Abstract
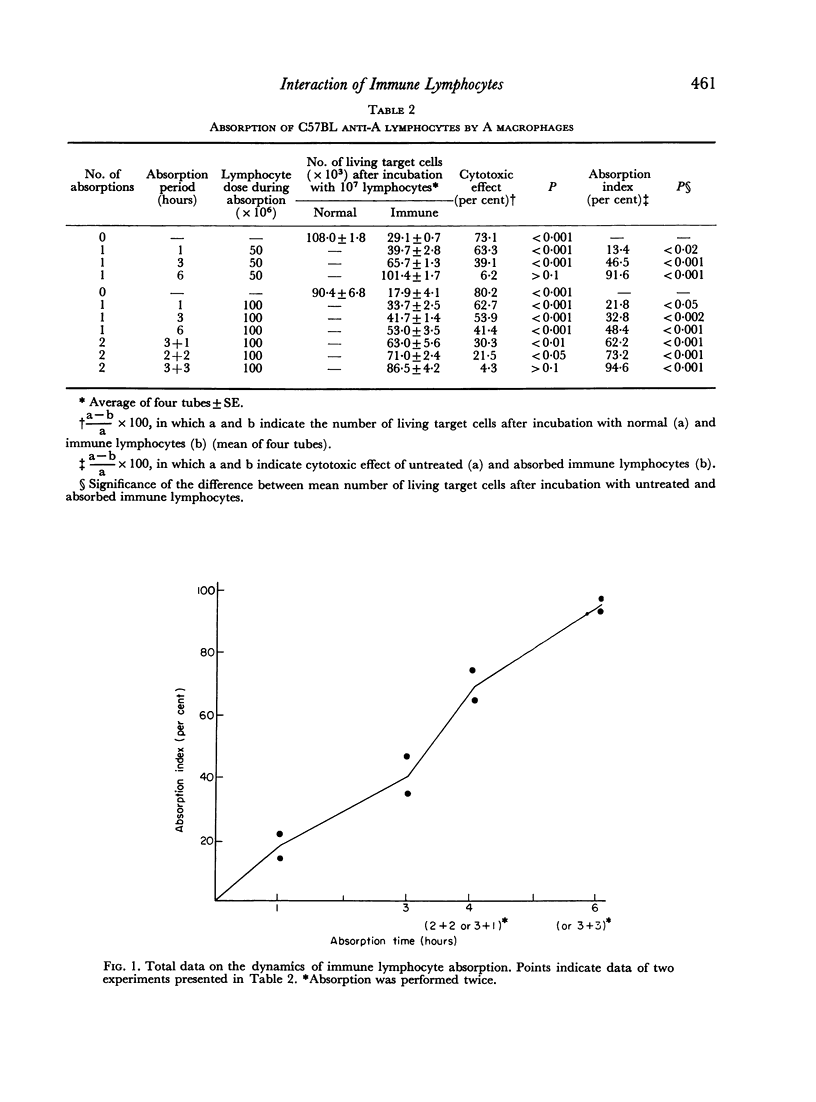
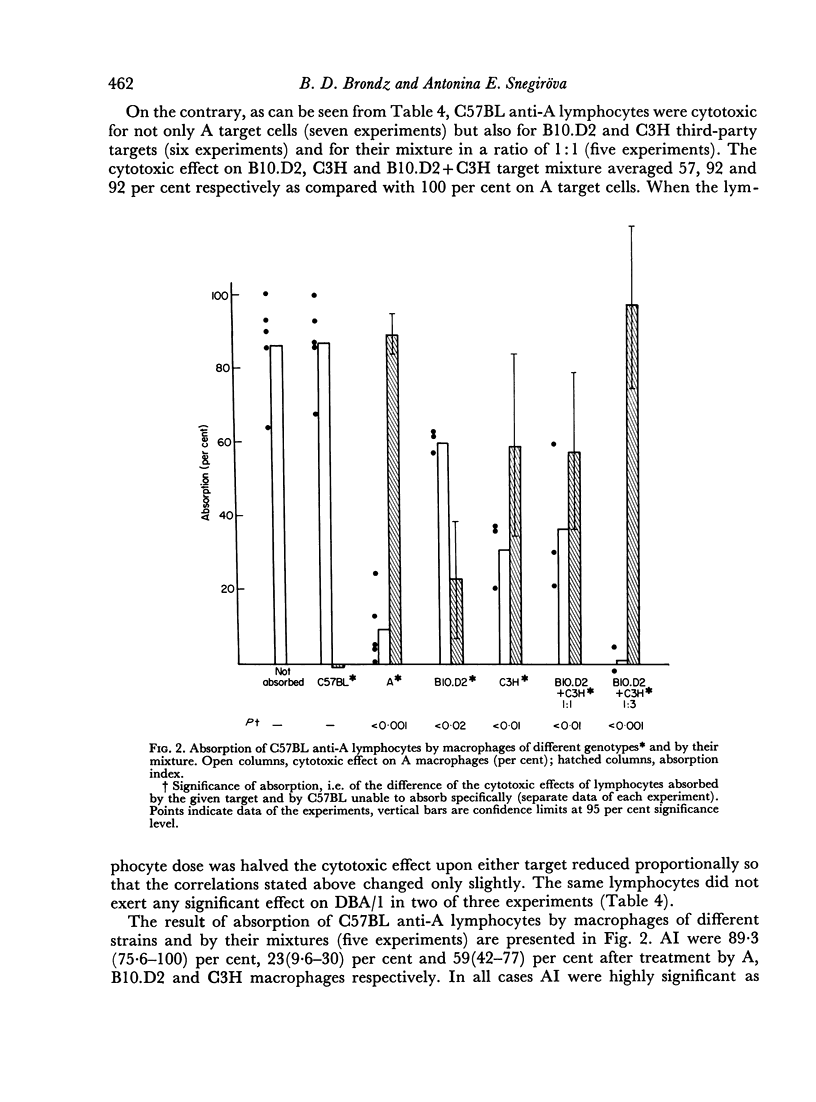
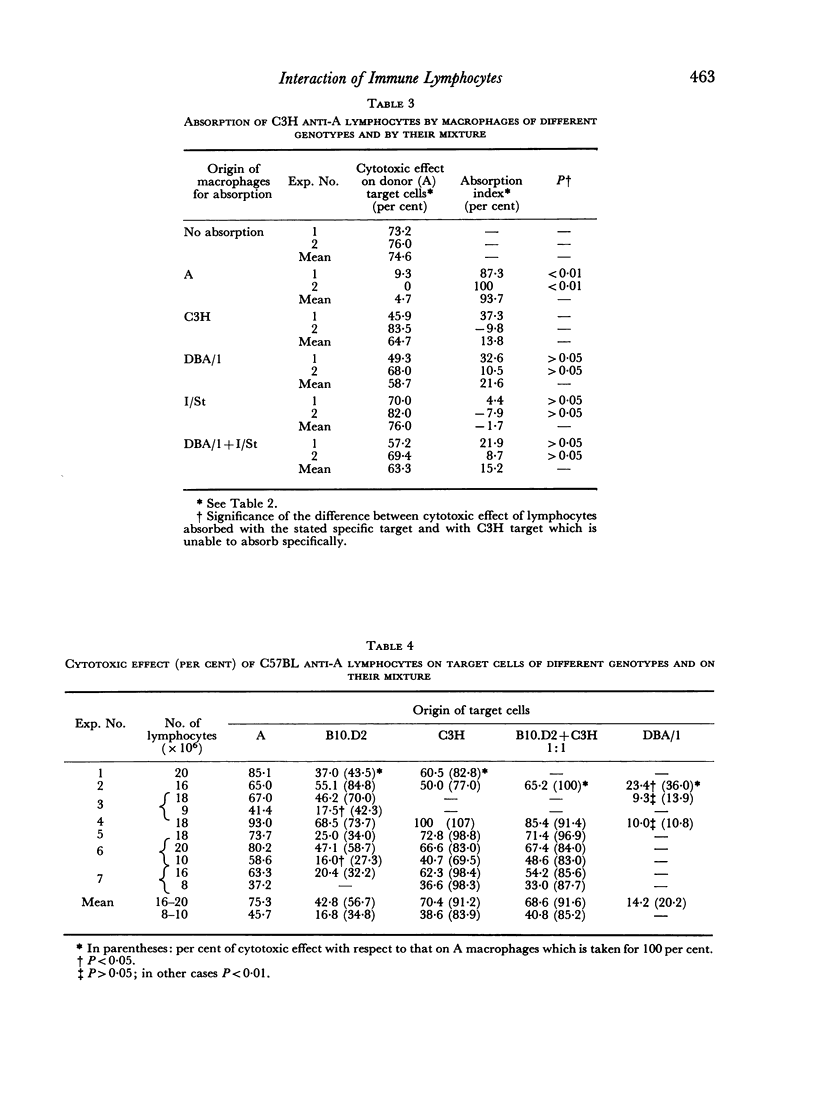
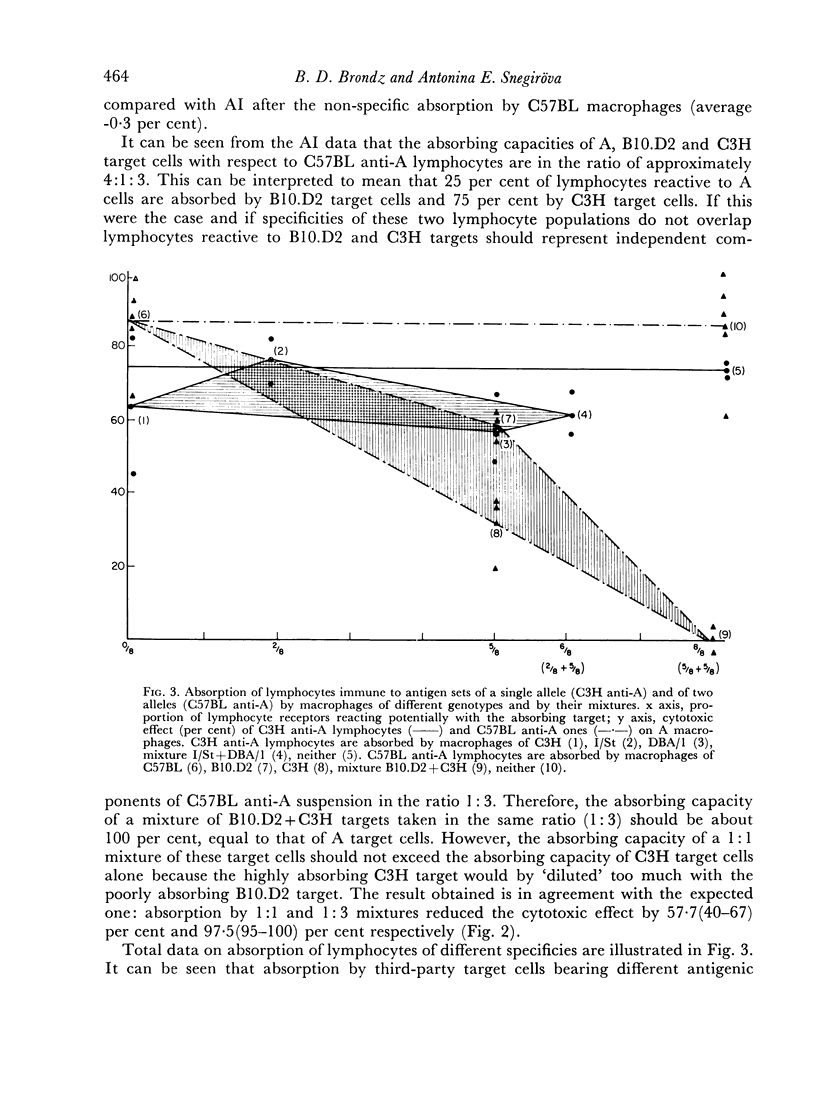
Optimum conditions have been found for a specific quantitative absorption of mouse lymphocytes on to allogeneic target cells. Using this technique, it has been established that C3H anti-A lymphocytes immune to the specificities of a single H-2 sub-locus (D) were absorbed neither on cells bearing only some of the components of the immunizing complex (DBA/1 and I/St targets) nor on their mixture. Conversely, C57BL anti-A lymphocytes immune to the specificities of two H-2 sub-loci (D and K) reacted separately with each of the components on corresponding third-party targets (B10.D2(H-2d) and C3H (H-2k) as shown both by the direct cytotoxic effect and by the quantitative absorption technique. The results of absorption of these lymphocytes on mixtures of B10.D2 and C3H target cells used in various proportions indicate that C57BL anti-A lymphocytes represent a mixture of two `polyvalent' populations in ratio of 1:3. This, together with the previous data indicates that `committed' lymphocytes may be `polyvalent' and that the initial recognition step of H-2 antigens may result from a direct contact between membranes of grafted cells and `unprimed' host lymphocytes. Structural matching of the membrane antigenic complex and of normal and immune lymphocytes is suggested as a decisive factor of immunological recognition initiation in a transplantation context.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Richter M. Cells involved in the immune response. XI. Identification of the antigen-reactive cell as the tolerant cell in the immunologically tolerant rabbit. J Exp Med. 1969 Jul 1;130(1):165–184. doi: 10.1084/jem.130.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRONDZ B. D. INTERACTION OF IMMUNE LYMPHOCYTES IN VITRO WITH NORMAL AND NEOPLASTIC TISSUE CELLS. Folia Biol (Praha) 1964;10:164–176. [PubMed] [Google Scholar]
- Birbeck M. S., Hall J. G. Transformation, in vivo, of basophilic lymph cells into plasma cells. Nature. 1967 Apr 8;214(5084):183–185. doi: 10.1038/214183a0. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Old L. J., Stockert E. An approach to the mapping of antigens on the cell surface. Proc Natl Acad Sci U S A. 1968 Jul;60(3):886–893. doi: 10.1073/pnas.60.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondz B. D. Complex specificity of immune lymphocytes in allogeneic cell cultures. Folia Biol (Praha) 1968;14(2):115–131. [PubMed] [Google Scholar]
- Brondz B. D., Golberg N. E. Further in vitro evidence for polyvalent specificity of immune lymphocytes. Folia Biol (Praha) 1970;16(1):20–28. [PubMed] [Google Scholar]
- COWLING D. C., QUAGLINO D. EFFECT OF SOME ANTIGENS ON LEUCOCYTE CULTURES. J Pathol Bacteriol. 1965 Jan;89:63–70. doi: 10.1002/path.1700890108. [DOI] [PubMed] [Google Scholar]
- Caron G. A. The effect of antigens in combination on lymphocyte transformation in vitro. Int Arch Allergy Appl Immunol. 1967;31(6):521–528. doi: 10.1159/000229897. [DOI] [PubMed] [Google Scholar]
- David J. R. Macrophage migration. Fed Proc. 1968 Jan-Feb;27(1):6–12. [PubMed] [Google Scholar]
- Davies D. A. The molecular individuality of different mouse H-2 histocompatibility specificities determined by single genotypes. Transplantation. 1969 Jul;8(1):51–70. doi: 10.1097/00007890-196907000-00007. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., McGREGOR D. D., COWEN D. M. Initiation of immune responses by small lymphocytes. Nature. 1962 Nov 17;196:651–655. doi: 10.1038/196651a0. [DOI] [PubMed] [Google Scholar]
- Henney C. S. The specificity of the early immune response. I. A comparison of the specificity of delayed hypersensitivity and humoral responses following immunization with human gammaG globulin. J Immunol. 1969 Sep;103(3):519–527. [PubMed] [Google Scholar]
- MEDAWAR P. B. The homograft reaction. Proc R Soc Lond B Biol Sci. 1958 Dec 4;149(935):145–166. doi: 10.1098/rspb.1958.0058. [DOI] [PubMed] [Google Scholar]
- Macher E., Chase M. W. Studies on the sensitization of animals with simple chemical compounds. XI. The fate of labeled picryl chloride and dinitrochlorobenzene after sensitizing injections. J Exp Med. 1969 Jan 1;129(1):81–102. doi: 10.1084/jem.129.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OORT J., TURK J. L. A HISTOLOGICAL AND AUTORADIOGRAPHIC STUDY OF LYMPH NODES DURING THE DEVELOPMENT OF CONTACT SENSITIVITY IN THE GUINEA-PIG. Br J Exp Pathol. 1965 Apr;46:147–154. [PMC free article] [PubMed] [Google Scholar]
- ROSENAU W., MOON H. D. Lysis of homologous cells by sensitized lymphocytes in tissue culture. J Natl Cancer Inst. 1961 Aug;27:471–483. [PubMed] [Google Scholar]
- SNELL G. D., HOECKER G., AMOS D. B., STIMPFLING J. H. A REVISED NOMENCLATURE FOR THE HISTOCOMPATIBILITY-2 LOCUS OF THE MOUSE. Transplantation. 1964 Nov;2:777–784. doi: 10.1097/00007890-196411000-00010. [DOI] [PubMed] [Google Scholar]
- STROBER S., GOWANS J. L. THE ROLE OF LYMPHOCYTES IN THE SENSITIZATION OF RATS TO RENAL HOMOGRAFTS. J Exp Med. 1965 Aug 1;122:347–360. doi: 10.1084/jem.122.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman S. F., Levine H. Immunochemical studies on delayed and arthus-type hypersensitivity reactions. I. The relationship between antigenic determinant size and antibody combining site size. J Immunol. 1967 Feb;98(2):211–219. [PubMed] [Google Scholar]
- Schlossman S. F., Yaron A. Immunochemical studies on the specificity of cellular and antibody-mediated immune reactions. Ann N Y Acad Sci. 1970 Feb 13;169(1):108–115. doi: 10.1111/j.1749-6632.1970.tb55975.x. [DOI] [PubMed] [Google Scholar]
- Sell S. Studies on rabbit lymphocytes in vitro. VI. The induction of blast transformation with sheep antisera to rabbit IgA and IgM. J Exp Med. 1967 Mar 1;125(3):393–400. doi: 10.1084/jem.125.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Takagi N., Yagi Y., Moore G. E., Pressman D. Immunoglobulin production in cloned sublines of a human lymphocytoid cell line. J Immunol. 1969 Jun;102(6):1388–1393. [PubMed] [Google Scholar]
- Taylor R. B. Immune paralysis of thymus cells by bovine serum albumin. Nature. 1968 Nov 9;220(5167):611–611. doi: 10.1038/220611a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Blyth JL NOWELL P. C. Quantitative studies on the mixed lymphocyte interaction in rats. 3. Kinetics of the response. J Exp Med. 1968 Nov 1;128(5):1157–1181. doi: 10.1084/jem.128.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]


