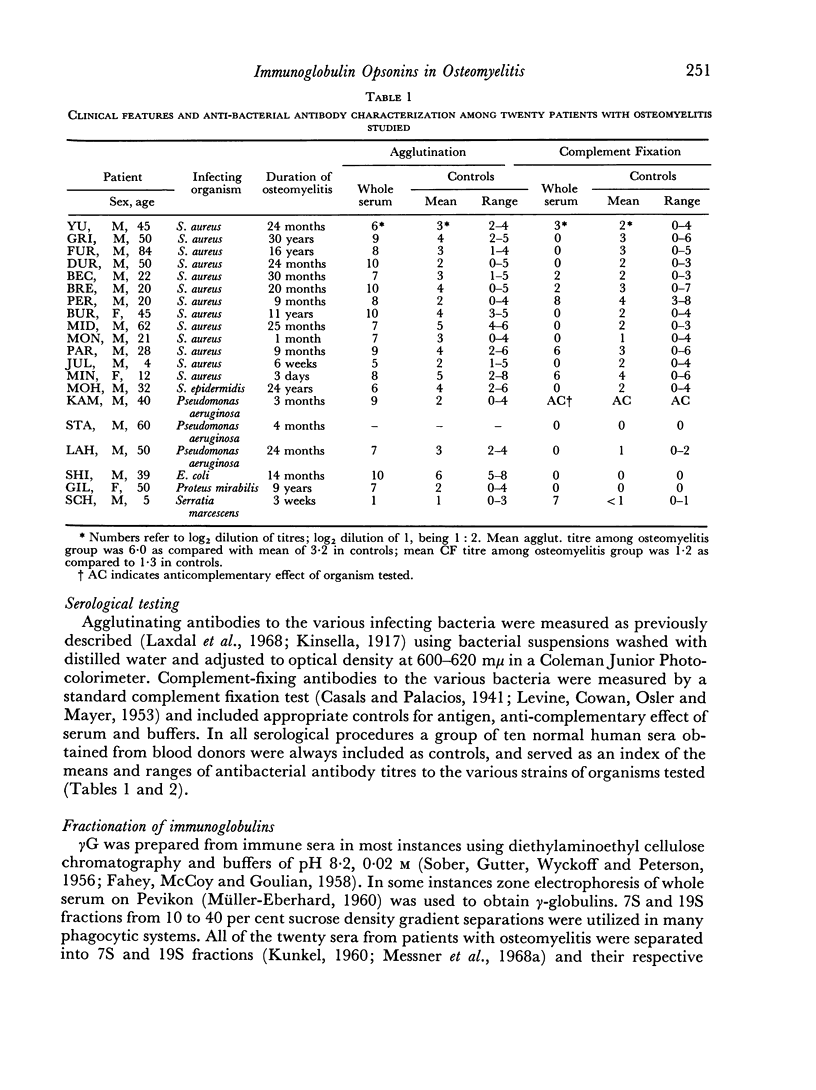
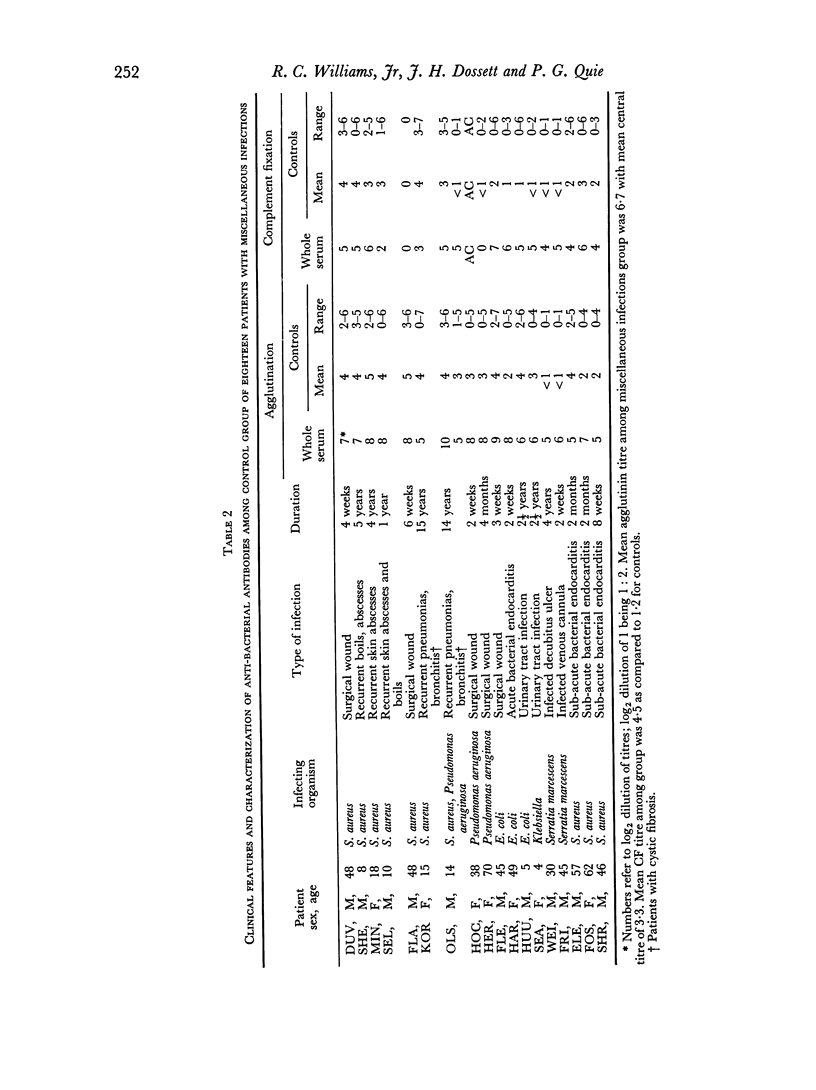
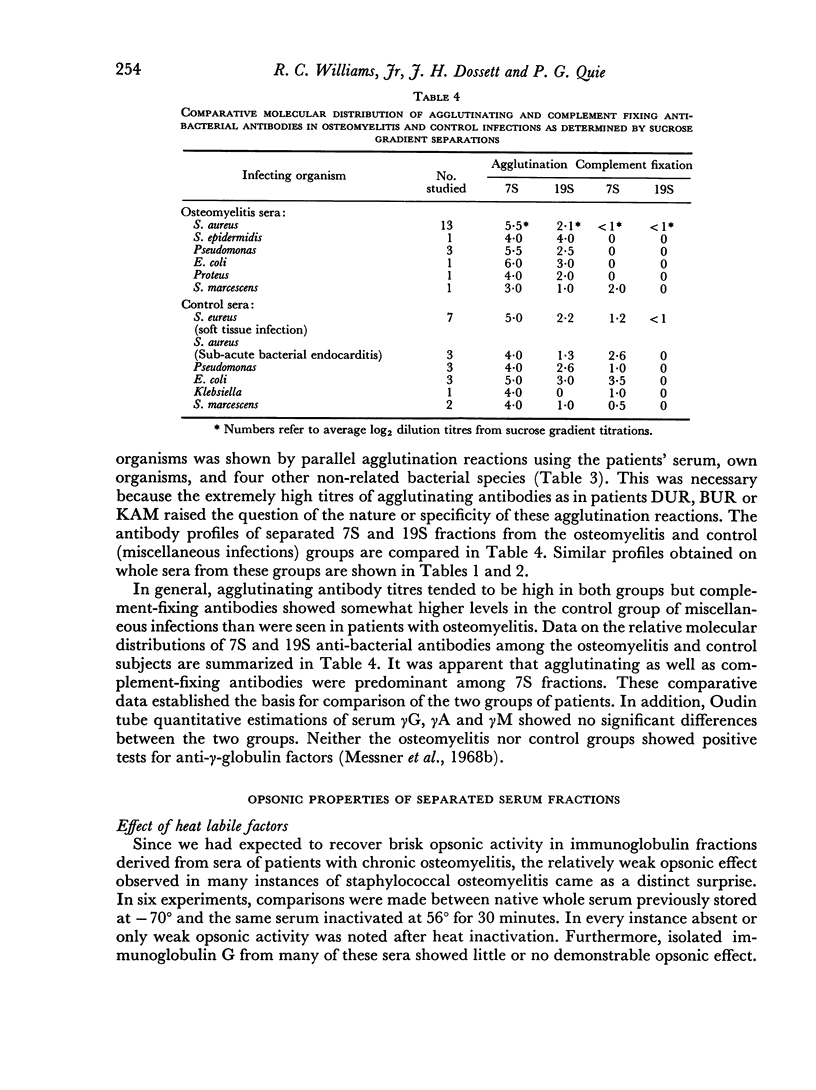
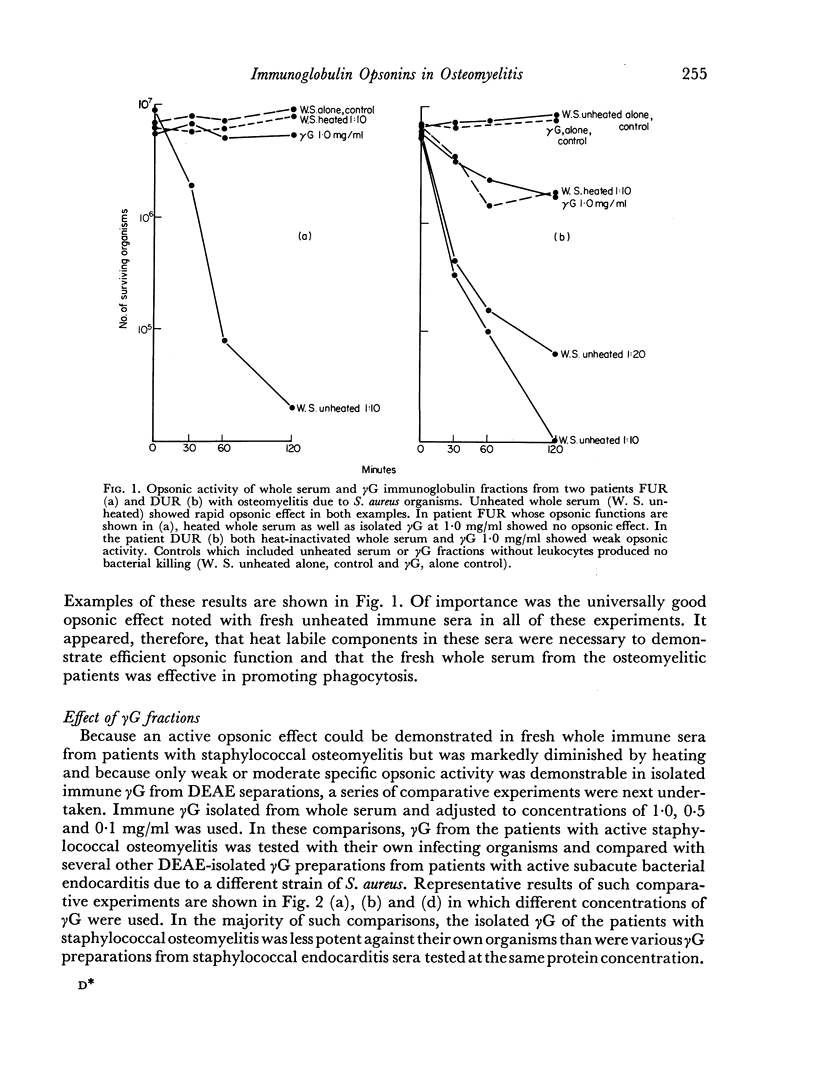
Abstract
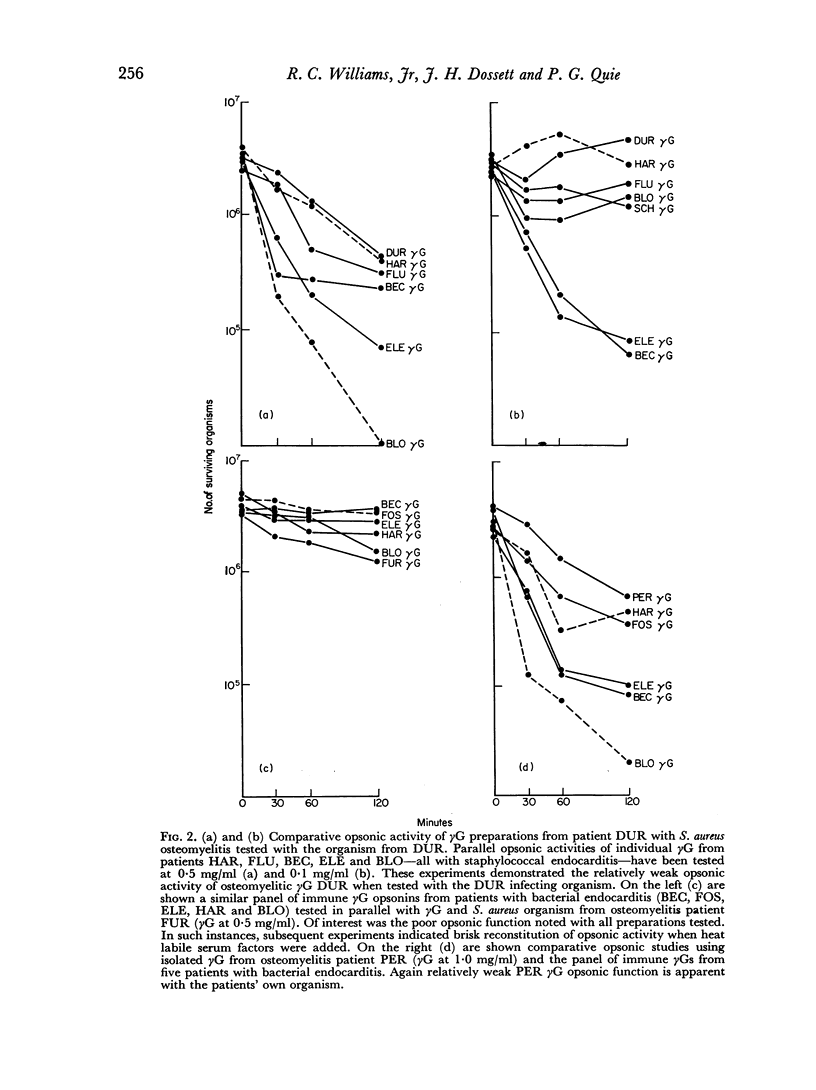
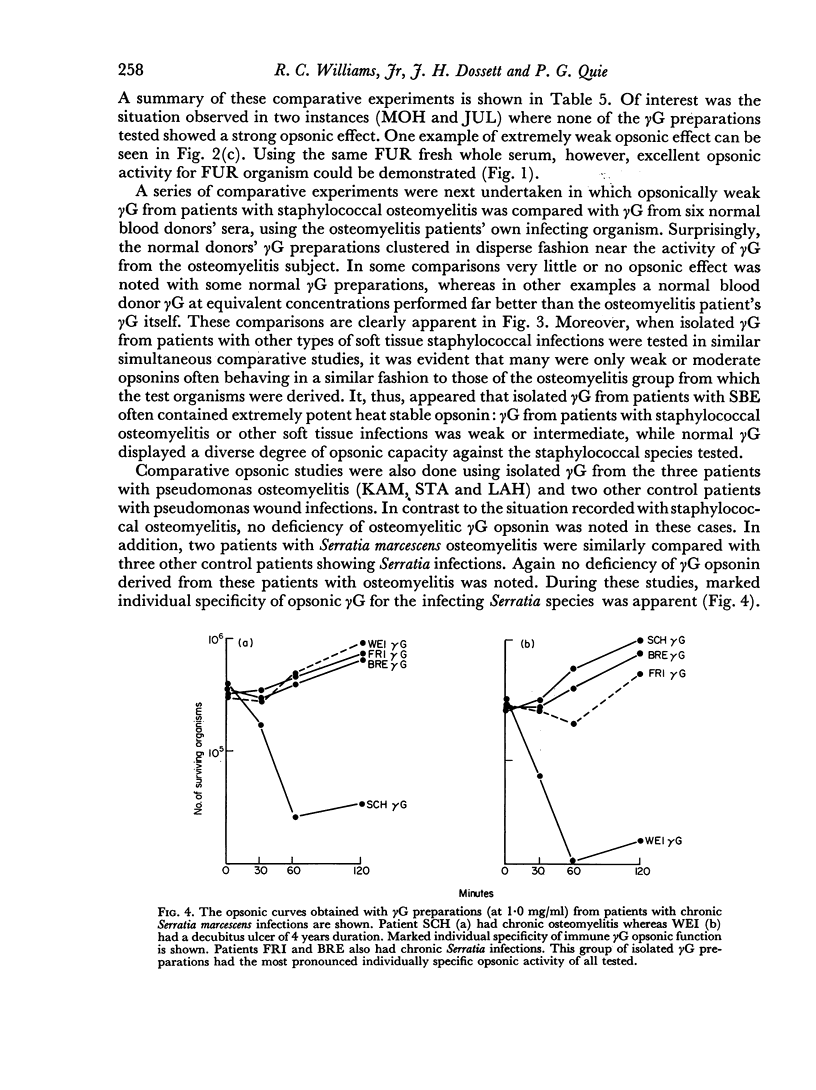
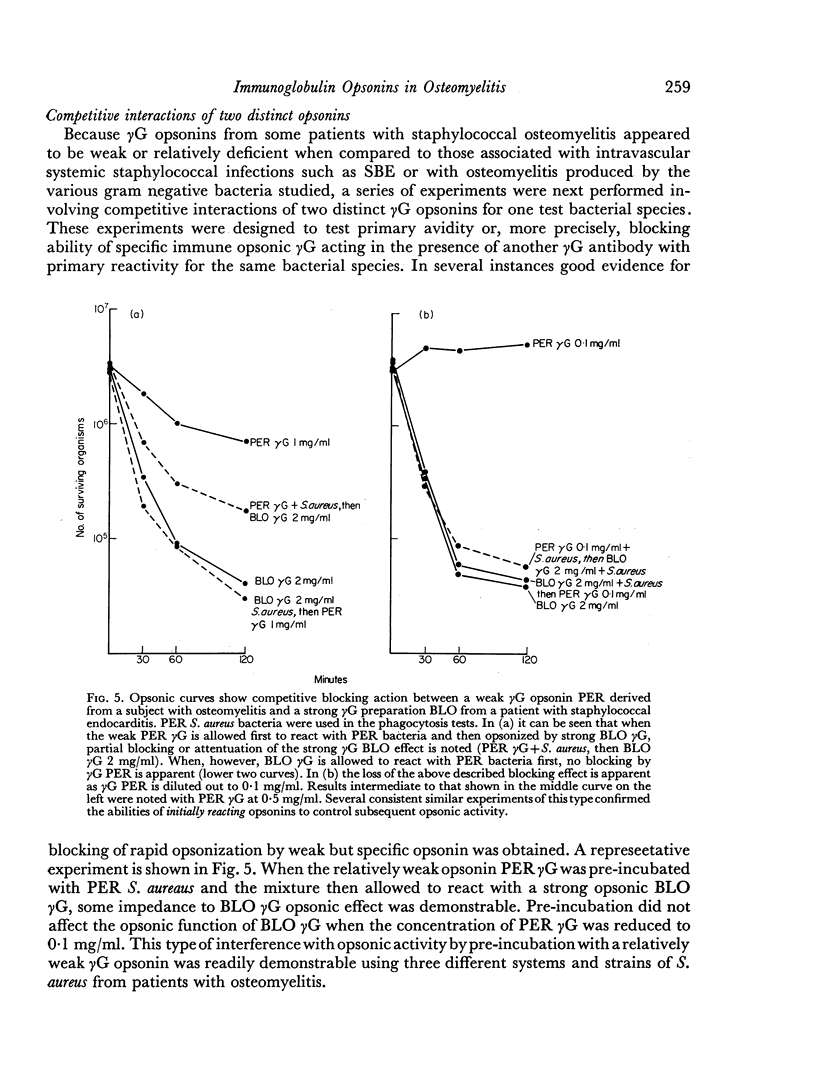
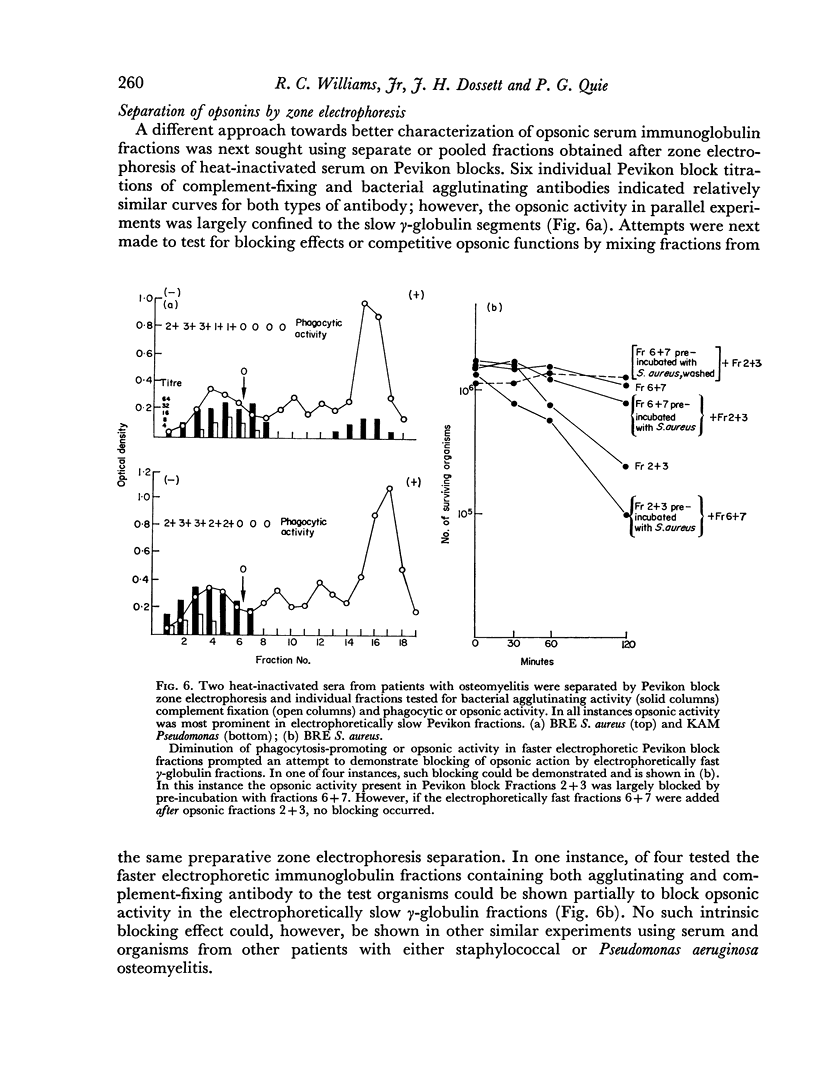
The opsonic activity of serum and isolated immunoglobulin fractions has been studied in twenty patients with osteomyelitis, using a quantitative assay which measures phagocytosis and killing of bacteria by human polymorphonuclear leukocytes. Opsonic functions as well as agglutinating and complement-fixing antibodies were compared with normal human sera as well as with a control group of eighteen patients with miscellaneous established severe infections. A surprising lack of heat stable γG opsonic activity, particularly for various species of Staphylococcus aureus, was documented among many patients with chronic active osteomyelitis. A similar apparent deficiency or low level of heat stable γG opsonin was also demonstrated among some of the controls with severe soft tissue infections. These weak opsonins showed a marked contrast to the potent heat stable γG opsonin activity previously documented in sub-acute bacterial endocarditis. Heat stable γG opsonin proved to be present in effective concentration in Gram-negative infections of bone and in the control group. Evidence was obtained for competitive blocking of phagocytic mechanisms by relatively weak immune γG opsonins allowed to react with bacteria prior to contact with more potent γG opsonins subsequently added to the test system. High titres of agglutinating anti-bacterial antibodies contrasted with relatively low levels of complement-fixing antibodies in many patients with chronic osteomyelitis. Opsonic activities of both 7S and 19S fractions of heat inactivated sera were studied in sera from osteomyelitis patients and control infections. When phagocytosis-promoting properties were not detectable in such fractions, opsonic capacity could be effectively restored by adding fresh serum devoid of anti-bacterial antibodies but possessing complement activity. Opsonic capacity of serum fractions then depended on the marked facilitative effect of heat labile factors. Activity ratios were derived of opsonic activity to actual amount of immunoglobulin present acting in concert with a constant amount of complement.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. V. The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. J Exp Med. 1951 Feb;93(2):107–120. doi: 10.1084/jem.93.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson D. K., Dunn A. W. Management of common bacterial infections of bones and joints. J Bone Joint Surg Am. 1967 Jan;49(1):164–182. [PubMed] [Google Scholar]
- FAHEY J. L., McCOY P. F., GOULIAN M. Chromatography of serum proteins in normal and pathologic sera: the distribution of protein-bound carbohydrate and cholesterol, siderophilin, thyroxin-binding protein, B12-binding protein, alkaline and acid phosphatases, radio-iodinated albumin and myeloma proteins. J Clin Invest. 1958 Feb;37(2):272–284. doi: 10.1172/JCI103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROST H. M., VILLANEUVA A. R., ROTH H. Pyogenic osteomyelitis: diffusion in live and dead bone with particular reference to the tetracycline antibiotics. Henry Ford Hosp Med Bull. 1960 Jun;8:255–262. [PubMed] [Google Scholar]
- GERLINGS-PETERSEN B. T., PONDMAN K. W. Erythrophagocytosis: A study of the antigen-antibody-complement reaction. Vox Sang. 1962 Nov-Dec;7:655–674. doi: 10.1111/j.1423-0410.1962.tb04594.x. [DOI] [PubMed] [Google Scholar]
- Gerlings-Petersen B. T., Pondman K. W. The relationship between complement, the heterogeneity of antibody and phagocytosis. Bibl Haematol. 1965;23:829–833. doi: 10.1159/000384374. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- KELLY P. J., MARTIN W. J., COVENTRY M. B. BACTERIAL ARTHRITIS OF THE HIP IN THE ADULT. J Bone Joint Surg Am. 1965 Jul;47:1005–1018. [PubMed] [Google Scholar]
- Laxdal T., Messner R. P., Williams R. C., Jr, Quie P. G. Opsonic, agglutinating, and complement-fixing antibodies in patients with subacute bacterial endocarditis. J Lab Clin Med. 1968 Apr;71(4):638–653. [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J. A new supporting medium for preparative electrophoresis. Scand J Clin Lab Invest. 1960;12:33–37. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Laxdal T., Quie P. G., Williams R. C., Jr Rheumatoid factors in subacute bacterial endocarditis--bacterium, duration of disease or genetic predisposition? Ann Intern Med. 1968 Apr;68(4):746–756. doi: 10.7326/0003-4819-68-4-746. [DOI] [PubMed] [Google Scholar]
- OUDIN J. B. Specific precipitation in gels and its application to immunochemical analysis. Methods Med Res. 1952;5:335–378. [PubMed] [Google Scholar]
- Quie P. G., Messner R. P., Williams R. C., Jr Phagocytosis in subacute bacterial endocarditis. Localization of the primary opsonic site to Fc fragment. J Exp Med. 1968 Oct 1;128(4):553–570. doi: 10.1084/jem.128.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS J. B., KENNY K., SUTER E. THE ISOLATION AND BIOLOGICAL ACTIVITIES OF RABBIT GAMMA M- AND GAMMA G-ANTI-SALMONELLA TYPHIMURIUM ANTIBODIES. J Exp Med. 1965 Aug 1;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Quie P. G. Studies of human C-reactive protein in an in vitro phagocytic system. J Immunol. 1968 Sep;101(3):426–432. [PubMed] [Google Scholar]
- Wollheim F. A., Williams R. C., Jr Immunoglobulin studies in six kindreds of patients with adult hypogammaglobulinemia. J Lab Clin Med. 1965 Sep;66(3):433–445. [PubMed] [Google Scholar]


