Abstract
Delayed onset muscle soreness (DOMS) is quite common, but the mechanism for this phenomenon is still not understood; even the existence of muscle tenderness (mechanical hyperalgesia) has not been demonstrated in experimental models. We developed an animal model of DOMS by inducing eccentric contraction (lengthening contraction, ECC) to the extensor digitorum longus muscle (EDL), and investigated the existence of mechanical hyperalgesia in the EDL by means of behavioural pain tests (Randall-Selitto test and von Frey hair test, applied to/through the skin on the EDL muscle) and c-Fos expression in the spinal dorsal horn. We found that the mechanical withdrawal threshold measured with the Randall-Selitto apparatus decreased significantly between 1 and 3 days after ECC, while that measured by von Frey hairs did not. The group that underwent stretching of the muscle only (SHAM group) showed no change in mechanical pain threshold in either test. These results demonstrated that the pain threshold of deep tissues (possibly of the muscle) decreased after ECC. c-Fos immunoreactivity in the dorsal horn (examined 2 days after ECC/SHAM exercise) was not changed by either ECC or compression (1568 mN) to the EDL muscle by itself, but it was significantly increased by applying compression to the EDL muscle 2 days after ECC. This increase was observed in the superficial dorsal horn of the L4 segment of the ipsilateral side, and was clearly suppressed by morphine treatment (10 mg kg−1, i.p.). These results demonstrated the existence of mechanical hyperalgesia in the muscle subjected to ECC. This model could be used for future study of the neural mechanism of muscle soreness.
Delayed onset muscle soreness (DOMS) is described as an unpleasant sensation or pain after unaccustomed strenuous exercise, and is quite common in humans (Armstrong, 1984). The most characteristic symptom in DOMS is tenderness, a kind of mechanical hyperalgesia, in the exercised muscle. It usually reaches a peak some 24–48 h after exercise in humans and disappears within 3–7 days (Armstrong, 1984; Newham, 1988; Graven-Nielsen & Arendt-Nielsen, 2003). There is usually no spontaneous pain (Graven-Nielsen & Arendt-Nielsen, 2003).
While the mechanism underlying DOMS remains unclear, eccentric muscular work (contraction while the muscle is being stretched) is known to cause DOMS more effectively than concentric work (Armstrong et al. 1983; Newham, 1988; Pyne, 1994). Eccentric exercise has been widely used in human and animal studies, and histological (Armstrong et al. 1983; McCurry & Faulkner, 1985; Friden & Lieber, 1998), ultrastructural (Newham et al. 1983; Ogilvie et al. 1988), biochemical (Armstrong et al. 1983; Ostrowski et al. 1998; Blais et al. 1999), and physical (Proske & Morgan, 2001) changes have been found. However, the mechanism of mechanical hyperalgesia has not yet been clarified, and even the existence of mechanical hyperalgesia (tenderness) itself, one of the most typical symptoms in DOMS, has not been confirmed in experimental animals. We could find just one paper reporting that reflex EMG activity from biceps femoris muscle, taken as an index of pain, was elicited after manual extension of the exercised paw in rabbits (Itoh & Kawakita, 2002).
The purpose of this study was to examine whether eccentrically exercised muscle is hyperalgesic to mechanical stimulation. To assess such hyperalgesia, we used withdrawal threshold from mechanical stimulation in awake animals and c-Fos protein expression in the spinal dorsal horn. c-Fos protein is well known to be induced after neural excitation in various nervous systems, and its expression in the superficial dorsal horn of the spinal cord has been used as a neural marker of pain since Hunt et al. (1987) reported that various kinds of noxious stimuli induced c-Fos protein in this region, which contains secondary neurones receiving nociceptive Aδ- and C-fibre inputs (Cervero & Connell, 1984; Sugiura et al. 1986; Mizumura et al. 1993; Ling et al. 2003).
Preliminary results have appeared in abstract form (Taguchi et al. 2003).
Methods
Animals
Fifty-four male Sprague-Dawley rats (SLC Inc., Japan) weighing ca 200 g (age 7 weeks) at the beginning of the experiments were used in this study, 26 for behavioural experiments and 28 for c-Fos experiments. The animals were kept two per cage under a 12 h light–dark cycle (light between 07.00 h and 19.00 h) in an air-conditioned room (22–24°C). They had food and water ad libitum throughout the experiment. All experimental procedures were approved by the Animal Care Committee, Nagoya University.
Exercise protocol
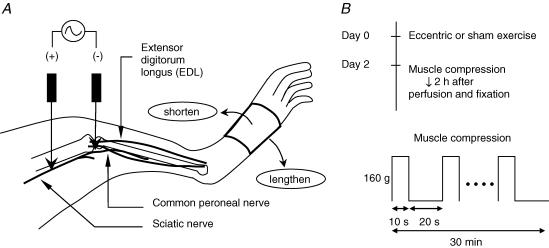
On day 0 the animals underwent either eccentric contraction (ECC) or stretching of the muscle (SHAM) under anaesthesia with sodium pentobarbital (50 mg kg−1, i.p.). Rectal temperature was kept in the physiological range (37–38°C) with a heating pad during the exercise period. Procedures for contracting and stretching the muscle are shown schematically in Fig. 1A. A pair of needle electrodes, insulated except for the tips, were transcutaneously inserted near the common peroneal nerve that innervates the extensor digitorum longus muscle (EDL). Repetitive contraction of the EDL was induced in ECC group rats by electrically stimulating the common peroneal nerve through these needle electrodes. Currents were supplied through an isolator (SS-202J, Nihon Kohden Corp., Japan) connected to an electrical stimulator (SEN-7203, Nihon Kohden Corp.). Before starting exercise, twitch threshold currents were determined. If the threshold current exceeded 100 μA, the electrodes were repositioned so that a lower threshold was obtained. The current applied during exercise in the ECC group was set at three times the twitch threshold. The stimulus parameter to induce tetanic contraction was a frequency of 50 Hz with pulse duration of 1 ms. The EDL muscle was stretched from the starting position (about 45 deg plantar-flexion) to maximal plantar-flexion (about 90 deg) over a 1 s period (‘lengthen’ in Fig. 1A) with the use of a linearized servomotor (CPL28T08B-06C2T, Oriental Motor Co. Ltd, Japan) and then returned to the starting position over 3 s (‘shorten’ in Fig. 1A). The movement of the servomotor was synchronized with the electrical stimulator so that the muscle was stretched while it was being activated. This pattern was repeated every 4 s for a total of 500 repetitions. The rats in the SHAM group received no current to their nerves; that is, the EDL was not activated at all during the exercise period, but simply stretched as described above. After the rats had recovered from anaesthesia following exercise or sham exercise, they behaved, ate and drank normally.
Figure 1. Schematic drawing of the experimental procedures.
A, methods of applying eccentric contraction to the extensor digitorum longus (EDL) muscle. One electrode was inserted transcutaneously near the sciatic nerve (+ pole) and the other near the common peroneal nerve (− pole). Repetitive contractions of the muscle were induced by cyclic electrical stimulation of the nerve. The hindpaw was fixed to a bar that was connected to the linearized motor, and it was pulled (‘lengthened’) synchronously with the muscle contraction so that the EDL muscle was stretched. Current intensity of the electrical stimulation was set at 3 times the threshold for twitch contraction, and frequency was set at 50 Hz to induce tetanic contraction. Contraction was repeated 500 times (1 s contraction followed by 3 s resting period). B, schedule of exercise and compression (upper panel), and the protocol of compression (lower panel) in the c-Fos experiment. Upper panel: on day 0 the animals underwent either eccentric contraction (ECC) or stretching of the muscle (SHAM). On day 2 the animals in the ECC and SHAM groups were each divided into two subgroups, one with and one without muscle compression. Lower panel: exercised muscle (EDL muscle) was compressed with a force of 160 g by Randall-Selitto apparatus for 10 s, and there was an interval of 20 s before the next compression. This session was repeated 60 times.
Pain tests
Randall-Selitto test
A Randall-Selitto apparatus (Ugo Basile, Italy) was used to measure the withdrawal threshold. The animals were restrained around the trunk with a towel to calm them, and treated gently during the experiments. A cone-shaped pusher with a rounded tip (diameter of the base: 9 mm) was applied to the belly of the EDL muscle through shaved skin. The rate of force application was set at 156.8 mN s−1 and there was a 2450 mN cut-off loading to avoid damaging the tissue. The intensity of pressure causing an escape reaction was defined as the withdrawal threshold. The tests were always done between 12.00 h and 15.00 h to avoid circadian fluctuations. Training sessions were carried out for 4 consecutive days to increase the sensitivity of the test (Taiwo et al. 1989). Measurements were performed 10 times at intervals of several minutes, and the mean value of the latter five trials was taken as the threshold. The experimenter was blind to which group an animal belonged.
von Frey hair test
To ensure that change in the withdrawal threshold measured by the Randall-Selitto apparatus was not the result of cutaneous mechanical hyperalgesia, the cutaneous mechanical pain threshold was measured with self-made von Frey hairs (VFHs, diameter: 0.5 mm, bending forces 37.5–707.3 mN in quasi-logarithmic order) because the mechanical strain induced by thin VFHs hardly reaches the deeper muscle layer (Takahashi et al. 2004). The rats were restrained at the trunk with a towel, as in the Randall-Selitto test, and each filament was applied to the skin on the exercised (EDL) muscle. The threshold force was determined by the method of limits. Briefly, a filament that was presumed painful was applied first. If an animal withdrew its leg, then weaker filaments were applied one by one until the animal did not show the withdrawal response. Then a stronger filament was applied again. This up and down procedure was repeated a few times to determine the withdrawal threshold. A filament was applied twice and if an animal showed at least one withdrawal response, this was taken as positive. Each filament was applied at intervals of a few seconds.
As repeated acid injections into gastrocnemius muscle caused bilateral secondary hyperalgesia of the plantar surface of the paw (Sluka et al. 2001), we also examined von Frey hair threshold of the plantar surface of the paw bilaterally.
Other procedures were the same as the Randall-Selitto test.
After completion of a series of behavioural pain tests, the animals used were killed by inhalation of a rising concentration of carbon dioxide.
c-Fos study
Exercise and compression protocol
On day 2 the animals in the ECC and SHAM groups were divided into two respective subgroups, with or without muscle compression. Thus, there was a total of four groups (n= 17): a sham exercised group without muscle compression on day 2 (SHAM, n= 4), a sham exercised group with muscle compression (SHAM + compression, n= 4), an eccentrically exercised group without muscle compression (ECC, n= 5), and an eccentrically exercised group with muscle compression (ECC + compression, n= 4). Compressive stimulation was applied to the exercised muscle through the skin on day 2. Compression with a force of 1568 mN was applied by the Randall-Selitto apparatus (Ugo Basile, Italy) for 10 s, followed by a 20 s rest period (Fig. 1B). This was repeated for 30 min under anaesthesia (sodium pentobarbital, 50 mg kg−1, i.p.). The force of 1568 mN is about 2 times higher than the withdrawal threshold in awake rats (described below).
Effect of morphine
In another series of experiments, the effect of morphine on c-Fos expression induced by ECC + compression was examined to make sure that c-Fos expression was induced by noxious inputs (Presley et al. 1990). Eleven animals were used in this series. The methods and interval for ECC and compression were the same as described above. Five animals received morphine (10 mg kg−1, dissolved in saline to give a concentration of 10 mg ml−1, i.p.) 20 min before compression of EDL, and the remaining six received saline (1 ml kg−1, i.p.) instead of morphine.
Immunohistochemistry
Two hours after the end of the muscle compression (or 2 days after the exercise session in groups without compression), the animals were deeply anaesthetized with sodium pentobarbital, and then perfused transcardially with 500 ml of 0.1 m phosphate-buffered saline (PBS, pH 7.4) followed by 500 ml of 4% paraformaldehyde in 0.1 m PBS. The spinal cord (L1–S1) was quickly removed, postfixed for 24 h in the same fixative, and then cryoprotected in 15% sucrose dissolved in PBS for 1 day, followed by 30% sucrose for 2 days. Transverse frozen sections (40 μm) of the spinal cord from L2–L6 were cut with a microtome, and every second section was used for analysis.
Immunohistochemical staining of c-Fos was performed according to the avidin–biotin peroxidase method (ABC method, Hsu et al. 1981). Briefly, following several rinses in PBS, endogenous tissue peroxidase activity was quenched by soaking the sections in 0.3% hydrogen peroxide solution in PBS for 30 min. The specimens were then treated in blocking solution, PBS containing 0.3% Triton X-100, and 5% normal goat serum (DGS00301, Cosmo Bio Co., Ltd, Japan) for 2 h. They were processed for immunohistochemistry by the free-floating ABC technique using polyclonal rabbit antibody to c-Fos (1: 5000, sc-52, Santa Cruz Biotechnology, USA) diluted with the blocking solution described above for 3 days at 4°C, biotinylated goat anti-rabbit immunoglobulin (1: 500, BA-1000, Vector Laboratories, USA) for 90 min, and avidin–biotin complex (PK-6100, Vector Laboratories) for 90 min at room temperature. Sections were washed with PBS between incubations. Finally, sections were reacted in a 0.05 m Tris-buffer (pH 7.6) containing 0.02% 3,3′-diamino-benzidine tetrahydrochloride (DAB), 0.02% nickel ammonium sulphate and 0.003% hydrogen peroxide for a few minutes at room temperature to produce a purple-black reaction product. The sections were washed in the Tris-buffer to stop the staining reaction, and then mounted on gelatin-coated slides, air-dried, cleaned in xylene and cover-slipped with mounting medium (Mount Quick, Daido Sangyo Co. Ltd, Japan).
For quantitative analysis, the number of c-Fos-ir neurones was counted under a light microscope at a magnifying power of 200. To study the laminar distribution of c-Fos, the spinal dorsal horn was divided into three specific regions based on the cytoarchitectonic organization reported by Molander et al. (1984): the superficial dorsal horn (SDH, laminae I–II), the proprius nucleus (PN, laminae III–IV), and the neck of the dorsal horn (NDH, laminae V–VI). The investigator responsible for counting the c-Fos-ir neurones was blinded to the group to which a section belonged. The number of c-Fos-ir neurones in an animal was divided by the number of sections counted and the result represented the average number of cells per section for a certain animal.
Statistical analyses
Results are expressed as mean ±s.e.m. One-way ANOVA followed by Bonferroni's multiple comparison test was performed to examine the change in the withdrawal threshold measured by the Randall-Selitto test. The withdrawal threshold measured by the Randall-Selitto test was compared on each day between SHAM and ECC groups using an unpaired t test. The non-parametric Friedman test followed by Dunn's post hoc test was used to compare the withdrawal threshold measured by the von Frey hair test, and the non-parametric Mann-Whitney test was used to compare the values after ECC between SHAM and ECC groups. We used these different statistical methods for the values obtained with the Randall-Selitto test and von Frey hair test, because the former values were continuous, whereas the latter were discontinuous and increased in logarithmic order. In the c-Fos study one-way ANOVA followed by Bonferroni's multiple comparison test was used to compare all pairs among the four groups. P < 0.05 was considered to be significant.
Results
Mechanical hyperalgesia
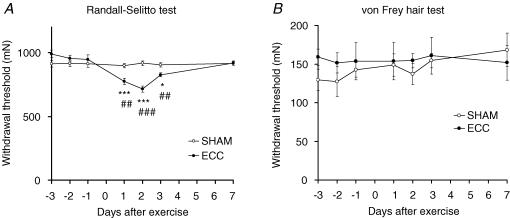
The mechanical threshold measured with the Randall-Selitto apparatus was almost stable before exercise, at 912.1 ± 27.3 mN for the sham exercised animals and 946.4 ± 36.7 mN for ECC animals 1 day before exercise. This value started to decrease in ECC animals 1 day after exercise, and reached a minimum 2 days after exercise (Fig. 2A). Despite this change in withdrawal threshold, no apparent changes in spontaneous behaviours were recognized. Decreased mechanical withdrawal threshold, although less intense, was still observed 3 days after exercise. Complete recovery was observed 7 days after exercise. The change in the withdrawal threshold in ECC was statistically significant 1, 2 and 3 days after exercise compared with that 1 day before exercise (day −1 in Fig. 2A, P < 0.05–0.001). The withdrawal threshold of the sham-exercised animals remained constant during the entire experimental period (Fig. 2A), and the withdrawal threshold of ECC animals was significantly lower than the sham-exercised group 1, 2 and 3 days after exercise (P < 0.01–0.001).
Figure 2. Decreased pain thresholds after ECC were found with Randall-Selitto test but not with von Frey hair test.
A, withdrawal thresholds of the eccentrically exercised muscle measured by Randall-Selitto apparatus (n= 6 for each group). Abscissa: days after exercise; ordinate: withdrawal threshold. Data from the ECC group, •; result of the SHAM group, ○. Randall-Selitto test showed decreased pain threshold after ECC. *P < 0.05, ***P < 0.001 compared with the value immediately before the day of exercise (−1 day). ##P < 0.01, ###P < 0.001 compared with the SHAM group on each day after treatment. B, withdrawal threshold for the skin over the exercised muscle measured by von Frey hairs (n= 7 for each group). The presentation is the same as in A. No significant differences were found between groups on each day, or between −1 day and days after ECC (non-parametric Friedman test followed by Dunn's multiple comparison test). These results suggest that the pain threshold was decreased in the deeper tissue, probably in the muscle.
Despite the decrease in mechanical withdrawal threshold, no pain-related behaviours such as licking, biting, or lifting of the exercised paw were observed, and the rats walked normally in their cage up to 7 days after eccentric exercise.
In contrast, the pain threshold at the skin over the EDL muscle, which was measured as the withdrawal threshold to VFH stimulation, showed no significant change after treatment (eccentric or sham muscle contraction) in either the ECC or the SHAM group (P > 0.05 when the thresholds after treatment were compared with that on day −1, Friedman test followed by Dunn's multiple comparison test; P > 0.05 when the threshold was compared between two groups on each day after treatment, non-parametric Mann-Whitney test, Fig. 2B), suggesting that the change of the withdrawal threshold measured with the Randall-Selitto apparatus represents the pain threshold change in deeper tissues, possibly the muscle.
The bilateral von Frey hair threshold of the plantar surface of the paw did not change significantly for at least 7 days after eccentric exercise (von Frey hair thresholds of the ipsilateral plantar surface of the skin were 237.9 ± 25.1, 249.8 ± 15.7 and 244.0 ± 25.6 mN immediately before (−1), 2 and 7 days after eccentric exercise, respectively, and those of the contralateral plantar surface of the skin were 244.0 ± 25.6, 256.2 ± 24.1 and 207.6 ± 24.2 mN, respectively, n= 7).
c-Fos study
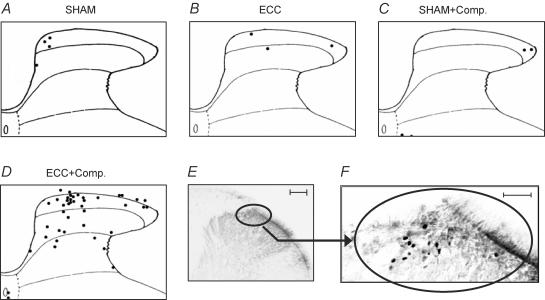
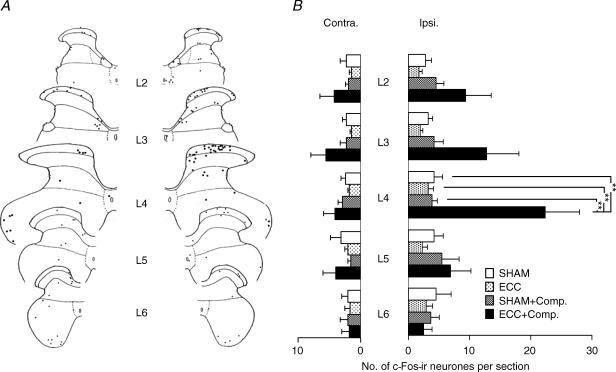
Representative sections of each group are shown in Fig. 3. c-Fos-ir was found in the nuclei of some neurones in the dorsal horn of the spinal cord (sample photograph of the ECC + compression group in Fig. 3F). The number of c-Fos-ir neurones in the dorsal horn was small in the SHAM group (Fig. 3A). Similarly, only a few neurones were labelled in the ECC and SHAM + compression groups (Fig. 3B and C). Thus, neither ECC 2 days before by itself nor the compression (two times the Randall-Selitto threshold) used in this experiment activated the nociceptive pathway significantly in anaesthetized animals. In the ECC + compression group, on the other hand, c-Fos immunoreactivity clearly increased, especially in the middle of the superficial dorsal horn corresponding to laminae I–II (Fig. 3D, E and F). A smaller number of c-Fos-ir neurones was seen also in the proprius nucleus in the case shown in Fig. 3. Figure 4 shows the segmental distribution of c-Fos-ir-positive neurones in one rat of the ECC + compression group. The most prominent increase in c-Fos expression was observed in the spinal cord at L4, and some expression was also found in L2–L3 spinal segments as seen in this case, but not L5 and L6 (Fig. 4A). On average in four cases, the number of c-Fos-ir-positive neurones in the entire dorsal horn (laminae I–VI) at the L4 segment on the ipsilateral side was significantly greater in the ECC + compression group than in the SHAM group (P < 0.01, one-way ANOVA followed by Bonferroni's multiple comparison test, Fig. 4B). It must be noted that the number of c-Fos-ir-positive neurones in the ECC + compression group was also greater than in the other two groups (P < 0.01, one-way ANOVA followed by Bonferroni's multiple comparison test, Fig. 4B). ECC and SHAM + compression groups had numbers of c-Fos-ir-positive neurones that were not significantly different from the SHAM group. At L2 and L3, the number of c-Fos-ir neurones tended to be greater in the ECC + compression group than in the other three groups, but none of the differences among the four groups were significant. The number of c-Fos-ir neurones occasionally appeared to be increased in the contralateral side in the ECC + compression group, especially in cases with larger numbers of c-Fos-ir neurones (example in Fig. 4A), but the change was not statistically significant compared with the other groups (Fig. 4B).
Figure 3. c-Fos-ir neurones in the ipsilateral dorsal horn of the spinal cord at L4.
A–D, camera lucida drawings of representative sections from four groups 2 days after treatment: SHAM group (A), ECC group (B), SHAM + compression group (C), and ECC + compression group (D). Labelled neurones are represented as black dots. Note that the number of labelled neurones increased in the ECC + compression group on the second day. This was especially marked in the superficial dorsal horn. E and F, photographs with different magnification of the same section as in D, showing c-Fos-ir neurones in the ipsilateral dorsal horn of the spinal cord at L4. Calibration bars: 100 μm in E and 50 μm in F.
Figure 4. Segmental distribution of the c-Fos-ir neurones in the dorsal horn of the spinal cord at L2–L6.
A, representative camera lucida drawing of the c-Fos-ir neurones in the dorsal horn of the spinal cord at L2–L6 in a rat with ECC + compression. Labelled neurones are represented as black dots. The largest number of labelled neurones was observed in the superficial dorsal horn of segment L4, and smaller numbers were also seen in segments L2 and L3. B, summary of segmental distribution of c-Fos-ir neurones in the spinal dorsal horn (L2–L6). Number of labelled neurones per section in the entire dorsal horn in each segment is shown. The number of animals used was 4 in SHAM, SHAM + compression, and ECC + compression groups, and 5 in ECC group. Note that there is a significant increase in the number of c-Fos-ir neurones at L4 in ECC + compression group compared with the other three groups (**P < 0.01). The number of c-Fos-ir neurones at L2 and L3 in ECC + compression group tended to be greater, but differences among the four groups were not significant.
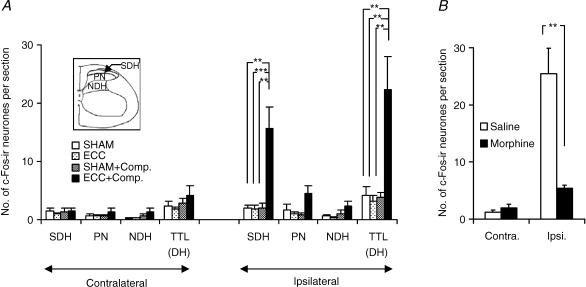
Closer inspection of the spinal dorsal horn at L4 (Fig. 5A) reveals a clear increase in the numbers of labelled neurones in the superficial dorsal horn (SDH, laminae I–II) in the ECC + compression group (ipsilateral SDH, Fig. 5A, 1.9 ± 0.6 neurones per section (n= 4) in the SHAM group, versus 15.6 ± 3.7 neurones per section (n= 4) in the ECC + compression group, P < 0.01). The number of c-Fos-ir neurones in the ECC + compression group was also significantly greater than in the other two groups (P < 0.01 and 0.001). Most c-Fos-ir neurones were found in the middle or medial half of the superficial dorsal horn (, and 4A) corresponding to the projection area of input from the common peroneal nerve (Brushart et al. 1981; Molander & Grant, 1986). The numbers of labelled neurones in the ECC and SHAM + compression groups were not significantly different from that in the SHAM group.
Figure 5. Number of c-Fos-ir neurones in the different areas of the spinal dorsal horn at L4 (A) and suppression of its increase by morphine (B).
In A, TTL (DH) represents total number of labelled neurones in the entire dorsal horn; SDH, superficial dorsal horn; PN, proprius nucleus; NDH, neck of the dorsal horn. The number of animals was 4 for SHAM, SHAM + compression, and ECC + compression groups, and 5 in ECC group. A significant increase in the number of c-Fos-ir neurones was observed in the superficial dorsal horn compared with each of the other three groups (**P < 0.01, ***P < 0.001). The numbers of c-Fos-ir neurones in the proprius nucleus and the neck of the dorsal horn appeared to be somewhat increased in the ECC + compression group, but the difference was not statistically significant. In B, filled columns show the number of c-Fos-ir neurones per section in the animals that received morphine (10 mg kg−1, i.p.) 20 min before compression of EDL muscle 2 days after ECC, and open columns show those in the control group that received saline instead of morphine. Number of animals was 5 for the morphine group, and 6 for the control group. Note that c-Fos immunoreactivity was clearly suppressed in the morphine-treated group (**P < 0.01, Mann-Whitney test).
There was a tendency for the number of c-Fos-ir neurones in the proprius nucleus to be larger in ECC + compression group (PN in Fig. 5A, 4.5 ± 1.4 neurones per section (n= 4) in the ECC + compression group versus 1.6 ± 1.0 neurones per section (n= 4) in the SHAM group), but the difference was not significant.
Effect of morphine
In this series of experiments, ECC + compression induced expression of c-Fos immunoreactivity in almost the same number of dorsal horn neurones (saline group, Fig. 5B) on the side ipsilateral to the stimulation as in the experiment described above. These neurones were found mainly in the superficial dorsal horn. Morphine treatment clearly decreased the number of c-Fos-ir neurones in the dorsal horn (morphine group in Fig. 5B, P < 0.01 compared with saline group).
Discussion
In the present study the mechanical pain threshold after ECC decreased when measured with the Randall-Selitto apparatus, but not when measured with VFHs. Because the tip diameter of the VFHs is small (0.5 mm), it is unlikely that the mechanical stress applied to the surface is transmitted deep into the muscle (Takahashi et al. 2004); thus the VFH measures the pain threshold of the skin. In contrast, the tip of the Randall-Selitto apparatus is much larger than that of von Frey hairs, and so the force applied can be transmitted deeper through the skin (Takahashi et al. 2004). Therefore, together with the absence of change in the VFH pain threshold, change in the pain threshold measured with the Randall-Selitto method is considered to represent the change in the pain threshold in the deeper tissue, probably the muscle. This change was observed 1 day after ECC and reached the lowest value 2 days after ECC, which is compatible with our everyday experience of delayed onset muscular soreness (Armstrong, 1984; Newham, 1988; Proske & Morgan, 2001).
The present results clearly demonstrated that c-Fos expression was increased only in the ECC + compression group. In the ECC group, there were a few labelled neurones in the dorsal horn at L4, but the number did not differ from the SHAM group. This observation is consistent with the fact that spontaneous pain is not prominent in DOMS 2 days after eccentric contraction (Graven-Nielsen & Arendt-Nielsen, 2003). Because c-Fos expression is known to be induced by many factors in corresponding neural pathways, one might expect that muscular contraction or stretching stimulation itself induces c-Fos. However, the expression levels of c-Fos protein in neurones usually peak about 2 h after neuronal excitation, and expression disappears 4–16 h following excitation (Menétrey et al. 1989). In the present study the animals underwent either muscle stretching or muscle contraction by electrical stimulation of the nerve 2 days before perfusion. Therefore, any c-Fos-ir expression that had been induced by these stimuli should have already vanished by the time the animals were perfused. In the SHAM + compression group, the number of labelled neurones did not differ from the SHAM group. This result suggests that muscle compression itself did not activate the pain pathway in the anaesthetized condition.
In contrast, the number of neurones with c-Fos-ir in the ECC + compression group significantly increased, and the increase was observed in the ipsilateral superficial dorsal horn at L4 when compared with the other three groups. The superficial dorsal horn is well known to be an important region where thinly myelinated and unmyelinated afferents from skin (Light & Perl, 1979; Sugiura et al. 1986), viscera (Cervero & Connell, 1984; Mizumura et al. 1993) and muscle (Brushart & Mesulam, 1980; Brushart et al. 1981; Mense & Craig, 1988; Ling et al. 2003) terminate. Brushart et al. found that muscle afferents of the rat common peroneal branch projected densely to the substantia gelatinosa (Brushart et al. 1981). Recently Ling et al. demonstrated, by intracellular injection of Phaseolus vulgaris leucoagglutinin to single C-afferent neurones in dorsal root ganglia, that unmyelinated (C) afferent fibres from the medial head of the gastrocnemius muscle run at the surface of the dorsal funiculus, giving off collaterals into laminae I and II, and sometimes into parts of lamina III (Ling et al. 2003). Thus, the prominent increase of c-Fos-ir in the superficial dorsal horn of the ECC + compression group and absence of increase in SHAM + compression group strongly suggest that the muscle was hyperalgesic to mechanical stimulation (existence of tenderness) 2 days after eccentric muscular work. This conclusion was supported by the fact that morphine treatment clearly decreased c-Fos immunoreactivity in the dorsal horn.
The increase of c-Fos-ir-positive neurones in the ECC + compression group was found mainly in L4, and some in L3–L2, spinal segments; no increase was found in L5 or further caudally. A previous report showed that the great majority of sensory neurones innervating EDL are located in L4 dorsal root ganglion (Peyronnard et al. 1986). Spinal terminations of the gastrocnemius muscle traced by the intracellular labelling technique were found rostral to the level where fibres entered into the spinal cord, but not caudal to it (personal communication from Dr Y. Sugiura, Department of Anatomy, Nagoya University School of Medicine, Nagoya, Japan). The present result is in agreement with this observation, and may suggest that muscle afferents terminate at the segment of entry and rostral but not caudal to it.
Tenderness induced by palpating the exercised muscle in DOMS might be conveyed in part by C-fibre afferents to the secondary neurones, since it is felt as diffuse and dull pain. In fact, there are C-fibre sensory receptors in the muscle that have nociceptive characteristics similar to cutaneous and visceral nociceptors, and that respond to various kinds of noxious stimuli (Kumazawa & Mizumura, 1977; Mense, 1977; Mense & Meyer, 1988). In addition, those receptors are sensitized to mechanical stimulation by inflammatory mediators such as bradykinin (Mense & Meyer, 1988). It has also been suggested that myelinated fibres are involved in DOMS (Weerakkody et al. 2001, 2003). Although there has been dispute as to whether an inflammatory process is involved in the underlying mechanism of DOMS (Smith, 1991), the possibility cannot be excluded that some inflammatory mediators or cytoplasmic components released as a result of the microinjury in the muscle after eccentric contraction may sensitize the nociceptors to mechanical stimulation. Since the mechanism of hyperalgesia to mechanical stimulation in general is still poorly understood, electrophysiological studies of muscle nociceptor activities may shed some light on this field. Our preliminary study showed increased mechanical response and decreased mechanical threshold in C-fibres in this model (Taguchi et al. 2004). The present animal model would seem to be useful in investigating the mechanisms of DOMS and the mechanism of mechanical hyperalgesia in general.
Acknowledgments
This work was supported in part by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare (H14-Choju-29), and by a Grant-in-Aid for Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology (No. 14657014).
References
- Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc. 1984;16:529–538. [PubMed] [Google Scholar]
- Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Blais C, Jr, Adam A, Massicotte D, Péronnet F. Increase in blood bradykinin concentration after eccentric weight-training exercise in men. J Appl Physiol. 1999;87:1197–1201. doi: 10.1152/jappl.1999.87.3.1197. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Henry EW, Mesulam MM. Reorganization of muscle afferent projections accompanies peripheral nerve regeneration. Neuroscience. 1981;6:2053–2061. doi: 10.1016/0306-4522(81)90043-9. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Mesulam MM. Transganglionic demonstration of central sensory projections from skin and muscle with HRP-lectin conjugates. Neurosci Lett. 1980;17:1–6. doi: 10.1016/0304-3940(80)90051-8. [DOI] [PubMed] [Google Scholar]
- Cervero F, Connell LA. Distribution of somatic and visceral primary afferent fibres within the thoracic spinal cord of the cat. J Comp Neurol. 1984;230:88–98. doi: 10.1002/cne.902300108. [DOI] [PubMed] [Google Scholar]
- Friden J, Lieber RL. Segmental muscle fiber lesions after repetitive eccentric contractions. Cell Tissue Res. 1998;293:165–171. doi: 10.1007/s004410051108. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen T, Arendt-Nielsen L. Induction and assessment of muscle pain, referred pain, and muscular hyperalgesia. Curr Pain Headache Rep. 2003;7:443–451. doi: 10.1007/s11916-003-0060-y. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedure. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Itoh K, Kawakita K. Effect of indomethacin on the development of eccentric exercise-induced localized sensitive region in the fascia of the rabbit. Jpn J Physiol. 2002;52:173–180. doi: 10.2170/jjphysiol.52.173. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977;273:179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Ling LJ, Honda T, Shimada Y, Ozaki N, Shiraishi Y, Sugiura Y. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in the guinea pig. J Comp Neurol. 2003;461:140–150. doi: 10.1002/cne.10619. [DOI] [PubMed] [Google Scholar]
- McCurry KK, Faulkner JA. Injury to skeletal muscle fibers of mice following lengthening contractions. J Appl Physiol. 1985;59:119–126. doi: 10.1152/jappl.1985.59.1.119. [DOI] [PubMed] [Google Scholar]
- Menétrey D, Gannon A, Levine JD, Basbaum AI. Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J Comp Neurol. 1989;285:177–195. doi: 10.1002/cne.902850203. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977;267:75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Craig AD. Spinal and supraspinal terminations of primary afferent fibers from the gastrocnemius-soleus muscle in the cat. Neuroscience. 1988;26:1023–1035. doi: 10.1016/0306-4522(88)90117-0. [DOI] [PubMed] [Google Scholar]
- Mense S, Meyer H. Bradykinin-induced modulation of the response behaviour of different types of feline group III and IV muscle receptors. J Physiol. 1988;398:49–63. doi: 10.1113/jphysiol.1988.sp017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumura K, Sugiura Y, Kumazawa T. Spinal termination patterns of canine identified A-δ and C spermatic polymodal receptors traced by intracellular labeling with phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1993;335:460–468. doi: 10.1002/cne.903350313. [DOI] [PubMed] [Google Scholar]
- Molander C, Grant G. Laminar distribution and somatotopic organization of primary afferent fibers from hindlimb nerves in the dorsal horn: a study by transganglionic transport of horseradish peroxidase in the rat. Neuroscience. 1986;19:297–312. doi: 10.1016/0306-4522(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230:133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- Newham DJ. The consequences of eccentric contractions and their relationship to delayed onset muscle pain. Eur J Appl Physiol. 1988;57:353–359. doi: 10.1007/BF00635995. [DOI] [PubMed] [Google Scholar]
- Newham DJ, McPhail G, Mills KR, Edwards RH. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983;61:109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Ogilvie RW, Armstrong RB, Baird KE, Bottoms CL. Lesions in the rat soleus muscle following eccentrically biased exercise. Am J Anat. 1988;182:335–346. doi: 10.1002/aja.1001820405. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Zacho M, Asp S, Pederson BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyronnard JM, Charron LF, Lavoie J, Messier JP. Motor, sympathetic and sensory innervation of rat skeletal muscles. Brain Res. 1986;373:288–302. doi: 10.1016/0006-8993(86)90343-4. [DOI] [PubMed] [Google Scholar]
- Presley RW, Menetrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990;10:323–335. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537:333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne DB. Exercise-induced muscle damage and inflammation: a review. Aust J Sci Med Sport. 1994;26:49–58. [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Smith LL. Acute inflammation: the underlying mechanism in delayed onset muscle soreness? Med Sci Sports Exerc. 1991;23:542–551. [PubMed] [Google Scholar]
- Sugiura Y, Lee CL, Perl ER. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Sato J, Mizumura K. Program No. 745.7. Abstract Viewer/Itinerary Planner. Washington,DC: Society for Neuroscience; Response to mechanical, chemical, and thermal stimulations of the muscle C-fiber sensory receptors after eccentric contraction in rats. [Google Scholar]
- Taguchi T, Tamura R, Sato J, Mizumura K. Program No. 66.5. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003. Existence of muscular tenderness revealed by behavioral pain test and c-Fos expression after eccentric contraction in rats. [Google Scholar]
- Taiwo YO, Coderre TJ, Levine JD. The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res. 1989;487:148–151. doi: 10.1016/0006-8993(89)90950-5. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Taguchi T, Itoh K, Nishimura N, Morisada M, Okada K, et al. Program No. 920.5. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2004. Measurement of the muscle pain by a transcutaneous pressure: Theoretical and experimental analyses. [Google Scholar]
- Weerakkody NS, Percival P, Hickey MW, Morgan DL, Gregory JE, Canny BJ, et al. Effects of local pressure and vibration on muscle pain from eccentric exercise and hypertonic saline. Pain. 2003;105:425–435. doi: 10.1016/S0304-3959(03)00257-4. [DOI] [PubMed] [Google Scholar]
- Weerakkody NS, Whitehead NP, Canny BJ, Gregory JE, Proske U. Large-fiber mechanoreceptors contribute to muscle soreness after eccentric exercise. J Pain. 2001;2:209–219. doi: 10.1054/jpai.2001.22496. [DOI] [PubMed] [Google Scholar]