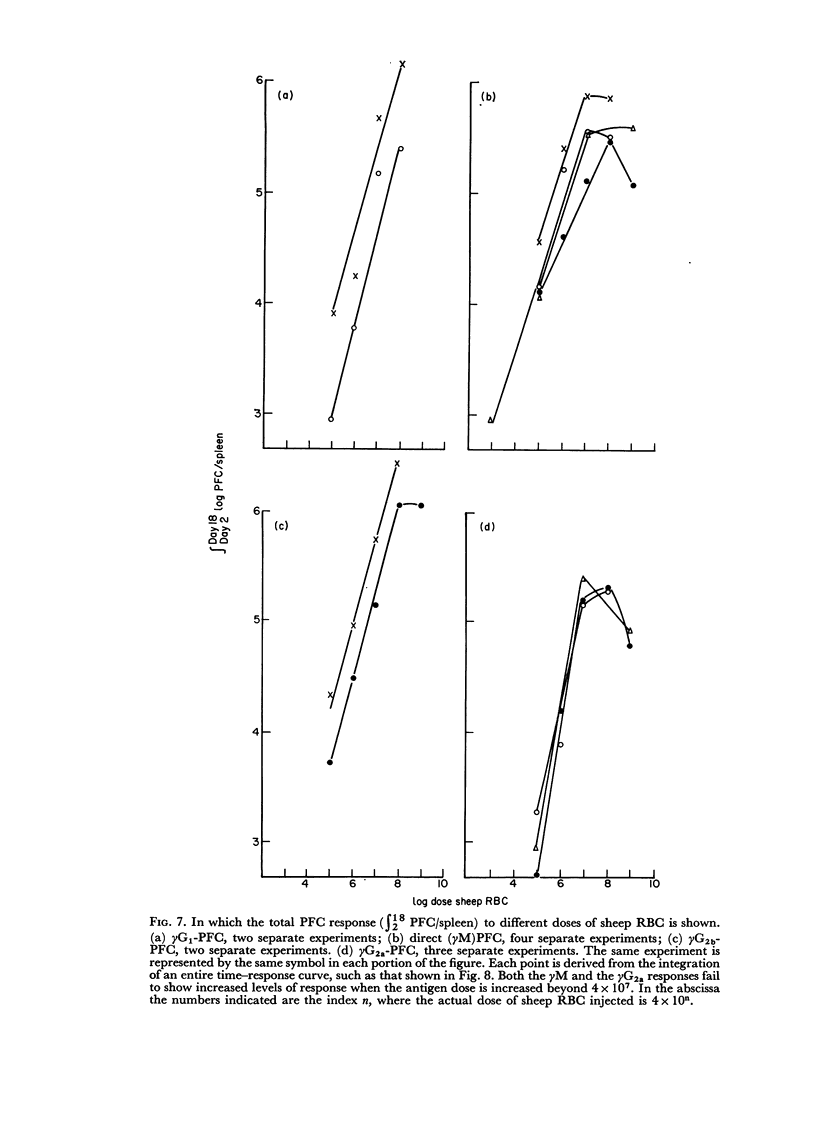
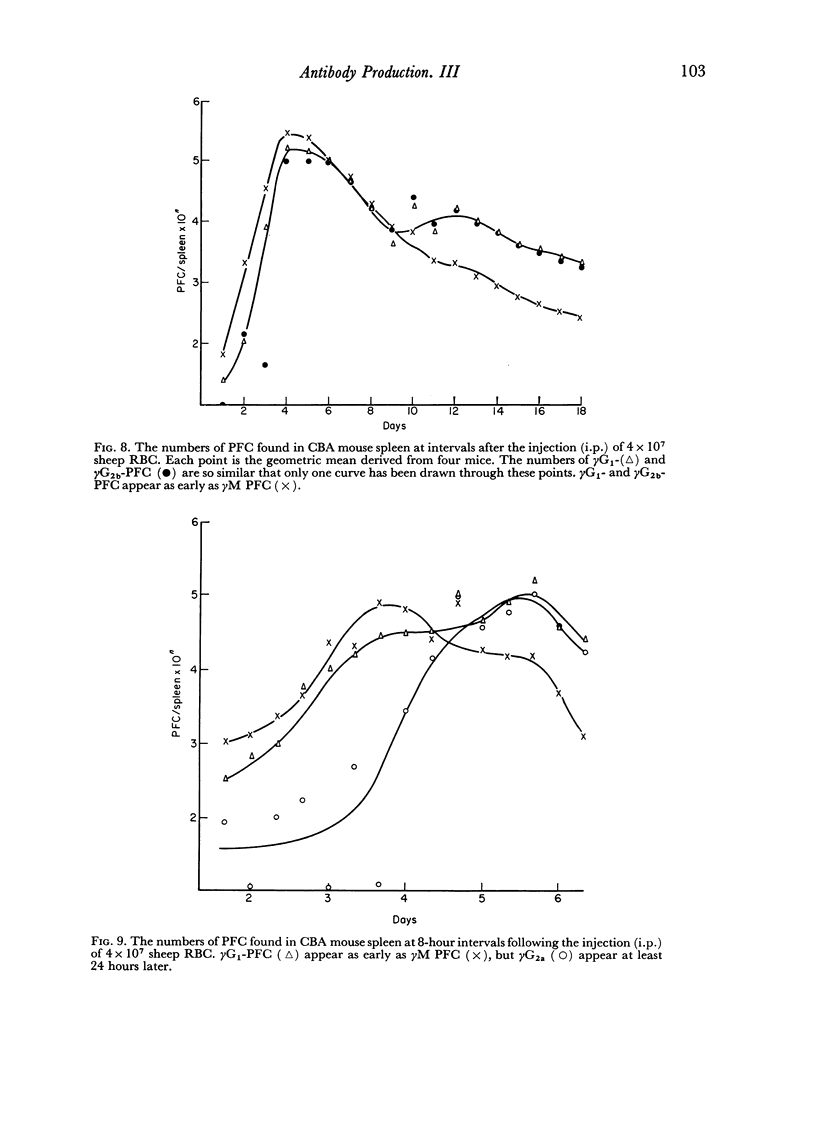
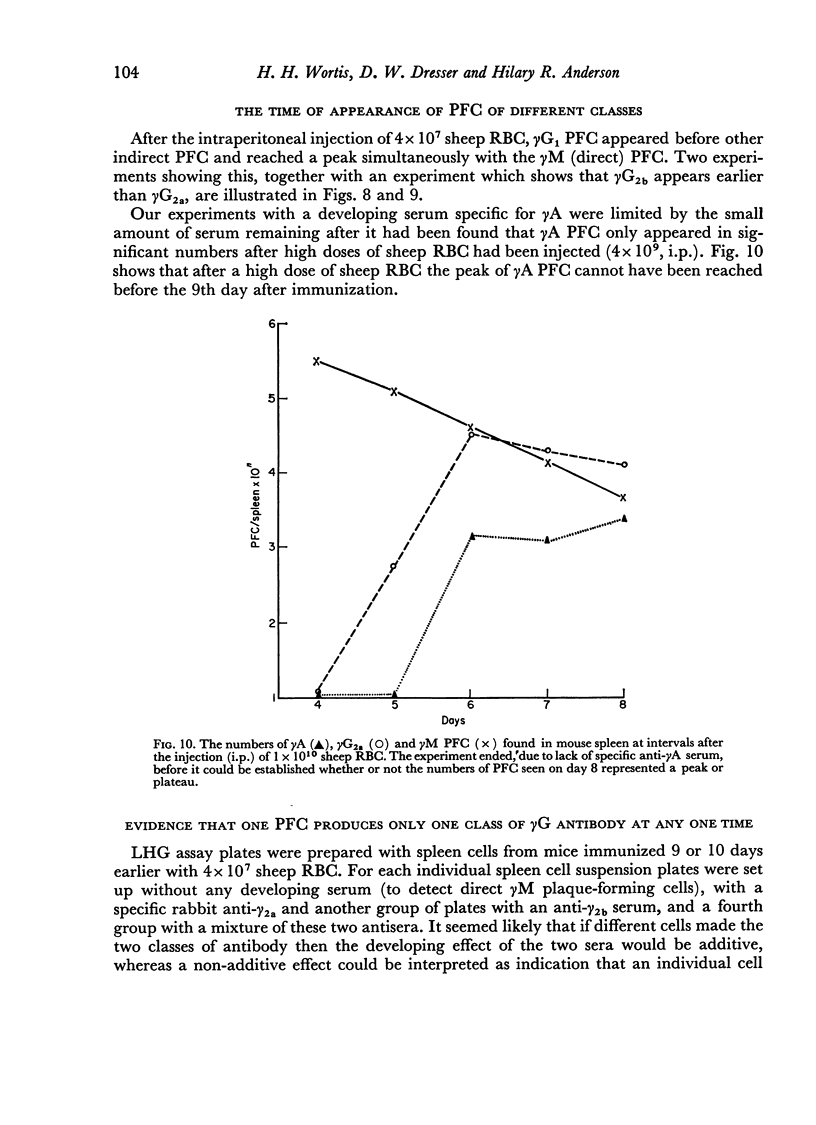
By the use of antiglobulin sera of known specificity mouse cells releasing γM, γG1, γG2a, γG2b or γA antibody to sheep erythrocytes can be identified in the LHG assay. The properties of these sera are described. Cells releasing γG1 or γG2b antibody appear as soon after immunization as cells releasing γM antibody. High doses of sheep RBC (1×1010) were needed to stimulate the appearance of γA releasing cells in the spleen after intraperitoneal immunization.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER F. L. STUDIES ON MOUSE ANTIBODIES. I. THE RESPONSE TO SHEEP RED CELLS. J Immunol. 1965 Jul;95:26–38. [PubMed] [Google Scholar]
- Barth W. F., McLaughlin C. L., Fahey J. L. The immunoglobulins of mice. VI. Response to immunization. J Immunol. 1965 Nov;95(5):781–790. [PubMed] [Google Scholar]
- Bauer D. C., Stavitsky A. B. ON THE DIFFERENT MOLECULAR FORMS OF ANTIBODY SYNTHESIZED BY RABBITS DURING THE EARLY RESPONSE TO A SINGLE INJECTION OF PROTEIN AND CELLULAR ANTIGENS. Proc Natl Acad Sci U S A. 1961 Oct;47(10):1667–1680. doi: 10.1073/pnas.47.10.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier G. M., Cebra J. J. Frequency distribution of alpha, gamma, kappa and lambda polypeptide chains in human lymphoid tissues. J Immunol. 1965 Aug;95(2):246–253. [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., WUNDERLICH J., MISHELL R. THE IMMUNOGLOBULINS OF MICE. I. FOUR MAJOR CLASSES OF IMMUNOGLOBULINS: 7S GAMMA-2-, 7S GAMMA-1-, GAMMA-1A (BETA-2A)-, AND 18S GAMMA-1M-GLOBULINS. J Exp Med. 1964 Aug 1;120:223–242. doi: 10.1084/jem.120.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., WUNDERLICH J., MISHELL R. THE IMMUNOGLOBULINS OF MICE. II. TWO SUBCLASSES OF MOUSE 7S GAMMA-2-GLOBULINS: GAMMA-2A- AND GAMMA-2B-GLOBULINS. J Exp Med. 1964 Aug 1;120:243–251. doi: 10.1084/jem.120.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZENBERG L. A., WARNER N. L., HERZENBERG L. A. IMMUNOGLOBULIN ISOANTIGENS (ALLOTYPES) IN THE MOUSE. I. GENETICS AND CROSS-REACTIONS OF THE 7S GAMMA-2A-ISOANTIGENS CONTROLLED BY ALLELES AT THE IG-1 LOCUS. J Exp Med. 1965 Mar 1;121:415–438. doi: 10.1084/jem.121.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer L. W., Borsos T., Rapp H. J., Vannier W. E. Heterogeneity of rabbit IgM antibody as detected by C'1a fixation. J Exp Med. 1968 Mar 1;127(3):589–603. doi: 10.1084/jem.127.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop N. E., Jr, Roeder E. M. Passive immune hemolysis: titration of hemolytic anti-protein antibody using bis-diazotized-benzidine to couple antigen to erythrocytes. Proc Soc Exp Biol Med. 1966 Feb;121(2):514–523. doi: 10.3181/00379727-121-30818. [DOI] [PubMed] [Google Scholar]
- INGRAHAM J. S., BUSSARD A. APPLICATION OF A LOCALIZED HEMOLYSIN REACTION FOR SPECIFIC DETECTION OF INDIVIDUAL ANTIBODY-FORMING CELLS. J Exp Med. 1964 Apr 1;119:667–684. doi: 10.1084/jem.119.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham J. S., Biegel A. A., Watanabe M. R., Todd C. W. Effect of anti-allotype sera on hemolytic plaque formation by single rabbit spleen cells. J Immunol. 1967 Nov;99(5):1023–1035. [PubMed] [Google Scholar]
- MELLORS R. C., KORNGOLD L. THE CELLULAR ORIGIN OF HUMAN IMMUNOGLOBULINS (GAMMA-2, GAMMA-1M, GAMMA-1A). J Exp Med. 1963 Sep 1;118:387–396. doi: 10.1084/jem.118.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan T., Peterson W. J. Further studies on the secondary antibody-forming potential of juvenile, young adult, adult, and aged mice. Dev Biol. 1966 Aug;14(1):112–129. doi: 10.1016/0012-1606(66)90008-x. [DOI] [PubMed] [Google Scholar]
- NOSSAL G. J., ADA G. L., AUSTIN C. M. ANTIGENS IN IMMUNITY. II. IMMUNOGENIC PROPERTIES OF FLAGELLA, POLYMERIZED FLAGELLIN AND FLAGELLIN IN THE PRIMARY RESPONSE. Aust J Exp Biol Med Sci. 1964 Jun;42:283–294. [PubMed] [Google Scholar]
- NOSSAL G. J., SZENBERG A., ADA G. L., AUSTIN C. M. SINGLE CELL STUDIES ON 19S ANTIBODY PRODUCTION. J Exp Med. 1964 Mar 1;119:485–502. doi: 10.1084/jem.119.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig V., Green I., Vassalli P., Benacerraf B. Changes in the proportion of guinea-pig gamma-1 and gamma-2 antibodies during immunization and the cellular localization of these immunoglobulins. Immunology. 1968 May;14(5):601–609. [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotz P. H., Colten H., Talal N. Mouse macroglobulin antibody to sheep erythrocytes: a non-complement-fixing type. J Immunol. 1968 Apr;100(4):752–755. [PubMed] [Google Scholar]
- Plotz P. H., Talal N., Asofsky R. Assignment of direct and facilitated hemolytic plaques in mice to specific immunoglobulin classes. J Immunol. 1968 Apr;100(4):744–751. [PubMed] [Google Scholar]
- SVEHAG S. E. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. III. SEQUENTIAL CHANGES IN ELECTROPHORETIC MOBILITY OF 19S AND 7S ANTIBODIES SYNTHESIZED BY RABBITS AFTER A SINGLE VIRUS INJECTION. J Exp Med. 1964 Feb 1;119:225–240. doi: 10.1084/jem.119.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzl J., Ríha I. Detection of cells producing 7S antibodies by the plaque technique. Nature. 1965 Nov 27;208(5013):858–859. doi: 10.1038/208858a0. [DOI] [PubMed] [Google Scholar]
- TORRIGIANI G., ROITT I. M. THE ENHANCEMENT OF 19S ANTIBODY PRODUCTION BY PARTICULATE ANTIGEN. J Exp Med. 1965 Jul 1;122:181–193. doi: 10.1084/jem.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBB T., LAPRESLE C. Study of the adsorption on and desorption from polystyrene-human serum albumin conjugates of rabbit anti-human serum albumin antibodies having different specificities. J Exp Med. 1961 Jul 1;114:43–50. doi: 10.1084/jem.114.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEILER R. J., HOFSTRA D., SZENTIVANYI A., BLAISDELL R., TALMAGE D. W. The inhibition of labeled antigen precipitation as a measure of serum gamma-globulin. J Immunol. 1960 Aug;85:130–137. [PubMed] [Google Scholar]
- WHITE R. G., JENKINS G. C., WILKINSON P. C. The production of skin-sensitizing antibody in the guinea-pig. Int Arch Allergy Appl Immunol. 1963;22:156–165. doi: 10.1159/000229362. [DOI] [PubMed] [Google Scholar]
- Walters C. S., Jackson A. L. Detection of gamma A antibody-releasing cells to erythrocyte and lipopolysaccharide antigens. J Immunol. 1968 Sep;101(3):541–545. [PubMed] [Google Scholar]
- Warner N. L., Herzenberg L. A., Goldstein G. Immunoglobulin isoantigens (allotypes) in the mouse. II. Allotypic analysis of three gammaG2-myeloma proteins from (NZB x BALB/c)F1 hybrids and of normal gammaG2-globulins. J Exp Med. 1966 Apr 1;123(4):707–721. doi: 10.1084/jem.123.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler E., Melletz E. W., Breuninger-Peck E. Facilitation of immune hemolysis by an interaction between red cell-sensitizing antibody and gamma-globulin allotype antibody. Proc Natl Acad Sci U S A. 1965 Nov;54(5):1310–1317. doi: 10.1073/pnas.54.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C., Fleming W. A., White R. G. The effect of adjuvants on biosynthesis of 19S and 7S antibody against bacteriophage phiX174 in the guinea-pig. Immunology. 1967 Dec;13(6):603–611. [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the localized haemolysis in gel (LHG) assay. II. Assay procedure. Immunology. 1968 Jan;14(1):69–79. [PMC free article] [PubMed] [Google Scholar]