Abstract
Mammalian bladder epithelium functions as an effective permeability barrier. We demonstrate here that this epithelium can also function as a secretory tissue directly involved in modifying urinary protein composition. Our data indicate that normal bovine urothelium synthesizes, as its major differentiation products, two well-known proteases: tissue-type plasminogen activator and urokinase, as well as a serine protease inhibitor, PP5. Moreover, we demonstrate that the urothelium secretes these proteins in a polarized fashion into the urine via a cAMP- and calcium-regulated pathway. Urinary plasminogen activators of ruminants are therefore urothelium derived rather then kidney derived as in some other species; this heterogeneity may have evolved in response to different physiological or dietary factors. In conjunction with our recent finding that transgenic mouse urothelium can secrete ectopically expressed human growth hormone into the urine, our data establish that normal mammalian urothelium can function not only as a permeability barrier but also as a secretor of urinary proteins that can play physiological or pathological roles in the urinary tract.
Mammalian urothelium lines the lower urinary tract and has evolved into a highly effective permeability barrier that can maintain steep chemical gradients (1). Thus, the permeability of urothelium to water, urea, ammonia, and protons is among the lowest in biological membranes (2). To perform this barrier function, urothelium makes extensive tight junctions and elaborates a highly specialized apical surface, which is almost completely covered with 0.2–0.5-μm rigid-appearing plaques (1, 3). These urothelial plaques consist of two-dimensional crystalline, hexagonal arrays of 16-nm particles composed of uroplakins, which represent major differentiation products of mammalian urothelium (4–9). Consistent with such a highly structured apical surface, which may seem incompatible with a secretory function, normal urothelium is not known to affect the composition of urine proteins, which are synthesized either by the liver (e.g., the major urinary protein complex of rodents), gaining access to the urine by glomerular filtration (10), or by the kidneys [uromodulin and epidermal growth factor (11, 12)]. We show here, however, that normal bovine urothelium serves as the main source of a major class of soluble, urine proteins including urokinase, tissue-type plasminogen activator, and a potent serine protease inhibitor, PP5. In conjunction with our recent finding that transgenic mouse urothelium can secrete human growth hormone into the urine (13), these results change our concept about the function of mammalian urothelium, which must now be considered not just as a permeability barrier but also as a possible source of urine proteins that may play important physiological or pathological roles in lower urinary tract.
Materials and Methods
Subtractive cDNA Library.
Bovine urothelial cDNAs were used as the “tester” (20 ng), whereas those from nonurothelial tissues were used as the “driver” (600 ng) to generate a urothelium-specific cDNA library by suppression-subtractive hybridization (14) by using a subtractive kit (CLONTECH). Southern blot was done by separating the PCR products on a 2% agarose gel, transferring the DNA to a nylon membrane, and hybridizing with a 32P-labeled cDNA probe. Northern blot was done by using 20 μg of total RNA. Probes were labeled with the Multiprime DNA-labeling system (Amersham Pharmacia). The PCR-amplified cDNA products of the subtractive library were packaged into PCRII vector by using a T/A cloning kit (Invitrogen) and were cloned. Plasmid DNA was isolated by using QIAprep spin miniprep kit (Qiagen, Chatsworth, CA). DNA sequencing was performed by dideoxynucleotide chain termination method (15) by using a T7 DNA sequence kit (Amersham Pharmacia).
Antibodies and Other Reagents.
The sources of antibodies and other reagents are as follows: a rabbit antiserum to total uroplakins (4); mouse monoclonal antibodies AE1 and AE3 to keratins (16); a 52-kDa human high molecular mass urokinase (uPA), a 65-kDa 2-chain human tissue-type plasminogen activator (tPA), a rabbit antiserum to mouse uPA, and mouse monoclonal antibodies to human tPA and uPA (American Diagnostica, Greenwich, CT); a rabbit antiserum to rat PP5 (a generous gift from W. Kisiel of the University of New Mexico); affinity-purified horseradish peroxidase-conjugated goat antibodies to mouse and rabbit IgG (ICN); and bovine plasminogen, thrombin, fibrinogen, and brefeldin A (Sigma).
Immunohistochemistry.
Tissue sections were incubated sequentially with 1% hydrogen peroxide in methanol to block the endogenous peroxidase, 2% goat serum, primary antibodies in 2% goat serum at 4°C overnight, and finally specific horseradish peroxidase-conjugated secondary antibodies. The sections were counterstained with hematoxylin, mounted in glycerin gelatin, and observed with a Zeiss microscope.
Cell and Organ Culture of Bovine Urothelium.
Bovine urothelial cells were cultured at 37°C in the presence of mitomycin C-treated NIH 3T3 feeder cells in DMEM containing 15% FCS/0.5 μg/ml hydrocortisone/5 ng/ml cholera toxin/5 μg/ml insulin/15 ng/ml epidermal growth factor (17). 3T3 feeder cells and any contaminating fibroblasts were removed by using 0.01% EDTA in PBS when urothelial cells reached ≈70% confluence. Conditioned media were prepared by incubating the cells with fresh DMEM (without serum) for 24 h, collecting the medium, and centrifuging it at 3,000 × g for 10 min to remove cell debris. Total cell lysates were prepared by dissolving the cells in a solution containing 0.1 M Tris·HCl (pH 7.4), 0.1% SDS, 0.1% Nonidet P-40. In some experiments, cells (5 days postconfluent and incubated overnight with growth medium without cholera toxin) were washed twice with a release buffer (10 mM Hepes, pH 7.0/150 mM NaCl/5 mM KCl) and incubated at 37°C for 30 min in the same buffer with or without 1.0 mM 8-BrcAMP or 1.0 mM calcium ionophore A23187. For assessment of polarized secretion, urothelial cells were plated in a (6-well) Transwell plate on 24-mm Nucleopore filters (0.4-mm pore size; Costar) that had been soaked in a serum-containing medium for 1 h. The cells were incubated with a serum-free medium for 24 h before the media were collected from the apical and basal compartments of the Transwell plate.
For organ culture, bladder mucosa was peeled from a fresh bovine bladder. The isolated mucosa consisting of the intact urothelium and some underlying stromal layers (total thickness ≈2 mm; surface area ≈5 × 5 cm) was rinsed with DMEM and sandwiched, urothelial side up, between two plastic (24-well) plates specially designed so that both the urothelial surface and the underlying mesenchymal tissue can be completely soaked in a serum-free DMEM medium.
Immunoblot and Fibrin Zymography.
Proteins were separated by SDS/PAGE on a 10% polyacrylamide gel (acrylamide/bisacrylamide ratio, 120:1) and electrophoretically transferred to nitrocellulose membrane. After incubation with 2% nonfat milk in PBS, the membrane was incubated with primary and horseradish peroxidase-conjugated secondary antibodies, treated with an enhanced chemiluminescence kit (Pierce), and exposed to Fuji x-ray film.
For zymography, an SDS gel was rinsed with 2.5% Triton X-100 (2 × 30 min) and PBS (10 min), laid carefully onto a fibrin–agarose indicator gel, sealed with plastic wrap, and incubated at 37°C. For in situ zymography, 0.5 ml of indicator gel solution was put on a 10-μm (unfixed and air-dried) frozen tissue section and immediately covered by a 25 × 25-mm glass coverslip. The section was incubated at 37°C in a humid chamber. In some experiments, the sections were preincubated with antibodies against tPA and/or uPA at room temperature for 30 min and washed with PBS 2 × 5 min before applying the indicator gel (18, 19). The fibrin–agarose indicator gel contained 5 μg/ml bovine plasminogen, 0.06 units/ml bovine thrombin, 1.8 mg/ml bovine fibrinogen, 3.2% nonfat milk, 1% agarose (20).
Results and Discussion
uPA and tPA as Major Differentiation Products of Bovine Urothelium.
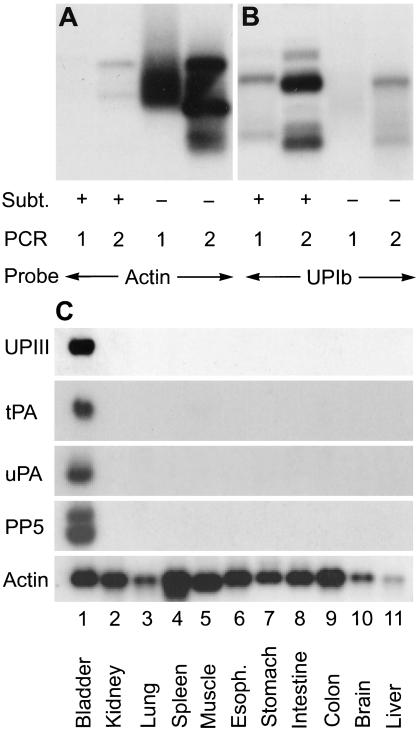
To study the differentiation process of bladder epithelium, we generated a subtraction cDNA library of normal bovine urothelium by suppression subtractive hybridization (14). Common messages were eliminated by hybridizing the cDNA of bovine urothelium with those of 10 other tissues including kidney, lung, spleen, skeletal muscle, esophagus, stomach, intestine, colon, brain, and liver. This resulted in an estimated >1,000-fold enrichment of urothelial-specific cDNA, as evidenced by a >10-fold increase in the cDNA of uroplakin Ib, a well-established urothelial marker (7), and a >100-fold reduction in actin cDNA (Fig. 1 A and B). The most abundant cDNAs in this enriched library encoded uroplakins (32 of a total of 140 clones), thus validating the effectiveness of the subtraction technique. The next four most abundant cDNAs encoded PP5, a Kunitz-type serine protease inhibitor that can inhibit plasmin (27 clones) (21, 22); keratin 19, which is one of the major urothelial keratins (10 clones) (17, 23); tPA (9 clones); and uPA (6 clones). Northern blotting showed that all four genes were, like uroplakin genes, so highly expressed in bovine urothelium that they seemed urothelium “specific” (Fig. 1C and data not shown).
Figure 1.
Enrichment of urothelium-specific cDNAs in a bovine urothelial subtraction library. Common cDNAs in bovine urothelium were eliminated by suppression subtractive hybridization (14). (A and B) Southern blot analyses of the subtraction products. The PstI-digested urothelial cDNA fragments before (−) or after (+) subtraction, which were PCR amplified once (marked 1) or twice (marked 2), were electrophoretically separated on an agarose gel and probed for (A) actin or (B) uroplakin Ib, a urothelial marker (7). Scanning of the autoradiograms showed a >100-fold reduction in actin and >10-fold enrichment in uroplakin cDNAs. (C) Northern blot analyses. Twenty micrograms of total RNAs from bovine urothelium and other tissues were fractionated by agarose gel electrophoresis and probed for uroplakin III, tPA, uPA, PP5 protease inhibitor, and actin (control).
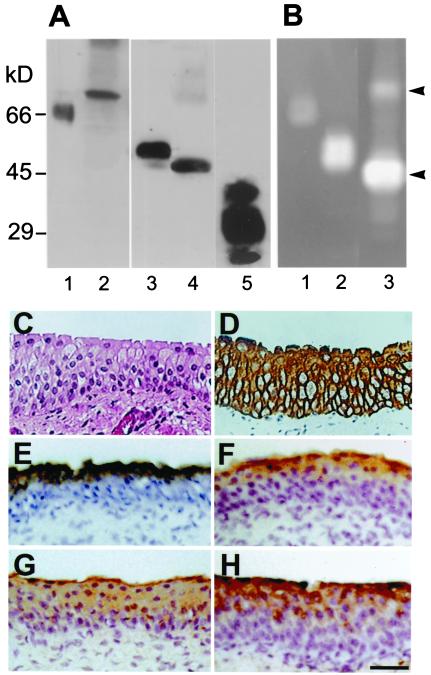
Given the current thought that tPA and uPA are synthesized and secreted into the urine mainly by the kidneys (24, 25), we were surprised to find that genes encoding plasminogen activators (PAs) and the PP5 protease inhibitor were so highly expressed in normal bovine urothelium. Immunoblot analysis confirmed, however, the presence of tPA (68 kDa), uPA (48 kDa), and PP5 (31 kDa) in the total protein extracts of (scraped) in vivo bovine urothelium (Fig. 2A). The two PAs were enzymatically active, as evidenced by their ability to activate plasminogen in zymography (20) (Fig. 2B). Immunolocalization showed that uPA and PP5 were associated with the upper cell layers (Fig. 2 G and H), whereas tPA was enriched in the superficial umbrella cells (Fig. 2F).
Figure 2.
Detection of PAs and PP5 in bovine urothelium. (A) Immunoblot analysis. Total bovine urothelial proteins (10 μg; lanes 2, 4, and 5), recombinant human (rh) tPA (66 kDa, 0.2 μg; lane 1), or rh-uPA (52 kDa, 0.2 μg; lane 3) produced in bacteria were separated by SDS/PAGE and immunoblotted with antibodies against tPA (1 and 2), uPA (3 and 4), and PP5 (5). Note the detection of bovine tPA (68 kDa), uPA (48 kDa), and PP5 (27, 31, and 35 kDa) in bovine urothelial extracts; the three PP5 bands reflect different degrees of glycosylation (ref. 50 and data not shown). The sizes of the human and bovine PAs are known to be slightly different. (B) Detection of fibrinolytic activities of rh-tPA (lane 1, 0.1 μg), rh-uPA (land 2, 0.1 μg), and a total bovine urothelial extract (lane 3, 10 μg) by zymography. The SDS gel was overlaid with a fibrin-containing indicator gel, incubated at 37°C for 1–3 h, followed by Coomassie blue staining of the indicator gel. Note the bovine tPA (68-kDa) and uPA (48-kDa) bands (arrowheads). (C–H) Immunolocalization of the PAs and PP5. Paraffin sections (5 μm) of bovine bladder were stained with hematoxylin and eosin (C), AE1 and AE3 antibodies to keratins (D) (16, 51), rabbit antitotal uroplakins (E) (4), mouse anti-tPA (F), rabbit anti-uPA (G), and rabbit anti-PP5 (H) (50). Note the staining of superficial cells by anti-tPA and anti-PP5 and the suprabasal cells by anti-uPA. (C–H) Same magnification. (Bar = 50 μm.)
Urothelial Secretion of Proteases and Inhibitor.
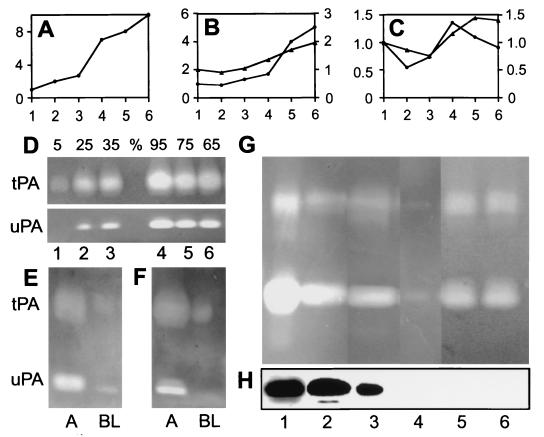
To examine in more detail the synthesis and secretion of PAs, we cultured bovine urothelial cells in the presence of 3T3 feeder cells. Under this condition, bovine urothelial cells formed stratified colonies and expressed uroplakins in the suprabasal cells (Fig. 3A; refs. 17 and 26). These cells also expressed tPA (Fig. 3B) and uPA (Fig. 3C) in a differentiation-dependent manner, consistent with the immunolocalization data (Fig. 2; see ref. 27). Although only ≈5% of the PA activities were secreted into the culture medium in 30 min, this secretion could be rapidly up-regulated to 25–35% by 8-Br-cAMP or calcium ionophore A23187 (Fig. 3D; also see E and F). This result is consistent with an earlier finding that tPA secretion by cultured human endothelial cells can be stimulated by cAMP (28, 29) and suggests that the secretion of PA by urothelium occurs via a regulated pathway, which may enable the enzymes to be acutely released in response to certain physiological stimuli such as wounding. But for this secretion to be functional in the urine, it must occur apically. Previous studies indicate that the polarity of PA secretion by epithelia is cell type dependent. Thus, human intestinal epithelial CaCO2 cells and HeLa cells secrete uPA basolaterally; canine kidney MDCK cells secrete uPA apically; and rat FRT thyroid cells secrete tPA apically (30, 31). That urothelial cells secreted both tPA and uPA in a polarized fashion mainly via their apical surface was demonstrated by the fact that >90% of the extracellular PAs were found in the apical compartment when the cells were grown on a Nucleopore filter in a dual compartment culture system (Fig. 3 E and F).
Figure 3.
Differentiation-dependent expression and apical secretion of plasminogen activators by cell- and organ-cultured bovine urothelium. (A–C) mRNA levels (relative to glyceraldehyde-3-phosphate dehydrogenase as assessed by Northern blot, ●) and PA activities (zymography, ▴) of uroplakin III (A), tPA (B), and uPA (C) in cultured bovine urothelial cells (17). Cells were harvested at 25% confluence (lane 1), 50% (lane 2), 100% (lane 3), 3 days postconfluence (pc) (lane 4), 6 days pc (lane 5), and 9 days pc (lane 6). Loading was normalized on the basis of cell numbers. Note the differentiation-dependent expression of UPIII and tPA and, less strikingly, of uPA. (D) Stimulation of PA secretion. Cultured bovine urothelial cells were treated with a control medium (lanes 1 and 4), 1 μM calcium ionophore A23187 (lanes 2 and 5), and 1 mM 8-Br-cAMP (lanes 3 and 6). Numbers above denote the percentages of PA activities in conditioned media (lanes 1–3) vs. cell lysates (lanes 4–6). (E) Polarized secretion of PAs. Bovine urothelial cells were cultured on a Nucleopore filter (Transwell, 0.4-mm pore size; Costar). A and BL denote the apical and basal-lateral compartments, respectively. The media were collected after 24 h in a control medium (E) or, after 15 min, in the presence of 1 mM of cAMP (F). Note that a great majority of the PAs were secreted apically and that the polarity was not affected by cAMP stimulation. (G) Secretion of tPA and uPA by organ-cultured bovine bladder mucosa into the overlying culture medium. Samples were 20 μg of total protein extract of in vivo bovine urothelium (lane 1), 15 μl of fresh bovine urine (lane 2); 20 μl of a medium that had been conditioned by organ-cultured bovine urothelium for 3 h (lane 3); 20 μl of medium conditioned in the presence of 5 μg/ml brefeldin A, a secretory inhibitor (lane 4) (32); 5 μg of total protein extract of 5-day postconfluent cell-cultured bovine urothelium (lane 5); and 20 μl of the culture medium that had been conditioned for 24 h by 5-day postconfluent cells (lane 6). Note the secretion of tPA and uPA by organ-cultured urothelium and its inhibition by BFA. (H) Detection of PP5 in the same samples as in G by immunoblotting. Note the detection of PP5 in urothelium, fresh urine, and in a medium conditioned by organ-cultured bovine urothelium; also note the absence of PP5 in cultured urothelial cells.
Because the physiological state of cultured cells can be very different from the in vivo tissues, we studied the secretion of PAs by normal bovine urothelium maintained in short-term organ culture. A piece of fresh bladder mucosa was sandwiched between two plastic plates with holes containing tissue culture media. A tPA/uPA activity ratio of ≈1/8 was observed in bovine urine (Fig. 3G, lane 2); a similar ratio was observed in normal bovine urothelial extract (lane 1) as well as in a medium that had been conditioned by organ-cultured bovine urothelium (lane 3). This apical secretion of the PAs was blocked by brefeldin A, an inhibitor of the secretory process (lane 4) (32), proving that the PAs in the culture medium were actively secreted rather than leaked from lysed cells. The PA concentrations in the cell and organ culture-conditioned media (50–200 μg of tPA and 100–150 μg of uPA/liter) were roughly equivalent to those of bovine urine (≈250 μg of tPA and ≈500 μg of uPA/liter), suggesting that urothelial secretion can account for most of the urinary PAs (Table 1). Finally, we showed that PP5, which was present in the urine (Fig. 3H, lane 2), was also secreted by organ-cultured urothelium into the medium (Fig. 3H, lane 3).
Table 1.
Concentrations of plasminogen activators in bovine urine, urothelia-conditioned media, and urothelial cells, as calculated from the zymography data
| Urine, μg/liter | Conditioned media,
μg/liter*
|
Urothelium, μg/mg cellular
protein
|
|||
|---|---|---|---|---|---|
| Organ culture | Cell culture | In vivo | Cell culture | ||
| uPA | 500† | 100 | 150 | 10 | 8 |
| tPA | 250 | 50 | 200 | 5 | 15 |
Because bovine bladder has an average surface area of ≅200 cm2, in contact with ≅5 liters of urine per day, these numbers were adjusted to be equivalent to media exposed to cultured urothelial cells at 37°C for 24 h, at a ratio of 25 ml/cm2 surface area of cells.
The synthesis of PAs and PP5 by cultured urothelial cells differed significantly from the organ-cultured urothelium. Thus cultured urothelial cells synthesized and secreted relatively more tPA (with a tPA/uPA activity ratio of 1:3 vs. 1:8 in organ culture). Such an altered tPA/uPA ratio may be caused by different tissue culture conditions and the accompanied changes in the differentiation state of the urothelial cells. In addition, the cultured urothelial cells completely suppressed the synthesis of PP5 (Fig. 3H, lanes 5 and 6). Because, as we suggested earlier (17), cultured urothelial cells mimic a regenerative urothelium, the down-regulation of the protease inhibitor in such a “healing” urothelium may lead to the enhancement of the proteolytic activities that are frequently associated with in vivo wound healing and tissue remodeling.
Species Variation in the Site of PA Synthesis.
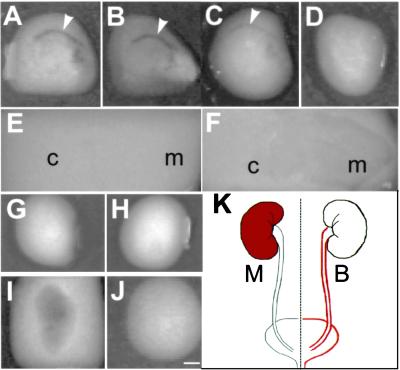
To determine the distribution of PAs in the bovine urinary tract, we performed in situ zymography by using unfixed cryo-tissue sections. As expected, bovine urothelium showed a high fibrinolytic activity as evidenced by its ability to produce a clear zone in an opaque fibrin-containing indicator gel (Fig. 4A); this activity could be blocked by a combination of antibodies to tPA and uPA (Fig. 4 B–D), indicating that these two enzymes could account for most of the detected fibrinolysis. Very little fibrinolytic activity was detected in bovine kidney sections even after prolonged incubation (Fig. 4 E and F). Similar results were obtained with the bladder and kidney tissues of sheep, another ruminant (data not shown). This situation is quite different from those of the mouse and human urinary tracts. Mouse urothelium had a low fibrinolytic activity (Fig. 4 G and H), whereas its kidneys had very high activities (Fig. 4 I and J), consistent with previous in situ hybridization data (33–35). Similarly, the reported PA content of normal human urothelium [60 ng tPA/mg and 2 ng uPA/mg total urothelial proteins (27, 36)] was several hundred-fold lower than that of normal bovine urothelium (Table 1). Together, these results indicate that although mouse and human urinary PAs are mainly kidney derived (Fig. 4 G–J; refs. 24 and 33), ruminant PAs are primarily urothelium-derived (Fig. 4 A–F; summarized in Fig. 4K).
Figure 4.
Distribution of plasminogen activator activities in bovine vs. mouse urinary tract. Frozen tissue sections (10 μm) were air dried and preincubated at 37°C for 30 min with PBS (A), anti-tPA (B), anti-uPA (C), and a mixture of anti-tPA and anti-uPA (D) before they were overlaid with an (opaque) fibrin-containing indicator gel and incubated at 37°C for 30 min. Fibrinolysis by plasminogen activator results in a clear zone. (A–F) Sections of bovine bladder (A–D) or kidney (E, F). Note the strong fibrinolytic activities in the urothelial zone (arrowheads) and their complete inhibition by a mixture of anti-tPA and anti-uPA. The bovine kidney sections were incubated with the indicator gel for a prolonged period of 3 h (E) and 8 h (F) to show the barely detectable PA activities. (G–J) Sections of mouse bladder (G and H) or kidney (I and J) that were overlaid with indicator gels with (G and I) or without (H and J) plasminogen. Note the strong fibrinolytic activities in mouse kidney (I) and their absence in mouse bladder (G). (K) Site of PA production (marked red) in the urinary tracts of mouse (M) and bovine (B). c, cortex; m, medulla. All pictures are of the same magnification. (Bar = 5 mm.)
Possible Functions of Urinary PAs.
Thus, bovine urothelium synthesizes and secretes into the urine large amounts of uPA and tPA as well as, importantly, a potent protease inhibitor: PP5. Also known as tissue factor pathway inhibitor-2, PP5 is a serine protease inhibitor consisting of three tandemly arranged Kunitz-type domains (21). PP5 can directly or indirectly inhibit kallikrein, plasmin, and PAs (22). The coexistence of PAs with PP5 in the urine suggests that their activities must be tightly regulated. That urine of all animal species examined contained significant amounts of fibrinolytic enzymes (Fig. 3; refs. 20 and 37) strongly suggests that these enzymes play important functional role(s) in the urinary tract. These roles have not yet been clearly defined, but they may include the following. First, they may be involved in extracellular proteolysis to prevent or circumvent the obstruction of the urinary tract because of protein precipitation or fibrin clot formation (33). Second, urinary stone formation or urolithiasis may involve the precipitation of a matrix of mucoproteins followed by crystallization of minerals onto this matrix. Urinary proteases may prevent the formation of protein nucleation/matrix (38). Third, urothelial desquamation is an important defense mechanism against bacterial attachment (39, 40), and PAs and their inhibitors seem to play a role in regulating desquamation (41). Fourth, PAs and their inhibitors may play a role in urothelial migration and tissue remodeling, as has been shown to occur in many cell types (42–44). Finally, urinary PAs and kallikrein, another urinary protease that can activate pro-uPA (45), can digest and inactivate the sodium channels on the urothelial surface, thereby playing a role in regulating sodium transport (46). That ruminant urinary PAs are synthesized by the urothelium, instead of the usual kidneys, suggests that PAs are not needed in the ruminant kidneys, possibly because of different dietary or physiological factors.
Concluding Remarks.
Although mammalian urothelium is thought to function mainly as an exceptionally effective permeability barrier that can withstand repeated stretching, we show here that bovine urothelium can, in addition, serve as a major supplier for a class of soluble urinary proteins. This establishes that bovine urothelium can actively modify the protein composition of the urine; that this polarized secretory process can be regulated by cAMP and calcium; and that urothelium can perhaps serve as a better transgenic “bioreactor” in secreting recombinant human proteins into the urine than previously thought feasible, at least when ruminants are used in such “biofarm” applications (13). That the secretory activity of urothelium is not limited to the ruminants is demonstrated by our recent finding that mouse urothelium ectopically expressing human growth hormone can secrete this protein into the urine (13). Taken together, these results indicate that mammalian urothelia in general can secrete urine proteins that may play physiological or pathological roles in the lower urinary tract (47). Additional data are needed to understand how proteins are secreted through the plaque-covered apical urothelial surface, e.g., whether the secretion occurs selectively at the interplaque or the so-called “hinge” areas (3, 48).
Acknowledgments
We thank Dr. Walter Kisiel of the University of New Mexico for an antiserum to PP5 and Dr. Gert Kreibich of New York University and Dr. Kenneth Fong of CLONTECH laboratories for helpful discussions. This work was supported by National Institutes of Health Grants DK39753, DK52206, DK57269 (to T.-T.S.), and EY06769 (to R.M.L.).
Abbreviations
- tPA
tissue-type plasminogen activator
- uPA
urokinase
- PP5
placenta protein 5
References
- 1.Hicks R M. Biol Rev Camb Philos Soc. 1975;50:215–246. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 2.Negrete H O, Lavelle J P, Berg J, Lewis S A, Zeidel M L. Am J Physiol. 1996;271:F886–F894. doi: 10.1152/ajprenal.1996.271.4.F886. [DOI] [PubMed] [Google Scholar]
- 3.Kachar B, Liang F, Lins U, Ding M, Wu X R, Stoffler D, Aebi U, Sun T-T. J Mol Biol. 1999;285:595–608. doi: 10.1006/jmbi.1998.2304. [DOI] [PubMed] [Google Scholar]
- 4.Wu X R, Manabe M, Yu J, Sun T-T. J Biol Chem. 1990;265:19170–19179. [PubMed] [Google Scholar]
- 5.Wu X R, Sun T-T. J Cell Sci. 1993;106:31–43. doi: 10.1242/jcs.106.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Lin J H, Wu X R, Kreibich G, Sun T-T. J Biol Chem. 1994;269:1775–1784. [PubMed] [Google Scholar]
- 7.Yu J, Lin J H, Wu X R, Sun T-T. J Cell Biol. 1994;125:171–182. doi: 10.1083/jcb.125.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X R, Lin J H, Walz T, Haner M, Yu J, Aebi U, Sun T-T. J Biol Chem. 1994;269:13716–13724. [PubMed] [Google Scholar]
- 9.Sun T-T, Liang F X, Wu X R. Adv Exp Med Biol. 1999;462:7–18. doi: 10.1007/978-1-4615-4737-2_1. [DOI] [PubMed] [Google Scholar]
- 10.Finlayson J S, Asofsky R, Potter M, Runner C C. Science. 1965;149:981–982. doi: 10.1126/science.149.3687.981. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann S, Metzger R, Bunnemann B. Histochemistry. 1990;94:517–523. doi: 10.1007/BF00272616. [DOI] [PubMed] [Google Scholar]
- 12.Lev-Ran A, Hwang D L, Ben-Ezra J, Williams L E. Clin Exp Pharmacol Physiol. 1992;19:667–673. doi: 10.1111/j.1440-1681.1992.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 13.Kerr D E, Liang F, Bondioli K R, Zhao H, Kreibich G, Wall R J, Sun T-T. Nat Biotechnol. 1998;16:75–79. doi: 10.1038/nbt0198-75. [DOI] [PubMed] [Google Scholar]
- 14.Diatchenko L, Lau Y F, Campbell A P, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov E D, Siebert P D. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng S C, Jarvinen M J, Nelson W G, Huang J W, Woodcock M J, Sun T-T. Cell. 1982;30:361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- 17.Surya B, Yu J, Manabe M, Sun T-T. J Cell Sci. 1990;97:419–432. doi: 10.1242/jcs.97.3.419. [DOI] [PubMed] [Google Scholar]
- 18.Schafer B M, Maier K, Eickhoff U, Todd R F, Kramer M D. Am J Pathol. 1994;144:1269–1280. [PMC free article] [PubMed] [Google Scholar]
- 19.Vassalli J D, Hamilton J, Reich E. Cell. 1977;11:695–705. doi: 10.1016/0092-8674(77)90086-1. [DOI] [PubMed] [Google Scholar]
- 20.Granelli-Piperno A, Reich E. J Exp Med. 1978;148:223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprecher C A, Kisiel W, Mathewes S, Foster D C. Proc Natl Acad Sci USA. 1994;91:3353–3357. doi: 10.1073/pnas.91.8.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen L C, Sprecher C A, Foster D C, Blumberg H, Hamamoto T, Kisiel W. Biochemistry. 1996;35:266–272. doi: 10.1021/bi951501d. [DOI] [PubMed] [Google Scholar]
- 23.Moll R, Franke W W, Schiller D L, Geiger B, Krepler R. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 24.Angles-Cano E, Rondeau E, Delarue F, Hagege J, Sultan Y, Sraer J D. Thromb Haemostasis. 1985;54:688–692. [PubMed] [Google Scholar]
- 25.Shapiro R L, Duquette J G, Nunes I, Roses D F, Harris M N, Wilson E L, Rifkin D B. Am J Pathol. 1997;150:359–369. [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Manabe M, Wu X R, Xu C, Surya B, Sun T-T. J Cell Biol. 1990;111:1207–1216. doi: 10.1083/jcb.111.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubeau L, Jones P A, Rideout W M d, Laug W E. Cancer Res. 1988;48:5552–5556. [PubMed] [Google Scholar]
- 28.Emeis J J, van den Eijnden-Schrauwen Y, van den Hoogen C M, de Priester W, Westmuckett A, Lupu F. J Cell Biol. 1997;139:245–256. doi: 10.1083/jcb.139.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegeman R J, van den Eijnden-Schrauwen Y, Emeis J J. Thromb Haemostasis. 1998;79:853–858. [PubMed] [Google Scholar]
- 30.Canipari R, Zurzolo C, Polistina C, Garbi C, Aloj L, Cali G, Gentile R, Nitsch L. Biochim Biophys Acta. 1992;1175:1–6. doi: 10.1016/0167-4889(92)90002-s. [DOI] [PubMed] [Google Scholar]
- 31.Ragno P, Estreicher A, Gos A, Wohlwend A, Belin D, Vassalli J D. Exp Cell Res. 1992;203:236–243. doi: 10.1016/0014-4827(92)90060-l. [DOI] [PubMed] [Google Scholar]
- 32.Donaldson J G, Finazzi D, Klausner R D. Nature (London) 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- 33.Sappino A P, Huarte J, Vassalli J D, Belin D. J Clin Invest. 1991;87:962–970. doi: 10.1172/JCI115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kristensen P, Eriksen J, Dano K. J Histochem Cytochem. 1991;39:341–349. doi: 10.1177/39.3.1899685. [DOI] [PubMed] [Google Scholar]
- 35.Larsson L I, Skriver L, Nielsen L S, Grondahl-Hansen J, Kristensen P, Dano K. J Cell Biol. 1984;98:894–903. doi: 10.1083/jcb.98.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasui Y, Suzumiya J, Marutsuka K, Sumiyoshi A, Hashida S, Ishikawa E. Cancer Res. 1989;49:1067–1070. [PubMed] [Google Scholar]
- 37.Hart D A, Rehemtulla A, Babins E M. Comp Biochem Physiol B Biochem Mol Biol. 1986;84:287–293. doi: 10.1016/0305-0491(86)90078-7. [DOI] [PubMed] [Google Scholar]
- 38.du Toit P J, Van Aswegen C H, Steinmann C M, Klue L, Du Plessis D J. Med Hypotheses. 1997;49:57–59. doi: 10.1016/s0306-9877(97)90253-x. [DOI] [PubMed] [Google Scholar]
- 39.Aronson M, Medalia O, Amichay D, Nativ O. Infect Immun. 1988;56:1615–1617. doi: 10.1128/iai.56.6.1615-1617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulvey M A, Lopez-Boado Y S, Wilson C L, Roth R, Parks W C, Heuser J, Hultgren S J. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 41.Lyons-Giordano B, Lazarus G S. Dev Biol. 1995;170:289–298. doi: 10.1006/dbio.1995.1215. [DOI] [PubMed] [Google Scholar]
- 42.Ossowski L, Biegel D, Reich E. Cell. 1979;16:929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- 43.Talhouk R S, Bissell M J, Werb Z. J Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romer J, Bugge T H, Pyke C, Lund L R, Flick M J, Degen J L, Dano K. Nat Med. 1996;2:725. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- 45.Ichinose A, Fujikawa K, Suyama T. J Biol Chem. 1986;261:3486–3489. [PubMed] [Google Scholar]
- 46.Lewis S A, Alles W P. Proc Natl Acad Sci USA. 1986;83:5345–5348. doi: 10.1073/pnas.83.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walther M M, Campbell W G, Jr, O'Brien D P d, Wheatley J K, Graham S D., Jr J Urol. 1987;137:764–768. doi: 10.1016/s0022-5347(17)44206-6. [DOI] [PubMed] [Google Scholar]
- 48.Liang F, Kachar B, Ding M, Zhai Z, Wu X R, Sun T-T. Differentiation. 1999;65:59–69. doi: 10.1046/j.1432-0436.1999.6510059.x. [DOI] [PubMed] [Google Scholar]
- 49.Declerck P J, Verstreken M, Collen D. Thromb Haemostasis. 1995;74:1305–1309. [PubMed] [Google Scholar]
- 50.Rao C N, Reddy P, Liu Y, O'Toole E, Reeder D, Foster D C, Kisiel W, Woodley D T. Arch Biochem Biophys. 1996;335:82–92. doi: 10.1006/abbi.1996.0484. [DOI] [PubMed] [Google Scholar]
- 51.Cooper D, Schermer A, Sun T-T. Lab Invest. 1985;52:243–256. [PubMed] [Google Scholar]