Abstract
Increases in the use and application of pyrethroid insecticides have resulted in concern regarding potential effects on aquatic ecosystems. Methods for the detection of pyrethroids in receiving waters are required to monitor environmental levels of these insecticides. One method employed for the identification of causes of toxicity in aquatic samples is the toxicity identification evaluation (TIE); however, current TIE protocols do not include specific methods for pyrethroid detection. Recent work identified carboxylesterase treatment as a useful method for removing/detecting pyrethroid-associated toxicity. The present study has extended this earlier work and examined the ability of carboxylesterase activity to remove permethrin- and bifenthrin-associated toxicity to Ceriodaphnia dubia and Hyalella azteca in a variety of matrices, including laboratory water, Sacramento River (CA, USA) water, and Salinas River (CA, USA) interstitial water. Esterase activity successfully removed 1,000 ng/L of permethrin-associated toxicity and 600 ng/L of bifenthrin-associated toxicity to C. dubia in Sacramento River water. In interstitial water, 200 ng/L of permethrin-associated toxicity and 60 ng/L of bifenthrin-associated toxicity to H. azteca were removed. The selectivity of the method was validated using heat-inactivated enzyme and bovine serum albumin, demonstrating that catalytically active esterase is required. Further studies showed that the enzyme is not significantly inhibited by metals. Matrix effects on esterase activity were examined with municipal effluent and seawater in addition to the matrices discussed above. Results confirmed that the esterase retains catalytic function in a diverse array of matrices, suggesting that this technique can be adapted to a variety of aquatic samples. These data demonstrate the utility of carboxylesterase treatment as a viable step to detect the presence of pyrethroids in receiving waters.
Keywords: Carboxylesterase, Pyrethroid, Toxicity identification evaluation, Ceriodaphnia dubia, Hyalella azteca
INTRODUCTION
The use of pyrethroid insecticides is increasing, which could result in a concomitant rise in their occurrence in receiving waters and sediments [1]. Pyrethroids are potentially toxic to a number of aquatic organisms, including many fish species as well as aquatic invertebrates [2–7]. Recent studies have identified pyrethroid-associated toxicity to aquatic organisms in sediments [4,5], interstitial waters [8–10], and storm-water runoff [3,11]. The effects of increasing pyrethroid usage on aquatic ecosystems therefore could be important [12] (http://www.ecologyandsociety.org/vol9/iss6/art1/), and receiving waters and sediments should be monitored for the presence of pyrethroids. However, current analytical techniques for pyrethroid detection are labor-intensive, time-consuming, and expensive [4,5,13]. The use of toxicity identification evaluation (TIE) methods is an efficient, routine technique to detect and identify many toxicants in environmental samples [14–16]. However, published U.S. Environmental Protection Agency (U.S. EPA) TIE protocols do not address the issue of pyrethroid detection specifically.
Previous work identified carboxylesterase activity as a useful tool for the detection of pyrethroids in receiving waters that appeared to be appropriate for use in TIE testing with Ceriodaphnia dubia [13]. These studies showed that the introduction of carboxylesterase to a water sample before the addition of test organisms was sufficient to remove pyrethroid-associated toxicity. Carboxylesterases are enzymes that hydrolyze ester-containing compounds (e.g., pyrethroids) to their corresponding acid and alcohol, which are usually detoxification products [17] (Fig. 1). Carboxylesterase activity is important for the metabolism and subsequent detoxification of many exogenous ester-containing compounds, including carbamates [18], organophosphates (OPs) [19], and pyrethroids [20].
Fig. 1.
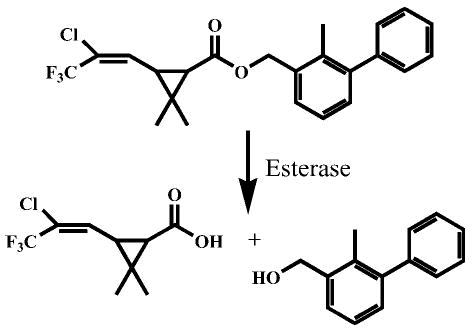
Esterase-mediated hydrolysis of the pyrethroid bifenthrin.
The present study was designed to further validate the use of esterase activity in TIE testing in multiple systems. Ideally, any method developed for pyrethroid detection would be functional for multiple organisms in a variety of matrices. This study extended earlier work to new matrices, Sacramento River (CA, USA) water and Salinas River (CA, USA) interstitial water, as well as to a new test species, Hyalella azteca. Two different pyrethroids were used, permethrin and bifenthrin, which were chosen based on their usage patterns [5] and rates of esterase-mediated hydrolysis [13]. Permethrin is one of the most commonly used pyrethroids in California (USA), and as such, the likelihood that this pyrethroid would be observed in aquatic sampling is increased (http://www.cdpr.gov). Bifenthrin is of concern to many groups performing sediment toxicity studies, and work has shown that bifenthrin toxicity can occur in interstitial water toxicity studies [5,21]. Previous work identified permethrin as being hydrolyzed relatively quickly by the porcine carboxylesterase employed in these studies, whereas bifenthrin was recalcitrant to esterase-mediated hydrolysis [13]. These two pyrethroids therefore represent the extremes of hydrolytic activity that the esterase most likely will encounter during a toxicity study. This project provides further evidence for the utility of carboxylesterase activity in detecting the presence of pyrethroid-associated toxicity in TIE testing systems.
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Aldrich Chemical (St. Louis, MO, USA) or Fisher Scientific (Pittsburgh, PA, USA) unless otherwise noted. Pesticide standards were acquired from Accustandard (New Haven, CT, USA). The esterase-inhibitor 1,1,1-trifluoro-3-octylthiol-propan-2-one (OTFP) was synthesized according to the methods described by Wheelock et al. [22]. Porcine liver esterase and bovine serum albumin (BSA) were purchased from Sigma Chemical (St. Louis, MO, USA). Esterase concentration is reported in terms of units of enzyme activity per milliliter of water, with an enzyme level of 2.5 × 10−3 units of esterase activity per milliliter of water defined as a 1x concentration for the purposes of this project. A unit of activity is the amount of enzyme that hydrolyzes 1.0 μmol of ethyl butyrate to butyric acid and ethanol per minute at pH 8.0 at 25°C (as defined by Sigma Chemical). Different preparations of enzyme can vary in their activity level, but the nomenclature of 1x was kept constant for all enzyme studies. Two different esterase preparations were used in the present study, an esterase solution from porcine liver in an ammonium sulfate suspension (3.2 M, pH 8.0, catalog no. E-2884; Sigma Chemical) and a crude lyophilized powder (<5% buffer salts, catalog no. E-3019; Sigma Chemical). Esterase activity and protein concentration vary with lot number and should be reported in all studies.
Toxicity testing
Studies with the cladoceran C. dubia, a freshwater invertebrate, were performed as detailed by Wheelock et al. [13] and are briefly described here. Ceriodaphnia dubia neonates (age, <24 h) were obtained from cultures maintained at AQUA-Science (Davis, CA, USA). Cultures were maintained at 25 ± 1°C, with a photoperiod of 16:8-h light:dark, and were fed a mixture of the green alga Selenastrum capricornutum (University of Texas Algae Type Collection, Austin, TX, USA) and blended trout chow (Silver Cup, Murray, UT, USA). Laboratory water consisted of reverse osmosis–treated well water amended with dry salts to U.S. EPA moderately hard specifications. Sacramento River water was collected from the Sacramento River at Freeport Marina (Sacramento, CA, USA).
The 48-h toxicity tests were conducted in 20-ml glass scintillation vials containing 18 ml of moderately hard dilution water. Test solutions were not renewed, and the organisms were not fed during the exposures. Mortalities were monitored daily. The concentrations required to cause an effect in 50% of the population (EC50) were calculated from the mortality data using a computer program (ToxCalc™, Ver 5.0.23; Tidepool Scientific, McKinnleyville, CA, USA). Insecticide working standards (100 μg/L) were prepared by dissolving the chemicals in high-performance liquid chromatography–grade methanol. Permethrin exposures were performed at 500, 750, and 1,000 ng/L, and bifenthrin exposures were conducted at 300, 450, and 600 ng/L. Methanol concentration in all test solutions was less than 0.1%.
Initial toxicity studies were performed with C. dubia using two different esterase preparations, an esterase solution in an ammonium sulfate suspension (activity = 1,840 U/ml [184 U/mg protein, 10 mg protein/ml], lot 102K7062; Sigma Chemical) and a lyophilized powder (activity = 20 U/mg, lot 039H7005; Sigma Chemical). Tolerance of C. dubia to esterase exposure was examined at 0x, 1x, 5x, 10x, 100x, 500x, and 1,000x. Subsequent exposures to the liquid preparation were performed at 0x, 1x, 5x, 10x, and 100x. Studies with the heat-inactivated enzyme and with the noncatalytic protein BSA were prepared exactly as described for the esterase, with concentrations reported in terms of x. In these cases, x refers to the equivalent level of protein that would be present in 1x of the esterase preparation. Heat-inactivated enzyme was prepared by heating the enzyme at 100°C for 4 min. The liquid enzyme stock solutions were prepared by diluting the neat enzyme 1:10 in ultrapure water (<1 μS/cm as prepared by ion-exchange treatment; Cole Parmer, Vernon Hills, IL, USA), giving a final enzyme dilution of 184 U/ml. The lyophilized enzyme was reconstituted in ultrapure water (1 mg/ml, 20 U/ml). Enzyme stock solutions were aliquotted into the test containers using a precision pipette (Rainin, Woburn, MA, USA) to achieve the desired enzyme concentration. Solutions were allowed to stand for 1 h before addition of the test organisms.
Hyalella azteca exposures were conducted at the University of California at Davis Marine Pollution Studies Laboratory. Amphipods were obtained from Chesapeake Cultures (Hayes, VA, USA) 48 h before test initiation, maintained at 23°C, and fed yeast–cerophyl–trout chow [23]. Exposures were conducted in 20-ml glass scintillation vials (three replicates) that had been preleached with freshwater for a minimum of 2 h and rinsed thoroughly. Test containers held 15 ml of treated sample and five 7- to 14-d-old amphipods, which were loaded in 0.5 ml of culture water. Exposures were conducted for 96 h at 23°C following U.S. EPA guidelines [24]. Water-quality parameters of dissolved oxygen, pH, and conductivity (uncorrected; i.e., not specific conductance) were measured using a Hach SensION© selective ion meter (Hach, Loveland, CO, USA) with appropriate electrodes at test initiation, and temperature was measured using a continuously recording thermograph and thermometer. Ammonia was measured with a colorimetric method (salicylate method) using a Hach 2310 spectrophotometer. Total ammonia was measured in terms of mg/L, and un-ionized ammonia was calculated from the total concentration using pH, temperature, and salinity (which was zero for these studies). Water hardness was approximately 80 mg/L as CaCO3, pH varied from 7.8 to 8.0, and un-ionized ammonia was 5.2 mg/L. Salinas River interstitial water was collected from a reference site near the city of Chualar (~20 km south of Salinas, CA, USA). No OPs were detected in this sample using enzyme-linked immunosorbent assays (data not shown).
Concentrations of bifenthrin and permethrin were prepared from 100 mg/L stock solutions. Superstock solutions were diluted to 100 μg/L secondary stock solutions in methanol, then diluted further to test concentrations using Marine Pollution Studies Laboratory well water. Permethrin exposures were conducted at 100, 150, and 200 ng/L and bifenthrin exposures at 20, 60, and 100 ng/L. A treatment blank was used to monitor for methanol-associated toxicity.
Initial experiments used a liquid preparation of enzyme (activity = 1,840 U/ml, lot 102K7062; Sigma Chemical) that was diluted to 10.03 U/ml. Tolerance of H. azteca to esterase was determined by exposing the amphipods to a range of esterase concentrations (0x, 100x, 500x, 1,000x, and 5,000x). Esterase stock solutions were prepared in glass volumetric flasks (545 μl of enzyme per 10 ml of Milli-Q® water, filtered at 18.2 MΩ at 25°C with a 25-μm filter; Millipore, Billerica, MA, USA). Subsequent experiments used a lyophilized powder enzyme preparation (activity = 20 U/mg solid, lot 123K7033; Sigma Chemical) that was used to create a stock solution of 1 mg/ml. Tolerance of H. azteca to exposure to the lyophilized enzyme was examined at 0x, 100x, 500x, 1,000x, and 5,000x. Additional experiments examining the ability of the esterase to remove pyrethroid-associated toxicity were conducted with the lyophilized esterase at 0x, 10x, 50x, 100x, and 500x. Neat liquid enzyme or enzyme stock solutions were transferred to volumetric flasks of spiked water and aliquotted to test containers by pouring (~10 ml per test container). The esterase was added to the water sample at least 1 h before introduction of the organisms. Mortality was then monitored daily for the duration of the study.
Enzyme assays
All esterase activity assays were conducted according to the methods described by Wheelock et al. [22] as adapted from those described by Ljungquist and Augustinsson [25] using the standard esterase substrate p-nitrophenyl acetate (PNPA). Assays were initiated by adding esterase to a flat-bottom, 96-well polystyrene microtiter plate (Dynex Technologies, Chantilly, VA, USA) in Tris buffer (0.1 M, pH 7.4) at 25°C, followed by addition of the substrate and analysis at 405 nm for 2.0 min in kinetic mode on a Spectramax 340PC plate reader (Molecular Devices, Sunnyvale, CA, USA). For inhibition assays, the esterase was incubated with the inhibitor for 10 min before the addition of substrate.
Matrix effects on esterase activity were examined over a 48-h period. Studies were initiated by adding 2 μl of esterase (1,840 U/ml, lot 102K7062; Sigma Chemical) to 10 ml of matrix in a 15-ml Falcon® Blue Max™ polypropylene conical tube (17 × 120 mm; Becton Dickinson Labware, Franklin Lakes, NJ, USA). Activity assays were conducted by removing 10-μl aliquots of each matrix and measuring esterase activity as described above. Final esterase concentration in all assays was 0.1 μg/ml (1.84 mU/ml). Several different matrices were examined, including sodium phosphate buffer (0.1 M, pH 7.4), Milli-Q water (Millipore), natural seawater (collected from Bodega Bay, CA, USA, and filtered to 0.45 μm), municipal effluent (collected from the Sacramento Regional Wastewater Treatment Plant, Sacramento, CA, USA), Sacramento River water (collected from the Sacramento River at Freeport Marina, Sacramento, CA, USA), and interstitial water extracted from two sediments from the lower Santa Maria River watershed (near Guadalupe, CA, USA). The characteristics of the sediment samples, Santa Maria and Orcutt Creek, as well as the precise location of sampling have been described by Anderson et al. [7]. Total organic carbon of the Orcutt Creek sediment was 0.95%, and that of the Santa Maria sediment was 1.1%. The Orcutt Creek sediment contained 0.322 μg/L of chlorpyrifos and the Santa Maria 0.589 μg/L.
The effect of metals on esterase activity was examined using 19 different metal cations that were purchased as the chloride salt. Stock solutions were prepared in Milli-Q water (0.1 M). Assays were initiated by adding esterase to a 96-well microtiter plate in Tris buffer (0.1 M, pH 7.4), followed by 2 μl of each metal (final metal concentration, 1 mM).
Inhibited esterase was generated for studies examining the need for catalytically active enzyme to remove pyrethroid-associated toxicity. To a 2.8-ml bottle of esterase (184 U/ml, 10 mg/ml, lot 102K7062; Sigma Chemical), 28 μl of a 50 mM stock solution of chlorpyrifos-oxon (in ethanol) were added to give a final concentration of 0.5 mM. An equivalent control bottle was prepared with 28 μl of ethanol. Following addition of the inhibitor, no esterase activity was detected by PNPA assay. The excess chlorpyrifos-oxon (not bound to the esterase) was then removed by filtering the enzyme mixture through a 3-ml, 50K cutoff Centricon® column (Millipore) at 6,000 rpm on a Sorvall® centrifuge (Kendro Laboratory, Asheville, NC, USA) for 20 min. After centrifugation, 2 ml of chilled phosphate buffer (0.1 M, pH 7.4) were added to the column, which was centrifuged again. This method was repeated eight times, until all chlorpyrifos-oxon was removed. Removal was verified by addition of 100 μl of the inhibited enzyme preparation to untreated enzyme and then assaying for enzyme activity with PNPA. Successful removal of the excess chlorpyrifos-oxon occurred when no esterase inhibition was observed. Control enzyme was manipulated identically, and no significant loss in activity was observed during the centrifugation process. The inhibited enzyme preparation was then stored at 4°C for approximately 12 h before use in toxicity assays. Studies were performed with an additional esterase-inhibitor OTFP identically as described for chlorpyrifos-oxon.
RESULTS
Toxicity testing with C. dubia
Results demonstrated that the liquid enzyme preparation was not overtly toxic at levels up to 100x (Table 1). However, at increased concentrations, significant toxicity was observed. The lyophilized powder did not exhibit any significant toxicity at enzyme levels up to 1,000x. Neither the heat-inactivated enzyme nor the protein BSA exhibited toxic effects at any of the concentrations tested.
Table 1.
Acute esterase toxicity to Ceriodaphnia dubiaa
| 48-h mortality (%)
|
|||
|---|---|---|---|
| Enzyme concentrationb | Liquidc | Lyophilizedd | BSAe |
| 0x | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 1x | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 5x | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 10x | 5 ± 5 | 0 ± 0 | 0 ± 0 |
| 100x | 10 ± 12 | 0 ± 0 | 0 ± 0 |
| 500x | 100 ± 0 | 0 ± 0 | 0 ± 0 |
| 1,000x | 100 ± 0 | 10 ± 12 | 0 ± 0 |
Data are the average of four replicates with five neonates ± standard deviation.
lx = 2.5 × 10−3 U/ml in laboratory water.
Ammonium sulfate suspension esterase preparation (lot 102K7062; Sigma Chemical, St. Louis, MO, USA).
Lyophilized esterase preparation (lot 039H7005; Sigma Chemical).
BSA = bovine serum albumin.
Permethrin-associated toxicity in laboratory water was partially removed at 5x and completely removed at 10x and 100x (Table 2). No concentration–response relationship was observed for the three different concentrations of permethrin examined. Similar effects were observed with the Sacramento River water; however, the enzyme data suggested increased efficacy in Sacramento River water versus laboratory water, demonstrating matrix-specific efficiency. A concentration–response relationship was observed at the 5x level, with increasing concentrations of permethrin causing increased mortality. Bifenthrin-associated toxicity was removed similarly to permethrin-associated toxicity, with 10x removing all toxicity in laboratory water (Table 2). However, 100x was necessary to remove all toxicity in Sacramento River water. Esterase removal of pyrethroid-associated toxicity was more efficient for permethrin in laboratory water but less efficient in Sacramento River water. Concentration–response relationships were observed for both matrices, with higher levels of bifenthrin eliciting increased toxicity.
Table 2.
Effect of liquid esterase on pyrethroid toxicity to Ceriodaphnia dubiaa
| 48-h mortality (%)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Active enzymec |
Inactive enzymed |
BSAe |
|||||||
| Pyrethroid (ng/L)b | 0xf | 1x | 5x | 10x | 100x | 10x | 100x | 10x | 100x |
| Permethrin | |||||||||
| Laboratory waterg | |||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 11 ± 19 | 0 | 0 | 0 |
| 500 | 100 | 100 | 60 ± 28 | 0 | 0 | 100 | 65 ± 10 | 85 ± 30 | 90 ± 11 |
| 750 | 100 | 100 | 75 ± 10 | 0 | 0 | 100 | 100 | 100 | 100 |
| 1,000 | 100 | 100 | 70 ± 38 | 0 | 0 | 100 | 100 | 100 | 100 |
| Sacramento River waterh | |||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 500 | 100 | 95 ± 10 | 0 | 0 | 0 | 100 | 60 ± 16 | 85 ± 10 | 85 ± 19 |
| 750 | 100 | 100 | 21 ± 28 | 0 | 0 | 100 | 100 | 100 | 100 |
| 1,000 | 100 | 100 | 75 ± 19 | 0 | 0 | 100 | 100 | 100 | 100 |
| Bifenthrin | |||||||||
| Laboratory waterg | |||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 300 | 100 | 100 | 0 | 0 | 5 ± 10 | 100 | 100 | 100 | 100 |
| 450 | 100 | 100 | 0 | 0 | 0 | 100 | 100 | 100 | 100 |
| 600 | 100 | 100 | 40 ± 43 | 0 | 0 | 100 | 100 | 100 | 100 |
| Sacramento River waterh | |||||||||
| 0 | 5± 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 ± 10 |
| 300 | 100 | 100 | 46 ± 37 | 5 ± 10 | 0 | 100 | 100 | 100 | 100 |
| 450 | 100 | 100 | 95 ± 10 | 45 ± 33 | 5 ± 10 | 100 | 100 | 100 | 100 |
| 600 | 100 | 100 | 100 | 79 ± 17 | 0 | 100 | 100 | 100 | 100 |
Data are the average of four replicates with five neonates ± standard deviation.
Pyrethroid concentrations are nominal water concentrations.
Esterase was prepared as described in Materials and Methods.
Esterase was heated at 100°C for 4 min.
Bovine serum albumin (BSA) was added at protein concentrations equivalent to those used in the esterase preparations.
1x dilution = 2.3 × 10−3 U/ml. A unit of activity is the amount of enzyme that hydrolyzes 1.0 μmol of butyrate to butyric acid and ethanol per minute at pH 8.0 at 25°C (as defined by Sigma Chemical, St. Louis, MO, USA).
Water amended to U.S. Environmental Protection Agency moderately hard specifications.
Sacramento River water was collected at Garcia Bend, near Sacramento (CA, USA).
Two different controls were used to verify that the observed toxicity removal was caused by catalytically active enzyme. Heat-inactivated enzyme did not remove any permethrin- or bifenthrin-associated toxicity at an esterase concentration of 10x (Table 2). At 100x, a slight reduction in permethrin-associated toxicity was observed at the lowest concentration of permethrin examined (500 ng/L). However, no reduction in bifenthrin-associated toxicity was observed at 100x (Table 2). Studies performed with BSA did not significantly remove permethrin- or bifenthrin-associated toxicity at 10x or 100x in either matrix examined (Table 2).
The lyophilized esterase preparation was examined for its ability to remove bifenthrin-associated toxicity (Table 3). At 50x or greater, all bifenthrin-associated toxicity was removed. Equivalent studies performed with BSA showed that no reduction in toxicity occurred up to 250x. However, at 250x, a significant reduction was found in toxicity (30% mortality) at the lowest concentration of bifenthrin examined (300 ng/L).
Table 3.
Efficacy of lyophilized esterase on removal of bifenthrin-associated toxicity to Ceriodaphnia dubiaa
| 48-h Mortality (%)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Esterasec |
Bovine serum albumind |
||||||||
| Bifenthrin (ng/L)b | 0xe | 10x | 50x | 100x | 250x | 10x | 50x | 100x | 250x |
| 0 | 0 | 0 | 0 | 0 | 0 | 5 ± 10 | 0 | 0 | 0 |
| 300 | 100 | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 30 ± 20 |
| 450 | 100 | 100 | 0 | 0 | 0 | 100 | 100 | 100 | 100 |
| 600 | 100 | 100 | 0 | 0 | 0 | 100 | 100 | 100 | 100 |
Data are the average of four replicates with five neonates ± standard deviation. Studies were performed in laboratory water amended to U.S. Environmental Protection Agency moderately hard specifications.
Bifenthrin concentrations are nominal water concentrations.
Esterase was prepared as described in Materials and Methods using lyophilized esterase (lot 039H7005; Sigma Chemical, St. Louis, MO, USA).
Bovine serum albumin (BSA) was added at protein concentrations equivalent to those used in the esterase preparations (250x BSA = 3.125 mg protein/ml).
1x dilution = 2.3 × 10−3 U/ml. A unit of activity is the amount of enzyme that hydrolyzes 1.0 μmol of butyrate to butyric acid and ethanol per minute at pH 8.0 at 25°C (as defined by Sigma Chemical).
Toxicity testing with H. azteca
Significant differences were observed in the acute toxicity of the two different esterase preparations to H. azteca (Table 4). The liquid preparation caused 37% mortality at 500x, whereas the lyophilized preparation exhibited 0% mortality. The maximum esterase concentration examined was 5,000x, which caused 100% mortality with the liquid preparation but only 53% with the lyophilized preparation.
Table 4.
Acute esterase toxicity to Hyalella aztecaa
| 96-h mortality (%)
|
||
|---|---|---|
| Enzyme concentrationb | Liquidc | Lyophilizedd |
| 0x | 0 ± 0 | 0 ± 0 |
| 100x | 0 ± 0 | 0 ± 0 |
| 500x | 37 ± 15 | 0 ± 0 |
| 1,000x | 35 ± 22 | 20 ± 20 |
| 5,000x | 100 ± 0 | 53 ± 12 |
Data are the average of three replicates with five amphipods ± standard deviation.
1x = 2.5 × 10−3 U/ml in laboratory water.
Ammonium sulfate suspension esterase preparation (lot 102K7062; Sigma Chemical, St. Louis, MO, USA).
Lyophilized esterase preparation (lot 123K7033; Sigma Chemical).
Initial studies with the liquid esterase showed significant mortality in the controls at enzyme concentrations greater than 100x (data not shown). However, at an esterase concentration of 100x in laboratory water, 67 and 20% mortality was still observed in exposures to 100 ng/L of bifenthrin and of permethrin, respectively. The decision therefore was made to use higher enzyme concentrations for the H. azteca studies. However, there was concern regarding the esterase-associated toxicity following exposure to the liquid enzyme preparation. This toxicity was attributed to elevated ammonia levels, which were detected in all samples and correlated directly with the concentration of enzyme added to the sample (Table 5). The lyophilized esterase preparation therefore was used for further studies with H. azteca. The lyophilized esterase removed essentially all permethrin-associated toxicity in laboratory water, with no observed concentration–response relationship (Table 6). The interstitial water studies showed that a concentration of 50x was necessary to remove all permethrin-associated toxicity. No significant enzyme-associated toxicity was observed in any of the studies. Esterase activity was not as efficient at removing bifenthrin-associated toxicity in laboratory water (Table 6). A significant reduction in bifenthrin-associated toxicity was found at the 20 ng/L dose, but only for the higher enzyme concentrations. Enzyme concentrations as high as 500x did not significantly reduce bifenthrin-associated toxicity at 60 and 100 ng/L. However, a significant reduction in toxicity was observed in the interstitial water dosed with 60 ng/L and 500x esterase. No significant mortality was observed in the controls.
Table 5.
Ammonia and pH comparison of liquid versus lyophilized esterasea
| Enzyme concentrationb |
|||
|---|---|---|---|
| Measured parameter | 0x | 100x | 500x |
| Liquid esterasec | |||
| pH | 8.17 | 8.06 | 7.91 |
| Total ammonia (mg/L) | 0.3 | 15 | 73.8 |
| Un-ionized ammonia (mg/L) | 0.02 | 0.80 | 2.84 |
| Lyophilized esterased | |||
| pH | 7.90 | 8.42 | 8.42 |
| Total ammonia (mg/L) | 0.2 | 0.4 | 0.2 |
| Un-ionized ammonia (mg/L) | 0.01 | 0.05 | 0.02 |
Values were measured during toxicity studies with Hyalella azteca.
1x dilution = 2.3 × 10−3 U/ml. A unit of activity is the amount of enzyme that hydrolyzes 1.0 μmol of butyrate to butyric acid and ethanol per minute at pH 8.0 at 25°C (as defined by Sigma Chemical, St. Louis, MO, USA).
Enzyme was from an ammonium sulfate suspension from porcine liver (3.2 M, pH 8.0, lot 102K7062; Sigma Chemical).
Enzyme was from a crude lyophilized powder (lot 123K7033; Sigma Chemical).
Table 6.
Effect of lyophilized esterase on pyrethroid toxicity to Hyalella aztecaa
| 96-h mortality (%) per enzyme concentrationc |
|||||
|---|---|---|---|---|---|
| Pyrethroid (ng/L)b | 0x | 10x | 50x | 100x | 500x |
| Permethrin | |||||
| Laboratory waterd | |||||
| 0 | 0 | 7 ± 12 | 7 ± 12 | 7 ± 12 | 0 |
| 100 | 73 ± 23 | 7 ± 12 | 7 ± 12 | 0 | 0 |
| 150 | 93 ± 12 | 0 | 0 | 7 ± 12 | 7 ± 12 |
| 200 | 100 | 0 | 0 | 7 ± 12 | 0 |
| Chualar interstitial watere | |||||
| 0 | 0 | 13 ± 12 | 7 ± 12 | 7 ± 12 | 0 |
| 100 | 93 ± 12 | 33 ± 23 | 0 | 0 | 0 |
| 150 | 100 | 80 ± 20 | 0 | 0 | 7 ± 12 |
| 200 | 100 | 93 ± 12 | 0 | 0 | 13 ± 23 |
| Bifenthrin | |||||
| Laboratory waterd | |||||
| 0 | 0 | 7 ± 12 | 7 ± 12 | 27 ± 31 | 0 |
| 20 | 85 ± 23 | 40 ± 20 | 0 | 13 ± 12 | 13 ± 23 |
| 60 | 100 | 100 | 87 ± 23 | 93 ± 12 | 93 ± 12 |
| 100 | 100 | 100 | 93 ± 12 | 100 | 100 |
| Chualar interstitial watere | |||||
| 0 | 0 | 13 ± 12 | 7 ± 12 | 7 ± 12 | 0 |
| 20 | 20 ± 0 | 93 ± 12 | 23 ± 9 | 7 ± 12 | 13 ± 12 |
| 60 | 100 | 100 | 100 | 87 ± 12 | 13 ± 12 |
| 100 | 100 | 100 | 100 | 100 | 67 ± 12 |
Data are the average of three replicates with five amphipods ± standard deviation. Esterase was prepared from a crude lyophilized powder as described in Materials and Methods (lot number 123K7033; Sigma Chemical, St. Louis, MO, USA).
Pyrethroid concentrations are nominal water concentrations.
1x dilution = 2.3 × 10−3 U/ml. A unit of activity is the amount of enzyme that hydrolyzes 1.0 μmol of butyrate to butyric acid and ethanol per minute at pH 8.0 at 25°C (as defined by Sigma Chemical).
Granite Canyon well water (Granite Canyon, CA, USA).
Chualar pore water was collected from a reference site on the Salinas River near the city of Chualar (CA, USA).
Esterase characterization
The time dependence of esterase activity was examined in several different matrices (Table 7). A phosphate buffer was most efficient at maintaining esterase activity over 48 h, with a 24% reduction in activity observed. Milli-Q water was essentially as efficient as phosphate buffer, with a 29% reduction in activity during the monitoring period. A significant reduction in activity was observed in seawater, with no detectable activity by 48 h. The other examined matrices performed similarly, with loss of all activity by 12 to 24 h. However, all examined matrices had greater than 50% remaining activity up to 2 h after test initiation.
Table 7.
Time dependence of esterase activity in various matricesa
| Interstitial water
|
|||||||
|---|---|---|---|---|---|---|---|
| Time (h) | Bufferb | Waterc | Municipal effluent | Sacramento River water | Santa Mariad | Orcutt Creeke | Seawater |
| 0 | 100 ± 12 | 89 ± 14 | 86 ± 21 | 132 ± 17 | 73 ± 10 | 83 ± 6 | 76 ± 18 |
| 1 | 103 ± 14 | 99 ± 20 | 78 ± 8 | 118 ± 1 | 65 ± 15 | 89 ± 4 | 90 ± 11 |
| 2 | 101 ± 18 | 101 ± 5 | 76 ± 11 | 108 ± 13 | 63 ± 21 | 82 ± 7 | 80 ± 6 |
| 4 | 102 ± 15 | 98 ± 6 | 71 ± 6 | 109 ± 13 | 49 ± 14 | 78 ± 4 | 78 ± 6 |
| 6 | 101 ± 21 | 93 ± 8 | 73 ± 7 | 116 ± 17 | 47 ± 17 | 76 ± 6 | 77 ± 7 |
| 8 | 94 ± 15 | 90 ± 4 | 61 ± 7 | 108 ± 14 | 36 ± 15 | 65 ± 7 | 70 ± 14 |
| 12 | 98 ± 16 | 94 ± 4 | 7 ± 4 | 96 ± 13 | NDf | 57 ± 5 | 59 ± 14 |
| 24 | 76 ± 18 | 74 ± 10 | ND | 3 ± 2 | ND | ND | 32 ± 11 |
| 36 | 77 ± 15 | 75 ± 7 | ND | ND | ND | ND | 11 ± 4 |
| 48 | 76 ± 12 | 71 ± 5 | ND | ND | ND | ND | ND |
All values are shown as the percentage of activity relative to initial buffer control (100 mM, pH 7.4, phosphate buffer) and are the average of three replicates ± standard deviation. Enzyme (0.1 μg/ml, 18.4 mU/ml) was incubated in each matrix at 25°C, and activity was measured using the substrate p-nitrophenyl acetate. A unit of activity is the amount of enzyme that hydrolyzes 1.0 μmol of butyrate to butyric acid and ethanol per minute at pH 8.0 at 25°C (as defined by Sigma Chemical, St. Louis, MO, USA). Sources of all water samples are described in Materials and Methods.
Sodium phosphate buffer (100 mM, pH 7.4).
Milli-Q® water (Millipore, Billerica, MA, USA).
Collected from the Santa Maria River near Guadalupe (CA, USA).
Collected from Orcutt Creek near Guadalupe (CA, USA).
ND = not detected.
Esterase inhibition studies performed with permethrin and two inhibitors showed that 10x catalytically active esterase removed all toxicity at three different pyrethroid concentrations (Table 8). Incubation of the enzyme with chlorpyrifos-oxon did not affect the ability of the esterase to reduce toxicity. However, use of the esterase-inhibitor OTFP resulted in a dose-dependent increase in permethrin-associated toxicity. Incubation of the esterase-inhibitor OTFP with the enzyme before test initiation reduced the enzyme’s capability to reduce pyrethroid toxicity in a dose-dependent manner.
Table 8.
Effect of enzyme inhibition on esterase efficacy in removing permethrin-associated toxicity to Ceriodaphnia dubiaa
| Esterase inhibitor | Permethrin (ng/L)b | No enzymec | Active enzymed | Inhibited enzymee |
|---|---|---|---|---|
| Chlorpyrifos-oxonf | 0 | 0 | 0 | 0 |
| 500 | 95 ± 10 | 5 ± 10 | 5 ± 10 | |
| 750 | 100 | 0 | 0 | |
| 1,000 | 100 | 0 | 0 | |
| OTFPg | 0 | 0 | 0 | 0 |
| 500 | 100 | 0 | 60 ± 0 | |
| 750 | 100 | 0 | 90 ± 12 | |
| 1,000 | 100 | 0 | 95 ± 10 |
Data are shown as the mean percentage mortality ± standard deviation for 48-h C. dubia toxicity using five neonates with four replicates.
Permethrin concentrations are nominal water concentrations.
Toxicity assays were performed in the absence of esterase, or 0x, where 1x dilution = 2.3 × 10−3 U/ml. A unit of activity is the amount of enzyme that hydrolyzes 1.0 μmol of butyrate to butyric acid and ethanol per minute at pH 8.0 at 25°C (as defined by Sigma Chemical, St. Louis, MO, USA).
Uninhibited enzyme (10x) was added to each container (liquid preparation, lot number 102K7062; Sigma Chemical).
The indicated inhibitor (10x) was added to the esterase at a final concentration of 0.5 mM.
The specific activity of the porcine esterase preparation before inhibition was 112.9 μmol/min/mg as determined by p-nitrophenyl acetate (PNPA) hydrolysis. Following inhibition with 0.5 mM chlorpyrifos-oxon and subsequent filtration-based inhibitor removal, no PNPA hydrolysis activity was detected. However, 72 h after toxicity assay initiation, the esterase specific activity had increased to 81.2 μmol/min/mg, indicating reactivation of the enzyme.
OTFP = 1,1,1-trifluoro-3-octylthiol-propan-2-one.
A series of 19 different metals were screened for their ability to inhibit esterase activity (Table 9). Only iron(III), zinc, rubidium, nickel, iron(II), and copper(II) significantly inhibited catalytic activity (p < 0.05). The greatest level of inhibition was observed using copper(II), with 31% inhibition. A positive control using diazinon-oxon, a known inhibitor of porcine esterase, showed 100% inhibition.
Table 9.
Effect of metals on esterase activitya
| Metalb | Specific activity (μmol/min/mg) | % Control |
|---|---|---|
| Controlc | 26.4 ± 0.7 | 100 |
| Barium | 29.0 ± 3.4 | 110 |
| Cerium(III) | 28.2 ± 2.3 | 107 |
| Cesium | 27.4 ± 1.4 | 104 |
| Calcium | 27.6 ± 1.8 | 104 |
| Lithium | 27.5 ± 0.4 | 104 |
| Copper(I) | 27.1 ± 3.3 | 103 |
| Manganese | 26.6 ± 1.7 | 101 |
| Cadmium | 26.0 ± 1.0 | 98 |
| Cobalt | 25.7 ± 1.5 | 97 |
| Lead(II) | 25.7 ± 0.9 | 97 |
| Mercury | 25.4 ± 0.2 | 96 |
| Iron(III) | 24.0 ± 0.5 | 91* |
| Potassium | 23.8 ± 3.4 | 90 |
| Zinc | 23.3 ± 1.0 | 88* |
| Rubidium | 23.3 ± 0.1 | 88* |
| Sodium | 22.2 ± 4.2 | 84 |
| Nickel | 19.5 ± 1.0 | 74* |
| Iron(II) | 19.1 ± 2.6 | 72* |
| Copper(II) | 18.2 ± 2.5 | 69* |
| Diazinon-oxon | 0.01 ± 0.0 | 0 |
Assays were performed in quadruplicate using the liquid esterase preparation (lot 102K7062; Sigma Chemical, St. Louis, MO, USA). Activity assays were performed with the substrate p-nitrophenyl acetate. An asterisk indicates that the value is significantly lower than control using Student’s t test (p < 0.05).
All metals were purchased as the chloride salt and were present at a final concentration of 1 mM.
Control is defined as the activity measured in sodium phosphate buffer (0.1 M, pH 7.4, 25°C) in the absence of any metals.
DISCUSSION
Esterase toxicity to C. dubia and H. azteca
The first issue examined was overt toxicity of the enzyme preparation to the testing organisms. Currently, two commercial preparations of the porcine esterase are available. Earlier studies with the enzyme reported use of an ammonium sulfate suspension [13]. The second enzyme preparation is available as a crude lyophilized powder, which has the advantage of decreased ammonium salts, reduced cost, and increased homogeneity. The hydrolytic activities of the two preparations differ by approximately one order of magnitude (lyophilized powder, 20 U/mg protein; liquid preparation, 184 U/mg protein). The main advantages of the liquid preparation include increased specific activity, ease of handling for addition to testing containers, and existing data regarding enzyme efficacy from previous studies (one laboratory involved in the present study, however, reported that handling of the lyophilized powder was easier). We evaluated both preparations for toxicity to the testing organisms. Initial studies evidenced no significant differences in toxicity up to 100x for both species. This level of enzyme was shown to be more than sufficient in previous studies for complete removal of pyrethroid-associated toxicity [13]. The liquid preparation therefore was used in further studies with C. dubia, mainly to enable comparisons with pre-existing data. However, subsequent studies with H. azteca using complicated matrices, such as interstitial water, demonstrated a need to increase the enzyme concentration above 100x to achieve removal of pyrethroid toxicity (data not shown). Toxicity studies showed that for enzyme concentrations greater than 100x, significantly greater mortality was associated with the liquid preparation versus the powder (Table 4). Therefore, studies with H. azteca were performed with the lyophilized powder preparation. The observed enzyme-associated toxicity at higher esterase concentrations was attributed to the presence of ammonia. Hyalella azteca have a higher ammonia tolerance than C. dubia, with 96-h median lethal concentration (LC50) values of 5.2 mg/L (B.M. Phillips, unpublished data) and 0.78 mg/L [15] of un-ionized ammonia, respectively. The 96-h LC50 values for H. azteca compared well to published values of 2.14 mg/L at pH 7.5 and 5.38 mg/L at pH 8.5 for hard water [26]. These data show the importance of validating the esterase treatment for each new organism and matrix, because sensitivity to esterase acute toxicity as well as enzyme efficacy likely will vary.
Toxicity studies with C. dubia
The esterase generally was very effective at removing both permethrin- and bifenthrin-associated toxicity. Reductions in permethrin-associated toxicity were greater than reductions in bifenthrin-associated toxicity. This finding most likely resulted from the increased hydrolytic rate of permethrin over bifenthrin [13]. Matrix effects were observed, in that permethrin removal in Sacramento River water was greater than that in laboratory water, possibly because of the absorption of permethrin by organic material in the river water. The effects observed with bifenthrin were the opposite, in that toxicity removal was lower in river water. These results are more difficult to explain. Permethrin has significantly greater water solubility than bifenthrin (5.5 vs 0.014 μg/L [27]); therefore, it would be expected that bifenthrin would be removed through hydrophobic interactions to a greater extent than permethrin. Given that essentially equivalent toxic units of each pyrethroid were used in the assays, the results were unexpected. Although variability was found at low enzyme activities, all pyrethroid-associated toxicity in both matrices was removed at an enzyme level of 10x. It is important to point out that the concentrations of pyrethroids used in this study were quite high and not necessarily environmentally relevant [5]. It therefore is possible that significantly lower levels of esterase could be employed in future assays. For applying this TIE method to an environmental sample, it will be desirable to use the greatest level of esterase possible while still maintaining selective removal of pyrethroid toxicity. This application will require a balance of esterase level with potential ammonia toxicity as well as overall protein level. As demonstrated in Table 3, higher levels of protein can result in nonselective removal of pyrethroid toxicity as observed with the 250x BSA preparation. The nonselective removal of pyrethroid toxicity is not necessarily a deterrent, because the assay results remain valid. The problem arises in the presence of other hydrophobic contaminants that could be removed from solution in addition to the pyrethroids, thereby convoluting the significance and meaning of the observed drop in toxicity. The issue of assay selectivity in the presence of high concentrations of esterase could be addressed, in part, by using other TIE testing protocols, such as employing piperonyl butoxide to detect the presence of OPs [14,15,28].
Toxicity studies with H. azteca
Observations with H. azteca were similar to those with C. dubia; however, it was necessary to increase the enzyme concentration to 500x to remove bifenthrin-associated toxicity. The lack of a strong concentration–response relationship between increased enzyme levels and bifenthrin toxicity was interesting. The 100x and 500x preparations were essentially equivalent in laboratory water, and the 500x preparation was slightly more efficient in interstitial water. The decreased efficacy of the esterase treatment with bifenthrin could, potentially, be addressed with modifications of the current testing methods. Currently, the esterase is added to the sample and incubated for approximately 1 h before the addition of the organisms. The length of incubation could be increased significantly in an attempt to hydrolyze a greater amount of pyrethroids. However, increasing the length of incubation to 3 h with the H. azteca studies did not make a significant difference in toxicity reduction (data not shown). Therefore, it may be necessary to incubate the esterase with the water sample for several hours or overnight. One could format the assay such that esterase was added to the sample either 12 or 24 h before the initiation of toxicity testing to provide the esterase with additional time to hydrolyze pyrethroids in the water sample. Our data show that even in the most challenging matrix examined during the present study, the Santa Maria interstitial water, significant esterase activity was present more than 8 h after enzyme addition (Table 7).
The enzyme consistently removed 20 ng/L of bifenthrin and up to 200 ng/L of permethrin in interstitial water. Given the challenging nature of the matrix, this observation is useful. Interstitial water can be a complicated matrix, containing fine particles, colloids, organic carbon, and so on [29]. These components can decrease pyrethroid bioavailability and be expected to result in reduced toxicity in interstitial water [5,29,30]. In the context of a TIE, any reduction in toxicity is beneficial and can be used in a weight-of-evidence approach to implicate a cause of toxicity. Even in the most complex system examined in the present study, which used a pyrethroid that is recalcitrant to esterase-induced hydrolysis and a complicated matrix, we were able to observe significant reductions in pyrethroid-associated toxicity. These observations suggest that this method will be useful for detecting the presence of pyrethroids in aquatic testing.
Esterase characterization
One of the vital points to establish in validating the application of esterase treatment for use in TIEs is the specificity of the method. In particular, it is necessary to show unequivocally that the esterase is catalytically removing the pyrethroids (i.e., through enzymatic hydrolysis) and not through nonspecific effects (i.e., adsorption to hydrophobic surfaces). Removal of pyrethroid toxicity through noncatalytic mechanisms is still useful. However, it eliminates the specificity of the method, because other hydrophobic contaminants most likely will be removed as well. Early work had shown that esterase treatment did not affect OP toxicity [13]. However, to demonstrate conclusively that the observed reduction in toxicity following enzyme addition was caused by catalytically active esterase, we performed a number of studies. Exposures conducted with heat-inactivated enzyme and BSA had essentially no effect on pyrethroid-associated toxicity. However, a slight reduction in toxicity was observed with the permethrin exposures, but not in the bifenthrin exposures. It would be expected that the increased hydrophobicity of bifenthrin would result in greater adsorption to the protein than for permethrin. That the opposite results were observed suggests that the reductions were caused by the presence of a small amount of catalytically active enzyme. It is possible that following heat inactivation, some of the protein was capable of refolding to regain some catalytic activity. This theory was supported further by the observation that BSA did not have any significant effects on either permethrin- or bifenthrin-associated toxicity. These data strongly indicate that catalytically active enzyme is essential for the reduction in toxicity.
These observations were verified further by inhibiting the enzyme with two known porcine esterase inhibitors, chlorpyrifos-oxon [13] and OTFP [22]. Interestingly, chlorpyrifos-oxon proved to be ineffective at inhibiting the esterase for extended intervals. Following addition of the inhibitor to the enzyme preparation, activity assays performed before initiation of the toxicity test indicated no remaining catalytic activity. However, activity assays performed at the end of the toxicity study (48 h later) showed that substantial catalytic activity had been restored (72% of control value) (Table 8). These data suggest that esterase inhibited with chlorpyrifos-oxon can be reactivated. These results may be explained by a greater stability of OTFP in our system compared to that of chlorpyrifos-oxon and/or that reactivation of the slow, tight-binding OTFP inhibition occurs more slowly than the reactivation of esterase phosphorylated by chlorpyrifos-oxon. A caution is that thione insecticides in the sample could generate oxon over a period of time. Under some conditions, running a simple colorimetric esterase assay before and after the bioassay could test if the esterase activity has been reduced by some contaminant in the sample water. Previous studies had reported that chlorpyrifos-oxon was a potent inhibitor of porcine esterase, with values for the concentration of inhibitor required to reduce enzyme velocity by 50% in the low nM range, depending on the substrate [13]. In that study, the inhibitor was incubated with the enzyme for only 10 min, as opposed to more than 48 h in the present work. This observation further shows the utility of using carboxylesterase activity to detect pyrethroid toxicity in receiving waters, because the presence of OPs may not inhibit the enzyme significantly over extended intervals. Inhibition of the esterase with OTFP significantly reduced the ability of the enzyme to remove permethrin-associated toxicity. These data suggest that OTFP forms a tighter association with the enzyme than with chlorpyrifos-oxon. Data for other carboxylesterases (e.g., juvenile hormone esterase) have shown that OTFP is a reversible inhibitor, with reactivation times on the order of days to weeks [31]. These results further support the hypothesis that catalytically active esterase is required to achieve removal of pyrethroid-associated toxicity.
A number of different matrices were examined for their effects on esterase activity. Strong matrix effects were observed, showing that each new matrix needs to be validated before the esterase can be used in routine toxicity testing. However, the key point is that current methods call for only a 1-h incubation of the esterase before addition of the test organisms. In every matrix examined, significant catalytic activity was present to at least 8 h after test initiation (Table 7). Therefore, in all matrices examined in the present study, loss of esterase activity most likely is not a significant issue. As expected, both phosphate buffer and Milli-Q water were the most efficient at preserving esterase activity. Interstitial water was the most challenging matrix because of the presence of organic material, but the esterase was still capable of reducing significant pyrethroid-associated toxicity.
The reduction of esterase catalytic efficiency in interstitial water suggests that applying these methods in TIEs with bulk sediment will be more challenging, because excessive particles most likely will reduce the enzyme’s efficiency. However, enzyme could be added to water overlying sediment in a static exposure to hydrolyze (i.e., detoxify) pyrethroids that diffused out of the sediments. Organisms would then be added after an appropriate interval. This method may not be useful for strictly benthic organisms, but it could have applicability to epibenthic organisms. It also could be useful for nektonic or planktonic organisms to determine if a bioavailable fraction of pyrethroid was diffusing from the sediments. For example, enzyme addition would work well in the sediment–water interface exposure system developed by Anderson et al. [32,33]. In this method, organisms are held above the sediment surface in a screened container, ensuring that only fluxed contaminants affect the organism. It is important that this method be developed for use with sediment organisms. Because of the hydrophobic nature of pyrethroids, these chemicals most likely will be detected in sediments and interstitial waters rather than in the water column [5,27]. University of California at Davis researchers have experimented with adding the enzyme to overlying water in H. azteca solid-phase tests and observed reductions in toxicity [8–10]. Currently, studies are underway to refine the method by testing it with pyrethroid-spiked sediments.
Very little metal-induced esterase inhibition was observed, with only copper(II), iron(II), and nickel causing significant decreases in esterase activity (p < 0.05). Such high levels of metals are unlikely to be observed in environmental samples (1 mM). In addition, much of the inhibitory activity was caused by solubility issues, because the limit of solubility of many of the metals examined in the present study was exceeded at 1 mM. Therefore, the presence of metals most likely will not be a major inhibitor of esterase activity in TIE testing. In addition, TIE protocols call for the use of ethylenediaminetetra-acetic acid as a chelating factor to detect the presence of divalent metal cations. Thus, the presence of many metals could be verified independently in a TIE protocol via the ethylenediaminetetra-acetic acid method or use of a cation solid-phase extraction column if metals were suspected (e.g., based on land-use practices where the sample was collected). In addition, the greatest level of inhibition observed was approximately 31% with copper(II), which means that 69% of esterase activity remained to hydrolyze pyrethroids. Taken together, these results strongly suggest that metals will not interfere with the use of esterase in TIE protocols.
Reproducibility of the esterase solution
An important issue in the use of esterase activity during routine toxicity testing is the homogeneity and reproducibility of the esterase preparation. Because the esterase used in this project is a commercial product, it is widely available to the research and toxicity testing communities. The manufacturer provides quality-assurance information for the product on the company website (http://www.sigmaaldrich.com). No expiration date for either esterase preparation has been established by the company. The activity of the enzyme has been reassayed every two to three years after the manufacturing date and reported to be essentially constant. However, a survey of the available esterase preparations from Sigma Chemical shows that enzyme activity of the commercial product has decreased steadily since the inception of this project. The currently available lot of the ammonium sulfate preparation has a reported activity of 130 U/mg, as opposed to 184 U/mg for the enzyme used in the present study. Our experiments have shown a slight loss in activity of the liquid preparation over six months when stored at 4°C (data not shown). However, an important issue is that the liquid preparation is a heterogeneous suspension. Therefore, transferring small amounts of the enzyme has increased variability. Experiments in which 2 μl of esterase were aliquotted to a sample container led to relative standard deviations (RSDs) as high as 21% (n = 3) as opposed to the transfer of 10 μl, which never exceeded 10% RSD (n = 3) (data not shown). Initial studies with H. azteca attempted to transfer small quantities of esterase directly from the vial to the testing container (data not shown). This method resulted in extremely high RSDs, and it was established that a single stock solution should be generated and larger volumes transferred to the testing containers. These techniques greatly reduced variation.
To compare vial-to-vial variability, we examined the esterase activity in five different bottles from the same lot number, all purchased at the same time, and found an approximately 11% RSD among the five bottles (data not shown). This deviation can easily be attributed to experimental variability, indicating that vial-to-vial reproducibility is acceptable. Esterase activity in these same five bottles was monitored weekly for six months, and no change in average percentage RSD was observed. Another important issue is the variability in activity between enzyme lots. Because these preparations come from different batches of porcine liver tissue, concern exists that isozyme abundance and activity may vary on a batch-to-batch basis. Indeed, some of our initial experiments observed differences in the ability to remove pyrethroid-associated toxicity (data not shown). Therefore, toxicity testing laboratories most likely will need to purchase a given amount of esterase and characterize its activity and pyrethroid-removal ability in their testing system. This characterization would remain constant as long as they had the same batch of enzyme, but it could be verified every few months for quality-control purposes with either a standard activity assay using an esterase substrate (e.g., PNPA) or in combination with organism exposure to a known concentration of pyrethroid. According to data supplied by the manufacturer as well as that generated in our laboratory, the activity of the enzyme should not decrease significantly during storage (under appropriate conditions). These procedures would enable the appropriate quality controls required in a toxicity testing laboratory.
Both enzyme preparations were effective at removing pyrethroid-associated toxicity, but elevated levels of ammonia associated with the liquid preparation are of some concern if high concentrations of esterase are required. The ammonia can be removed with a quick buffer-exchange step. An advantage of a clean-up step would be the ability to concentrate the enzyme further and to increase the specific activity. This step would enable the addition of higher levels of active esterase while maintaining relatively low levels of overall protein. Filtering of the enzyme preparation through a 50K Centricon filter served to increase the specific activity (data not shown). The enzyme also could be dialyzed before use or ethanol-precipitated to remove ammonium sulfate. A number of other manipulations are possible that could increase the efficacy of the current assay, but a trade-off exists between efficiency and efficacy. An advantage of the current method is the ease of application, but some laboratories might not have the equipment necessary to implement these additional procedures. Based on the present results, addition of a clean-up step does not appear to be necessary, but it could become useful in the presence of more complicated matrices. In the future, the use of sol gel–encapsulated esterase or of polyethylene glycol–or glutaraldehyde-stabilized esterase could be employed to increase the robustness of the procedure.
Other esterases also are available commercially, including a rabbit liver esterase that has increased activity compared to the lyophilized porcine preparation (75 U/mg vs 20 U/mg for the preparations used in the present study; Sigma Chemical). The main disadvantage of the rabbit enzyme is the cost, which is approximately 80-fold greater than that of the porcine enzyme. Another possibility for improving the efficacy of the method would be to move away from a crude enzyme preparation and use a purified carboxylesterase. The identification and isolation of a pyrethroid-hydrolyzing esterase has been reported in the literature [34]. If this type of esterase could be expressed and purified on a large scale and made commercially available, it could be an ideal solution for use in a TIE. The esterase also could be prepared in a low-ammonium-salt buffer to prevent the complications associated with ammonia toxicity. This solution is the most attractive in terms of producing the optimal assay. However, a number of scientific and commercial hurdles need to be overcome before it could be practical.
Application of esterase activity in TIE studies
The present study has demonstrated that the inclusion of esterase activity in phase I TIE protocols has the promise of being a useful assay. These early studies were performed with pyrethroid concentrations that most likely are much greater than the levels that would be experienced in environmental settings. It was deemed to be advantageous to use elevated pyrethroid concentrations for a number of reasons. First and foremost, it was necessary to achieve essentially 100% mortality in the testing organisms to demonstrate that the organisms could be rescued from toxicity. Second, because the main effort of this work was to demonstrate the efficacy of the esterase preparation, it was thought that performing the studies at greater pyrethroid concentrations would show better proof of concept. If the esterase is effective at removing multiple toxic units (defined as 100/EC50) of pyrethroid toxicity, it also should be effective at reducing toxicity at lower pyrethroid concentrations. In all cases during the present study, the esterase demonstrated the greatest efficacy at reducing toxicity from lower pyrethroid concentrations relative to higher concentrations (Tables 2, 3, and 6). Therefore, this trend is expected to continue at even lower, more environmentally realistic pyrethroid concentrations.
It is vital that future studies validate the specificity of the esterase treatment for the reduction of pyrethroid toxicity. Results of previous studies with OPs (diazinon and chlorpyrifos) suggested that the method was selective [13]. However, these were isolated studies and were not performed within the context of a full phase I TIE. Therefore, it is advised that validation studies work on establishing the specificity of the method. This objective could be accomplished using real-world samples that have been shown (via analytical confirmation) to contain pyrethroids and/or spiking studies. The study should be conducted with a range of partially to very toxic concentrations of chemicals other than pyrethroids, to which the carboxylesterase can be added to ascertain whether the enzyme/protein removes toxicity to the test organisms in a nonspecific manner. These experiments should be performed with a range of commonly occurring toxicants in aqueous samples that possess different physicochemical properties and different modes/mechanisms of action. For example, the inclusion of metals (e.g., copper and zinc), OPs, carbamates, and other physical stressors along with the pyrethroids would be useful for establishing the specificity of the esterase treatment. By performing these studies with multiple organisms in a variety of matrices, such as interstitial water and agricultural or storm-water runoff, the utility and specificity of the method could be established.
This method probably will be most applicable at the Phase I stage of a TIE. Previous work described a flowchart for how an esterase treatment could be included into the TIE process [13]. It may not be necessary to employ the esterase treatment for all TIE samples, but it could be used for samples with an origin that has resulted in a high likelihood of pyrethroids. For example, if the sample is from agricultural runoff or sediment from sites of known agricultural drainage, then including the esterase treatment step in the TIE process is logical. If the presence of pyrethroids is indicated based on the TIE process, then confirmation studies could be performed with more advanced (and expensive) techniques, such as mass spectrometry. An additional advantage of the esterase treatment method is that it enables toxicity testing laboratories to perform the initial tests for pyrethroids in-house rather than sending samples out to external laboratories for analytical analyses. Following the successful completion of the validation studies with the real-world TIE samples discussed above, it will be necessary to develop a protocol for the inclusion of the esterase treatment that is suitable for U.S. EPA approval. The first steps toward this protocol have been established by the present as well as previous studies [13]. Completion of the validation studies described above as well as development of the methods for standardizing the commercial esterase preparation are the next logical steps in this process.
CONCLUSION
The ability of an esterase treatment to detect/remediate pyrethroid-associated toxicity in three different matrices using two different organisms was determined. Results indicate that carboxylesterase addition would be a useful technique for inclusion in TIEs, allowing for the selective removal of pyrethroid-associated toxicity. However, concerns remain about the standardization of the method from batch-to-batch and across different laboratories. The present study shows that the esterase preparation is robust and not significantly affected by the presence of metals or OPs. In addition, it is effective in a variety of matrices, including interstitial water. Further attempts to refine the assay could extend the length of time that the esterase is incubated with the sample before addition of the organism. Increased incubation times likely will increase both esterase efficacy as well as potentially reduce the level of esterase required in the assay. It also would be interesting to examine the utility of the esterase treatment in additional matrices with other organisms. In particular, it would be beneficial to format the assay for use in vertebrate assays, such as those with fat-head minnows (Pimephales promelas), a standard U.S. EPA test species, and it would be valuable to extend this assay to estuarine or marine organisms, such as the estuarine amphipod Eohaustorius estuarius. Given the efficacy of the esterase in seawater, applying this treatment to these system most likely will be straightforward. Further studies also should focus on the applicability of the method for use in sediment TIEs, because the majority of the pyrethroid toxicity is expected to be found in sediments rather than the water column. The use of carboxylesterase activity to remove/detect pyrethroid-associated toxicity in aquatic and sediment samples is a simple, cost-effective, and mechanistically based assay that can be formatted readily to a range of matrices and testing organisms. This method should be useful in monitoring for the presence of pyrethroids in receiving waters, interstitial waters, and potentially, in bulk sediments.
Acknowledgments
C.E. Wheelock was supported by National Institutes of Health (NIH) postdoctoral training grant T32 DK07355-22 and a Japanese Society for the Promotion of Science postdoctoral fellowship. The present work was supported, in part, by National Institute of Environmental Health Sciences (NIEHS) grant R37 ES02710, NIEHS Superfund grant P42 ES04699, NIEHS Center for Environmental Health Sciences grant P30 ES05707, and NIH/National Institute of Allergy and Infectious Diseases grant U01 AI058267. Funding also was provided by the State Water Resources Control Board (Proposition 13 Pesticide Research and Identification of Source, and Mitigation Grant Agreements 04-195-552-0 and 04-135-55-20). The authors thank two anonymous reviewers for providing valuable criticism of the manuscript.
References
- 1.Epstein L, Bassein S, Zalom FG. Almond and stone fruit growers reduce OP, increase pyrethroid use in dormant sprays. Calif Agric. 2000;54:14–19. [Google Scholar]
- 2.Denton DL, Wheelock CE, Murray S, Deanovic LA, Hammock BD, Hinton DE. Joint acute toxicity of esfenvalerate and diazinon to fathead minnow (Pimephales promelas) larvae. Environ Toxicol Chem. 2003;22:336–341. [PubMed] [Google Scholar]
- 3.Werner I, Zalom FG, Oliver MN, Deanovic LA, Kimball TS, Henderson JD, Wilson BW, Krueger W, Wallender WW. Toxicity of storm-water runoff after dormant spray application in a French prune orchard, Glenn County, California, USA: Temporal patterns and the effect of ground covers. Environ Toxicol Chem. 2004;23:2719–2726. doi: 10.1897/03-572. [DOI] [PubMed] [Google Scholar]
- 4.Weston DP, You J, Lydy MJ. Distribution and toxicity of sediment-associated pesticides in agriculture-dominated water bodies of California’s Central Valley. Environ Sci Technol. 2004;38:2752–2759. doi: 10.1021/es0352193. [DOI] [PubMed] [Google Scholar]
- 5.Amweg EL, Weston DP, Ureda NM. Use and toxicity of pyrethroid pesticides in the Central Valley, California, USA. Environ Toxicol Chem 24:966–972. Correction. 2005;24:1300–1301. doi: 10.1897/04-146r1.1. [DOI] [PubMed] [Google Scholar]
- 6.Wheelock CE, Eder KJ, Werner I, Huang H, Jones PD, Brammell BF, Elskus AA, Hammock BD. Individual variability in esterase activity and CYP1A levels in Chinook salmon (Oncorhynchus tshawytscha) exposed to esfenvalerate and chlorpyrifos. Aquat Toxicol. 2005;74:172–192. doi: 10.1016/j.aquatox.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson BS, Phillips BM, Hunt JW, Huntley SA, Worcester K, Kapellas N, Tjeerdema RS. Evidence of pesticide impacts in the Santa Maria River watershed (California, USA) Environ Toxicol Chem. 2006;25:1160–1170. doi: 10.1897/05-231r.1. [DOI] [PubMed] [Google Scholar]
- 8.Huntley SA, Phillips BM, Anderson BS, Hunt JW, Tjeerdema RS, Richard N, Worcester K. 2004. Causes of sediment toxicity in the lower Santa Maria River watershed (California, USA). Proceedings, Society of Environmental Toxicology and Chemistry, Portland, OR, USA, November 14–18, p 75.
- 9.Phillips BM, Huntley SA, Anderson BS, Hunt JW, Tjeerdema RS, Carpio-Obeso M, Connor V. 2005. Causes of toxicity in the New River. Proceedings, Northern California Society of Environmental Toxicology and Chemistry, Berkeley, CA, USA, May 3–4, p 29.
- 10.Phillips BM, Anderson BS, Hunt JW, Tjeerdema RS, Worcester K, Rowan J, Carpio-Obeso M, Richard N. 2005. Characterization and identification of pyrethroid toxicity in sediment: A review of TIE case studies. Proceedings, Northern California Society of Environmental Toxicology and Chemistry, Berkeley, CA, USA, May 3–4, p 17.
- 11.Werner I, Deanovic LA, Hinton DE, Henderson JD, de Oliveira GH, Wilson BW, Krueger W, Wallender WW, Oliver MN, Zalom FG. Toxicity of stormwater runoff after dormant spray application of diazinon and esfenvalerate (Asana) in a French prune orchard, Glenn County, California, USA. Bull Environ Contam Toxicol. 2002;68:29–36. doi: 10.1007/s00128-001-0215-7. [DOI] [PubMed] [Google Scholar]
- 12.Lydy MJ, Beldin J, Wheelock CE, Hammock BD, Denton DL. Challenges in regulating pesticide mixtures. Ecology and Society. 2004;9:1. [Google Scholar]
- 13.Wheelock CE, Miller JL, Miller MG, Shan G, Gee SJ, Hammock BD. Development of toxicity identification evaluation procedures for pyrethroid detection using esterase activity. Environ Toxicol Chem. 2004;23:2699–2708. doi: 10.1897/03-544. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Environmental Protection Agency. 1991. Methods for aquatic toxicity identification evaluations. Phase I toxicity characterization procedures. EPA-600/6-91/003. Office of Research and Development, Duluth, MN.
- 15.U.S. Environmental Protection Agency. 1993. Methods for aquatic toxicity identification evaluations: Phase II toxicity identification procedures for samples exhibiting acute and chronic toxicity. EPA-600/R-92/080. Office of Research and Development, Duluth, MN.
- 16.U.S. Environmental Protection Agency. 1993. Methods for aquatic toxicity identification evaluations: Phase III toxicity confirmation procedures for samples exhibiting acute and chronic toxicity. EPA-600/R-92/081. Office of Research and Development, Duluth, MN.
- 17.Wheelock CE, Shan G, Ottea JA. Overview of carboxylesterases and their role in metabolism of insecticides. J Pestic Sci. 2005;30:75–83. [Google Scholar]
- 18.Gupta RC, Dettbarn WD. Role of carboxylesterases in the prevention and potentiation of N-methylcarbamate toxicity. Chem-Biol Interact. 1993;87:295–303. doi: 10.1016/0009-2797(93)90057-6. [DOI] [PubMed] [Google Scholar]
- 19.Casida JE, Quistad GB. Organophosphate toxicology: Safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- 20.Abernathy CO, Casida JE. Pyrethroid insecticides: Esterase cleavage in relation to selective toxicity. Science. 1973;179:1235–1236. doi: 10.1126/science.179.4079.1235. [DOI] [PubMed] [Google Scholar]
- 21.Phillips BM, Anderson BS, Hunt JW, Nicely PA, Tjeerdema RS, Rowan J, Crane D, Mekebri A. 2003. Preliminary identification of bifenthrin toxicity to Hyalella azteca in sediment from the Central Valley, California. Proceedings, Society of Environmental Toxicology and Chemistry, Austin, TX, USA, November 9–13, p 136.
- 22.Wheelock CE, Severson TF, Hammock BD. Synthesis of new carboxylesterase inhibitors and evaluation of potency and water solubility. Chem Res Toxicol. 2001;14:1563–1572. doi: 10.1021/tx015508+. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Environmental Protection Agency. 2000. Methods for measuring the toxicity and bioaccumulation of sediment-associated contaminants with freshwater invertebrates. EPA 600/R-99/064. Office of Research and Development, Washington, DC.
- 24.U.S. Environmental Protection Agency. 2002. Methods for measuring acute toxicity of effluents and receiving water to freshwater and marine organisms. EPA 821/R-02/021. Office of Research and Development, Washington, DC.
- 25.Ljungquist A, Augustinsson KB. Purification and properties of two carboxylesterases from rat-liver microsomes. Eur J Biochem. 1971;23:303–313. doi: 10.1111/j.1432-1033.1971.tb01622.x. [DOI] [PubMed] [Google Scholar]
- 26.Ankley GT, Schubauer-Berigan MK, Monson PD. Influence of pH and hardness on toxicity of ammonia to the amphipod Hyalella azteca. Can J Fish Aquat Sci. 1995;52:2078–2083. [Google Scholar]
- 27.Laskowski DA. 2002. Physical and chemical properties of pyrethroids. In Ware GW, ed, Reviews of Environmental Contamination and Toxicology, Vol 174. Springer-Verlag, New York, NY, USA, pp 49–170. [DOI] [PubMed]
- 28.Ankley GT, Dierkes JR, Jensen DA, Peterson GS. Piperonyl butoxide as a tool in aquatic toxicological research with organophosphate insecticides. Ecotoxicol Environ Saf. 1991;21:266–274. doi: 10.1016/0147-6513(91)90065-w. [DOI] [PubMed] [Google Scholar]
- 29.Williamson B, Burgess RM. 2003. Sediment and pore-water chemistry. In Carr RS, Nipper M, eds, Porewater Toxicity Testing: Biological, Chemical, and Ecological Considerations. SETAC, Pensacola, FL, USA, pp 63–94.
- 30.Landrum PF, Nihard SR, Eadie BJ, Herche LR. Reductions in bioavailability of organic xenobiotics to Pontoporeia hoyi in the presence of humic and fulvic materials and natural dissolved organic matter. Environ Toxicol Chem. 1985;4:459–467. [Google Scholar]
- 31.Abdel-Aal YAI, Hammock BD. Transition state analogs as ligands for affinity purification of juvenile hormone esterase. Science. 1986;233:1073–1075. doi: 10.1126/science.3738525. [DOI] [PubMed] [Google Scholar]
- 32.Anderson BS, Hunt JW, Hester MM, Phillips BM. 1996. Assessment of sediment toxicity at the sediment–water interface. In Ostrander GK, ed, Techniques in Aquatic Toxicology. Lewis, Boca Raton, FL, USA, pp 609–624.
- 33.Anderson BS, Hunt JW, Phillips BM, Fairey R, Puckett HM, Stephenson M, Taberski K, Newman J, Tjeerdema RS. Influence of sample manipulation on contaminant flux and toxicity at the sediment–water interface. Mar Environ Res. 2001;51:191–211. doi: 10.1016/s0141-1136(00)00034-9. [DOI] [PubMed] [Google Scholar]
- 34.Stok J, Huang H, Jones PJ, Wheelock CE, Morisseau C, Hammock BD. Identification, expression and purification of a pyrethroid hydrolyzing carboxylesterase from mouse liver microsomes. J Biol Chem. 2004;279:29863–29869. doi: 10.1074/jbc.M403673200. [DOI] [PubMed] [Google Scholar]


