Abstract
The translation of nerve transmission to Ca2+ signals in urinary bladder smooth muscle (UBSM) is incompletely understood. Thus, we sought to characterize Ca2+ signals in strips of UBSM loaded with the Ca2+-sensitive fluorescent dye, fluo-4, using laser scanning confocal microscopy. Two types of Ca2+ signals occurred spontaneously and could be evoked with field stimulation: large, rapid, global Ca2+ transients termed ‘global Ca2+ flashes’, and much smaller, localized Ca2+ transients. Global Ca2+ flashes were inhibited by the L-type voltage-dependent Ca2+ channel (VDCC) inhibitor, diltiazem and with P2X receptor blockade. Simultaneous intracellular recordings and Ca2+ measurements indicated that these events are caused by Ca2+ influx through VDCCs during action potentials. Small, local Ca2+ transients occurred spontaneously, and their frequency could be elevated with field stimulation. Atropine, an inhibitor of muscarinic receptors, did not affect these local Ca2+ transients. However, the desensitizing P2X receptor agonist α,β-methylene ATP, and the purinergic antagonist, suramin, effectively inhibited the local Ca2+ transients. The frequency of these ‘purinergic Ca2+ transients’ was increased about 7-fold by a 10 s stimulus train (1 Hz). The amplitude, duration at one-half amplitude and the spatial spread of the evoked purinergic Ca2+ transients were F/Fo= 2.4 ± 0.13, 111.7 ± 9.3 ms and 14.0 ± 1.0 μm2, respectively. Tetrodotoxin inhibited evoked purinergic Ca2+ transients, indicating that they were dependent on nerve fibre activation. Purinergic Ca2+ transients were not dependent on VDCC activity. Neither 2-APB, an inhibitor of inositol 1,4,5-triphosphate (Ins(1,4,5)P3) (IP3)-induced Ca2+ release, nor ryanodine inhibited the purinergic Ca2+ transients. We have identified two novel Ca2+ signals in rat UBSM. Large, rapid, global Ca2+ flashes that represent Ca2+ influx through VDCCs during action potentials, and local, purinergic Ca2+ transients that represent Ca2+ entry through P2X receptors. Our results indicate that purinergic Ca2+ transients evoked by release of ATP from nerve varicosities are elementary signals in the process of nerve-smooth muscle communication.
The function of the urinary bladder is to relax and store urine during filling, and contract forcefully to empty the bladder during micturition. These seemingly disparate roles are achieved through complex interactions between the autonomic nervous system and urinary bladder smooth muscle (UBSM). UBSM in adult rats is well innervated, receiving approximately 16 000 afferent and efferent axons from ganglion neurones (Gabella, 1999). The bundles of nerve fibres branch repeatedly in UBSM and eventually become single fibres containing varicosities. Located within the varicosities are clear vesicles, or a combination of clear vesicles and dense-cored vesicles (Gabella, 1999) containing neurotransmitters, including acetylcholine (ACh) and ATP or a related purine (Hoyle & Burnstock, 1993; Gabella, 1995). Nerve stimulation evokes the release of ACh and ATP (Dowdall et al. 1974; Kasakov & Burnstock, 1982; Theobald & de Groat, 1989; Silinsky & Redman, 1996). ACh binds to muscarinic receptors, stimulating the production of inositol 1,4,5-triphosphate [Ins (1,4,5)P3] (IP3) and the release of Ca2+ from the sarcoplasmic reticulum. ATP binds to, and opens purinergic receptors allowing the influx of cations, including Ca2+ and Na+ (Isenberg et al. 1992; Evans et al. 1996).
Local Ca2+ signals in smooth muscle, in the form of Ca2+ waves, were first observed in electrically stimulated rat tail arteries (Iino et al. 1994). The release of noradrenaline from surrounding nerve fibres induced Ca2+ waves through the activation of (IP3)-mediated Ca2+ release. Stationary, local Ca2+ signals (Ca2+ sparks), caused by local Ca2+ release through a cluster of ryanodine receptors (RyRs), were subsequently detected in smooth muscle (Nelson et al. 1995).
Transient increases in Ca2+ in smooth muscle have been recorded following the activation of purinerigic receptors. In UBSM, the activation of purinergic receptors induced excitatory junctional potentials (EJPs) and Ca2+ transients (Bramich & Brading, 1996; Hashitani et al. 2000). Recently, Ca2+ imaging techniques revealed fast Ca2+ events in smooth muscle following nerve stimulation. Field stimulation of the vas deferens evoked local, transient Ca2+ events in smooth muscle cells (neuroeffector Ca2+ events). These events were mediated by postsynaptic smooth muscle P2X receptors activated by ATP released at synaptic varicosities (Brain et al. 2002, 2003). When the nerve terminals and the neuroeffector Ca2+ events were both visualized, it was found that the neuroeffector Ca2+ events occurred immediately beneath stimulated sympathetic varicosities. Similar, transient Ca2+ events evoked with field stimulation were found in small rat mesenteric arteries and were termed ‘junctional Ca2+ transients’ (Lamont & Wier, 2002; Lamont et al. 2003). Line scan images showed that junctional Ca2+ transients were evoked immediately following a Ca2+ increase in adjacent nerve fibres. Pharmacological studies showed that junctional Ca2+ transients were mediated through the activation of purinergic receptors (Lamont & Wier, 2002; Lamont et al. 2003).
In the present study, we used a combination of intracellular microelectrode recordings and a laser scanning confocal microscope to image fast Ca2+ events in UBSM. Two new Ca2+ events whose frequencies are elevated with nerve stimulation, were detected in rat UBSM: (1) large, rapid, global Ca2+ flashes that encompass the entire smooth muscle fibre, and are caused by Ca2+ influx through L-type voltage-dependent Ca2+ channels (VDCCs) during an action potential, and (2) much smaller, localized purinergic Ca2+ transients. Although similar in appearance to RyR-mediated Ca2+ sparks (Nelson et al. 1995; Herrera et al. 2001; Heppner et al. 2003), these purinergic Ca2+ transients are larger and longer than Ca2+ sparks and represent a novel elementary Ca2+ signal mediated by the activation of P2X receptors. Purinergic Ca2+ transients probably play a critical, initial role in nerve-evoked excitation–contraction coupling in UBSM.
Methods
Tissue preparation
Adult male rats were killed by pentobarbital sodium (130 mg (kg body weight)−1), followed by thoracotomy in accordance with the guidelines for the use and care of laboratory animals (NIH publication 85-23, 1985) and as approved by the Institutional Animal Use and Care Committee of the University of Vermont. The urinary bladder was quickly removed and placed in cold Hepes-buffered saline. Strips of UBSM (approximately 0.5 mm thick × 1 mm wide × 5 mm long) were carefully removed from the serosal surface with sharp scissors and pinned to small sylgard blocks. The sylgard blocks with the attached UBSM strips were placed tissue side down, in a chamber specially designed to measure rapid Ca2+ responses. To allow fresh physiological saline solution (PSS) to flow under and around the tissue strip, thin spacers (0.2 mm) were placed on either side of the tissue. The tissue was superfused (1–2 ml min−1) with a PSS at 37°C. For the experiments quantifying the small, localized Ca2+ transients, sucrose (12%) was added to the superfusing PSS to reduce tissue movement (Foster et al. 1989; Heppner et al. 1997). Sucrose was not added to the PSS in the simultaneous measurement of Ca2+ and membrane potential with microelectrodes, or in experiments involving the large, rapid global Ca2+ events. Drugs were dissolved in the superfusing salt solution and applied for at least 20 min prior to image acquisition unless otherwise indicated.
Ca2+ imaging and analysis
Strips of UBSM were imaged with a laser scanning confocal microscope (OZ; Noran Instruments) attached to a Nikon diaphot microscope. A Nikon ×60 water immersion objective (NA 1.2) was used to visualize the tissue. Images were acquired using an Intervision software package controlled by a Silicon graphics Workstation (O2). To visualize Ca2+ events in UBSM, the tissue was placed in a Hepes solution containing the Ca2+ fluorescent dye, fluo-4 AM (10 μm) (Molecular Probes; Eugene, OR, USA) and pluronic acid (2.5 μg ml−1; Molecular Probes). To facilitate loading of the dye, the tissue was kept in the dark for 60 min at 21–23°C followed by a wash in normal Hepes buffer (21–23°C) before placing the tissue in PSS. All experiments were conducted in PSS (37°C). A krypton–argon laser was used to excite fluo-4 and the emitted light was captured at wavelengths >500 nm. For most experiments, images were acquired at 30 images s−1 (every 33.33 ms). The size of the field was 116 × 109 μm (512 × 480 pixels). To measure latency, images were acquired at 240 images s−1, an acquisition rate that yields an interimage interval of 4.13 ms. Image analysis was conducted offline using customized software written in our laboratory (Dr Adrian Bonev). Evoked Ca2+ transients were detected by measuring an increase in the fractional fluorescence of Ca2+F/Fo= 1.3, that could be distinguished above the background noise. Baseline fluorescence was measured by averaging at least 10 images with no Ca2+ transients. Ca2+ transients were measured using a 1.5 × 1.5 μm box (7 × 7 pixels) and analysed as previously described (Perez et al. 1999; Jaggar & Nelson, 2000; Wellman et al. 2001) using customized software written in our laboratory.
Field stimulation
The nerves in the strip were excited with platinum electrodes placed in the recording chamber, which were attached to the output of the stimulus isolation unit of a Grass S44 stimulator. Voltages used to evoke Ca2+ transients ranged from 30 to 100 V. A frequency of 1 Hz and a pulse duration of 0.2 ms was used throughout this study. Ca2+ transients were evoked with a train of stimuli applied for 10 s at a frequency of 1 Hz. This relatively low frequency allowed individual Ca2+ events to be identified, and facilitated quantification of these events during the stimulus train. Global Ca2+ events were evoked by increasing the stimulating voltage until threshold was reached. The smaller evoked Ca2+ transients were visualized by reducing the stimulating voltage just below threshold for the global Ca2+ events.
Microelectrode intracellular recording
To record voltage from UBSM cells, strips of tissue were prepared as above for Ca2+ imaging. Standard microelectrode techniques were used. Oxygenated PSS was superfused over the tissue strips at 1–2 ml min−1 (37°C). Microelectrodes were pulled on a Flaming/Brown gas puller (Sutter Instruments, Novato, CA, USA) and had resistances of 60–80 MΩ when filled with 2.0 m KCl. The transmembrane potential was acquired using an Axoclamp 2A (Axon Instruments, Union City, CA, USA) and recorded using Axotape software (Axon Instruments). The membrane potential was measured as the difference between the reference electrode in the bath and the intracellular microelectrode.
Experimental design
Each experiment consisted of three to five 15 s recordings from the same field during which a 10 s stimulus train (1 Hz) was applied. One to three files were recorded under control conditions and after drug application. The time interval between each file was at least 5 min. The tissue was incubated for at least 20 min in drug-containing solutions before test stimuli were applied.
Drugs and solutions
UBSM strips were removed from the urinary bladder in Hepes solution with the following composition (mm): NaCl, 134; KCl, 6; MgCl2, 1; CaCl2, 2; Hepes, 10; glucose, 10; (pH 7.4). NaOH was used to adjust the pH of Hepes solutions. Evoked Ca2+ transients were measured in PSS with the following composition (mm): NaCl, 119; KCl, 4.7; NaHCO3, 23.8; KH2PO4, 1.2; CaCl2, 1.6; MgCl2, 1.2, EDTA, 0.023; and glucose, 11.0 (pH 7.4). PSS was continuously bubbled with 95% O2–5% CO2. For experiments using nominal Ca2+-free solution, Ca2+ was omitted from the superfusing PSS. 2-Aminethoxydiphenyl-borate (2-APB) was obtained from Tocris (Ellisville, MO, USA), ryanodine was obtained from LC laboratories (Woburn, MA, USA) and diltiazem, α,β-methylene ATP, suramin, atropine, and tetrodotoxin (TTX) were obtained from Sigma (St Louis, MO, USA).
Statistics
Data were normalized to control, and expressed as mean ±s.e.m. At least three paired experiments were conducted for each paradigm using at least three rats. n refers to the number of evoked Ca2+ transients or the number of preparations as indicated in the text. To determine significance, Ca2+ events under control conditions were compared to Ca2+ events following drug treatment using a paired student's t test on the raw data. Significance was determined at P≤ 0.05.
Results
Rapid, global Ca2+ events in UBSM are caused by Ca2+ influx during an action potential
UBSM strips, loaded with fluo-4, appeared as dark grey, homogenous bundles when examined with a laser scanning confocal microscope. In a single field, several bundles of UBSM were often seen lying in parallel. Alongside and between the bundles of smooth muscle were thin, very bright fibres that usually ran in parallel with the smooth muscle bundles. Based on their location and morphology, these fibres were probably nerve processes innervating the smooth muscle (Gabella, 1995; Gabella & Davis, 1998; Drake et al. 2003).
In PSS, smooth muscle bundles displayed repetitive, large, very rapid increases in Ca2+ that spread quickly along the length of the bundle. These Ca2+ events occurred spontaneously or could be evoked with field stimulation. We termed these large, rapid, global Ca2+ events ‘global Ca2+ flashes’. These events were sensitive to P2X receptor inhibition. Treatment with α,β-methylene ATP (10 μm), a P2X receptor agonist, which desensitizes P2X receptors, and suramin (10 μm), an inhibitor of P2X receptors, significantly decreased the number of spontaneous global Ca2+ flashes to 20.0 ± 13.0% of control (n= 6 preparations; P < 0.05) and also significantly decreased the number of global Ca2+ flashes evoked by stimulation to 36.3 ± 12.0% of control (n= 8 preparations; P < 0.05). This suggests that P2X receptor activation contributes to spontaneous and evoked global Ca2+ transients.
To identify the origin of the global Ca2+ flashes, a single muscle bundle was impaled with a microelectrode to record changes in the membrane potential. Simultaneous recordings of Ca2+-activated fluorescence changes and membrane potential showed that each global Ca2+ flash was evoked by a single action potential (Fig. 1). Since the upstroke of the action potential is dependent on Ca2+ influx through VDCCs, the global Ca2+ flash should be reduced when these channels are blocked. Diltiazem, a reversible inhibitor of VDCCs, markedly decreased these events (Fig. 2). For example, in one preparation, 68 global Ca2+ flashes were recorded in PSS during a 15 s recording using 1 Hz stimulation. Diltiazem (100 μm; 20 min) eliminated these events. Following a 35 min rinse in PSS, 70 global Ca2+ flashes were recorded. Simultaneous recordings of Ca2+ fluorescence and membrane potential from bundles of UBSM, along with the sensitivity of the global Ca2+ flashes to an inhibitor of VDCCs indicate that each global Ca2+ flash results from Ca2+ influx during an action potential. Immediately following the global Ca2+ flashes, the UBSM would contract suggesting that the VDCC-mediated Ca2+ influx is causally related to UBSM contraction.
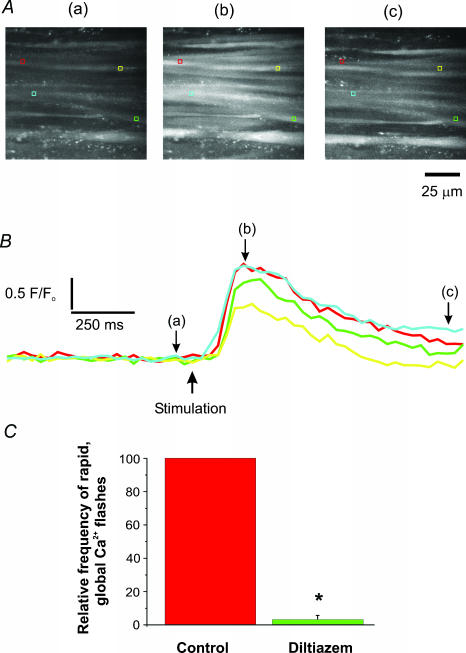
Figure 1. Simultaneous recording of Ca2+ and voltage from a single bundle of UBSM.
A, images recorded from UBSM in normal PSS (37°C) loaded with fluo-4. The muscle bundles are orientated horizontally. Images were acquired at 30 images s−1. The letters above each image correspond to changes in Ca2+-activated fluorescence in B (upper trace) and were recorded before (a), during (b) and after (c) a spontaneous action potential in the UBSM bundle impaled with the microelectrode. B, simultaneous recordings of changes in Ca2+-activated fluorescence (upper trace) and voltage (lower trace) from a single bundle of UBSM. Changes in Ca2+-activated fluorescence were measured from the red box in A located on the impaled muscle bundle. The microelectrode impalement site is indicated by the green rectangle. Notice that each of the three action potentials induced a simultaneous increase in the Ca2+-activated fluorescence. The large increase in Ca2+-activated fluorescence from a single muscle bundle during an action potential is also shown above in Ab.
Figure 2. Global Ca2+ flashes in UBSM recorded during field stimulation.
A, average images (acquired at 30 images s−1) showing bundles of smooth muscle before (a), approximately 100 ms following a single stimulus pulse (b), and approximately 1 s following field stimulation (c). Several bundles demonstrated an increase in Ca2+-activated fluorescence changes following stimulation. B, the changes in Ca2+-activated fluorescence were recorded from the regions of interest (coloured boxes) in A showing a rapid increase in Ca2+-activated fluorescence and a slow return to resting Ca2+ levels following field stimulation. Letters a, b and c correspond to the images in A. C, the relative number of global Ca2+ flashes from paired experiments recorded during field stimulation in control and with diltiazem (100 μm). Inhibition of VDCCs significantly decreased the number of these global Ca2+ flashes. *P < 0.05, n= 3 preparations.
Nerve stimulation elevates the frequency of local Ca2+ transients
In addition to the global Ca2+ flashes, smaller, more localized Ca2+ transients were also evident in UBSM. These localized Ca2+ transients became more apparent when the global Ca2+ flashes were inhibited with either VDCC blockers or when sucrose (12%) was added to the superfusing PSS to restrict tissue movement. The frequency of these small Ca2+ transients could be elevated by low-frequency field stimulation (1 Hz) (Fig. 3) (movie file provided as supplemental data). To evoke these small Ca2+ transients with field stimulation, the stimulating voltage was decreased to a level just below the threshold needed to induce global Ca2+ flashes. To measure the effect of field stimulation on the frequency of these events, Ca2+ transients were recorded from the same field before and after stimulation (10 s at 1 Hz). Nerve stimulation significantly elevated the frequency of the Ca2+ transients about 7-fold, from 23 ± 8.4 to 156 ± 35.9 events (P < 0.05; n= 4 preparations).
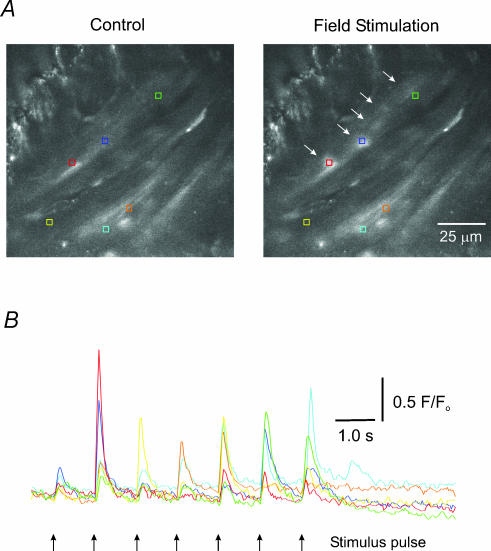
Figure 3. Local Ca2+ responses in UBSM evoked with field stimulation.
A, images of UBSM show smooth muscle (acquired at 30 images s−1) bundles running diagonally from the lower left to the upper right of each image before (left panel) and after (right panel) a single stimulus pulse. Coloured boxes enclose regions of interest where evoked Ca2+ responses occur, and correspond to the coloured traces below in B. The white arrows in the right panel show Ca2+ responses occurring in response to a single stimulus and correspond to the second stimulus pulse from the left in B. B, changes in Ca2+-activated fluorescence in response to seven stimulus pulses (1 Hz). Note that with each pulse a Ca2+ transient occurs in some of the selected regions of interest (movie file provided as supplemental data).
Purinergic receptor, but not muscarinic receptor, activation mediates the localized Ca2+ transients
Parasympathetic nerve fibres innervating UBSM corelease ACh and ATP in response to nerve stimulation (Kasakov & Burnstock, 1982; Theobald & de Groat, 1989). Activation of muscarinic receptors induces IP3-mediated release of Ca2+ from internal stores. ATP activates P2X receptor channels which permit the influx of Na+ and Ca2+ ions into UBSM cells. To study the link between nerve transmission and evoked Ca2+ transients, we used selective pharmacological inhibitors of muscarinic and purinergic receptors. Atropine (10 μm), which inhibits muscarinic receptors, did not significantly affect the frequency of evoked local Ca2+ transients (Fig. 4). To assess the contribution of P2X receptors to the evoked Ca2+ transients, we used compounds that inhibit P2X receptor function. Suramin (10 μm) and α,β-methylene ATP (10 μm) nearly abolished the local Ca2+ transients evoked with field stimulation (Fig. 4). In addition, P2X receptor inhibition significantly decreased the number of spontaneous Ca2+ transients to 19.0 ± 7.0% of control (20 s file; α,β-methylene ATP, 10 μm and suramin, 10 μm) (P < 0.05; n= 4 preparations). These results indicate that the majority of spontaneous Ca2+ transients and evoked Ca2+ transients are purinergic in nature and probably reflect the influx of Ca2+ into UBSM through P2X receptors. We refer to these events as ‘purinergic Ca2+ transients’ hereafter.
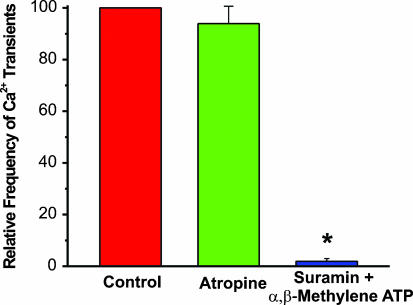
Figure 4. Inhibition of P2X receptors, but not muscarinic receptors, significantly decreased the frequency of evoked Ca2+ transients.
Column graph showing no change in evoked Ca2+ transients with muscarinic receptor inhibition by atropine (10 μm), but nearly complete abolition of these events when P2X receptors are blocked with suramin (10 μm) and α,β-methylene ATP (10 μm). To account for the variability in the number of events between different preparations, the data were normalized to control. *P < 0.05, n= 3 preparations.
Latency of purinergic Ca2+ transients from onset of nerve stimulation
To explore the relationship between nerve stimulation and purinergic Ca2+ transients, the time between nerve stimulation and the appearance of the local Ca2+ event (latency) was determined by acquiring images at the rate of 240 images s−1. At this acquisition rate, the time interval between images was 4.13 ms. Figure 5A and B shows the onset and decay of two Ca2+ events following a single stimulus. The purinergic Ca2+ transients occurred as early as 8 ms after a stimulus, with most Ca2+ events occurring 12–16 ms following a stimulus (Fig. 5C). These purinergic Ca2+ transients often appeared to originate from nerve fibres, and occurred repeatedly from the same site.
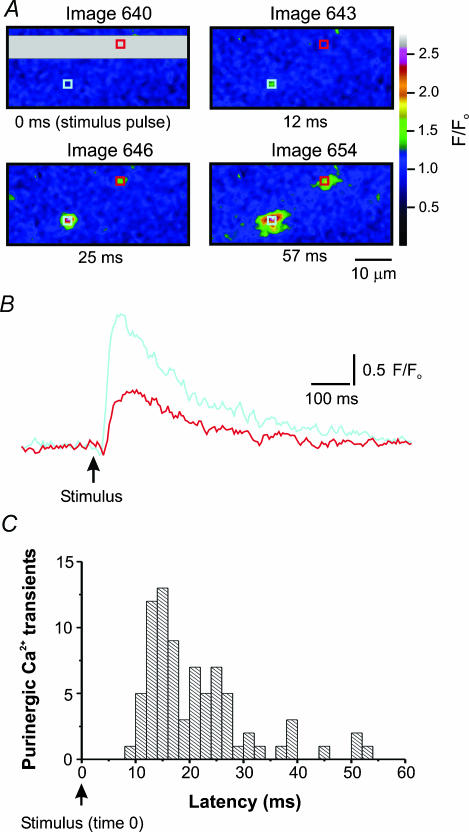
Figure 5. Field stimulation (1 Hz) evokes purinergic Ca2+ transients in UBSM.
A, four panels showing the stimulus flash followed by the onset of purinergic Ca2+ transients. The beginning of the grey region (upper left corner of grey area) of the upper left panel shows the precise time the stimulus was delivered to the tissue. About 12 ms following the stimulus (upper right panel), a purinergic Ca2+ transient appears in the light blue region of interest, followed 25 ms after the stimulus by a second purinergic Ca2+ transient in the red region of interest (lower left panel). The lower right panel shows the purinergic Ca2+ transients near their peak. B, the changes in Ca2+-activated fluorescence from the regions of interest in A before and after a stimulus pulse. Notice the rapid onset and slow decay of these purinergic Ca2+ transients. C, the latency of purinergic Ca2+ transients is displayed graphically. The earliest purinergic Ca2+ transients were recorded about 8 ms after the stimulus and reached a peak between 12 and 16 ms after stimulation.
TTX inhibits purinergic Ca2+ transients evoked with field stimulation
Since both UBSM and nerve fibres could potentially be activated by field stimulation, a pulse duration of 0.2 ms was used to selectively activate nerve fibres (Hashitani et al. 2000; Herrera et al. 2000). However, to ensure that the purinergic Ca2+ transients were not caused by direct activation of UBSM, tetrodotoxin (TTX) 2 μm was added to the superfusing solution to inhibit voltage-gated Na+ channels located on nerve processes. In paired experiments, the number of spontaneous purinergic Ca2+ transients recorded during 20 s files was not significantly affected by TTX (2 μm) (TTX 109.0 ± 24.0% of control, n= 4 preparations). In contrast, TTX (2 μm), significantly decreased the frequency of evoked purinergic Ca2+ transients (TTX 17.0 ± 8.0% of control, P < 0.05, n= 4 preparations), indicating that evoked purinergic Ca2+ transients were dependent on the activation of nerve fibres.
Purinergic Ca2+ transients do not depend on Ca2+ influx through VDCCs
The opening of VDCCs allows Ca2+ influx into UBSM. It is possible that localized Ca2+ entry through VDCCs into UBSM contributes to purinergic Ca2+ transients. To examine this possibility, we applied the VDCC inhibitor, diltiazem (100 μm), before field stimulation (1 Hz). Inhibiting VDCCs did not significantly reduce the frequency (diltiazem, 87.0 ± 7.0% of control, n= 3 preparations) or amplitude (control, 2.0 ± 0.04 F/F0, n= 246 Ca2+ events; diltiazem, 2.0 ± 0.03 F/F0, n= 310 Ca2+ events) of the purinergic Ca2+ transients, indicating that Ca2+ influx through VDCCs does not contribute to purinergic Ca2+ transients.
P2X receptor channels are permeable to both Ca2+ and Na+ ions. Therefore, purinergic Ca2+ transients should depend on external Ca2+. Removing external Ca2+ significantly decreased the frequency of purinergic Ca2+ transients. In the absence of stimulation, the frequency of spontaneous purinergic Ca2+ transients was significantly decreased in Ca2+-free external solution to 14.0 ± 9.0% of control (n= 4 preparations; P < 0.05). Returning Ca2+ to the external solution increased the frequency of purinergic Ca2+ transients to control levels (106.0 ± 17.0% of control, n= 4 preparations). The frequency of evoked purinergic Ca2+ transients was also inhibted significantly in Ca2+-free solution to 15.0 ± 5.0% of control (n= 4 preparations). When Ca2+ was returned to the external solution, the frequency of evoked purinergic Ca2+ transients returned to 77.0 ± 10.0% of control. Although it is likely that the release of transmitter from nerve terminals would also be blocked, these findings indicate that purinergic Ca2+ transients are dependent on external Ca2+ and suggest that internal Ca2+ release does not underlie these events.
Ca2+ release through IP3 receptors and RyRs does not contribute to the purinergic Ca2+ transients
In smooth muscle, IP3-mediated Ca2+ release produces Ca2+ waves (Iino et al. 1993), and Ca2+ release through RyRs can produce Ca2+ sparks which activate large-conductance potassium channels and lead to membrane hyperpolarization (Nelson et al. 1995; Jaggar et al. 2000; Herrera et al. 2001; Heppner et al. 2003). To study the contribution of Ca2+ release from internal stores to purinergic Ca2+ transients, we examined purinergic Ca2+ transients following inhibition of IP3 receptors with 2-APB (100 μm), and inhibition of RyRs with ryanodine (10 μm). The inhibition of IP3 receptors did not significantly alter the frequency (2-APB, 81.0 ± 10.0% of control, n= 4 preparations) or amplitude (control, 2.0 ± 0.02 F/F0, n= 711 purinergic Ca2+ transients; 2-APB, 2.0 ± 0.02 F/F0, n= 541 purinergic Ca2+ transients) of purinergic Ca2+ transients. Similarly, the inhibition of RyRs did not significantly alter the frequency (ryanodine 106.0 ± 10.0% normalized to control, n= 4 preparations) or amplitude of purinergic Ca2+ transients (control, 1.9 ± 0.18 F/F0, n= 339 purinergic Ca2+ transients; ryanodine, 1.9 ± 0.18 F/F0, n= 639 purinergic Ca2+ transients). These findings suggest that Ca2+ release from internal stores does not significantly contribute to purinergic Ca2+ transients.
Ca2+ sparks and purinergic Ca2+ transients represent two discrete elementary Ca2+ events in UBSM
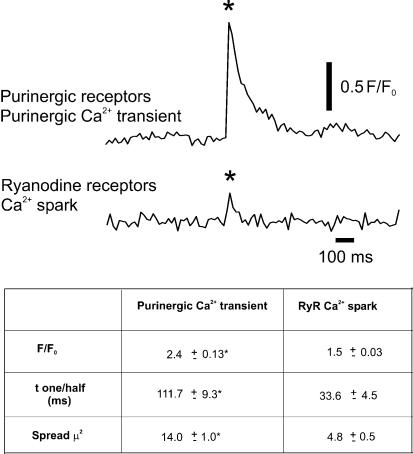
Ca2+ sparks are localized, transient events mediated by the release of sarcoplasmic reticulum Ca2+ through RyRs in smooth muscle (Nelson et al. 1995; Jaggar et al. 2000), including UBSM (Imaizumi et al. 1999; Kotlikoff et al. 1999; Herrera et al. 2001; Ohi et al. 2001). Purinergic Ca2+ transients are also brief, localized Ca2+ events, but represent Ca2+ influx through purinergic receptors. In the presence of ryanodine (10 μm), which blocks Ca2+ sparks, a population of larger events remained that were evoked by field stimulation. These events were sensitive to the purinerigic receptor inhibitors, α,β-methylene ATP (10 μm) and suramin (10 μm). Inhibition of purinergic receptors left a population of Ca2+ events that were smaller in amplitude and frequency (Ca2+ sparks). When the kinetics of each type of elementary Ca2+ transient were measured, we found that the purinergic Ca2+ transients were larger in amplitude, duration and spread compared to Ca2+ sparks (Fig. 6).
Figure 6. Ca2+-activated fluorescence for purinergic Ca2+ transients and Ca2+ sparks in UBSM.
The upper trace represents a purinergic Ca2+ transient (*) through P2X receptors recorded in the presence of ryanodine (10 μm) to inhibit Ca2+ sparks. The lower trace represents a Ca2+ spark (*) recorded in the presence of suramin (10 μm) and α,β-methylene ATP (10 μm) to block P2X receptors. Kinetic data from these traces show that purinergic Ca2+ transients mediated through P2X receptors are larger in amplitude and duration than Ca2+ sparks. In the table * indicates a significant difference (P < 0.05) between purinergic Ca2+ transients and RyR Ca2+ sparks.
Discussion
In this study, we identified three different Ca2+ signals in UBSM: (1) global Ca2+ flashes, which are large, rapid Ca2+ signals that arise from Ca2+ influx through VDCCs during an action potential, last for several seconds, and occupy the entire smooth muscle cell; (2) purinergic Ca2+ transients, which are smaller, local Ca2+ transients in UBSM mediated by Ca2+ influx through purinergic receptor channels; (3) Ca2+ sparks, which are localized Ca2+ events mediated by Ca2+ release through RyRs.
Global Ca2+ flashes represent Ca2+ influx during action potentials
Global Ca2+ flashes in UBSM occurred spontaneously, were evoked with field stimulation, and were sensitive to the inhibition of P2X receptors. These events travelled along the length of the UBSM bundle and were immediately followed by tissue contraction. These events were dependent on the influx of Ca2+ through VDCCs, since they were substantially reduced by the VDCC blocker, diltiazem (see Figs 1 and 2). This view is supported by the finding that the upstroke of the action potential is mediated by Ca2+ influx through VDCCs (Klockner & Isenberg, 1985; Mostwin, 1986; Heppner et al. 1997; Hashitani et al. 2000; Hashitani & Brading, 2003a,b) and is consistent with the observation that action potentials occur spontaneously in UBSM (Creed et al. 1983; Heppner et al. 1997; Hashitani et al. 2000, 2001; Hashitani & Brading, 2003a,b). Simultaneous recordings of voltage and Ca2+ in the guinea-pig UBSM revealed a Ca2+ transient associated with each action potential similar to the findings of the present study (Hashitani et al. 2001, 2004a,b). The block of Ca2+ global flashes by purinergic receptor inhibitors indicates that the activation of purinergic receptors is involved in the generation of VDCC-dependent action potentials and global Ca2+ flashes.
Nerve stimulation evokes purinergic Ca2+ transients
The frequency of purinergic Ca2+ transients increased with field stimulation when the pulse duration was selective for nerve fibres. When conduction along nerve fibres was blocked by inhibiting voltage-gated Na+ channels with TTX, evoked purinergic Ca2+ transients were not detected. These results indicate that the activation of nerve fibres in the UBSM significantly elevates the frequency of purinergic Ca2+ transients.
A property of synaptic transmission is latency, or the brief delay between presynaptic cell stimulation and the response in the postsynaptic cell. The latency of purinergic Ca2+ transients recorded with line scan confocal imaging was <3 ms in mesenteric arteries (Lamont & Wier, 2002), and about 6 ms in the vas deferens (Brain et al. 2002). Using high-speed confocal imaging (240 images s−1), events could be detected as soon as 8 ms following the stimulus, with the peak latency of purinergic Ca2+ transients in UBSM at 12–16 ms. There was approximately 4.1 ms between images at an acquisition rate of 240 images s−1, and therefore, the maximum error in latency would be 4.1 ms. Therefore, the latency of purinergic Ca2+ transients in UBSM was consistent with the delay found in other smooth muscle tissues. This result, in conjunction with the inhibitory effect of TTX, indicates that purinergic Ca2+ transients result from nerve fibre stimulation and synaptic transmission, and not direct stimulation of UBSM.
External Ca2+, but not VDCCs, is necessary to evoke purinergic Ca2+ transients
The purinergic Ca2+ transients should be dependent on external Ca2+, since P2X receptors are highly permeable to Ca2+. Removing Ca2+ from the external solution significantly decreased the frequency of purinergic Ca2+ transients. This effect was largely reversible, since the frequency of purinergic Ca2+ transients increased with the re-introduction of Ca2+ to the external medium. The removal of external Ca2+ could also inhibit the release of ATP from nerve terminals and thereby decrease purinergic Ca2+ transients. Our experiments do not differentiate between these two possibilities. However, if the removal of external Ca2+ had no effect on purinergic Ca2+ transients, then this would argue against Ca2+ influx through P2X receptor channels.
Another possibility is that Ca2+ influx through VDCCs contributes to the purinergic Ca2+ transients. VDCCs in UBSM (Creed et al. 1983; Heppner et al. 1997; Herrera et al. 2001; Herrera & Nelson, 2002; Hashitani & Brading, 2003a,b) are voltage sensitive and play a key role in mediating Ca2+ entry into UBSM cells, such as would occur during an action potential (Klockner & Isenberg, 1985; Mostwin, 1986; Heppner et al. 1997; Hashitani & Brading, 2003b). However, Ca2+ influx through VDCCs does not appear to contribute to purinergic Ca2+ transients for several reasons: (1) the duration of our stimulating pulse (0.2 ms) is selective for nerve fibres, and not likely to depolarize UBSM; (2) inhibition of VDCCs with diltiazem did not significantly decrease the frequency of purinergic Ca2+ transients; (3) the spatial spread of Ca2+ was local, and did not include the entire cell as would be expected with VDCC activation. Therefore, in this study, diltiazem-sensitive VDCCs do not appear to contribute to the purinergic Ca2+ transients.
Purinergic Ca2+ transients do not require Ca2+release from internal stores
Ca2+ stores in the sarcoplasmic reticulum are critical to many cellular functions and underlie Ca2+ transient events such as Ca2+ sparks and Ca2+ waves. Ca2+ sparks are generated by the release of Ca2+ through RyRs located on the sarcoplasmic reticulum (Nelson et al. 1995; Herrera et al. 2001; Heppner et al. 2003). Ca2+ waves are generated by the activation of IP3 receptors (Iino et al. 1993). Activation of muscarinic receptors leads to the production of IP3. Both M2 and M3 receptor subtypes are found in the urinary bladder. The M3 receptor is coupled to the Gq family of proteins and generates the production of IP3 and internal Ca2+ release (Caulfield, 1993). The M3 subtype mediates contraction in the urinary bladder (Hegde & Eglen, 1999) and could trigger the release of Ca2+ from the sarcoplasmic reticulum. However, the frequency of purinergic Ca2+ transients was not reduced when muscarinic receptors were blocked with atropine, suggesting that muscarinic receptor activation does not contribute to the initiation of the purinergic Ca2+ transients in UBSM.
Inhibition of Ca2+ release through RyRs (ryanodine, 10 μm) or through IP3 receptors (2-APB, 100 μm) and block of the production of IP3 by inhibition of the muscarinic receptor (atropine, 10 μm) did not alter the frequency of purinergic Ca2+ transients in UBSM. Similar results were also observed in arteries. Gitterman & Evans (2001) found that Ca2+-induced Ca2+ release was not involved in P2X-receptor-mediated contractions in rat mesenteric arteries, but that all the Ca2+ for contraction enters through P2X receptors. Therefore, in UBSM the release of Ca2+ from internal stores is not crucial to the generation of the purinergic Ca2+ transients.
Purinergic Ca2+ transients are mediated through purinergic receptors
P2X receptors belong to a family of ATP-sensitive ligand-gated cation channels (P2X1–P2X7) formed by the assembly of 3–6 individual subunits (Khakh et al. 2001). In the cat UBSM, the P2X2 receptor is the predominant subtype followed by the P2X1 receptor (Birder et al. 2004). However, in rodents the homomeric P2X1 receptor subtype is clearly associated with the membranes of UBSM (Lee et al. 2000; Elneil et al. 2001; Vial & Evans, 2000) and underlies the P2X receptor activity in UBSM as demonstrated in P2X1 receptor-deficient mice (Vial & Evans, 2000). Although some studies found no evidence of P2X1 clustering in rat or human urinary bladders (Elneil et al. 2001), other studies employing rat urinary bladder demonstrated P2X1 receptors distributed in clusters on smooth muscle membranes adjacent to nerve varicosities (Hansen et al. 1998; Dutton et al. 1999; Yunaev et al. 2000). These studies provide anatomical evidence of nerve fibre varicosities and P2X1 receptors, possibly in clusters, adjacent to one another.
Activation of P2X receptors allows the influx of cations, including Ca2+ and Na+ ions, into the cell. Recent Ca2+ imaging studies identified ATP-evoked Ca2+ events in the vas deferens (Brain et al. 2002, 2003) and in mesenteric arterial smooth muscle (Lamont & Wier, 2002; Lamont et al. 2003). In the vas deferens, stimulation of sympathetic fibres released ATP that activated P2X receptors. The Ca2+ influx through the P2X receptor was termed ‘neuroeffector Ca2+ transients’ (Brain et al. 2002, 2003). Similarly, in mesenteric artery smooth muscle, Ca2+ transients, termed ‘junctional Ca2+ transients’ were evoked by the activation of P2X receptors located on the smooth muscle, by ATP released from sympathetic fibres. This influx of Ca2+ through the P2X receptors in mesenteric arteries comprises the junctional Ca2+ transient (Lamont & Wier, 2002; Lamont et al. 2003). The kinetics of these Ca2+ events are similar to the evoked purinergic Ca2+ transients presently described in UBSM. Since evoked Ca2+ transients are blocked by inhibitors of P2X receptors, it is likely that the activation of a cluster of P2X1 receptors by nerve-evoked release of ATP underlies the evoked Ca2+ transients observed in the present study (Fig. 7). In UBSM, voltage recordings revealed excitatory junction potentials (EJPs), which are brief membrane potential depolarizations mediated by P2X receptors. EJPs initiate action potentials in UBSM, transient increases in Ca2+ that are followed by contractions (Bramich & Brading, 1996; Hashitani et al. 2000). Since the activation of P2X receptor channels permits the influx of both Ca2+ and Na+ ions it is likely that the purinergic Ca2+ transients detected in the present study represent the Ca2+ component of the EJP. Consistent with this hypothesis is the finding that global Ca2+ transients, which represent Ca2+ influx during an action potential, are significantly inhibited when purinergic Ca2+ transients are blocked. This suggests that the activation of purinergic receptors through EJPs is involved in the initiation of VDCC-dependent action potentials and global Ca2+ transients.
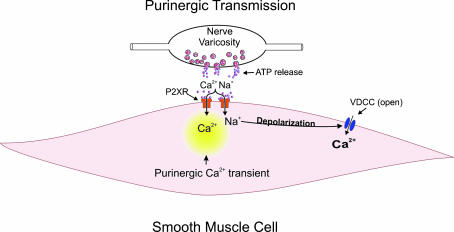
Figure 7. Illustration showing the origin of the purinergic Ca2+ transients mediated by P2X receptors.
ATP released from parasympathetic nerve varicosities activates P2X receptors located on UBSM. Ca2+ influx through the P2X receptors underlies the purinergic Ca2+ transients. The membrane potential depolarization arising from Ca2+ and Na+ influx through P2X receptors would open VDCCs allowing significant Ca2+ influx into the cell.
Two elementary localized Ca2+ events in UBSM: purinergic Ca2+ transients and Ca2+ sparks
UBSM exhibits two distinct, localized, transient, elementary Ca2+ events: purinergic Ca2+ transients and Ca2+ sparks. Although similar in appearance, the origin, kinetics, and function of these events are quite different. Purinergic Ca2+ transients, mediated by external Ca2+ influx through purinergic receptors, have significantly larger amplitudes, longer decay times and greater spatial spread than Ca2+ sparks mediated by Ca2+ efflux through RyRs located on the sarcoplasmic reticulum (Fig. 6). Ca2+ sparks are known to have a ‘braking’ effect on cell excitability by activating large-conductance Ca2+-sensitive K+ channels in arterial smooth muscle (for review see Jaggar et al. 2000), and have a similar role in UBSM (Imaizumi et al. 1999; Herrera et al. 2001; Heppner et al. 2003). Purinergic Ca2+ transients may have just the opposite effect. P2X receptors are non-selective cation channels that exhibit nearly equal permeability to Na+ and Ca2+ ions (Schneider et al. 1991). Under physiological conditions, Ca2+ ions comprise about 6–8% of the cation influx, with the balance comprised mainly of Na+ (Schneider et al. 1991). The activation of a significant number of P2X clusters could trigger sufficient Na+ and Ca2+ entry to depolarize the membrane potential and activate VDCCs. Subsequent activation of VDCCs would contribute the large amounts of Ca2+ that underlie the rising phase of action potentials in UBSM and cause contraction (Creed et al. 1983; Klockner & Isenberg, 1985; Heppner et al. 1997; Hashitani et al. 2000, 2001; Herrera et al. 2001).
Clinical conditions increase purinergic activity in the urinary bladder
During certain clinical conditions the contribution of purinergic pathways to excitation–contraction coupling in UBSM is altered. During pregnancy in rats, the composition of purinergic receptors beneath varicosities in the urinary bladder is changed. There is a decrease in clusters of P2X1, P2X2, P2X3, and P2X5, and an increase in subtypes P2X4, and P2X6 (Yunaev et al. 2000). In normal human UBSM, purinergic pathways appear to play a minor role. Normally, the density of P2X receptors in human urinary bladders is low compared to rodent urinary bladders (Bo & Burnstock, 1995). However, in unstable, symptomatically obstructed urinary bladders, P2X1 receptor subtype expression is significantly increased (for review see Boselli et al. 2001), and patients with idiopathic detrusor instability have a significant purinergic component of nerve-mediated contractions that is absent in normal human bladders (O'Reilly et al. 2002). This suggests a plasticity of P2X receptor expression that alters urinary bladder function. Our approach provides the means to examine changes in purinergic signalling at the elementary level in UBSM following outlet obstruction.
This study identifies two distinct types of Ca2+ signals exhibited in UBSM using confocal miscroscopy combined with fast Ca2+ imaging techniques: global Ca2+ flashes and novel, smaller, localized purinergic Ca2+ transients. The global Ca2+ flashes represent Ca2+ influx during action potentials. The smaller, localized purinergic Ca2+ transients are not RyR-mediated Ca2+ sparks, but instead represent Ca2+ influx through P2X receptors located on UBSM cells. These localized purinergic Ca2+ transients may represent the initial, crucial steps in the nerve-evoked cascade of events that leads to increases in intracellular Ca2+ and contraction of UBSM.
Acknowledgments
This study was supported in part by grants fromthe NIH to M. T. Nelson (DK53832 and DK065947). We would like to thank Drs David Hill-Eubanks, Jessica Filosa, Stephen Straub and Kevin Thorneloe for critically reading this manuscript and providing useful suggestions.
Supplementary material
The online version of this paper can be accessed at: DOI: 10.1113/jphysiol.2004.077826
http://jp.physoc.org/cgi/content/full/jphysiol.2004.077826/DC1 and contains supplemental material consisting of a movie clip from Fig. 3 showing purinergic Ca2+ transients in urinary bladder smooth muscle evoked by field stimulation (1 Hz). Notice that numerous purinergic Ca2+ transients occurr in response to each stimulus.
This material can also be found at:
http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp740/tjp740sm.htm
References
- Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford APDW, de Groat WC, Burnstock G. Atlerations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–F1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- Bo X, Burnstock G. Characterization and autoradiographic localization of [3H]α,β-methylene adenosine 5′-triphosphate binding sites in human urinary bladder. Br J Urol. 1995;76:297–302. doi: 10.1111/j.1464-410x.1995.tb07704.x. [DOI] [PubMed] [Google Scholar]
- Boselli C, Govoni S, Condino AM, D'Agostino G. Bladder instability: a re-appraisal of classical experimental approaches and development of new therapeutic strategies. J Autonomic Pharmacol. 2001;21:219–229. doi: 10.1046/j.1365-2680.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- Brain KL, Cuprian AM, Williams DJ, Cunnane TC. The sources and sequestration of Ca2+ contributing to neuroeffector Ca2+ transients in the mouse vas deferens. J Physiol. 2003;553:627–635. doi: 10.1113/jphysiol.2003.049734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain KL, Jackson VM, Trout SJ, Cunnane TC. Intermittent ATP release from nerve terminals elicits focal smooth muscle Ca2+ transients in mouse vas deferens. J Physiol 541. 2002;3:849–862. doi: 10.1113/jphysiol.2002.019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramich NJ, Brading AF. Electrical properties of smooth muscle in the guinea-pig urinary bladder. J Physiol. 1996;492:185–198. doi: 10.1113/jphysiol.1996.sp021300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors—characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Creed KE, Ishikawa S, Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol. 1983;338:149–164. doi: 10.1113/jphysiol.1983.sp014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdall MJ, Boyne AF, Whittaker VP. Adenosine triphosphate; a constituent of cholinergic synaptic vesicles. Biochem J. 1974;140:1–12. doi: 10.1042/bj1400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MJ, Gardner BP, Brading AF. Innervation of the detrusor muscle bundle in neurogenic detrusor overactivity. BJU Int. 2003;91:702–710. doi: 10.1046/j.1464-410x.2003.04186.x. [DOI] [PubMed] [Google Scholar]
- Dutton J, Hansen MA, Balcar VJ, Barden JA, Bennett MR. Development of P2X receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J Neurocytol. 1999;28:4–16. doi: 10.1023/a:1007043132537. [DOI] [PubMed] [Google Scholar]
- Elneil S, Skepper JN, Kidd EJ, Williamson JG, Ferguson DR. Distribution of P2X1 and P2X3 receptors in the rat and human urinary bladder. Pharmacol. 2001;63:120–128. doi: 10.1159/000056122. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol. 1996;497:413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CD, Fujii K, Kingdon J, Brading AF. The effect of cromakalim on the smooth muscle of the guinea-pig urinary bladder. Br J Pharmacol. 1989;99:281–291. doi: 10.1111/j.1476-5381.1989.tb11952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. The structural relation between nerve fibres and muscle cells in the urinary bladder of the rat. J Neurocytol. 1995;24:159–187. doi: 10.1007/BF01181533. [DOI] [PubMed] [Google Scholar]
- Gabella G. Structure of the intramural nerves of the rat bladder. J Neurocytol. 1999;28:615–637. doi: 10.1023/a:1007084130642. [DOI] [PubMed] [Google Scholar]
- Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol. 1998;27:141–155. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- Gitterman DP, Evans RJ. Nerve evoked P2X receptor contractions of rat mesenteric arteries; dependence on vessel size and lack of role of L-type calcium channels and calcium induced calcium release. Br J Pharmacol. 2001;132:1201–1208. doi: 10.1038/sj.bjp.0703925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MA, Balcar VJ, Barden JA, Bennett MR. The distribution of single P2X1-receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J Neurocytol. 1998;27:529–539. doi: 10.1023/a:1006908010642. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol. 2003a;140:146–158. doi: 10.1038/sj.bjp.0705319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003b;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004a;141:183–193. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Bramich NJ, Hirst GDS. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol 524. 2000;2:565–579. doi: 10.1111/j.1469-7793.2000.t01-2-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol 530. 2001;2:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004b;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999;64:419–428. doi: 10.1016/s0024-3205(98)00581-5. [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate the repolarization of the action potential in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Herrera GM, Bonev AD, Hill-Eubanks D, Nelson MT. Ca2+ sparks and KCa channels: Novel mechanisms to relax urinary bladder smooth muscle. In: Atala A, Slade D, editors. Bladder Disease Research Concepts and Clinical Applications. New York: Kluwer Academic/Plenum Publishers; 2003. pp. 347–357. [Google Scholar]
- Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279:R60–R68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2001;280:C481–C490. doi: 10.1152/ajpcell.2001.280.3.C481. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle CH, Burnstock G. Postganglionic efferent transmission in the bladder and urethra. In: Maggi CA, editor. Nervous Control of the Urogential System. Chur, Switzerland: Harwood Academic Publishers; 1993. pp. 349–381. [Google Scholar]
- Iino M, Kasai H, Yamazawa T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. EMBO J. 1994;13:5026–5031. doi: 10.1002/j.1460-2075.1994.tb06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Yamazawa T, Miyashita Y, Endo M, Kasai H. Critical intracellular Ca2+ concentration for all-or-none Ca2+ spiking in single smooth muscle cells. EMBO J. 1993;13:5287–5291. doi: 10.1002/j.1460-2075.1993.tb06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y, Ohi H, Yamamura H, Ohya S, Muraki K, Watanabe M. Ca2+ spark as a regulator of ion channel activity. Japan J Pharmacol. 1999;80:1–8. doi: 10.1254/jjp.80.1. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Ganitkevich VYa, Schneider P. Ca2+ influx through voltage- and purinoceptor-operated channels estimated from [Ca2+]c signals (myocytes from guinea-pig urinary bladder) Adv Exp Med Biol. 1992;311:369–371. doi: 10.1007/978-1-4615-3362-7_41. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1528–C1589. doi: 10.1152/ajpcell.2000.279.5.C1528. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Kasakov L, Burnstock G. The use of the slowly degradable analog, α,β-methylene ATP, to produce desensitization of the P2-purinoceptor; effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982;86:291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PPA. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- Klockner U, Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig) Pflugers Arch. 1985;405:329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Kotlikoff MI, Herrera G, Nelson MT. Calcium permeant ion channels in smooth muscle. Rev Physiol Biochem Pharmacol. 1999;134:147–199. doi: 10.1007/3-540-64753-8_4. [DOI] [PubMed] [Google Scholar]
- Lamont C, Vainorius E, Wier WG. Purinergic and adrenergic Ca2+ transients during neurogenic contractions of rat mesenteric small arteries. J Physiol. 2003;549:801–808. doi: 10.1113/jphysiol.2003.043380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont C, Wier WG. Evoked and spontaneous purinergic junctional Ca2+ transients (jCaTs) in rat small arteries. Circ Res. 2002;91:454–456. doi: 10.1161/01.res.0000035060.98415.4b. [DOI] [PubMed] [Google Scholar]
- Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol. 2000;163:2002–2007. [PubMed] [Google Scholar]
- Mostwin JL. The action potential of guinea pig bladder smooth muscle. J Urol. 1986;135:1299–1303. doi: 10.1016/s0022-5347(17)46079-4. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Ohi Y, Yamamura H, Nagano N, Ohya S, Muraki K, Watanabe M, Imaizumi Y. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol. 2001;534:313–326. doi: 10.1111/j.1469-7793.2001.t01-3-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly BA, Kosaka AH, Knight GF, Chang TK, Ford AP, Rymer JM, Popert R, Burnstock G, McMahon SB. P2X receptors and their role in female idiopathic detrusor instability. J Urol. 2002;167:157–164. [PubMed] [Google Scholar]
- Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Hopp HH, Isenberg G. Ca2+ influx through ATP-gated channels increments [Ca2+]i inactivates ICa in myocytes from guinea-pig urinary bladder. J Physiol. 1991;440:479–496. doi: 10.1113/jphysiol.1991.sp018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky EM, Redman RS. Synchronous release of ATP and neurotransmitter within milliseconds of a motor nerve impulse in the frog. J Physiol. 1996;492:815–822. doi: 10.1113/jphysiol.1996.sp021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald RJ, Jr, de Groat WD. The effects of purine nucleotides on transmission in vesical parasympathetic ganglia of the cat. J Autonom Pharmacol. 1989;9:167–181. doi: 10.1111/j.1474-8673.1989.tb00208.x. [DOI] [PubMed] [Google Scholar]
- Vial C, Evans RJ. P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor reponses in the mouse urinary bladder smooth muscle. Br J Pharmacol. 2000;131:1489–1495. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman GC, Cartin L, Eckman DM, Stevenson AS, Saundry CM, Lederer WJ, Nelson MT. Membrane depolarization, elevated Ca2+ entry, and gene expression in cerebral arteries of hypertensive rats. Am J Physiol Heart Circ Physiol. 2001;281:H2559–H2567. doi: 10.1152/ajpheart.2001.281.6.H2559. [DOI] [PubMed] [Google Scholar]
- Yunaev MA, Barden JA, Bennett MR. Changes in the distribution of different subtypes of P2X receptor clusters on smooth muscle cells in relation to nerve varicosities in the pregnant rat urinary bladder. J Neurocytol. 2000;29:99–108. doi: 10.1023/a:1007152428481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.