Abstract
Extracellular nucleotides are important regulators of epithelial ion transport, frequently exerting their action from the luminal side. Luminal P2Y receptors have previously been identified in rat distal colonic mucosa. Their activation by UTP and ATP stimulates K+ secretion. The aim of this study was to clarify which of the P2Y receptor subtypes are responsible for the stimulated K+ secretion. To this end P2Y2 and P2Y4 knock-out mice were used to measure distal colonic ion transport in an Ussing chamber. In mouse (NMRI) distal colonic mucosa, luminal UTP and ATP with similar potency induced a rapid and transient increase of the transepithelial voltage (Vte) (UTP: from −0.81 ± 0.23 to 3.11 ± 0.61 mV, n = 24), an increase of equivalent short circuit current (Isc) by 166.9 ± 22.8 μA cm−2 and a decrease of transepithelial resistance (Rte) from 29.4 ± 2.4 to 23.5 ± 2.0 Ω cm2. This effect was completely inhibited by luminal Ba2+ (5 mm, n = 5) and iberiotoxin (240 nm, n = 6), indicating UTP/ATP-stimulated K+ secretion. RT-PCR analysis of isolated colonic crypts revealed P2Y2, P2Y4 and P2Y6 specific transcripts. The luminal UTP-stimulated K+ secretion was still present in P2Y2 receptor knock-out mice, but significantly reduced (ΔVte: 0.83 ± 0.26 mV) compared to wild-type littermates (ΔVte: 2.08 ± 0.52 mV, n= 9). In P2Y4 receptor knock-out mice the UTP-induced K+ secretion was similarly reduced. Luminal UTP-stimulated K+ secretion was completely absent in P2Y2/P2Y4 double receptor KO mice. Basolateral UTP showed no effect. In summary, these results indicate that both the P2Y2 and P2Y4 receptors are present in the luminal membrane of mouse distal colonic mucosa, and stimulation of these receptors leads to K+ secretion.
P2 or nucleotide receptors are strongly expressed in all transporting epithelia and regulate ion secretion and absorption (Dubyak, 1999; Lazarowski & Boucher, 2001; Leipziger, 2003). Frequently, these membrane receptors are expressed both in the basolateral and luminal membranes. The luminal expression of P2 receptors is a phenomenon unique to epithelial organs (Leipziger, 2003). The activation of P2 receptors by extracellular nucleotides like ATP or UTP commonly influences ion transport processes. As a general theme, P2 receptor activation can trigger two different effects: (1) activation of ion (and water) secretion, and (2) inhibition of Na+ absorption. Pro-secretory effects include the activation of Cl− secretion in human respiratory epithelium (Mason et al. 1991), activation of HCO3− secretion in mouse gallbladder epithelium (Clarke et al. 2000) and stimulation of K+ secretion in rat distal colon (Kerstan et al. 1998), Necturus gallbladder (Cotton & Reuss, 1991) and human airway epithelium (Clarke et al. 1997). The inhibition of Na+ absorption was found in different steroid-sensitive epithelia and involves ENaC channels (Mall et al. 2000; Lehrmann et al. 2002a,b).
Mammalian P2 receptors are subdivided into metabotropic P2Y and ionotropic P2X receptors. Currently, eight different P2Y have been identified: five of them are linked to Gq proteins (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11) and three have been shown to be Gi-coupled (P2Y12, P2Y13, P2Y14) (Abbracchio et al. 2003). There is clear evidence for expression of P2Y1, P2Y2, P2Y4 and P2Y6 receptors in intact epithelial tissues (Ralevic & Burnstock, 1998; Dubyak, 2003; Leipziger, 2003). In addition, seven different P2X receptors are described in mammalian cells (P2X1–7) (North, 2002).
In many epithelial tissues luminal UTP is a lead agonist in the alteration of ion transport (Leipziger, 2003), and exclusively stimulates the P2Y2 and the P2Y4 receptors indicating that activation of either one may be responsible for the effect (Ralevic & Burnstock, 1998). In respiratory epithelium, the generation of the P2Y2 receptor knock-out (KO) mouse helped to show that the major luminal P2Y receptor involved in the activation of chloride secretion was the P2Y2 subtype (Cressman et al. 1999). Not all epithelia express this receptor subtype luminally but still respond vividly to the addition of luminal UTP. Mouse jejunum displays a luminal UTP-stimulated Cl− secretion, which was not affected in P2Y2 receptor KO mice (Cressman et al. 1999; Robaye et al. 2003). Using P2Y4 KO mouse it was recently established that the P2Y4 receptor is responsible for the UTP-stimulated Cl− secretion. Thus, the use of P2 receptor knock-out mice has become a powerful tool to investigate which receptors are expressed in a given tissue (Dubyak, 2003). In the present study we use this strategy to investigate the effect of luminal nucleotides on ion transport in mouse distal colon. Specifically, we address the question, Do luminal UTP and ATP stimulate K+ secretion as shown previously in rat distal colonic mucosa (Kerstan et al. 1998)? In this study we show that both luminal P2Y2 and luminal P2Y4 receptors are functionally expressed in mouse distal colon, and that their activation induces a transient K+ secretion.
Methods
Mouse strains
All handling and use of animals complied with Danish animal welfare regulations. In the present study different mouse strains were used (Table 1). Previous studies investigating P2 receptor effects on renal ion transport used in-house-bred NMRI mice (Department of Physiology, University of Freiburg) (Lehrmann et al. 2002a). In the first series of experiments in this paper also NMRI mice were used (Taconic, Lille Skensved, Denmark). The P2Y2 receptor knock-out mouse was generously provided as KO breeder pairs (on a B6D2 genetic background) by Dr B. H. Koller (University of North Carolina, Chapel Hill, USA). B6D2 P2Y2−/− mice (original P2Y2 KO) were then crossed with the SV129 mouse strain, generating B6D2/SV129 P2Y2+/+ (P2Y2 WT) and B6D2/SV129 P2Y2−/− (P2Y2 KO) littermates. The mouse P2Y4 receptor gene is localized on the X-chromosome. CD1/SV129 P2Y4+/o (P2Y4 WT) and CD1/SV129 P2Y4−/o (P2Y4 KO) littermates were generated as previously described (Robaye et al. 2003).
Table 1.
Summary of different mice used and their respective genetic background
| Mice used | Genetic background |
|---|---|
| NMRI | NMRI |
| Original P2Y2 KO | B6D2 |
| P2Y2 WT and KO | B6D2/SV129 |
| P2Y4 WT and KO | CD1/SV129 |
| P2Y2/P2Y4 WT and DKO | B6D2/SV129/CD1 |
Generation of P2Y2/P2Y4 double receptor knock-out mice
Double knock-out mice were generated by breeding P2Y4+/− females with P2Y2−/− (P2Y2 KO) males. From their offspring, P2Y2+/−/P2Y4+/− females were selected and further crossed with P2Y2+/− males in order to generate P2Y2+/+/P2Y4o/+ and P2Y2−/−/P2Y4o/− male littermates. Throughout this article, wild-type males P2Y2+/+/P2Y4o/+ will be referred to as P2Y2/P2Y4 WT and double knock-out males P2Y2−/−/P2Y4o/− as P2Y2/P2Y4 DKO.
Genotyping of P2Y2 and P2Y4 receptor knock-out mice
Genotyping was performed from extracted DNA from clipped tails. For the P2Y2 mice genotyping, a triple primer genomic PCR was used. The two different forward primers had the following sequences: 5′-GTCACGCGCACCCTCTACTA-3′ (identifying the WT allele) and 5′-GGGGAACTTCCTGACTAGG-3′ (identifying the inserted neo-cassette in the disrupted allele). The reverse primer for both alleles had the following sequence: 5′-GTCGGGTGCACTGCCTTTCT-3′. For the P2Y4 mouse genotyping two genomic PCRs were used. The wild type allele was detected using the primers 5′-AGTAGAGGTTCCAGTAGAAA-3′ and 5′-GACTCCTTGCTATTCACAT-3′ while the mutated allele was amplified with the primers 5′-CGAAGTTATATTAAGGGTTC-3′ and 5′-TAATCGGTCACCCTCA-3′. For the P2Y2/P2Y4 WT and DKO genotyping, the previous primers and methods were used.
Ussing chamber experiments
Mice (age 4–20 weeks) were killed by decapitation. Only the distal 2 cm of mouse colon was used. The muscular layers were gently removed and a piece was mounted in an Ussing Chamber with an aperture of 0.126 cm2. The two halves of the chamber were perfused continuously by a bubble lift system. The solutions on the two sides were composed as follows (mm): NaCl 120; NaHCO3 25; K2HPO4 1.6; KH2PO4 0.4; calcium gluconate 1.3; MgCl2 1; d-glucose 5, indomethacin (5 μm). The reservoirs were bubbled with carbogen (5% CO2 and 95% O2) and kept at 37°C by water jackets. The measurements were performed in ‘open-circuit’ mode. Transepithelial voltage (Vte) was referred to the serosal side and transepithelial resistance (Rte) was calculated from the voltage deflections (ΔVte) induced by short current pulses (25 μA, 0.6 s) (Lohrmann et al. 1995). These deflections were corrected for those obtained with the empty chamber. The equivalent short circuit current (Isc) was obtained by Ohm's law from Vte/Rte. The calculated Isc changes were derived from peak values. Initially, tetrodotoxin (TTX, 1 μm) was added to the serosal side to inhibit possible secretory activation by the enteric nervous system or other autonomous nerve cells. Subsequently, amiloride (100 μm) was added to the mucosal perfusate to abolish any rheogenic Na+ absorption. After an equilibration period (30–60 min) UTP or other agonists and antagonists were added to the mucosal side. In the experiments performed on low Na+ diet mice, the animals were fed a special diet containing (mg kg−1): Na+ 136; Cl− 178.5; K+ 7170 (Altromin, Lage, Germany).
Crypt preparation
Mice (age 4–20 weeks) were killed by decapitation (without anaesthesia). The preparation of colonic crypts was similar to that described by Siemer & Gögelein (1992) and Diener et al. (1989). A 4 cm piece of mouse distal colon was everted and rinsed with ice-cold Ca2+-free Ringer-type solution with the following composition (mm): NaCl 127; KCl 5; sodium pyruvate 5; d-glucose 5; 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (Hepes) 10; ethylenediaminetetraacetic acid (EDTA) 5; MgCl2 1. Both ends were tied to obtain a sac preparation. This sac was filled with the same Ca2+-free solution. The sacs were then incubated in the above mentioned solution for 10 min at 37°C. Isolated crypts were obtained by shaking the sacs.
RT-PCR analysis of P2Y receptors in isolated mouse colonic crypts
RT-PCR analysis was used to investigate if specific mRNAs for the mouse P2Y1, P2Y2, P2Y4 and P2Y6 receptors are present in mucosal epithelial cells. To this end, total RNA was extracted from approximately 500 isolated colonic crypts. Total RNA was transcribed into cDNA using reverse transcriptase. Primer selection was based on published mouse sequences for the different P2Y receptors (Table 2).
Table 2.
Summary of used primer sequences
| P2Y receptor | Forward primer 5′–3′ | Reverse primer 5′–3′ | Expected band size of amplicon (bp) |
|---|---|---|---|
| P2Y1 | TCTTGGGCTGTTATGGAT | TCTCAGGGATGTCTTGTG | 481 |
| P2Y2 | GTCACGCGCACCCTCTACTA | TCGGGTGCACTGCCTTTCTT | 548 |
| P2Y4 | CTTTGGCTTTCCCTTCTTGA | GTCCGCCCACCTGCTGAT | 427 |
| P2Y6 | GCCCTGTGCTGGAGACCTTC | CATGGCCCCAGTGACAAACA | 226 |
Solutions and chemicals
Tetrodotoxin (TTX) was purchased from Latoxan (Rosans, France). All other chemicals were of the highest grade of purity available and were obtained from Sigma-Aldrich Denmark A/S (Vallensbaek, Denmark) and Merck (Darmstadt, Germany).
Statistics
Data are shown as mean values ±s.e.m. (n), where n refers to the number of mucosal preparations or investigated crypts. Student's paired and unpaired t test was used to compare mean values within one experimental series. A P-value of < 0.05 was accepted as indicating statistical significance.
Results
Luminal ATP and UTP induce a rapid and transient increase of positive equivalent Isc in mouse distal colonic mucosa
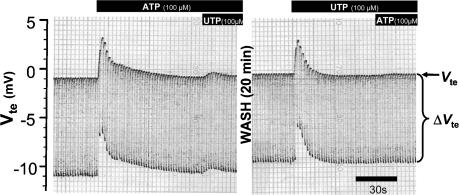
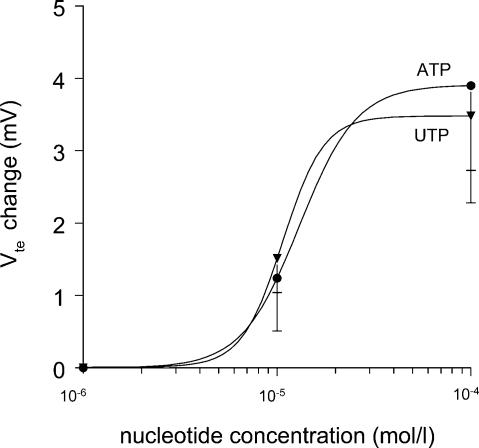
Initially, we set out to determine if the response to luminal nucleotides is similar in mouse colonic mucosa to that described for rat tissue (Kerstan et al. 1998). The experiments were performed in NMRI mice. Figure 1 shows an original trace from an Ussing chamber experiment in which ATP (100 μm), followed by UTP (100 μm), was applied to the luminal side of the mucosa. The resting transepithelial voltage (Vte) was close to −1 mV. In the entire experimental series the mean Vte was −1.00 ± 0.17 mV and the mean calculated transepithelial resistance (Rte) was 29.7 ± 2.2 Ω cm2 (n = 24). Application of ATP led to a very rapid (within 3 s) and transient change of polarity of the transepithelial voltage from approximately −1 mV to +3 mV (mean peak change of Vte from a resting −1.00 ± 0.17 to +3.11 ± 0.83 mV under stimulation, n = 24). Rte decreased from a prestimulatory value of 29.7 ± 2.2 to 24.1 ± 1.5 Ω cm2 (n = 24). The calculated Isc increase amounted to 148.3 ± 26.3 μA cm−2. After approximately 1 min, UTP was also added onto the mucosal surface. This induced only a very small deflection of Vte (0.43 ± 0.09 mV, n = 22). Subsequently, the tissue was gently and thoroughly washed and a second testing of luminal nucleotides was undertaken after approx. 20–30 min, with the application order of the luminal nucleotides reversed. In this second series, luminal UTP stimulated a rapid and transient deflection of Vte from a mean value of −0.81 ± 0.23 to 3.11 ± 0.61 mV and a decrease in Rte from 29.4 ± 2.4 to 23.5 ± 2.0 Ω cm2 (n = 24). Again, a subsequent addition of in this case luminal ATP was almost without effect (Vte change 0.32 ± 0.10 mV, n = 24). The concentration response curves for the luminal ATP- and UTP-mediated effect were investigated in a separate series of experiments in NMRI mice and are shown in Fig. 2. Both agonists were of similar potency. The data were fitted with the Hill equation and the EC50 values of 13 μmol l−1 for ATP and 10 μmol l−1 for UTP were calculated. Neither luminal adenosine nor UDP produced any effects (data not shown). This pharmacological profile strongly suggests that either activation of a P2Y2 or a P2Y4 receptor underlies this effect.
Figure 1. Effect of luminal ATP and UTP on ion transport in mouse distal colonic mucosa (NMRI mice).
Original recording of transepithelial voltage (Vte) and transepithelial voltage changes (ΔVte). The upper line shows Vte and the bandwidth of voltage deflections reflects the transepithelial resistance (Rte, see Methods). Application of luminal ATP or UTP to the luminal side led to a transient deflection of Vte to lumen-positive values and a decrease of Rte.
Figure 2. Concentration response relationship of luminal UTP- and ATP-induced transepithelial voltage deflections.
Both nucleotides showed a similar potency with an EC50 value of 13 μm for ATP and 10 μm for UTP.
The UTP and ATP effects are inhibited by luminal Ba2+ and iberiotoxin
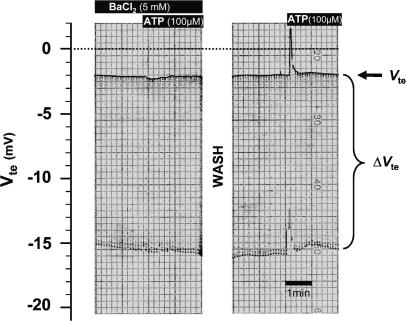
A change of Vte to lumen-positive values with a concomitant decrease in Rte may, in principle, be the result of either luminal hyperpolarizing conductances or basolateral depolarizing conductances. A luminal hyperpolarization could result from K+ or Cl− channel opening in the luminal membrane or a basolateral depolarization from opening of cationic or Cl− channels in the basolateral membrane. The following experiments were performed in NMRI mice to elucidate which ion conductances were activated by luminal ATP. Extrapolating from our previous rat data (Kerstan et al. 1998), it can be assumed that a luminal K+ channel was responsible for the observed effect. In strictly paired experiments shown in Fig. 3, the ATP (100 μm) effect was investigated in the presence and absence of luminal Ba2+ (5 mm) in NMRI mice. Both Vte and Rte effects of luminal ATP were almost completely blocked by luminal Ba2+ (voltage change with Ba2+, 0.38 ± 0.33 mV; voltage change after Ba2+ wash-out, 2.84 ± 0.58 mV; n = 5). Similar results were obtained when UTP was used as luminal agonist (data not shown). Importantly, luminal iberiotoxin (IBTX, 240 nm) also completely inhibited the UTP-stimulated Vte deflection (voltage change without IBTX, 5.25 ± 1.25 mV; voltage change with IBTX, 0.83 ± 0.36 mV; n = 6). These results strongly indicate that luminal UTP and ATP activate a luminal BK channel and thus K+ secretion.
Figure 3. Effect of luminal Ba2+ on luminal ATP-induced transepithelial voltage effect in mouse distal colonic mucosa.
Original recording of transepithelial voltage (Vte) and transepithelial voltage changes (ΔVte). The upper line shows Vte and the bandwidth of voltage deflections reflects the transepithelial resistance (Rte). Luminal Ba2+ completely inhibited the ATP-induced Vte deflections. After wash-out this effect was fully reconstituted.
PCR analysis reveals the presence of different P2Y receptors in isolated rat colonic crypts
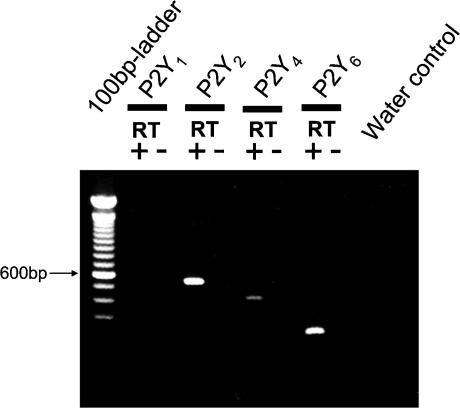
The above-described data strongly indicated that luminal ATP and UTP mediate their effect via P2Y2 or P2Y4 receptors. To investigate this further, the presence of specific P2Y1, P2Y2, P2Y4 and P2Y6 receptor mRNA in mouse colonic crypts of NMRI mice was determined. As shown in Fig. 4, specific P2Y receptor mRNA transcripts were found for the P2Y2, P2Y4 and the P2Y6 receptors (for details see Methods). Similar results were obtained in three different RNA extractions from isolated mouse colonic crypts. These results are consistent with the hypothesis that either a P2Y2 or a P2Y4 receptor mediates the effects observed in the Ussing chamber.
Figure 4. RT-PCR identification of different P2Y receptors mRNA transcripts from isolated distal colonic crypts of NMRI mice.
Positive results were found for the P2Y2, the P2Y4 and the P2Y6 receptors.
Reduced K+ secretory response in P2Y2 receptor KO mice
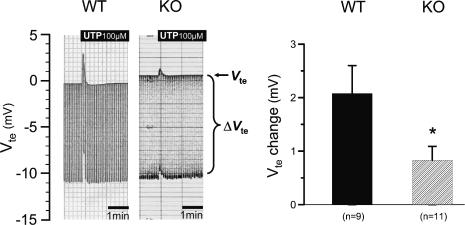
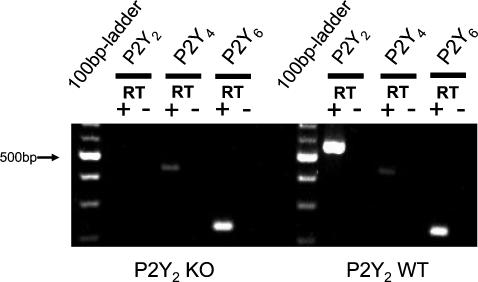
To explore this in detail, the recently generated P2Y2 receptor KO (original P2Y2 KO, genetic background: B6D2) mouse was used. Breeder pairs of these mice were set to reproduction. Mice of either sex were investigated at the same age as the NMRI mice. In the population of original P2Y2 KO mice, all animals responded with a pronounced response to luminal ATP and UTP. Application of ATP led to a rapid and transient change of polarity of the transepithelial voltage from a resting value of −0.30 ± 0.49 mV to +2.85 ± 1.11 mV under stimulation. Rte decreased from a prestimulatory value of 41.3 ± 5.4 Ω cm2 to 37.8 ± 4.4 Ω cm2 (n = 9). Similarly, the application of luminal UTP led to a rapid and transient change of polarity of the transepithelial voltage from a resting value of −0.35 ± 0.28 mV to +4.16 ± 1.04 mV under stimulation. Rte decreased from a prestimulatory value of 46.2 ± 5.6 Ω cm2 to 38.4 ± 3.6 Ω cm2 (n = 10). These results are similar to those measured in NMRI mice and therefore may indicate that a P2Y2 receptor is not linked to the activation of K+ secretion. This issue was further tested by comparing P2Y2 receptor KO mice with their littermate WT controls. This was done by crossing the original B6D2 P2Y2 KO mice with SV129 WT mice. The F2 generation (genetic background: B6D2/SV129) was used for functional experiments. The Ussing chamber results of these experiments are shown in Fig. 5. There is a clear difference between P2Y2 KO mice and their corresponding WT littermates. In WT and P2Y2 KO mice, UTP led to a rapid deflection by 2.08 ± 0.52 mV (n = 9) and 0.83 ± 0.26 mV (n = 11), respectively. In each P2Y2 receptor KO mouse, luminal UTP (100 μm) induced a smaller but still significant effect. These data strongly suggest that the P2Y2 receptor mediates part of the luminal nucleotide-mediated K+ secretory response, but yet another receptor may be present to account for the remaining part. We subsequently returned to the RT-PCR analysis and questioned which of the UTP- or UDP-sensitive P2Y receptors are detectable in P2Y2 KO mice (genetic background: B6D2/SV129). The representative gel in Fig. 6 shows no evidence for P2Y2 receptor-specific mRNA, but shows that the P2Y4 and the P2Y6 receptor mRNAs continue to be present. These results support the hypothesis that the P2Y4 receptor mediates the residual K+ secretory effect.
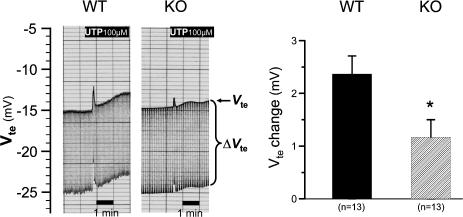
Figure 5. Effect of luminal UTP in P2Y2 receptor KO and WT littermates.
Two original recordings of transepithelial voltage (Vte) and transepithelial voltage changes (ΔVte) in KO and WT mice. The upper line shows Vte and the bandwidth of voltage deflections reflects the transepithelial resistance (Rte). The right panel shows the summary of all experiments.
Figure 6. RT-PCR identification of different mouse P2Y receptors mRNA transcripts from isolated distal colonic crypts from P2Y2 KO and WT mice.
Positive results were found for the P2Y4 and the P2Y6 receptors in WT and KO. P2Y2 receptor transcripts were absent in the KO mouse and present in the WT mouse.
Reduced K+ secretory response in P2Y4 receptor KO mice
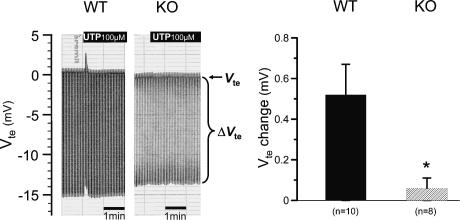
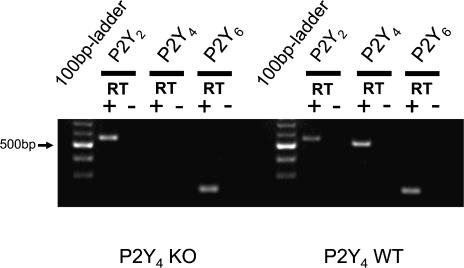
Obviously, the above-mentioned results imply that the P2Y4 receptor may also be expressed in the luminal membrane of distal mouse colonic mucosa. The recently generated P2Y4 KO mice (genetic background: CD1/SV129) (Robaye et al. 2003) were used for these experiments following the same approach as for the P2Y2 KO mice. In P2Y4 WT littermates, luminal UTP induced the well described rapid and transient change of polarity of the Vte from a resting value of −0.47 ± 0.25 mV to +0.06 ± 0.33 mV (n = 10) (Fig. 7). However, the magnitude of the UTP-induced Vte deflection was significantly smaller compared to both the NMRI mice (Figs 1 and 3) and the P2Y2 WT mouse (Fig. 5). Luminal UTP showed no effect in P2Y4 KO mice (resting −0.21 ± 0.18 mV to −0.15 ± 0.17 mV, n = 8). In one single experiment in a P2Y4 KO mouse, a very small Vte deflection of 0.40 mV was observed. These results support the hypothesis that the luminal membrane also expresses P2Y4 receptors. However, if both receptors are expressed in the luminal membrane one would expect a residual effect of luminal UTP in the P2Y4 KO mice. Figure 8 shows the RT-PCR results from isolated colonic crypts of a P2Y4 KO mouse (genetic background: CD1/SV129) indicating the absence of P2Y4 transcripts but the presence of P2Y2 and P2Y6 mRNA transcripts. Thus, the P2Y2 receptor continues to be expressed in the P2Y4 KO mouse.
Figure 7. Effect of luminal UTP in P2Y4 receptor knock-out mice in comparison to wild-type littermates.
Two original recordings of transepithelial voltage (Vte) and transepithelial voltage changes (ΔVte) in KO and WT mice. The upper line shows Vte and the bandwidth of voltage deflections reflects the transepithelial resistance (Rte). The right panel shows the summary of all experiments.
Figure 8. RT-PCR identification of different mouse P2Y receptor mRNA transcripts from isolated distal colonic crypts of P2Y4 KO and WT mice.
Positive results were found for the P2Y2 and the P2Y6 receptors in WT and KO. P2Y4 receptor transcripts were absent in the KO and present in the WT mice.
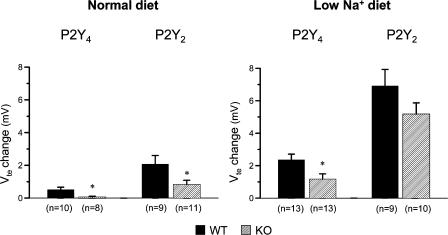
In the case of P2Y4 receptor WT mice, the nucleotide-induced effect is very small and may not allow us to observe any potential residual P2Y2 receptor-mediated response in the P2Y4 KO littermates. To address this issue, the conditions were manipulated in order to see an augmented luminal nucleotide-stimulated response. Recently, the BK channel was identified as the channel responsible for the nucleotide-induced K+ secretion (Leipziger et al. 2003). BK channels are activated both by depolarization and increases in [Ca2+]i. Depolarization of the cells reduces the level of [Ca2+]i needed to activate BK channels (Kanazirska et al. 1995). A low Na+ diet for 2 weeks enhances the ENaC-mediated electrogenic Na+ absorption in an aldosterone-dependent fashion, and thereby significantly depolarizes the luminal membrane of mouse distal colon (Will et al. 1985). Figure 9 shows two original Ussing chamber traces of mouse distal colonic mucosa from a P2Y4 WT and a corresponding P2Y4 KO littermate (genetic background: CD1/SV129). Electrogenic Na+ absorption under these conditions is greatly increased as seen by the large lumen-negative Vte close to −15 mV in both traces. In the entire series of experiments, this augmented amiloride-inhibitable Na+ absorption amounted to 657.0 ± 118.8 μA cm−2 (n = 10) in P2Y4 WT mice and to 667.1 ± 181.3 μA cm−2 (n = 11) in P2Y4 KO mice. Under these conditions, the luminal UTP-stimulated Vte deflection in P2Y4 WT mice was significantly increased to 2.37 ± 0.34 mV, as compared to mucosa from animals on a normal diet. Importantly, all P2Y4 KO mice now responded with a K+ secretory response (ΔVte change: 1.17 ± 0.33 mV) upon nucleotide stimulation. Figure 10 summarizes the results of all experimental series. Interestingly, in all investigated mice (P2Y2 WT and KO, P2Y4 WT and KO), the K+ secretory response was significantly increased in animals on a low Na+ diet, supporting the hypothesis that a low Na+ diet, in general, facilitates luminal nucleotide stimulated BK-dependent K+ secretion.
Figure 9. Effect of luminal UTP in P2Y4 receptor knock-out mice in comparison to wild-type littermates treated on a low Na+ diet for 2 weeks.
Two original recordings of transepithelial voltage (Vte) and transepithelial voltage changes (ΔVte) in KO and WT mice. The upper line shows Vte and the bandwidth of voltage deflections reflects the transepithelial resistance (Rte). Note the significantly more lumen negative Vte values in both original traces as compared to those in Figs 1, 3, 5 and 7. Importantly, in all P2Y4 KO mice, luminal UTP always induced the rapid and transient Vte deflection. The right panel shows the summary of 13 mouse pairs.
Figure 10. Summary of all UTP-stimulated K+ secretory effects (rapid and transient Vte deflections) in P2Y2 and P2Y4 WT and KO mice on normal and low Na+ diet.
Note the significant up-regulation of this effect in all tested animals after the treatment on a low Na+ diet.
Furthermore, these results indicate that in mice lacking P2Y4 receptors, a significant fraction of the luminal UTP-stimulated K+ secretory response is reduced, and that a significant residual UTP-mediated K+ secretion remains. This remaining K+ secretion is likely to be mediated via a luminal P2Y2 receptor. In summary, these results are consistent with the interpretation that both a luminal P2Y2 and a luminal P2Y4 receptor are linked to the activation of a transient K+ secretion.
Abolished UTP-stimulated K+ secretory response in P2Y2/P2Y4 double receptor knock-out mice
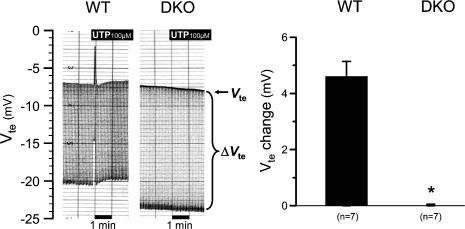
To investigate the above-stated hypothesis we generated P2Y2/P2Y4 double receptor KO mice (DKO) (genetic background: B6D2/SV129/CD1) (see Methods). Upon simple inspection, P2Y2/P2Y4 DKO mice showed no gross abnormalities. Male P2Y2/P2Y4 WT and DKO mice were investigated at the age of 4–20 weeks. As shown above, the luminal nucleotide-stimulated K+ secretory response is significantly up-regulated in mice treated on a low Na+ diet. Therefore, these experiments were conducted after the animals had received a low Na+ diet for 21 days. In the population of P2Y2/P2Y4 WT, all animals responded with a pronounced response to luminal UTP (Fig. 11). Application of UTP led to a rapid and transient change of the transepithelial voltage from a prestimulatory value of −8.76 ± 1.40 mV to −4.16 ± 1.25 mV under peak stimulation (n = 7). In sharp contrast, addition of luminal UTP to the colonic mucosa of P2Y2/P2Y4 DKO mice showed no effect (ΔVte: 0.00 ± 0.05 mV, n = 7). These results prove that both P2Y2 and P2Y4 receptors are expressed in the luminal membrane of mouse distal colonic mucosa and mediate the luminal nucleotide-stimulated K+ secretion.
Figure 11. Absence of luminal UTP-stimulated K+ secretion in P2Y2/P2Y4 double receptor KO mice in comparison to wild-type littermates.
Two original recordings of transepithelial voltage (Vte) and transepithelial voltage changes (ΔVte) in DKO and WT mice. The upper line shows Vte and the bandwidth of voltage deflections reflects the transepithelial resistance (Rte). The right panel shows the summary of 7 mouse pairs.
No effect of basolateral UTP on transepithelial ion transport in mouse distal colonic mucosa
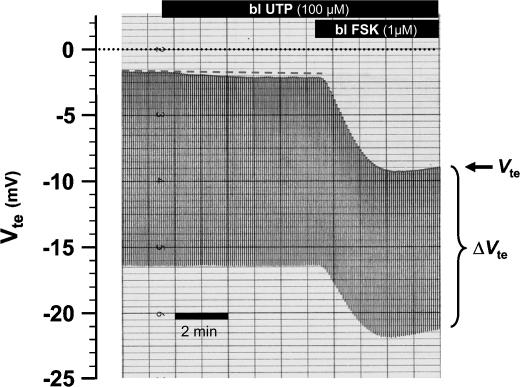
Basolateral nucleotides have previously been shown to regulate ion transport in the intestine. For example, basolateral ADP and ATP stimulate NaCl secretion via a P2Y1 receptor and basolateral UDP stimulated NaCl secretion via a P2Y6 receptor (Leipziger et al. 1997; Köttgen et al. 2003). Here, the effect of basolateral UTP on mouse distal colon was tested (genetic background: CD1/SV129). A representative trace from a P2Y4 WT is shown in Fig. 12. Basolateral UTP stimulated only a minimal deflection to more lumen-negative Vte values. In the entire series, Vte changed by 0.06 ± 0.04 mV to more lumen negative values upon stimulation with basolateral UTP. Only two out of five experiments showed this very small effect. Similarly, basolateral UTP in the P2Y2 WT mouse was ineffective (Vte change 0.34 ± 0.24 mV, n = 5). The subsequent addition of forskolin shows the well-known effect on Cl− secretion with a large change of Vte to lumen-negative values. These results indicate that basolateral UTP does not stimulate the observed K+ secretion induced by the addition of UTP to the luminal side.
Figure 12. No effect of basolateral UTP in a P2Y4 receptor WT mouse.
An original recording of transepithelial voltage (Vte) and transepithelial voltage changes (ΔVte). The upper line shows Vte and the bandwidth of voltage deflections reflects the transepithelial resistance (Rte). The dashed line represents the baseline before addition of basolateral UTP.
Discussion
K+ secretion via P2Y2 and P2Y4 receptors
Epithelia commonly respond to luminal nucleotides, such as ATP and UTP, modifying ion transport processes (Leipziger, 2003). UTP specifically activates the so-called ‘pyrimidine’ receptors P2Y2 and P2Y4 (Ralevic & Burnstock, 1998). It remains a difficult task to unravel which of these receptors actually mediates a given response, since specific antagonists are pending for these receptors (Ralevic & Burnstock, 1998). Therefore, the pharmacological profile regarding different agonists has been applied to differentiate these receptors. It was found that the human P2Y2 receptor is equally activated by ATP and UTP and not by ADP or UDP. In contrast, the human P2Y4 receptor displays a preference for UTP, with ATP being two orders of magnitude less potent (Nichols et al. 1996; Kennedy et al. 2000). In an earlier study in rat colonic mucosa, it was found that UTP and ATP stimulated a transient K+ secretion with similar potency (Kerstan et al. 1998). We assumed that a luminal P2Y2 receptor mediated this K+ secretion. Shortly after, however, it was recognized that the discriminative pharmacological profile for the human P2Y2 and P2Y4 receptors does not apply for rodent orthologues, as they were shown to be similarly activated by UTP and ATP (Bogdanov et al. 1998; Kennedy et al. 2000). Therefore, the question which of the receptors mediates distal colonic K+ secretion remained unanswered. A similar conflict of receptor identification was recognized in the gerbil stria vascularis of the inner ear. In an initial study, the authors identified a P2U receptor (later renamed P2Y2 (Fredholm et al. 1997)) responsible for inhibition of K+ secretion, similarly activated by ATP and UTP (Liu et al. 1995; Marcus et al. 1997; Marcus & Scofield, 2001). Nonetheless, it must be appreciated that any previously described P2U receptor-mediated effect in rodents may reflect either the P2Y2 and/or the P2Y4 receptor. Recent immunohistochemical and functional data suggest that, in the inner ear stria marginal cells and vestibular dark cells, the P2Y4 receptor leads to the observed effect (Marcus & Scofield, 2001; Sage & Marcus, 2002).
In airway epithelium, results from the P2Y2 KO mouse led to the identification of a luminal P2Y2 receptor important for nucleotide-induced Cl− secretion (Cressman et al. 1999). This study has also shown that the P2Y2 receptor is not involved in the luminal stimulation of Cl− secretion in other tissues, like the small intestine. A recent publication using a P2Y4 KO mouse identified a luminal P2Y4 receptor as responsible for UTP-activated Cl− secretion in the small intestine (Robaye et al. 2003). This study is the first to show a clear-cut biological effect of the P2Y4 receptor.
In our study, we show that luminal UTP/ATP activates a prompt and transient opening of luminal K+ channels in mouse distal colon, similar to that shown in rat. The observed Vte deflection is caused by opening of luminal K+ channels because the effect is luminal Ba2+ and iberiotoxin sensitive.
In a preliminary account, we have shown that the luminal nucleotide-stimulated K+ secretion is mediated via BK channels (Leipziger et al. 2003). In the present study, the use of P2Y2 and P2Y4 KO mice reveals that both P2Y2 and P2Y4 receptors mediate this K+ secretion. This conclusion is based on the finding that a significant part of the nucleotide-induced K+ secretion remained in P2Y2 KO mice. This residual component is therefore likely to be mediated via the other UTP receptor (P2Y4). Indeed, this K+ secretion was absent in P2Y4 receptor KO mice. These results are interesting and support the notion that the P2Y4 receptor is also expressed in the luminal membrane of mouse distal colon. However, the absence of a UTP effect in P2Y4 KO mice is puzzling. If both receptors are present, one would expect a remaining UTP effect in the P2Y4 KO mouse. The small size of the K+ secretory response in the P2Y4 WT may have hindered the detection of a potential P2Y2 receptor-mediated effect in the KO littermate. It is noteworthy that in one out of eight experiments, we did observe a very small Vte deflection in a P2Y4 KO mouse.
The magnitude of the Vte deflection varied between the different mouse strains. The UTP-induced Vte deflection was 3.92 ± 0.61 mV in NMRI mice and 4.51 ± 1.03 mV in B6D2 mice. However, in the B6D2/SV129 (P2Y2) strain, the Vte effect was smaller (2.08 ± 0.52 mV). In the CD1/129 (P2Y4) strain, the Vte effect was even smaller amounting to 0.52 ± 0.15 mV. The reason for this difference is unknown, but the genetic background of the different mice may give rise to the variability of this phenotype. The magnitude of the Vte signal is likely to be quantitatively correlated to the different elements involved in this signal transduction. These may include the amount of luminal P2Y2 or P2Y4 receptor proteins, the magnitude of the subapical intracellular Ca2+ signal or the density of BK channels. Further work will be needed to define this issue. We then tried to find conditions in which the luminal UTP/ATP-stimulated K+ secretion could be augmented. Since BK channels are both Ca2+ and depolarization activated, we searched for a way to depolarize the luminal membrane. This was successfully achieved by putting the mice on a low Na+ diet which will increase distal colonic ENaC-mediated Na+ absorption (Will et al. 1985) inducing a depolarization of the luminal membrane. Under these conditions the luminal UTP-stimulated K+ secretion was significantly augmented in all the tested mice, i.e. P2Y4 WT and KO and P2Y2 WT and KO. The most important finding is that a residual UTP-stimulated K+ secretion was always present in P2Y4 KO mice. This is consistent with the hypothesis that the P2Y2 receptor mediates the remaining effect. The absence of the luminal UTP-stimulated K+ secretion in P2Y2/P2Y4 DKO mice, in our opinion, proves that both receptors are expressed in the luminal membrane of mouse distal colonic mucosa.
Luminal localization of P2Y2 and P2Y4 receptors
We propose that both P2Y2 and P2Y4 receptors are localized in the luminal membrane of distal colonic mucosa. This is based on the observation that only luminal addition of nucleotides elicits K+ secretion, together with the rapid nature of the Vte deflection. Basolateral UTP had no effect. A potential leak of luminally applied agonist to the basolateral side and subsequent activation of basolateral P2Y receptors would be expected to elicit an effect with a significant delayed time course. Intriguingly, a basolateral [Ca2+]i elevating agonist would be expected to stimulate the basolateral SK4 channel, resulting in a more lumen-negative Vte. This was not observed. The tight distal epithelium of distal colon represents a significant diffusion barrier.
Nonetheless, immuno-histochemical proof is pending. Our own attempts to localize the P2Y2 receptor were unsuccessful. These findings are the first indication that the P2Y2 and the P2Y4 receptors are localized in the same luminal membrane of an epithelial preparation. In this study, the question of basolateral localization of the different P2Y receptors is not addressed.
The transient nature of the luminal nucleotide-stimulated K+ secretion
In the present study, a significant variation of the luminal nucleotide-mediated Vte deflection was observed. It is noteworthy that the effect is transient. A similar case was observed in rat tissue (Kerstan et al. 1998). The question at the time was whether a rapid breakdown of the nucleotide could underlie the fast deactivating time course. For that purpose, a continuously perfused Ussing chamber, assuring stable agonist concentrations, was utilized. The time course of the effect was not changed by this manoeuvre and thus it was concluded that a rapid ‘desensitization’ of the mechanism occurred. Interestingly, a recent study investigated the molecular prerequisites for P2Y4 receptor desensitization and identified three distinct C-terminal serine residues important for internalization of the receptor (Brinson & Harden, 2001). These results were compared to the P2Y6 receptor, which has a slower desensitization kinetic and lacks the relevant C-terminal end (Robaye et al. 1997; Brinson & Harden, 2001). It is speculated that the transient nature of this secretory response is due to a specific desensitization feature of the P2Y4 and the P2Y2 receptors. Although not investigated explicitly in this study, ‘re-sensitization’ of the effect normally occurred after 30–45 min (wash of the Ussing chamber and 30 min equilibration). Another important aspect of the transient nature of this response is likely to be related to the assumed local nucleotide-stimulated [Ca2+]i transient. We speculate that this [Ca2+]i transient has a similar time course to that observed in the Vte signal.
Other regulated ion conductances by luminal nucleotides
Inspection of Fig. 9 indicates that in addition to the described UTP-stimulated K+ secretion, other ion transport processes are regulated. It is apparent that shortly after the Vte peak a secondary decrease of Vte arises. This is due to the inhibition of electrogenic Na+ absorption and not discussed here (Lehrmann et al. 2002a). One may also question if luminal nucleotides are able to activate CFTR-mediated Cl− secretion, as shown for rat jejunum (Robaye et al. 2003). In our data, a major activation of Cl− secretion was not apparent. Inhibition of K+ secretion with Ba2+ or iberiotoxin did not unmask Cl− secretion. It is puzzling as to why the jejunum and the distal colon should behave differently. We speculate that the answer is hidden in the difference of experimental conditions of the two studies. In our study, we used basolateral TTX (1 μm) in order to reduce any pro-secretory effects, which may occur via the enteric nervous system or other neuronal elements intrinsic to the gut wall. Thus, our conditions favour a more complete deactivation of Cl− secretion in which CFTR, in a bottleneck-like fashion, determines Cl− secretion. In the intact mammalian colon, CFTR is the exclusive luminal Cl− exit pathway and needs preactivation before manoeuvres known to increase the driving force, i.e. opening of K+ channels, can stimulate Cl− secretion (Greger et al. 1997). This is nicely illustrated in a study on T84 cells (Stutts et al. 1995) or in data from CF KO mice (Colledge et al. 1995). It is possible that in the study on jejunum some CFTR preactivation was present to allow for luminal UTP-mediated Cl− secretion via an increase of K+ channel-dependent driving force.
Physiological role of luminal P2 receptor-mediated ion transport
To understand the physiological relevance of luminal P2 receptors, the question of a natural or pathological source for extracellular nucleotides has to be addressed. Either the extracellular nucleotide could originate from an external source (e.g. colonic bacteria) or could be released from the epithelium itself. Evidence for a bacterial source in the gut is pending. Interestingly, a recent study showed that enteropathogenic E. coli triggered the release of ATP from different cell lines including the colonic T84 cell line. Subsequently, the released ATP was broken down to adenosine, which stimulated chloride secretion via A2b receptors on the luminal side of T84 cells (Crane et al. 2002). The authors suggest that the E. coli stimulated release of nucleotides could account for the enteropathogen-induced diarrhoea. The common denominator for secretory epithelia like the airways, the conjunctiva of eye or the small and large intestine is that activation of luminal P2 receptors can stimulate Cl−, K+ or HCO3− secretion or inhibit Na+ absorption, resulting in an increase in the amount of fluid on the luminal surface of the epithelium. This mechanism could therefore serve to remove noxious particles by flushing away a damaging environment. Luminal nucleotides have consequently been implicated as elements of an innate defense mechanism of outer epithelial surfaces (Lazarowski & Boucher, 2001; Leipziger, 2003).
Acknowledgments
We gratefully acknowledge the expert technical assistance of Henriette Bjørn Rasmussen, Edith Bjørn Møller and Ann-Charlotte Andersen. We would also like to thank Ernst Martin Füchtbauer who helped with the genotyping of the P2Y2 knock-out mice. This study was supported by the Danish Medical Research Council, the Helga and Peter Korning Foundation and the Portuguese Foundation for Science and Technology.
References
- Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br J Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinson AE, Harden TK. Differential regulation of the uridine nucleotide-activated P2Y4 and P2Y6 receptors. SER-333 and SER-334 in the carboxyl terminus are involved in agonist-dependent phosphorylation desensitization and internalization of the P2Y4 receptor. J Biol Chem. 2001;276:11939–11948. doi: 10.1074/jbc.M009909200. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Chinet TC, Boucher RC. Extracellular ATP stimulates K+ secretion across human airway epithelium. Am J Physiol. 1997;272:L1084–L1091. doi: 10.1152/ajplung.1997.272.6.L1084. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Harline MC, Gawenis LR, Walker NM, Turner JT, Weisman GA. Extracellular UTP stimulates electrogenic bicarbonate secretion across CFTR knockout gallbladder epithelium. Am J Physiol Gastrointest Liver Physiol. 2000;279:G132–G138. doi: 10.1152/ajpgi.2000.279.1.G132. [DOI] [PubMed] [Google Scholar]
- Colledge WH, Abella BS, Southern KW, Ratcliff R, Jiang C, Cheng SH, MacVinish LJ, Anderson JR, Cuthbert AW, Evans MJ. Generation and characterization of a delta F508 cystic fibrosis mouse model. Nat Genet. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- Cotton CU, Reuss L. Electrophysiologic effects of extracellular ATP on Necturus gallbladder epithelium. J General Physiol. 1991;97:949–971. doi: 10.1085/jgp.97.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JK, Olson RA, Jones HM, Duffey ME. Release of ATP during host cell killing by enteropathogenic E. coli and its role as a secretory mediator. Am J Physiol Gastrointest Liver Physiol. 2002;283:G74–G86. doi: 10.1152/ajpgi.00484.2001. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Lazarowski ER, Homolya L, Boucher RC, Koller BH, Grubb BR. Effect of loss of P2Y2 receptor gene expression on nucleotide regulation of murine epithelial Cl− transport. J Biol Chem. 1999;274:26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- Diener M, Rummel W, Mestres P, Lindemann B. Single chloride channels in colon mucosa and isolated colonic enterocytes of the rat. J Membr Biol. 1989;108:21–30. doi: 10.1007/BF01870422. [DOI] [PubMed] [Google Scholar]
- Dubyak GR. Focus on ‘multiple functional P2X and P2Y receptors in the luminal and basolateral membranes of pancreatic duct cells’. Am J Physiol. 1999;277:C202–C204. doi: 10.1152/ajpcell.1999.277.2.C202. [DOI] [PubMed] [Google Scholar]
- Dubyak GR. Knock-out mice reveal tissue-specific roles of P2Y receptor subtypes in different epithelia. Mol Pharmacol. 2003;63:773–776. doi: 10.1124/mol.63.4.773. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, Schwabe U, Williams M. Towards a revised nomenclature for P1 and P2 receptors. Trends Physiol Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger R, Bleich M, Leipziger J, Ecke D, Mall M, Kunzelmann K. Regulation of ion transport in colonic crypts. News Physiol Sci. 1997;12:62–66. [Google Scholar]
- Kanazirska MP, Vassilev PM, Ye CP, Francis JE, Brown EM. Intracellular Ca2+-activated K+ channels modulated by variations in extracellular Ca2+ in dispersed bovine parathyroid cells. Endocrinology. 1995;136:2238–2243. doi: 10.1210/endo.136.5.7720673. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Qi AD, Herold CL, Harden TK, Nicholas RA. ATP, an agonist at the rat P2Y4 receptor, is an antagonist at the human P2Y4 receptor. Mol Pharmacol. 2000;57:926–931. [PubMed] [Google Scholar]
- Kerstan D, Gordjani N, Nitschke R, Greger R, Leipziger J. Luminal ATP induces K+ secretion via a P2Y2 receptor in rat distal colonic mucosa. Pflugers Arch. 1998;436:712–716. doi: 10.1007/s004240050693. [DOI] [PubMed] [Google Scholar]
- Köttgen M, Loffler T, Jacobi C, Nitschke R, Pavenstadt H, Schreiber R, Frische S, Nielsen S, Leipziger J. P2Y6 receptor mediates colonic NaCl secretion via differential activation of cAMP-mediated transport. J Clin Invest. 2003;111:371–379. doi: 10.1172/JCI16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC. UTP as an extracellular signaling molecule. News Physiol Sci. 2001;16:1–5. doi: 10.1152/physiologyonline.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- Lehrmann H, Thomas J, Kim SJ, Jacobi C, Leipziger J. Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol. 2002a;13:10–18. doi: 10.1681/ASN.V13110. [DOI] [PubMed] [Google Scholar]
- Lehrmann H, Wittekindt O, Leipziger J. Luminal P2Y2 receptor-mediated inhibition of electrogenic Na+ absorption in mouse distal colon. Pflugers Arch. 2002b;443:S358. [Google Scholar]
- Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- Leipziger J, Kerstan D, Nitschke R, Greger R. ATP increases [Ca2+]i and ion secretion via a basolateral P2Y receptor in rat distal colonic mucosa. Pflugers Arch. 1997;434:77–83. doi: 10.1007/pl00008079. [DOI] [PubMed] [Google Scholar]
- Leipziger J, Matos J, Sausbier M, Ruth P. Abolished colonic K+ secretion in Maxi K+ channel knock-out mice. Pflugers Arch. 2003;445:S26. (abstract) [Google Scholar]
- Liu J, Kozakura K, Marcus DC. Evidence for purinergic receptors in vestibular dark cells and strial marginal cell epithelia of the gerbil. Auditory Neurosci. 1995;1:331–340. [PMC free article] [PubMed] [Google Scholar]
- Lohrmann E, Burhoff I, Nitschke RB, Lang HJ, Mania D, Englert HC, Hropot M, Warth R, Rohm W, Bleich M, Greger R. A new class of inhibitors of cAMP-mediated Cl− secretion in rabbit colon, acting by the reduction of cAMP-activated K+ conductance. Pflugers Arch. 1995;429:517–530. doi: 10.1007/BF00704157. [DOI] [PubMed] [Google Scholar]
- Mall M, Wissner A, Gonska T, Calenborn D, Kuehr J, Brandis M, Kunzelmann K. Inhibition of amiloride-sensitive epithelial Na+ absorption by extracellular nucleotides in human normal and cystic fibrosis airways. Am J Respir Cell Mol Biol. 2000;23:755–761. doi: 10.1165/ajrcmb.23.6.4207. [DOI] [PubMed] [Google Scholar]
- Marcus DC, Scofield MA. Apical P2Y4 purinergic receptor controls K+ secretion by vestibular dark cell epithelium. Am J Physiol Cell Physiol. 2001;281:C282–C289. doi: 10.1152/ajpcell.2001.281.1.C282. [DOI] [PubMed] [Google Scholar]
- Marcus DC, Sunose H, Liu J, Shen Z, Scofield MA. P2U purinergic receptor inhibits apical IsK/KvLQT1 channel via protein kinase C in vestibular dark cells. Am J Physiol. 1997;273:C2022–C2029. doi: 10.1152/ajpcell.1997.273.6.C2022. [DOI] [PubMed] [Google Scholar]
- Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RA, Watt WC, Lazarowski ER, Harden TK. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: Identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharm. 1996;50:229. [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Robaye B, Boeynaems JM, Communi D. Slow desensitization of the human P2Y6 receptor. European J Pharmacol. 1997;329:231–236. [PubMed] [Google Scholar]
- Robaye B, Ghanem E, Wilkin F, Fokan D, Van Driessche W, Schurmans S, Boeynaems J-M, Beauwens R. Loss of nucleotide regulation of epithelial chloride transport in the jejunum of P2Y4-null mice. Mol Pharmacol. 2003;64:777–783. doi: 10.1124/mol.63.4.777. [DOI] [PubMed] [Google Scholar]
- Sage CL, Marcus DC. Immunolocalization of P2Y4 and P2Y2 purinergic receptors in strial marginal cells and vestibular dark cells. J Membr Biol. 2002;185:103–115. doi: 10.1007/s00232-001-0116-z. [DOI] [PubMed] [Google Scholar]
- Siemer C, Gögelein H. Activation of nonselective cation channels in the basolateral membrane of rat distal colon crypt cells by prostaglandin E2. Pflugers Arch. 1992;420:319–328. doi: 10.1007/BF00374465. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Lazarowski ER, Paradiso AM, Boucher RC. Activation of CFTR Cl− conductance in polarized T84 cells by luminal extracellular ATP. Am J Physiol. 1995;268:C425–C433. doi: 10.1152/ajpcell.1995.268.2.C425. [DOI] [PubMed] [Google Scholar]
- Will PC, Cortright RN, DeLisle RC, Douglas JG, Hopfer U. Regulation of amiloride-sensitive electrogenic sodium transport in the rat colon by steroid hormones. Am J Physiol. 1985;248:G124–G132. doi: 10.1152/ajpgi.1985.248.1.G124. [DOI] [PubMed] [Google Scholar]