Abstract
A procedure based on the rate of appearance of plaque-forming cells (PFC) in agarose was used to measure the relative rates of antibody synthesis and release by cells making antibody specific for Type III pneumococcal polysaccharide (SSS-III). The rate of antibody synthesis and release by SSS-III-specific PFC was directly related to the immunizing dose employed; maximal values were obtained with mice given an optimally immunogenic dose (0.5 μg) of SSS-III. However, dose-dependent reductions, not only in the magnitude of the antibody response, but also in the rate of antibody synthesis and release by specific PFC, were noted in mice receiving doses greater than 0.5 μg. The latter suggests that a decrease in the rate of antibody synthesis and release by antibody-forming cells may be an initial step in the induction of immunological paralysis by high doses of SSS-III.
Increases in the magnitude of the serum antibody and the PFC response were associated with corresponding increases in the rate of antibody synthesis and release by SSS-III-specific PFC following immunization; this suggests that cell differentiation—rather than proliferation—plays a major rôle in the development of the antibody response to SSS-III. In contrast, the results obtained in similar studies with sheep erythrocytes indicate that cell proliferation influences to a greater degree the magnitude of the antibody response elicited to this antigen.
Full text
PDF

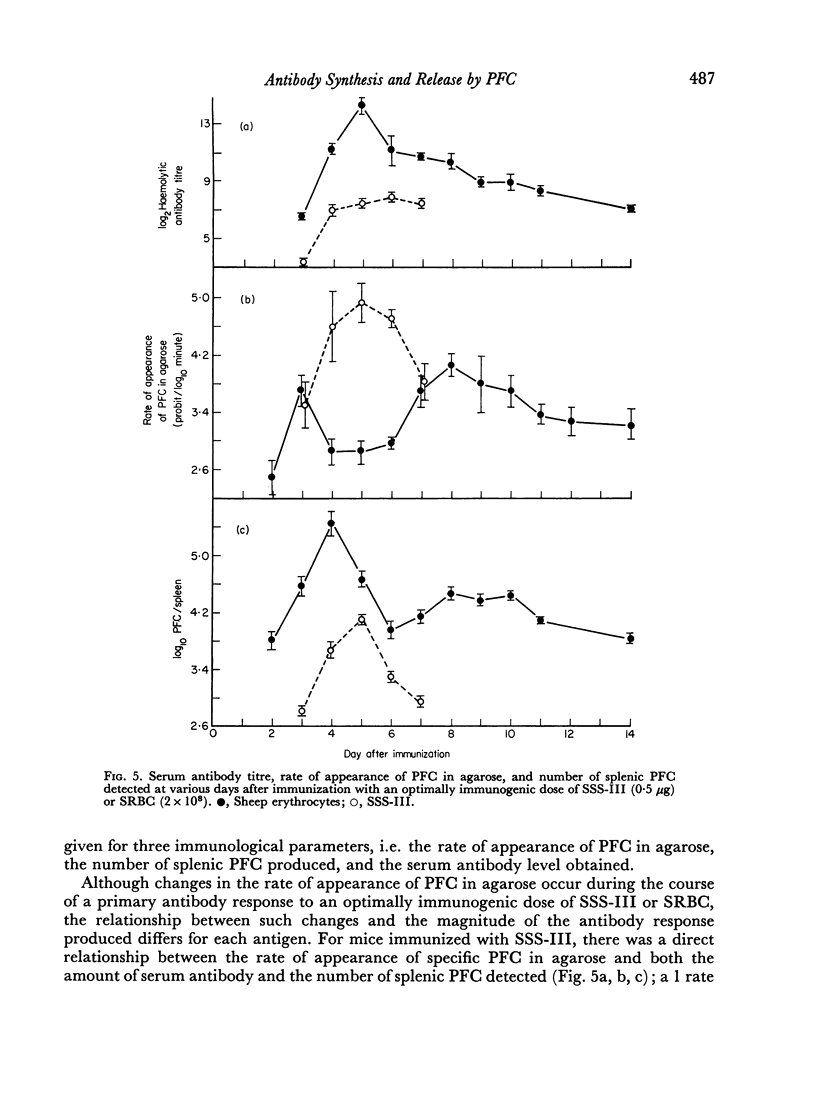
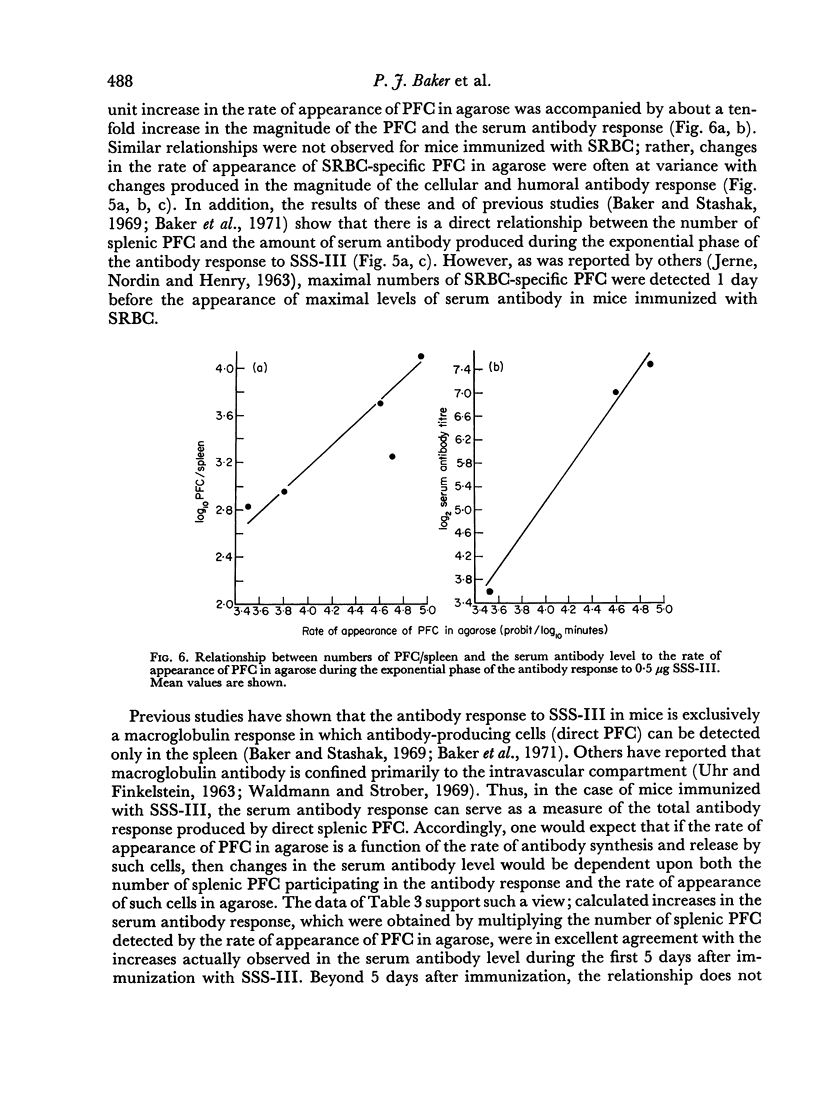




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Bernstein M., Pasanen V., Landy M. Detection and enumeration of antibody-producing cells by specific adherence of antigen-coated bentonite particles. J Immunol. 1966 Dec;97(6):767–777. [PubMed] [Google Scholar]
- Baker P. J., Landy M. Brevity of the inductive phase in the immune response of mice to capsular polysaccharide antigens. J Immunol. 1967 Oct;99(4):687–694. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W., Mishell R. I. Cell populations and cell proliferation in the in vitro response of normal mouse spleen to heterologous erythrocytes. Analysis by the hot pulse technique. J Exp Med. 1967 Sep 1;126(3):443–454. doi: 10.1084/jem.126.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN H. DISTRIBUTION OF ANTIBODY PLAQUE FORMING CELLS IN VARIOUS TISSUES OF SEVERAL STRAINS OF MICE INJECTED WITH SHEEP ERYTHROCYTES. Proc Soc Exp Biol Med. 1964 Nov;117:526–530. doi: 10.3181/00379727-117-29628. [DOI] [PubMed] [Google Scholar]
- Fitch F. W., Roseman J. M., Rowley D. A., Berenbaum M. C. Cell respiration as a requirement for antibody release in vitro. Nature. 1968 Jun 8;218(5145):972–973. doi: 10.1038/218972a0. [DOI] [PubMed] [Google Scholar]
- HELMREICH E., KERN M., EISEN H. N. Observations on the mechanism of secretion of gamma-globulins by isolated lymph node cells. J Biol Chem. 1962 Jun;237:1925–1931. [PubMed] [Google Scholar]
- Howard J. G., Elson J., Christie G. H., Kinsky R. G. Studies on immunological paralysis. II. The detection and significance of antibod-forming cells in the spleen during immunological paralysis with type 3 pneumococcal polysaccharide. Clin Exp Immunol. 1969 Jan;4(1):41–53. [PMC free article] [PubMed] [Google Scholar]
- Koros A. M., Mazur J. M., Mowery M. J. Radioautographic studies of plaque-forming cells. I. Antigen-stimulated proliferation of plaque-forming cells. J Exp Med. 1968 Aug 1;128(2):235–257. doi: 10.1084/jem.128.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEEPER C. A., SEASTONE C. V. MECHANISMS OF IMMUNOLOGIC PARALYSIS BY PNEUMOCOCCAL POLYSACCHARIDE. I. STUDIES OF ADOPTIVELY ACQUIRED IMMUNITY TO PNEUMOCOCCAL INFECTION IN IMMUNOLOGICALLY PARALYZED AND NORMAL MICE. J Immunol. 1963 Sep;91:374–377. [PubMed] [Google Scholar]
- Paul W. E., Benacerraf B., Siskind G. W., Goidl D. A., Reisfeld R. A. The anamnestic antibody response to type 3 specific pneumococcal polysaccharide. J Exp Med. 1969 Jul 1;130(1):77–89. doi: 10.1084/jem.130.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Siskind G. W., Benacerraf B., Ovary Z. Secondary antibody responses in haptenic systems: cell population selection by antigen. J Immunol. 1967 Oct;99(4):760–770. [PubMed] [Google Scholar]
- Plotz P. H., Talal N., Asofsky R. Assignment of direct and facilitated hemolytic plaques in mice to specific immunoglobulin classes. J Immunol. 1968 Apr;100(4):744–751. [PubMed] [Google Scholar]
- Sterzl J., Ríha I. Detection of cells producing 7S antibodies by the plaque technique. Nature. 1965 Nov 27;208(5013):858–859. doi: 10.1038/208858a0. [DOI] [PubMed] [Google Scholar]
- Storb U., Chiller J. M., Weiser R. S. Influence of metabolic inhibitors on the capacity of spleen cells to form hemolytic plaques and rosettes. Proc Soc Exp Biol Med. 1969 Jul;131(3):835–840. doi: 10.3181/00379727-131-33989. [DOI] [PubMed] [Google Scholar]
- Strander H. Studies on cells producing haemolytic antibodies against sheep red blood cells in short-term experiments in vitro. Immunology. 1966 Jan;10(1):45–55. [PMC free article] [PubMed] [Google Scholar]
- UHR J. W., FINKELSTEIN M. S. Antibody formation. IV. Formation of rapidly and slowly sedimenting antibodies and immunological memory to bacteriophage phi-X 174. J Exp Med. 1963 Mar 1;117:457–477. doi: 10.1084/jem.117.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]
- Wortis H. H., Dresser D. W., Anderson H. R. Antibody production studied by means of the localized haemolysis in gel (LHG) assay. 3. Mouse cells producing five different classes of antibody. Immunology. 1969 Jul;17(1):93–110. [PMC free article] [PubMed] [Google Scholar]


