Abstract
Two-pore (2-P) domain potassium channels are implicated in the control of the resting membrane potential, hormonal secretion, and the amplitude, frequency and duration of the action potential. These channels are strongly regulated by hormones and neurotransmitters. Little is known, however, about the mechanism underlying their regulation. Here we show that phosphatidylinositol 4,5-bisphosphate (PIP2) gating underlies several aspects of 2-P channel regulation. Our results demonstrate that all four 2-P channels tested, TASK1, TASK3, TREK1 and TRAAK are activated by PIP2. We show that mechanical stimulation may promote PIP2 activation of TRAAK channels. For TREK1, TASK1 and TASK3 channels, PIP2 hydrolysis underlies inhibition by several agonists. The kinetics of inhibition by the PIP2 scavenger polylysine, and the inhibition by the phosphatidylinositol 4-kinase inhibitor wortmannin correlated with the level of agonist-induced inhibition. This finding suggests that the strength of channel PIP2 interactions determines the extent of PLC-induced inhibition. Finally, we show that PIP2 hydrolysis modulates voltage dependence of TREK1 channels and the unrelated voltage-dependent KCNQ1 channels. Our results suggest that PIP2 is a common gating molecule for K+ channel families despite their distinct structures and physiological properties.
K+ channels are the largest and most diverse family of ion channels. They fall into three main groups based on their structural properties: voltage-gated (6 transmembrane or 6TM), inward rectifiers (2TM) and two-pore domain (4TM) channels. While all these channels are highly selective for K+ over other ions, they exhibit distinct physiological and biophysical properties.
Recently, two-pore (2-P) domain potassium channels have been shown to serve as molecular determinants of several leak K+ currents. Leak K+ currents serve to establish the resting membrane potential and modify the duration, frequency and amplitude of action potentials. The activity of 2-P domain channels is strongly regulated by a number of agents such as protons, protein kinases, and temperature (Lopes et al. 2000; Kim et al. 2001). In addition, three members of this family TREK1 (KCNK2), TREK2 (KCNK10) and TRAAK (KCNK4) are shown to be mechanosensitive. They are also targets of receptor-mediated regulation by neurotransmitters and hormones (for review see Lesage & Lazdunski, 2000; Goldstein et al. 2001; Patel & Honore, 2001). This modulation appears to be important for a number of physiological processes, including aldosterone secretion (Czirjak et al. 2000) and regulation of neuronal activity (Talley et al. 2000; Brickley et al. 2001). Some members of this ion channel family are activated by volatile anaesthetics (Patel et al. 1999; Sirois et al. 2000), and contribute to the effect of these pharmacological agents on neural activity (Sirois et al. 2000).
Modulation of channel activity by phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2 or PIP2) has recently emerged as a crucial aspect of ion channel regulation (Hilgemann & Ball, 1996; Baukrowitz et al. 1998; Huang et al. 1998; Sui et al. 1998; Shyng & Nichols, 1998; Zhang et al. 1999; Kobrinsky et al. 2000; Chuang et al. 2001; Runnels et al. 2002; Wu et al. 2002; for review see Hilgemann et al. 2001). Autonomic receptors (α1-adrenergic and M1/M3 muscarinic) and angiotensin II receptors (AT1), among others, can couple to G proteins of the Gq/G11 family and, when activated, stimulate phospholipase C (PLC). Since PLC hydrolyses PIP2, its stimulation causes local reduction of PIP2 levels in the plasma membrane (Stauffer et al. 1998; Varnai & Balla, 1998; van der Wal et al. 2001). For both inwardly rectifying (Kir) and voltagegated KCNQ potassium channels, agonist-induced inhibition can be observed when the levels of the phospholipid fall below a critical level for maintenance of channel activity (Baukrowitz et al. 1998; Xie et al. 1999; Kobrinsky et al. 2000; Zhang et al. 2003). Four members of the 2-P domain family, TASK1 (KCNK3), TASK3 (KCNK9), TREK1 (KCNK2) and TREK2 (KCNK10) have been reported to be inhibited by agonists that activate Gq/G11 proteins both in native and recombinant systems (Talley et al. 2000; Millar et al. 2000; Czirjak et al. 2001; Czirjak & Enyedi, 2002; Talley & Bayliss, 2002; Chemin et al. 2003). Here we show that TASK1, TASK3 and TREK1, but not TRAAK, are inhibited by muscarinic M1 stimulation. Although agonist-induced inhibition of TASK1 channels depended on PLC activation, downstream signals (inositol 1,4,5-trisphosphate, cytoplasmic Ca2+ and diacylglycerol) did not mediate the inhibition (Czirjak et al. 2001). Recently it was suggested that agonist-induced inhibition of TASK1 and TASK3 channels was mediated by PIP2 hydrolysis, but TREK1 and TREK2 current inhibition was mediated by a different mechanism (Chemin et al. 2003). In contrast, our results indicate that PIP2 hydrolysis underlies ACh-induced inhibition of TREK1 as well as TASK1 and TASK3 channels. In addition, we show that PIP2 activation of TRAAK channels is dependent on mechanical stimulation.
The present study demonstrates that PIP2 activation is a conserved property of members of the 2-P potassium channel family. We show that all tested members of the 2-P domain potassium channel family were activated by PIP2. The extent of agonist-induced inhibition observed by stimulation of Gq/G11 coupled receptors correlated well with both the kinetics of block by the PIP2 scavenger polylysine (poly Lys) and the extent of inhibition by the phosphatidylinositol 4-kinase (PI 4-kinase) inhibitor wortmannin. These findings suggested a direct role of PIP2 hydrolysis in agonist-induced inhibition and showed that the extent of inhibition depended on the sensitivity of the channel for PIP2. In addition, we show that PIP2 hydrolysis changed the voltage dependence of TREK1 channels. We also demonstrated the involvement of PIP2 in the regulation of 2-P channels by mechanical stimulation. Our findings indicate that PIP2 is critical for both activity and modulation of 2-P domain channels. In addition, we show that PIP2 modulates the voltage dependence of both 2-P channels and voltage-gated KCNQ channels, despite their distinct structures and physiological properties. These results suggest a general role for PIP2 in the regulation of all major families of K+ channels.
Methods
Molecular biology
The human TASK1, rat TASK3, mouse TREK1 and the mouse TRAAK cDNA constructs were subcloned into the pEXO vector (Duprat et al. 1997). The human KCNQ1, human KCNE1, human M1 muscarinic receptor and the human type I IP3 5-phosphatase were subcloned into the pGEMHE plasmid vector (Liman et al. 1992). Both vectors contain the 5′- and 3′-untranslated region of the Xenopusβ-globin gene to obtain optimal expression in Xenopus oocytes. The cDNA was linearized and cRNA was prepared using the Ambion mMESSAGE mMACHINE T7 kit. Point mutants were produced by Pfu based mutagenesis using the QuikChange™ kit (Stratagene Inc., La Jolla, CA, USA).
Electrophysiology
Xenopus oocytes were surgically removed from adult females under anaesthesia (0.4% 3-aminobenzoic acid ethyl ester). Frogs were humanely killed after the final oocyte collection. The experiments were carried out with the approval of the local animal care committee (IACUC). Oocytes were isolated using collagenase digestion and injected with 0.5–15 ng of cRNA in 50 nl of sterile water. Macropatch measurements in the inside-out configuration were performed as described (Rohacs et al. 2002). Electrodes for oocyte experiments contained (mm): 96 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, 5 Hepes, pH 7.4. For assessment of kinetics of block, inside-out patches were perfused with poly Lys and PIP2 antibody (PIP2Ab) in FVPP solution (mm: 96 KCl, 5 EDTA, 10 Hepes, 5 NaF, 3 Na3VO4, 10 Na2PO7, pH 7.4 with NaOH), which retarded hydrolysis of PIP2 and thus stabilized currents (Huang et al. 1998). For the PIP2 activation measurements the perfusion solution contained (mm): 96 KCl, 5 EGTA, 10 Hepes, pH 7.40 (bath solution). Unless otherwise specified, a ramp protocol from −100 to +100 mV was used (1 mV ms−1) with a holding potential of −80 mV. Negative pressure applied to the inside-out patches was measured using a BIO-TEK (Winooski, VT, USA) pneumatic transducer tester. Whole-oocyte currents were measured by conventional two-microelectrode voltage-clamp as described (Czirjak et al. 2001) unless specified otherwise.
Poly Lys (Sigma, St Louis, MO, USA) with an average molecular weight of 8 kDa was used. Poly Lys has a limited solubility in FVPP solutions; a saturated solution was made by adding 30 μg ml−1 poly Lys and centrifuging the solution (Rohacs et al. 2002). DiC8 PI(4,5)P2 was purchased from Echelon Research Laboratories Inc. (Salt Lake City, UT, USA) and from Cayman Chemical (Ann Arbor, MI, USA). DiC8 PIP2 was dissolved in the bath solution to the indicated concentrations. PIP2 antibody (Assay Designs, Ann Arbor, MI, USA) was diluted 1: 50 in FVPP solutions. Arachidonyl stearyl (AASt) PI(4,5)P2 was purchased from Roche Molecular Biochemicals and was dissolved in water and sonicated as described (Rohacs et al. 2002). Diacylglycerol analogues 1-stearoyl-2-arachidonoyl-sn-glycerol (SAG) and 1-stearoyl-2-linoleoyl-sn-glycerol (SLG) were purchased from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA, USA). They were dissolved in DMSO (20 mm) and stored at −80°C. Before each experiment SAG and SLG were dissolved in bath solution described earlier, and sonicated for 30 min. DMSO was included in all other solutions for these experiments to avoid any artifact caused by the solvent.
Error bars in the figures represent s.e.m. Each experiment shown or described was performed on a minimum of five oocytes. Student's unpaired t test was used to assess statistical significance. In Fig. 4D and E, inhibition was calculated by extrapolating the current levels before and after SAG or SLG to the period during the drug application, and comparing this level to the actual current level.
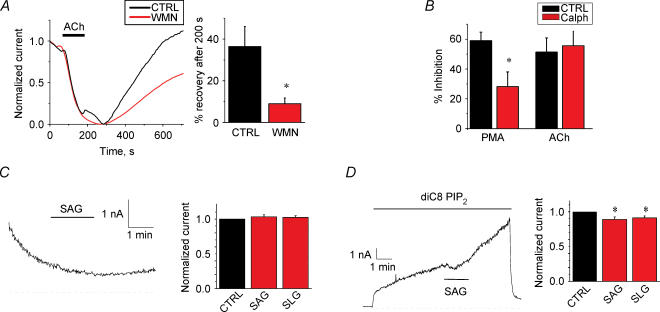
Figure 4. Modulation of TREK1 channels by PIP2.
A, effect of wortmannin on whole-cell current recovery from carbachol inhibition for TREK1 channels. Left, a typical normalized recovery from inhibition for TREK1 channels. Right, summary data for recovery of inhibition after 200 s of removal of ACh in the presence or absence of wortmannin (n= 16). Oocytes were treated with 10 μm wortmannin during the recovery and for at least 10 min prior to ACh application. B, effect of the PKC inhibitor calphostin-C (10 μm) on ACh-induced inhibition. Summary data of whole-cell current inhibition by either 10 nm PMA or 10 μm ACh in either presence or absence of calphostin-C (n= 5–7). C, effect of 10 μm SAG and 10 μm SLG on TREK1 currents on inside-out macropatches. Left, a typical inside-out macropatch current at +90 mV; SAG was applied as indicated. Establishment of the inside-out configuration is indicated by the arrow. Right, the summary data of effects of SAG and SLG on basal current. D, effects of 10 μm SAG and 10 μm SLG on PIP2-activated TREK1 currents on inside-out macropatches. Left, a typical inside-out macropatch current at +90 mV, 25 μm diC8 PIP2 and SAG were applied as indicated. Right, summary data of effects of SAG and SLG on inside-out macropatch currents activated by diC8 PI(4,5)P2.
Results
TASK1, TASK3, TREK1 and TRAAK channels are activated by PIP2
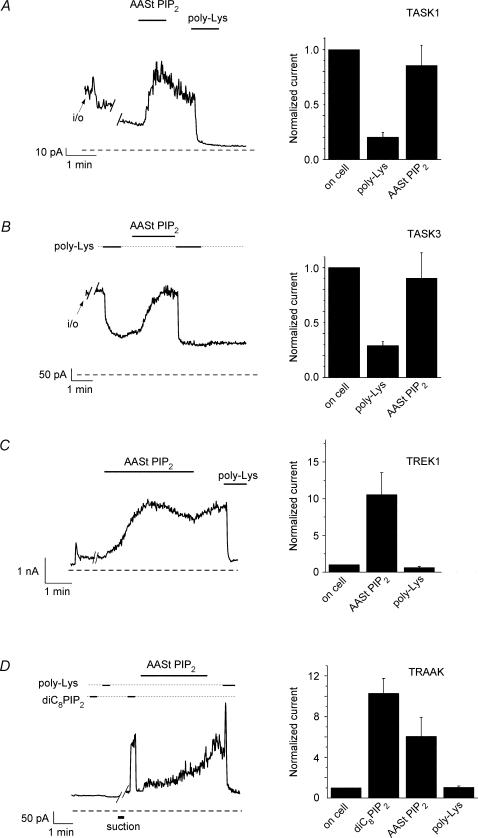
We investigated the effect of PIP2 on four 2-P domain K+ channels expressed in Xenopus oocytes (Fig. 1). For TASK1, following excision of macropatches in the inside-out mode, K+ currents substantially decreased (ran down). Channel run-down has been correlated to PIP2 hydrolysis for Kir channels (Hilgemann & Ball, 1996; Huang et al. 1998; Zhang et al. 1999). Application of 25 μm arachidonyl stearyl (AASt) PIP2 activated these channels (Fig. 1A). Subsequent application of polylysine (poly Lys), which acts as a PIP2 scavenger (Huang et al. 1998; Lopes et al. 2002; Rohacs et al. 2002), caused rapid and persistent current inhibition. TASK3 currents showed less pronounced run-down after excision, were persistently blocked by poly Lys application and were activated by AASt PIP2 (5 μm) (Fig. 1B).
Figure 1. Activation of 2-P domain channels by PIP2.
A, left, representative trace of TASK1 currents in an inside-out macropatch, measured at +100 mV. Applications of 25 μm AASt PIP2 and 3 μg ml−1 poly Lys are indicated by the horizontal lines. Right, statistical summary of the data. The average current level in the cell-attached configuration was 65 ± 21 pA (n= 3). B, left, a representative trace for TASK3 currents is shown, measured at +40 mV. Applications of 5 μm AASt PIP2 and 3 μg ml−1 poly Lys are indicated by the horizontal lines. Right, statistical summary of the data, normalized to the cell-attached current level. The average current in the cell-attached level was 580 ± 160 pA (n= 8). C, left, representative trace of TREK1 currents measured in macropatches at +100 mV. Applications of 2.5 μm AASt PIP2 and 3 μg ml−1 poly Lys are indicated by the horizontal lines. Right, statistical summary of the data (n= 6–10), normalized to the cell-attached level. The average current in the cell-attached level was 143 ± 35 pA (n= 10). D, left, representative trace for TRAAK currents is shown, measured in macropatches at +100 mV. Applications of 100 μm diC8 PIP2, 5 μm AASt PIP2 and 3 μg ml−1 poly Lys are indicated by the horizontal lines. Right, statistical summary of the data (n= 4), normalized to the cell-attached current level. The average current in the cell-attached level was 80 ± 9 pA (n= 8).
TREK1 and TRAAK are mechanosensitive members of the 2-P domain channel family. We tested whether these channels were also activated by PIP2. We expressed TREK1 and TRAAK channels in Xenopus oocytes and measured channel activity in inside-out macropatches. As the representative traces in Fig. 1C and D show, PIP2 application activated both channels. For TREK1 in most cases (n= 7 out of 10) activation of the channel occurred without application of a mechanical stimulus (suction) after seal formation (Fig. 1C). In the remaining three patches, PIP2 activation could be observed only after the application of a mechanical stimulus to the patch following the seal formation (data not shown). For these experiments, a −16 mmHg negative pressure was applied to the patch pipette (tip diameter 4–5 μm) for 40–70 s. For TRAAK, currents were elicited by PIP2 in most cases only after a mechanical stimulus had been applied (Fig. 1D, n= 4 out of 5). In one patch, activation of TRAAK currents occurred without application of a mechanical stimulus (data not shown). To test PIP2 sensitivity before and after mechanical stimulation, we used short chain (diC8), water soluble PIP2, which activates ion channels in a reversible fashion (Rohacs et al. 2002, 2003; Zhang et al. 2003). DiC8 PIP2 activated these channels to a comparable extent to AASt PIP2, but with faster kinetics. Interestingly, the effect of suction on PIP2 sensitivity persisted even after relieving application of the negative pressure. It should be noted, however, that mild negative pressure (2–5 mmHg) was necessary for seal formation in all experiments, and therefore it cannot be excluded that this promoted PIP2 activation in cases where further suction was not required in the inside-out patch configuration. Suction applied to the patch pipette also increased channel activity in the cell-attached configuration with intact PIP2 levels (data not shown). In non-injected oocytes 100 μm diC8 PIP2 did not activate any current before or after application of negative pressure (n= 6).
TASK1, TASK3 and TREK1, but not TRAAK are sensitive to agonist-induced PIP2 hydrolysis
Several G-protein coupled receptors are known to couple to PLC through Gq/G11. Upon receptor stimulation PLC hydrolyses PIP2. Hydrolysis of PIP2 inhibited the activity of several PIP2-dependent channels (Baukrowitz et al. 1998; Xie et al. 1999; Kobrinsky et al. 2000; Zhang et al. 2003). The extent of current inhibition observed is dependent on the strength of channel–PIP2 interactions (Kobrinsky et al. 2000; Zhang et al. 2003; Lopes et al. 2002). As we have seen, TASK1, TASK3 and TREK1 channels are activated by PIP2, whereas activation of TRAAK channels by PIP2 generally happened only after mechanical stimulation. To test whether hydrolysis of PIP2 inhibits these channels, we coexpressed all four channels with different Gq coupled receptors and measured the degree of inhibition upon agonist stimulation.
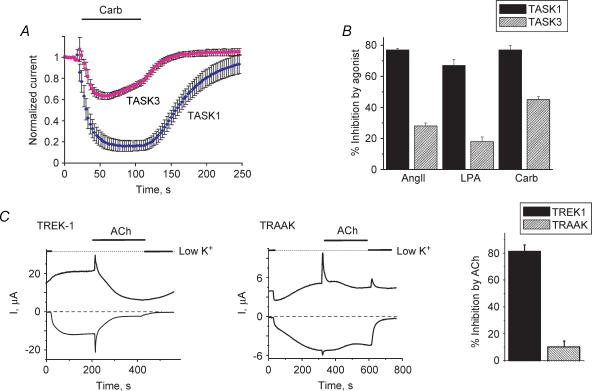
We coexpressed TASK1 or TASK3 channels with either muscarinic (M1) or angiotensin II (AT1a) receptors in Xenopus oocytes. Channel inhibition upon activation of either of these heterologously expressed receptors or the endogenous lysophosphatidic acid (LPA) receptor was seen for both channels (Fig. 2A and B). The extent of agonist-induced inhibition in all cases was lower for TASK3 channels than for TASK1 channels. TREK1 or TRAAK channels were coexpressed with M1 receptors in Xenopus oocytes. TREK1 was potently inhibited by ACh application. TRAAK channels on the other hand were only minimally inhibited (10%) by the agonist-induced PIP2 hydrolysis (Fig. 2C). The mechanism of this small inhibition is unclear; it is unlikely to be mediated by PIP2 depletion. Endogenous Ca2+-activated Cl− currents were used to monitor M1 receptor expression. The Cl− current develops quickly after ACh application, but inactivates rapidly, not contributing significantly to the currents measured in the later phases of the experiment. ACh inhibition of TREK1 channels is not affected by coexpression of M1 receptors with IP3 phosphatase, which abolishes IP3-induced Ca2+ release and consequently the Cl− currents (data not shown). We attempted to stimulate whole-cell TRAAK currents in Xenopus oocytes by low osmolarity, shear-stress and direct mechanical stimulation (gentle prodding of the oocyte with thin forceps). None of these interventions resulted in increased current, due probably to the convoluted nature of the oocyte membrane or the presence of the vitelline layer. Therefore we were not able to test in the whole-cell configuration whether mechanical stimulation confers sensitivity to agonist-induced PIP2 hydrolysis.
Figure 2. Inhibition of 2-P domain channels by agonist-induced PIP2 hydrolysis.
TASK1 and TASK3 currents were assessed by two-electrode voltage clamp. A, normalized effect of 1 μm carbachol application on whole-cell currents measured at −100 mV in 80 mm K+ extracellular solution, average of n= 5–6 cells. B, summary data for current inhibition by various agonists: angiotensin II (10 nm); lysophosphatidic acid (0.5 μm) and carbachol (1 μm) (for experiments done as in A). C, left, representative trace of the effect of 10 μm ACh on whole-cell TREK1 currents measured at +100 and −100 mV using a ramp protocol. Centre, representative trace of the effect of ACh on whole-cell TRAAK currents measured at +100 and −100 mV using a ramp protocol. Right, summary of the ACh-induced inhibition measured at −100 mV (n= 6 and n= 8 for TREK1 and TRAAK, respectively). Current level was estimated using the difference between current measured in high K+ (mm: 96 KCl, 1 NaCl, 1 MgCl2, 5 Hepes, pH 7.4) and current measured in low K+ (mm: 2 KCl, 95 NaCl, 1 MgCl2, 5 Hepes, pH 7.4) solutions at −100 mV. Note activation and fast inactivation of native Cl− current by ACh indicating expression of M1 receptors despite the lack of TRAAK inhibition.
Strength of channel–PIP2 interactions with TASK and TREK-1 channels correlate with kinetics of recovery from inhibition
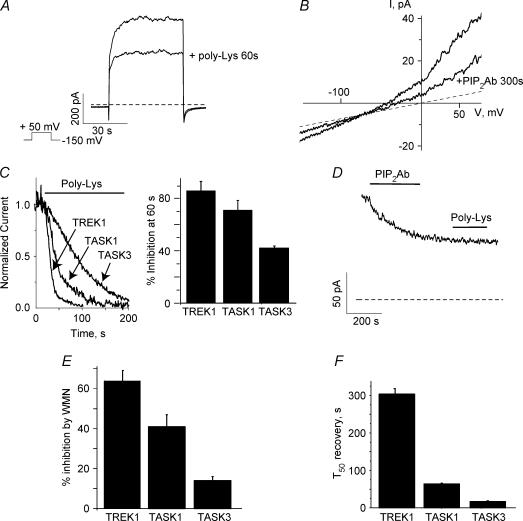
Poly Lys or PIP2Ab (an antibody against PI(4,5)P2) competes with channels for PIP2, thus scavenging PIP2 away from channels and reducing current levels (Huang et al. 1998; Lopes et al. 2002; Rohacs et al. 2002; Zhang et al. 2003). Slower inhibition by PIP2 scavengers is likely to reflect higher binding affinity of the channel to PIP2 or alternatively, stronger allosteric coupling between PIP2 molecules and the channel. Regardless of the precise mechanism, kinetics of current inhibition by PIP2 scavengers reflects a different apparent affinity of the channels for PIP2. We often refer to this apparent affinity as ‘strength of channel–PIP2 interactions’ (Zhang et al. 1999, 2003; Kobrinsky et al. 2000; Lopes et al. 2002; Rohacs et al. 2002). To assess the apparent channel affinity for PIP2 we measured the rate of current inhibition by poly Lys (Fig. 3A–C) and quantified the kinetics of the effect by measuring the percentage inhibition of the current after a 60 s poly Lys application (Fig. 3C). All three channels were fully inhibited by poly Lys suggesting that PIP2 is required for channel activity and that PIP2 electrostatically interacts with both channels. Spontaneous hydrolysis of PIP2 by lipid phosphatases in excised patches causes run-down of PIP2-sensitive channels (Hilgemann & Ball, 1996; Huang et al. 1998; Zhang et al. 1999). To avoid run-down, this set of experiments were performed in FVPP solution (see Methods) to inhibit lipid phosphatases and prevent breakdown of endogenous phosphoinositides throughout the experiment (Huang et al. 1998; Lopes et al. 2002). TASK3 channels often exhibited some run-down after excision even in the presence of FVPP solutions. Experiments were performed after currents were stable. We found that the kinetics of poly Lys inhibition were the fastest for TREK1, followed by TASK1, then TASK3 channels. These findings suggest that the interactions with PIP2 are the weakest for TREK1 channels, followed by TASK1 and TASK3 channels (Fig. 3C). We also tested the effect of PIP2Ab on TASK3 channels. PIP2Ab inhibited the channel with very slow kinetics, where the time to inhibit 50% of channel activity (T50) was 290 ± 50 s (n= 5) (Fig. 3D). In addition, PIP2Ab prevented poly Lys from inhibiting the channel (Fig. 3D, n= 6). Our results suggest that poly Lys inhibits the channel by specifically competing for PIP2 binding and not by scavenging other negatively charged lipids. These data also suggest that PIP2Ab does not inhibit completely channel activity.
Figure 3. Kinetics of inhibition of TASK1 and TASK3 channels by the PIP2 scavenger poly Lys correlates with wortmannin inhibition and recovery from agonist-induced inhibition.
A, typical response of TASK3 channels measured in oocyte macropatches to a depolarizing voltage step to +50 mV from a −150 mV holding voltage before and after 60 s application of poly Lys. B, typical response of TASK3 channels measured in oocyte macropatches to a voltage ramp (0.25 mV ms−1) before and after 300 s of PIP2Ab application. C, TREK1, TASK1 and TASK3 currents were measured in oocyte macropatches. Left, typical inhibition of either TREK1, TASK1 or TASK3 currents by poly Lys; right, summary data of kinetics of inhibition by poly Lys (30 μg ml−1, see Methods) for TREK1 (n= 5) TASK1 (n= 7) and TASK3 (n= 5) channels. D, typical effect of poly Lys application after PIP2Ab application. After PIP2Ab is bound to PIP2, poly Lys application had no effect on TASK3 channels (n= 6). E, summary data for inhibition of the whole-cell current by 60–90 min treatment with 10 μm wortmannin (n= 10–18). F, average whole-cell current recovery from carbachol inhibition for TREK1, TASK1 and TASK3 channels.
Wortmannin at low nanomolar concentrations specifically blocks phosphoinositide 3-kinases (PI 3-kinases) but at micromolar concentrations it also blocks the activity of several PI 4-kinases (Nakanishi et al. 1995). Since PI 4-kinase is required for the synthesis of PI(4,5)P2, its inhibition interferes with replenishment of PI(4,5)P2, thus decreasing PI(4,5)P2 levels (Zhang et al. 2003). Micromolar but not low nanomolar concentrations of wortmannin have been shown to affect TASK1 channel activity (Czirjak et al. 2001). Incubation of oocytes expressing either TREK1, TASK1 or TASK3 with wortmannin (10 μm) for 60–90 min suppressed channel activity. Wortmannin showed the smallest effect on TASK3 channels followed by TASK1 and TREK1 channels (Fig. 3E). These data together with the difference in the kinetics of poly Lys block, indicate that the order of the strength of channel–PIP2 interactions is TASK3, TASK1 and TREK1 channels.
There is a clear correlation between the slower poly Lys inhibition kinetics (Fig. 3C), the weaker wortmannin inhibition (Fig. 3E), and the smaller agonist-induced inhibition observed for TASK3 channels when compared to TASK1 channels (Fig. 2A and B). These results indicate that the level of agonist-induced inhibition of these channels correlates with the strength of interaction of the channel with PIP2, and suggest therefore that the inhibition of TASK channels is mediated by PIP2 hydrolysis.
We also measured the recovery from inhibition by ACh. The recovery of TASK1 channels from inhibition was shown to be affected by wortmannin at micromolar concentrations implicating PIP2 hydrolysis as the mechanism that underlies this inhibition (Czirjak et al. 2001). Similarly, recovery from PIP2 hydrolysis mediated agonist-induced inhibition of KCNQ (Suh & Hille, 2002; Zhang et al. 2003) and Kir (Xie et al. 1999) channels was inhibited by micromolar concentrations of wortmannin. These results suggest that for PIP2-sensitive channels, recovery from agonist-induced inhibition is dependent on PIP2 resynthesis. We found that the recovery from inhibition was the slowest for TREK1 channels, followed by TASK1 and TASK3 channels (Fig. 3F), further supporting the idea that the agonist-induced inhibition is mediated by PIP2 hydrolysis.
We attempted to identify molecular determinants of PIP2 interactions with 2-P channels. First we truncated the whole C-terminus of TASK3 channels, and this channel was still activated by PIP2 (data not shown). In addition we mutated several conserved positive residues in TASK3 (K137A, K144D–K145A and R150A) in the coupling region between the 2nd and 3rd TM domains. None of these mutations increased the level of agonist-induced inhibition suggesting they are not PIP2-interacting residues (data not shown). An increase in the agonist-induced inhibition would be expected if the residue was interacting with PIP2 and the mutant channel presented weaker channel–PIP2 interactions.
Agonist-induced inhibition of TREK1 currents is not mediated by protein kinase C or diacylglycerol
Agonist induced inhibition of TASK1 channels has been shown not to be mediated by downstream effectors of PLC activation (Czirjak et al. 2001). Our data indicate that this inhibition is mediated by PIP2 depletion. It has recently been suggested that the mechanism underlying agonist-induced inhibition was different for TASK and TREK channels (Chemin et al. 2003). Our data showing that TREK1 channels are activated by PIP2 and inhibited by PIP2 scavengers (Fig. 1D) suggest otherwise. Furthermore, incubation of oocytes for 2–3 h with 10 μm wortmannin, a concentration that inhibits PI 4-kinases (Nakanishi et al. 1995; Zhang et al. 2003), decreased TREK1 currents, consistent with PIP2 being necessary for maintaining channel activity (Fig. 3E). In addition, shorter incubations with 10 μm wortmannin (10–15 min) slowed recovery from ACh-induced inhibition (Fig. 4A), suggesting that PIP2 hydrolysis mediates the inhibition for these channels. Generally, these shorter incubations seem to affect replenishment of PIP2 without a significant effect on basal PIP2 levels.
TREK1 channels were also reported to be inhibited by protein kinase C (PKC) (Fink et al. 1996). To test whether PKC contributes to the agonist-induced inhibition we measured ACh inhibition in the presence of the specific PKC inhibitor calphostin-C (10 μm). Phorbol 12-myristate 13-acetate (PMA)-induced, but not ACh-induced, inhibition of TREK1 currents was diminished by calphostin-C treatment (Fig. 4B). Our data suggest that inhibition by PKC phosphorylation does not underlie agonist-induced inhibition. It was recently suggested that agonist-induced inhibition of TREK1 channels by metabotropic glutamate receptors is mediated by diacylglycerol (DAG) (Chemin et al. 2003). In this study, the DAG analogues SAG (10 μm) and SLG (10 μm) directly inhibited TREK2 and TREK1 currents previously activated by arachidonic acid (AA). To test whether DAG inhibits TREK1 currents in the absence of exogenously applied AA, we examined the effect of SAG (10 μm) and SLG (10 μm) on TREK1 current after patch excision or after activation by diC8 PIP2. Figure 4C left panel shows a representative TREK1 current trace in an inside-out patch from Xenopus oocytes shortly after seal formation. TREK1 currents usually showed an immediate increase after excision (not shown), followed by run-down (Fig. 4C). SAG and SLG showed no inhibition in these patches (n= 4 for each DAG analogue). Figure 4D shows a representative measurement in which TREK1 currents were re-activated by diC8 PIP2. Both SAG and SLG showed a mild but statistically significant inhibition, averaging around 10% (for measurement of inhibition see Methods). This small inhibition is unlikely, however, to account for the marked inhibition by M1 receptor activation.
Our data suggest that depletion of PIP2 by PLC accounts for the agonist-induced inhibition of TREK1 as well as TASK1 and TASK3 channels.
Agonist-induced PIP2 hydrolysis and PIP2 applications shift voltage sensitivity of TREK1 channels
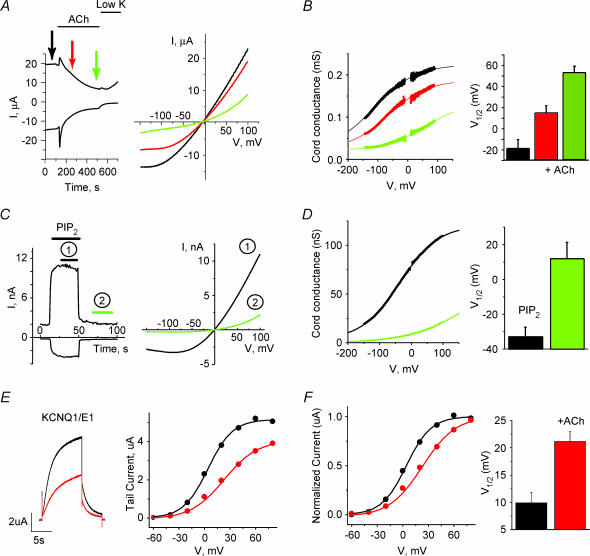
2-P domain K+ channels are generally open at all membrane potentials, but their activity has also been shown to be regulated by voltage. Most 2-P domain channels, including TASK and TREK, present an instantaneous current component and a second fast activation component in response to depolarizing steps (Lopes et al. 2000; Bockenhauer et al. 2001; Maingret et al. 2003) (see also Fig. 3A). The voltage sensitivity of TREK1 channels has been shown to be regulated by PKA phosphorylation and partially by external Mg2+ ions (Bockenhauer et al. 2001; Maingret et al. 2003). We studied the ability of agonist-induced PIP2 hydrolysis to regulate the voltage dependence of 2-P domain channels. The measurements were performed in constant external Mg2+ concentration. Figure 5A left panel shows the time course of M1 receptor mediated inhibition of TREK1 currents in Xenopus oocytes at +80 and −150 mV. The right panel shows individual representative traces in response to a slow voltage ramp (0.1 mV ms−1), which allowed measurements of steady-state current at a range of voltages before and after ACh application. Endogenous K+ currents were negligible compared to the expressed TREK1 currents measured in this voltage range. We assessed the voltage dependence by measuring the chord conductance of the current versus voltage plot. We measured currents at high external [K+]. This chord conductance reflects the ability of the channel to open at different voltages. Figure 5B left panel shows a plot of the chord conductance measured using the current–voltage relationships in Fig. 5A. The voltage dependence of TREK1 currents was affected by ACh suggesting that PIP2 hydrolysis modulates the voltage dependence of this channel. The right panel shows calculated V1/1 values as the voltage where conductance was half-way between the conductance at +100 mV and −100 mV (n= 12). Because of the limited voltage range where currents could be measured, it was not possible to fit all data with a Boltzmann equation. An equivalent and significant shift in V1/1 values during ACh stimulation was obtained for current–voltage traces (n= 6–9) that could be well fitted with a Boltzmann curve (R2 > 0.9) (V1/1 (in mV): before ACh: −74 ± 11; 1 min after ACh: 7 ± 22; steady state inhibition: 124 ± 40).
Figure 5. Agonist-induced PIP2 hydrolysis and PIP2 applications shift the voltage dependence of TREK1 and KCNQ1/KCNE1 channels.
A, left, time course of whole-cell currents for oocytes expressing TREK1 channels at +80 and −100 mV during ACh application in high K+ (96 mm) solution. Right, whole-cell currents in response to a slow voltage ramp (0.1 mV ms−1) before and after 1 min (red) and steady-state (green) ACh inhibition. B, left, plot of the chord conductance (I/V) measured using the current–voltage relationship shown in A. Continuous lines represent a Boltzmann fit of the data. Right, summary data of the effects of ACh on V1/1. V1/1 was estimated by the conductance half-way between the conductance at −100 and +100 mV (n= 12). C, left, activation of TREK1 currents in inside-out macropatches by 100 μm DiC8 PIP2 at +90 and −90 mV in symmetrical high K+ (96 mm) solutions. Right, currents in response to a slow voltage ramp (0.16 mV ms−1) during (black) and after (green) application of PIP2. D, left, plot of the chord conductance (I/V) measured using the current–voltage relationship shown in C. Continuous lines represent a Boltzmann fit of the data. Right, summary data of effects of the application of DiC8 PIP2 on V1/1 estimated by the voltage where the conductance was half-way between of the conductance measured at +100 and −150 mV (n= 6). E, left, representative current traces of KCNQ1/KCNE1 channels, activated by a voltage step from the holding potential of −80 mV to +60 mV and then −40 mV before and after the application of ACh. Right, current–voltage relationship measured from the tail currents at −40 mV after steps to different voltages. F, left, normalized current–voltage relationship before and after the application of ACh. Right, summary data (n= 15). All conductance measurements were fit by a Boltzmann equation (continuous line).
To further test whether PIP2 directly modulates voltage dependence of this channel, we measured the effect on the TREK1 voltage dependence in excised inside-out patches before and after PIP2 applications. The outwardly rectifying current present after the washout of 100 μm diC8 PIP2 is reflecting the activity of TREK-1 due to endogenous PIP2 present in the patch and residual effect of the diC8 PIP2 applied. We observed a left shift in the voltage dependence of TREK1 currents in response to diC8 PIP2. Figure 5C shows the time course of the activation of TREK1 currents by the water-soluble diC8 PIP2 analogue (100 μm). Figure 5C (left panel) shows averaged ramp currents from the periods. Figure 5D (left panel) shows the chord conductance of the ramp traces. The right panel shows summary of V1/1 values (n= 6), calculated as the voltage where conductance was half-way between the conductance at +100 mV and −150 mV. Endogenous currents in non-injected oocytes were negligible compared to TREK1 currents.
Our data indicate that the presence of PIP2 is required for the voltage-dependent activation of the channel at more hyperpolarized voltages. For channels where PIP2 is removed either by agonist-induced PIP2 hydrolysis or wash out of PIP2, a stronger depolarization is necessary to activate the channel.
Agonist-induced PIP2 hydrolysis inhibits current and shifts voltage dependence of KCNQ1/KCNE1 channels
We compared the effects of ACh on TREK1 with the effect of PIP2 hydrolysis in the voltage-gated KCNQ1/KCNE1 channel. PIP2 has been shown to activate KCNQ channels and its hydrolysis to underlie inhibition by agonist activation of PLC (Zhang et al. 2003). As for the TREK1 channels, ACh-induced PIP2 hydrolysis inhibited maximal KCNQ1/KCNE1 channel conductance and shifted the voltage dependence of activation towards more depolarized voltages (Fig. 5E and F). Our data are consistent with a recent report showing that application of PIP2 to excised patches expressing KCNQ1/KCNE1 channels shifted their voltage dependence to the left (Loussouarn et al. 2003). These channels were coexpressed with IP3 phosphatase to minimize Ca2+-dependent Cl− currents and Ca2+ modulation. These results suggest a conserved role of PIP2 as a gating molecule for structurally unrelated K+ channel families.
Discussion
The role of PIP2 as a second messenger that directly regulates transmembrane effector proteins is being increasingly appreciated (Hilgemann et al. 2001). In the present study, we have characterized the effects of PIP2 on members of the 2-P domain K+ channel family. We have shown that all four members of this family tested are activated by PIP2. Our data indicate that hydrolysis of PIP2 mediates inhibition of channel activity by hormones and neurotransmitters. In addition, we show that PIP2 hydrolysis modulates voltage dependence of 2-P channels. We also demonstrate that agonist-induced PIP2 hydrolysis modulates voltage dependence of both 2-P and voltage-gated KCNQ channels. Our results suggest that PIP2 is a common gating molecule for K+ channels and that its function is conserved despite the lack of homology between the putative PIP2-binding cytoplasmic domains of the different K+ channel families.
For TASK1 channels, PLC activation was shown to be required for the agonist-induced inhibition and downstream signals did not mediate the inhibition. In addition, wortmannin, at concentrations inhibiting PIP2 replenishment in the cell membrane, was shown to retard recovery from carbachol-induced inhibition (Czirjak et al. 2001). Although these results are consistent with PIP2 hydrolysis underlying the inhibition, they did not provide direct evidence for this mechanism. Recently, direct activation of TASK3 channels by PIP2 has been demonstrated (Chemin et al. 2003). It was suggested that PIP2 depletion underlies PLC-mediated inhibition of TASK, but not TREK, channels. Here we provide several lines of evidence that agonist-induced inhibition of TASK1 and TASK3 as well as TREK1 channels is mediated by PIP2 depletion. First TASK1, TASK3 and TREK1 channels are all activated by PIP2 (Fig. 1). Second, activity of all three channels is blocked by PIP2 scavengers (Fig. 1). Third all three channels are inhibited by wortmannin at concentrations that inhibit PI 4-kinases, depleting PIP2 (Fig. 3E). Fourth, wortmannin retards recovery of TREK1 channels from ACh-induced inhibition (Fig. 4A), similarly to TASK1 (Czirjak et al. 2001) and TASK3 (Chemin et al. 2003) channels. Finally, we show that the downstream products of PIP2 hydrolysis, DAG and PKC, do not underlie agonist-induced inhibition of TREK1 channels (Fig. 4B–D). Our results also indicate that IP3 does not mediate agonist-induced inhibition, as coexpression of IP3 phosphatase, eliminating the Ca2+-activated Cl− current, did not affect the M1-induced inhibition on TREK1 currents (data not shown). In conclusion, our results indicate that agonist-induced inhibition of TASK and TREK channels is mediated by depletion of PIP2.
Our data also show that the kinetics of poly Lys block of TREK1 and TASK channels (Fig. 3C) correlate with the level of agonist-induced inhibition (Fig. 2) and the kinetics of recovery from inhibition (Fig. 3F). In addition, effects of wortmannin on TREK1 and TASK channel activity (Fig. 3E) also correlate with agonist-induced inhibition. These results indicate that TASK3 channels have the strongest interaction with PIP2 followed by TASK1 and TREK1 and that the extent of agonist-induced inhibition by depletion of PIP2 is determined by the strength of channel–PIP2 interactions.
Inwardly rectifying K+ channels are best characterized in their interactions with PIP2 (Huang et al. 1998; Rohacs et al. 2003; Lopes et al. 2002). The highly conserved C and N terminal cytoplasmic portions of these channels presumably form a well-defined binding site that is formed by positively charged residues (Lopes et al. 2002). Mutations in the PIP2-interacting positive residues of these channels confer lower PIP2 affinity, resulting in either non-functional channels, or channels with lower activity and higher sensitivity to inhibition by agonist-induced PIP2 hydrolysis (Zhang et al. 1999; Kobrinsky et al. 2000; Lopes et al. 2002). The cytoplasmic domains of 2-P domain channels show a much lower level of conservation than those of Kir channels. As described earlier, our initial attempt to test potential PIP2 interacting sites comparable to those found in other channels did not prove successful. A five residue region (VLRFMT) in the proximal C-terminus has been implicated in agonist- and halothane-induced inhibition. Mutation of the positively charged Arg residue reduced agonist-induced inhibition by TRH and did not seem to inhibit basal current level (Talley & Bayliss, 2002). This suggests increased apparent PIP2 affinity, similarly to the I229L mutant in Kir3.4 channels (Zhang et al. 1999; Kobrinsky et al. 2000). Therefore this residue is unlikely to be a direct PIP2-interacting residue. Although unlikely, it is possible that PIP2 activates these channels through an intermediate PIP2 binding protein. This putative protein has to be membrane bound, show different apparent PIP2 affinities when bound to different channels and not be affected by the deletion of the whole C-terminus of the channel.
Mechanosensitive channels are sensitive to non-specific membrane perturbations. It could therefore be possible that incorporation of excess PIP2 into the membrane changes the physical properties of the membrane and affects channel activity. Our data, however, suggest that the effects of PIP2 are not mediated by such non-specific membrane perturbations. First, such perturbations should not be caused by the scavenging of PIP2 by poly Lys. Second, PIP2 does not activate stretch-activated channels, known to be present in the oocyte membrane in non-injected oocytes. Finally, since PIP2 is a minor (∼1%) component of the plasma membrane, decreasing its levels by wortmannin and ACh is highly unlikely to change the mechanical properties of the membrane significantly. Therefore, the correlation between whole-cell (Figs 2C, 3E, 4A and 5A and B) and excised-patch measurements (Figs 1C and 5C and D) on TREK1 channels strongly suggests that PIP2 activation does not proceed through non-specific membrane perturbation when PIP2 is directly applied to the patches.
We have also shown that PIP2 plays an important role in the regulation of 2-P channels by mechanical stimulation. TRAAK channels were generally only activated by PIP2 after applying negative pressure to the membrane patch. This raises the possibility that pressure regulates these channels by allowing the otherwise silent PIP2 interactions to exert their stimulatory effect.
Potassium channels inhibit cell excitability by holding the membrane potential below the firing threshold for action potential. Leak potassium channels contribute to the resting membrane potential and generally maintain it close to the potassium equilibrium potential. A decrease in leak potassium conductance is thought to increase cell excitability. The mechanism underlying this increase in excitability is believed to be a simple decrease in channel conductance and increased contribution of other ion channels, which will tend to depolarize the cell. Here we show that hormones and neurotransmitters not only decrease channel conductance but also modulate their voltage dependence. This change in the channel voltage dependence is also expected to contribute to changes in cell excitability.
We show that PIP2 hydrolysis inhibited channel activity and shifted the voltage dependence of both the 2-P domain TREK1 channel and the voltage-gated KCNQ1/KCNE1 channel. Our data are consistent with a recent report showing that addition of excess PIP2 to inside-out patches shifts the voltage sensitivity of KCNQ1/KCNE1 channels (Loussouarn et al. 2003). The nature of the voltage sensor of the 2-P domain K+ channels is not known, but positive charges outside the transmembrane domains, may serve this function, provided they are embedded in the membrane. For both channel families we observed a decrease in channel activity and shift on the voltage dependence towards more depolarized potentials by PIP2 hydrolysis. This conserved mechanism suggests a common role for PIP2 in setting the voltage sensitivity of different K+ channel families. Furthermore, PIP2 constitutes a common regulatory molecule for the activity of all three major K+ channel families, underlining a general role for PIP2 in the gating of K+ channels.
Acknowledgments
We thank Dr Bensheng Liu, Ms Elizabeth Findeis and Ms Irén Veres for preparing oocytes; Dr M. Lazdunski and Dr E. Honoré for providing cDNA clones for TASK1, TREK1 and TRAAK; Dr Cristophe Erneux for providing the type-1 IP3 5-phosphatase clone; and Dr J. Yang for critically reading the manuscript. This work was supported by NIH grant HL59949 to D.E.L., grants OTKA (T046954) and ETT-085/2003 to P.E. D.E.L is an Established Investigator of the American Heart Association (AHA). T.R. was supported by a postdoctoral fellowship from the Revson Foundation and a Scientist Development Grant (0330224 N) from AHA. C.B.L. was supported by a postdoctoral fellowship and a Scientist Development Grant from AHA.
References
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Bockenhauer D, Zilberberg N, Goldstein SA. KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci. 2001;4:486–491. doi: 10.1038/87434. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Chemin J, Girard C, Duprat F, Lesage F, Romey G, Lazdunski M. Mechanism undelying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J. 2003;22:5403–5411. doi: 10.1093/emboj/cdg528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5),P2 mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol. 2002;16:621–629. doi: 10.1210/mend.16.3.0788. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Petheo GL, Spat A, Enyedi P. Inhibition of TASK-1 potassium channel by phospholipase C. Am J Physiol Cell Physiol. 2001;281:C700–C708. doi: 10.1152/ajpcell.2001.281.2.C700. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Ball R. Regulation of cardiac Na+/Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE 2001. 2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Gnatenco C, Kim D. Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channels by pressure, free fatty acids and alkali. Pflugers Arch. 2001;442:64–72. doi: 10.1007/s004240000496. [DOI] [PubMed] [Google Scholar]
- Kobrinsky E, Mirshahi T, Zhang H, Jin T, Logothetis DE. Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+ current desensitization. Nature Cell Biol. 2000;2:507–514. doi: 10.1038/35019544. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Lopes CM, Gallagher PG, Buck ME, Butler MH, Goldstein SA. Proton block and voltage gating are potassium-dependent in the cardiac leak channel Kcnk3. J Biol Chem. 2000;275:16969–16978. doi: 10.1074/jbc.M001948200. [DOI] [PubMed] [Google Scholar]
- Lopes CMB, Zhang H, Rohacs T, Jin T, Logothetis DE. Alterations in conserved Kir channel–PIP2 interactions underlie channelopathies. Neuron. 2002;34:933–944. doi: 10.1016/s0896-6273(02)00725-0. [DOI] [PubMed] [Google Scholar]
- Loussouarn G, Park KH, Bellocq C, Baro I, Charpentier F, Escande D. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 2003;22:5412–5421. doi: 10.1093/emboj/cdg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Honore E, Lazdunski M, Patel AJ. Molecular basis of the voltage-dependent gating of TREK-1, a mechano-sensitive K+ channel. Biochem Biophys Res Commun. 2003;292:339–346. doi: 10.1006/bbrc.2002.6674. [DOI] [PubMed] [Google Scholar]
- Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci U S A. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci U S A. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Jin T, Ramdya PP, Molnar Z, Logothetis DE. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc Natl Acad Sci U S A. 2003;100:745–750. doi: 10.1073/pnas.0236364100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Lopes C, Mirshahi T, Jin T, Zhang H, Logothetis DE. Assaying phosphatidylinositol bisphosphate regulation of potassium channels. Meth Enzymol. 2002;345:71–92. doi: 10.1016/s0076-6879(02)45008-2. [DOI] [PubMed] [Google Scholar]
- Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Lei Q, Talley EM, Lynch C, III, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5),P2 concentration monitored in living cells. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- Sui JL, Petit Jacques J, Logothetis DE. Activation of the atrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci U S A. 1998;95:1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal J, Habets R, Varnai P, Balla T, Jalink K. Monitoring agonist-induced phospholipase C activation in live cells by fluorescence resonance energy transfer. J Biol Chem. 2001;276:15337–15344. doi: 10.1074/jbc.M007194200. [DOI] [PubMed] [Google Scholar]
- Wu L, Bauer CS, Zhen XG, Xie C, Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5),P2. Nature. 2002;419:947–952. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- Xie LH, Horie M, Takano M. Phospholipase C-linked receptors regulate the ATP-sensitive potassium channel by means of phosphatidylinositol 4,5-bisphosphate metabolism. Proc Natl Acad Sci U S A. 1999;96:15292–15297. doi: 10.1073/pnas.96.26.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CMB, Jin T, Logothetis DE. PIP2 activates KCNQ channels and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nature Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]