Abstract
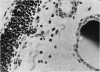
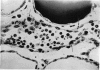
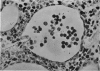
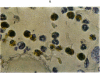
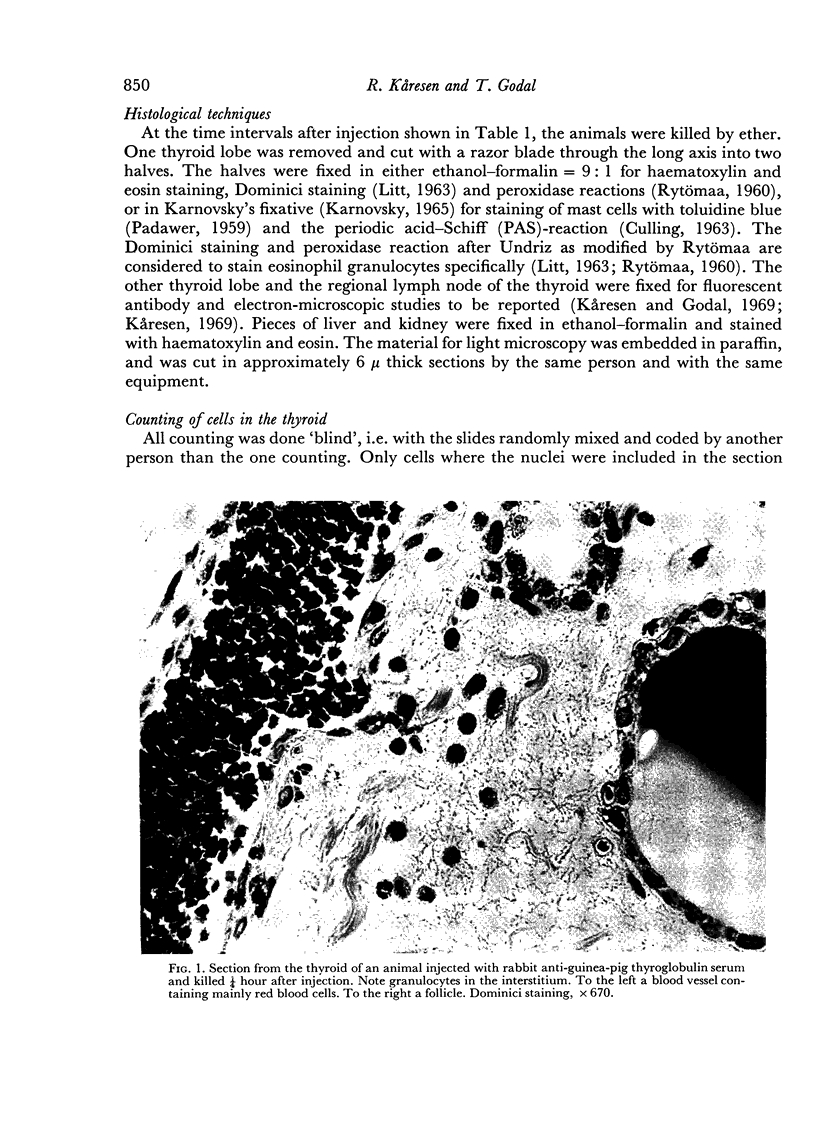
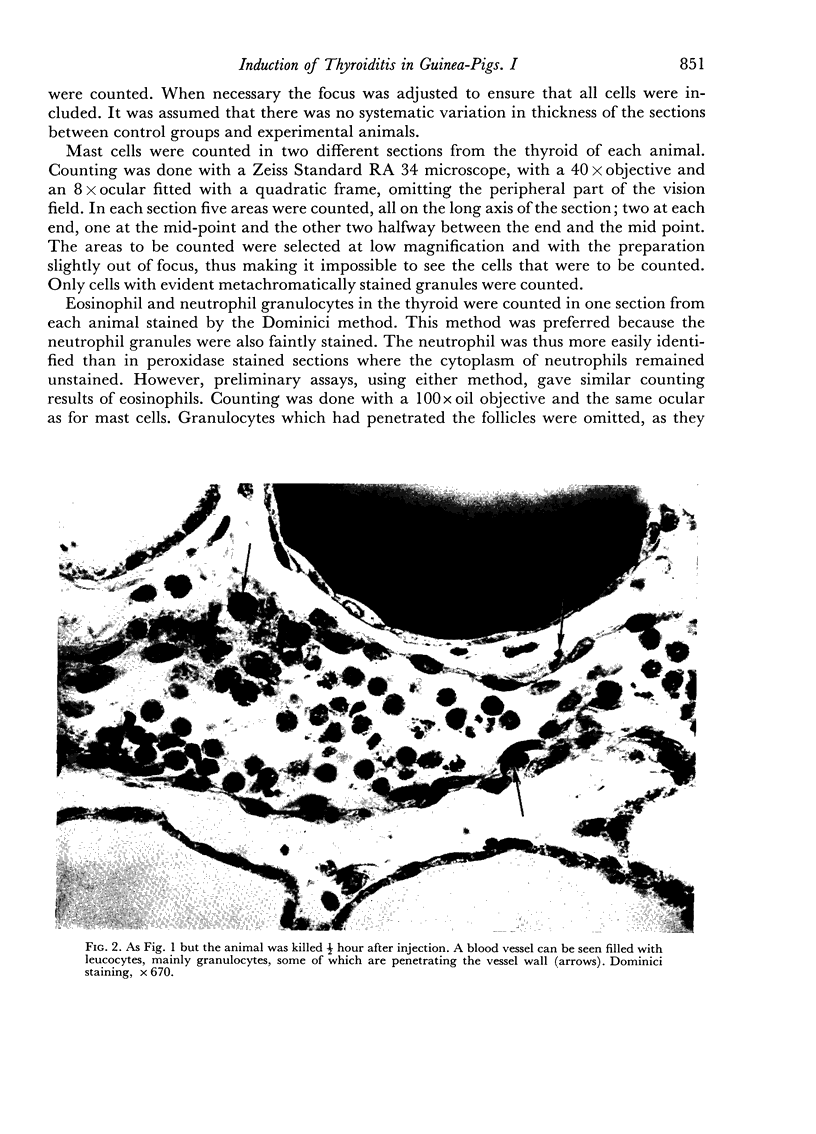
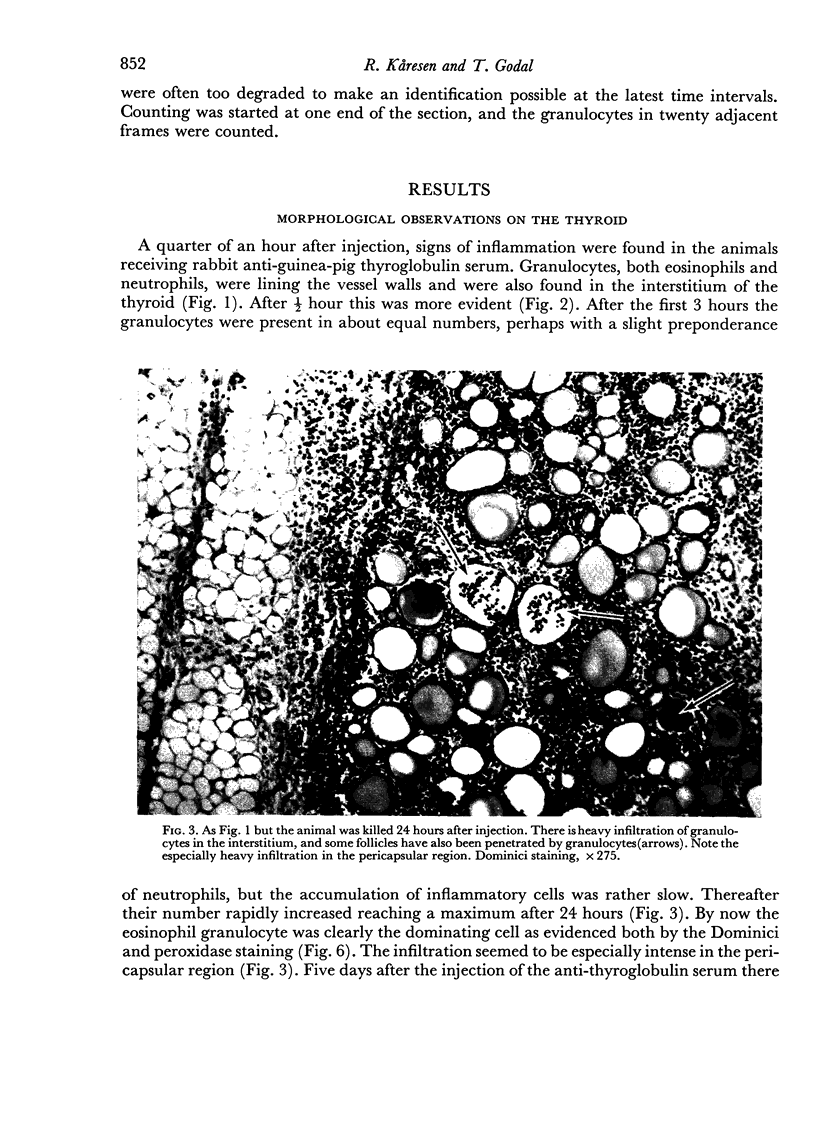
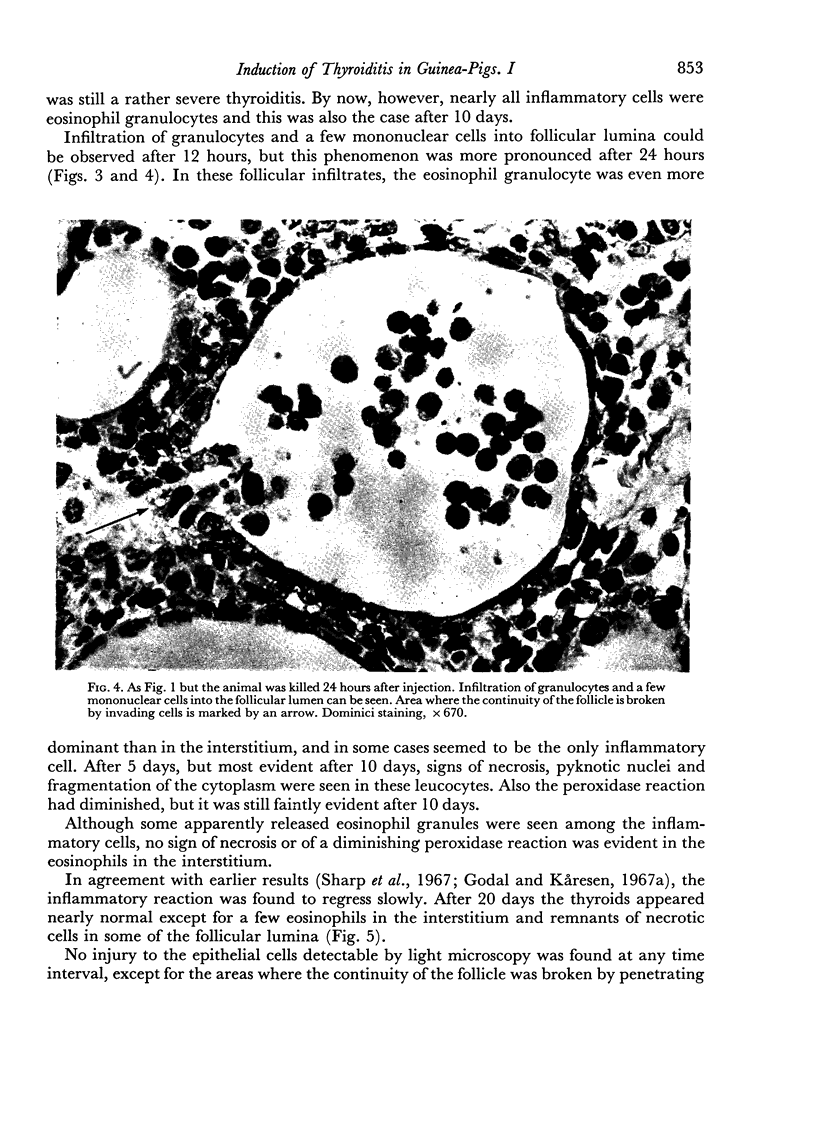
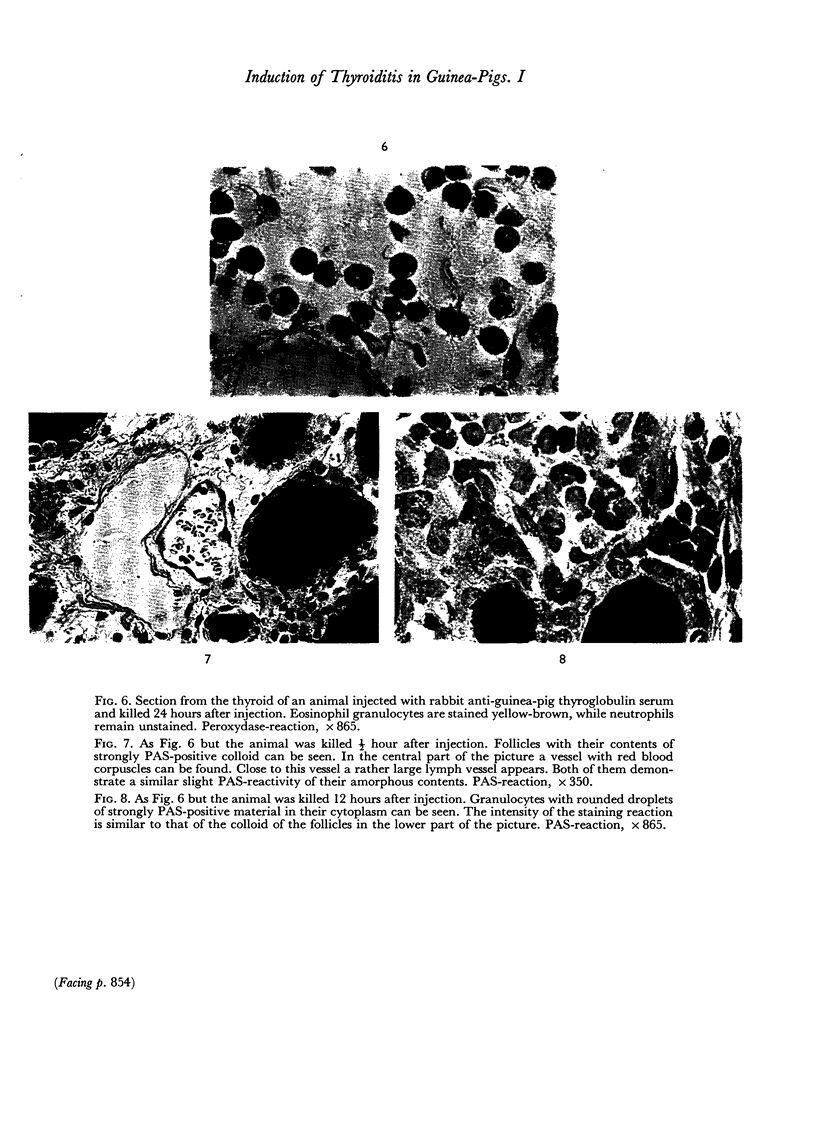
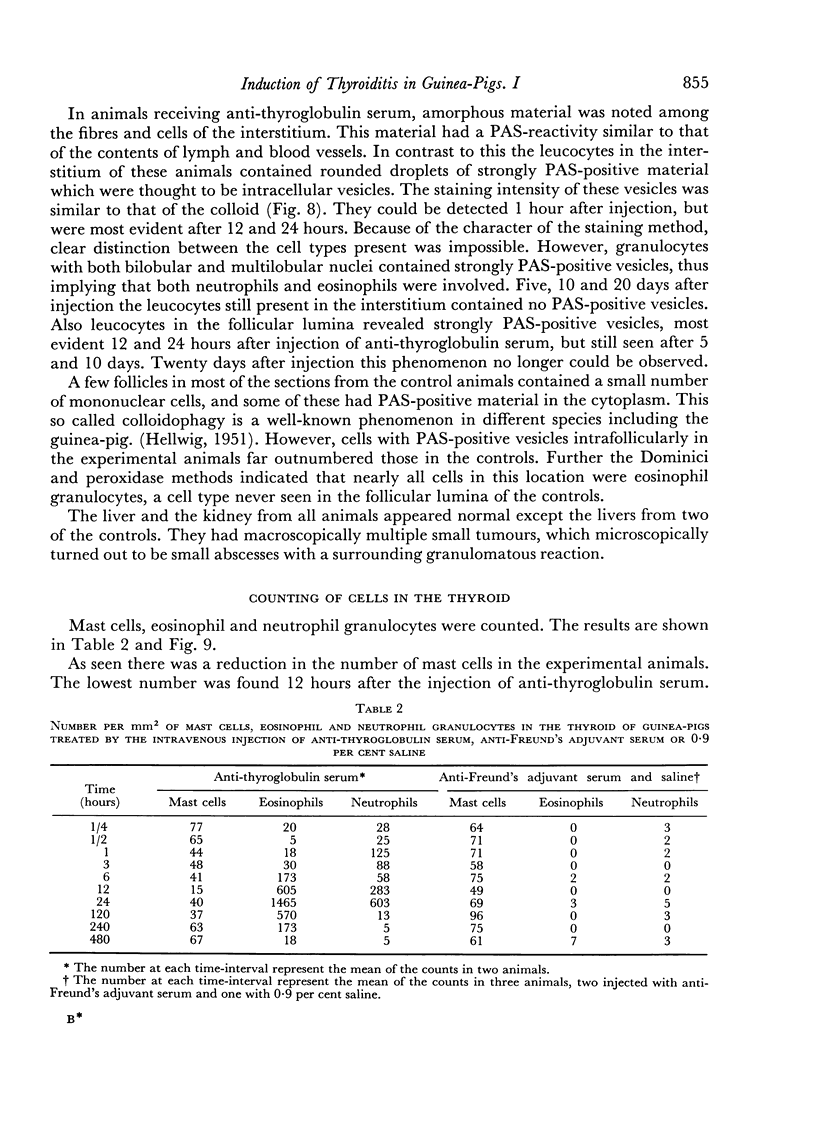
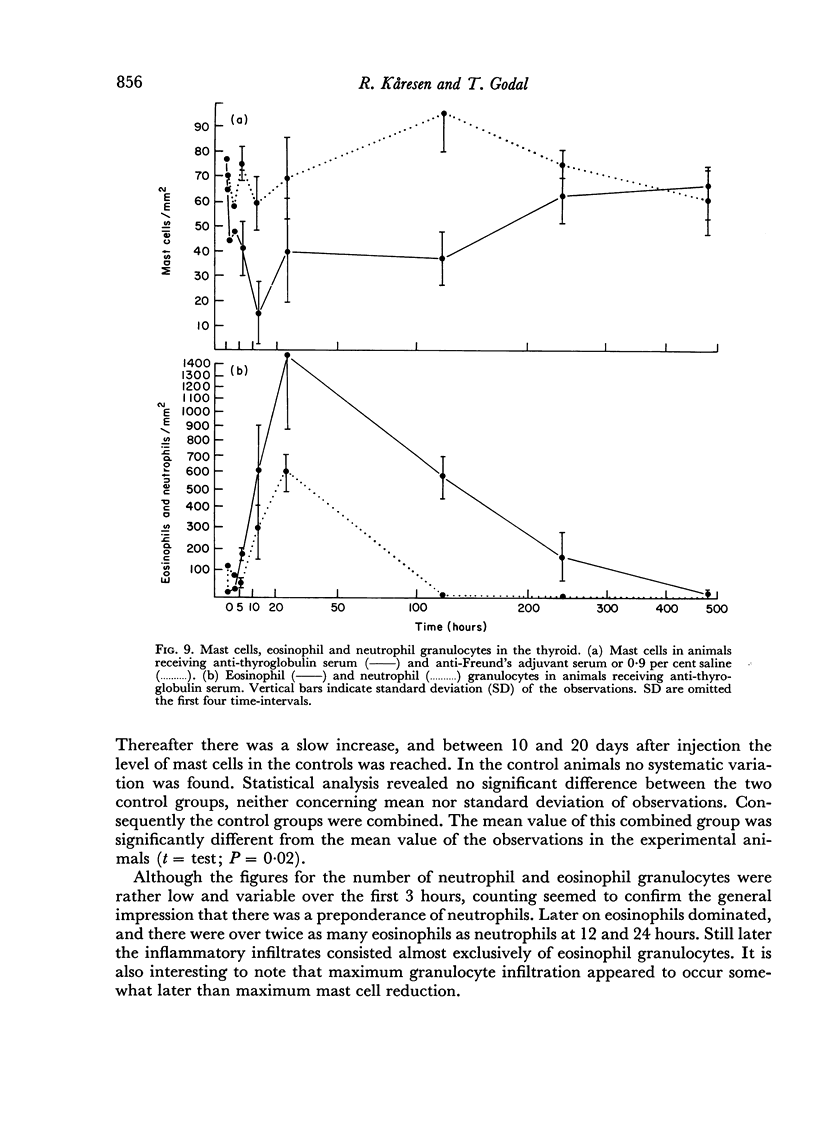
An inflammatory reaction in the thyroid of guinea-pigs, induced by intravenous injection of rabbit anti-guinea-pig thyroglobulin serum, has been studied at time intervals ranging from ¼ hour to 20 days after injection. Specific staining methods for eosinophils have been used to demonstrate that the inflammatory infiltrate mainly consisted of eosinophil granulocytes, but that neutrophil granulocytes were also present at the earliest time intervals. PAS-staining revealed that the granulocytes contained rounded droplets of material with a PAS-reactivity comparable to that of the colloid. This was most clearly seen 12 and 24 hours after injection. The possibility that this material represents phagocytosed thyroglobulin perhaps in an antigen—antibody complex has been suggested. The number of mast cells was counted and degranulation, which was apparently most extensive 12 hours before maximum granulocyte infiltration, was observed. Possible mechanisms involved in eosinotaxis and uptake of antigen—antibody complexes in the granulocytes have been discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHER G. T., HIRSCH J. G. MOTION PICTURE STUDIES ON DEGRANULATION OF HORSE EOSINOPHILS DURING PHAGOCYTOSIS. J Exp Med. 1963 Aug 1;118:287–294. doi: 10.1084/jem.118.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. T., McGovern V. J. Mast cell changes in rats with eosinophilia. J Pathol Bacteriol. 1968 Jan;95(1):217–224. doi: 10.1002/path.1700950126. [DOI] [PubMed] [Google Scholar]
- BOREUS L. O., CHAKRAVARTY N. The histamine content of guinea pig mast cells. Experientia. 1960 May 15;16:192–192. doi: 10.1007/BF02178981. [DOI] [PubMed] [Google Scholar]
- COCHRANE C. G., WEIGLE W. O., DIXON F. J. The role of polymorphonuclear leukocytes in the initiation and cessation of the Arthus vasculitis. J Exp Med. 1959 Sep 1;110:481–494. doi: 10.1084/jem.110.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. G., SAPP T. M. Experimental eosinophilia. IV. Eosinotactic influences of polysaccharides. Exp Mol Pathol. 1963 Feb;2:74–82. doi: 10.1016/0014-4800(63)90009-1. [DOI] [PubMed] [Google Scholar]
- COHEN S. G., SAPP T. M., GALLIA A. R. Experimental eosinophilia V. Specificity of regional lymph node responses to antigen-antibody systems. Proc Soc Exp Biol Med. 1963 May;113:29–32. doi: 10.3181/00379727-113-28266. [DOI] [PubMed] [Google Scholar]
- Chapman J. S., Clark J. Chemical stimulus to eosinophils. Am J Clin Pathol. 1968 Jun;49(6):815–820. doi: 10.1093/ajcp/49.6.815. [DOI] [PubMed] [Google Scholar]
- Cohen S. G., Sapp T. M. Experimental eosinophilia. 8. Cellular responses to altered globulins within cutaneous tissue. J Allergy. 1965 Sep-Oct;36(5):415–422. doi: 10.1016/0021-8707(65)90135-8. [DOI] [PubMed] [Google Scholar]
- Daniel P. M., Pratt O. E., Roitt I. M., Torrigiani G. The release of thyroglobulin from the thyroid gland into thyroid lymphatics; the identification of thyroglobulin in the thyroid lymph and in the blood of monkeys by physical and immunological methods and its estimation by radioimmunoassay. Immunology. 1967 May;12(5):489–504. [PMC free article] [PubMed] [Google Scholar]
- Eriksen J. Immunochemical studies on some serological cross-reactions in the Klebsiella group. 12. Serological reactions with the acidic capsular polysaccharides from Klebsiella type 3(c) as antigen. Acta Pathol Microbiol Scand. 1965;64(4):522–526. doi: 10.1111/apm.1965.64.4.522. [DOI] [PubMed] [Google Scholar]
- Gigli I., Nelson R. A., Jr Complement dependent immune phagocytosis. I. Requirements for C'1, C'4, C'2, C'3. Exp Cell Res. 1968 Jul;51(1):45–67. doi: 10.1016/0014-4827(68)90158-4. [DOI] [PubMed] [Google Scholar]
- HELLWIG C. A. Colloidophagy in the human thyroid gland. Science. 1951 Jun 22;113(2947):725–726. doi: 10.1126/science.113.2947.725. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H., MOTA I. The mechanism of anaphylaxis: specificity of antigen-induced mast cell damage in anaphylaxis in the guinea pig. Immunology. 1959 Jan;2(1):31–43. [PMC free article] [PubMed] [Google Scholar]
- Keller H. U., Sorkin E. Chemotaxis of leucocytes. Experientia. 1968 Jul 15;24(7):641–652. doi: 10.1007/BF02138287. [DOI] [PubMed] [Google Scholar]
- Keller R. Interrelations between different types of cells. II. Histamine-release from the mast cells of various species by cationic polypeptides of polymorphonuclear leukocyte lysosomes and other cationic compounds. Int Arch Allergy Appl Immunol. 1968;34(2):139–144. [PubMed] [Google Scholar]
- Kåresen R., Godal T. Induction of thyroiditis in guinea-pigs by intravenous injection of rabbit anti-guinea-pig thyroglobulin serum. II. Studies with fluorescent antibody technique. Immunology. 1969 Dec;17(6):863–874. [PMC free article] [PubMed] [Google Scholar]
- LITT M. STUDIES IN EXPERIMENTAL EOSINOPHILIA. VI. UPTAKE OF IMMUNE COMPLEXES BY EOSINOPHILS. J Cell Biol. 1964 Nov;23:355–361. doi: 10.1083/jcb.23.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITT M. Studies in experimental eosinophilia. III. The induction of peritoneal eosinophilia by the passive transfer of serum antibody. J Immunol. 1961 Nov;87:522–529. [PubMed] [Google Scholar]
- LITT M. Studies in experimental eosinophilia. V. Eosinophils in lynph nodes of guinea pigs following primary antigenic stimulation. Am J Pathol. 1963 May;42:529–549. [PMC free article] [PubMed] [Google Scholar]
- Nakamura R. M., Weigle W. O. In vivo behavior of homologous and heterologous thyroglobulin and induction of immunologic unresponsiveness to heterologous thyroglobulin. J Immunol. 1967 Apr;98(4):653–662. [PubMed] [Google Scholar]
- PADAWER J. A stain for mast cells and its application in various vertebrat43es and in a mastocytoma. J Histochem Cytochem. 1959 Sep;7:352–353. doi: 10.1177/7.5.352. [DOI] [PubMed] [Google Scholar]
- Parish W. E., Coombs R. R. Peripheral blood eosinophilia in guinea-pigs following implantation of anaphylactic guinea-pig and human lung. Br J Haematol. 1968 Apr;14(4):425–445. doi: 10.1111/j.1365-2141.1968.tb06994.x. [DOI] [PubMed] [Google Scholar]
- Roberts A. N. Rapid uptake of tritiated antigen by mouse eosinophils. Nature. 1966 Apr 16;210(5033):266–269. doi: 10.1038/210266a0. [DOI] [PubMed] [Google Scholar]
- SABESIN S. M. A function of the eosinophil: phagocytosis of antigen-antibody complexes. Proc Soc Exp Biol Med. 1963 Mar;112:667–670. doi: 10.3181/00379727-112-28134. [DOI] [PubMed] [Google Scholar]
- SPEIRS R. S., SPEIRS E. E. CELLULAR REACTIONS TO REINJECTION OF ANTIGEN. J Immunol. 1964 Apr;92:540–549. [PubMed] [Google Scholar]
- Scherer J., Janoff A. Mediators of inflammation in leukocyte lysosomes. VII. Observations on mast cell-rupturing agents in different species. Lab Invest. 1968 Feb;18(2):196–202. [PubMed] [Google Scholar]
- Sharp G. C., Wortis H. H., Dunmore B. The biological effects of anti-thyroid antibodies. Thyroid eosinophilia following passive transfer of anti-thyroglobulin antibody. Immunology. 1967 Jul;13(1):39–48. [PMC free article] [PubMed] [Google Scholar]