Abstract
A simplified cell culture system was developed to study neuronal plasticity. As changes in synaptic strength may alter network activity patterns, we grew hippocampal neurones on a microelectrode array (MEA) and monitored their collective behaviour with 60 electrodes simultaneously. We found that exposure of the network for 15 min to the GABAA receptor antagonist bicuculline induced an increase in synaptic efficacy at excitatory synapses that was associated with an increase in the frequency of miniature AMPA receptor-mediated EPSCs and a change in network activity from uncoordinated firing of neurones (lacking any recognizable pattern) to a highly organized, periodic and synchronous burst pattern. Induction of recurrent synchronous bursting was dependent on NMDA receptor activation and required extracellular signal-regulated kinase (ERK)1/2 signalling and translation of pre-existing mRNAs. Once induced, the burst pattern persisted for several days; its maintenance phase (> 4 h) was dependent on gene transcription taking place in a critical period of 120 min following induction. Thus, cultured hippocampal neurones display a simple, transcription and protein synthesis-dependent form of plasticity. The non-invasive nature of MEA recordings provides a significant advantage over traditional assays for synaptic connectivity (i.e. long-term potentiation in brain slices) and facilitates the search for activity-regulated genes critical for late-phase plasticity.
Synaptic connections are not static but undergo use-dependent modifications leading to strengthening or weakening of their efficacy (Ito, 1989; Bliss & Collingridge, 1993; Stevens & Sullivan, 1998; Malenka & Nicoll, 1999; Bi & Poo, 2001). This phenomenon, commonly referred to as neuronal or synaptic plasticity, is thought to play an important role in learning and memory (Milner et al. 1998). A particularly well studied form of neuronal plasticity is long-term potentiation (LTP) of the CA3–CA1 synapses in the hippocampus, which is readily induced in hippocampal slices by a high frequency stimulation of the Schaffer collaterals that synapse onto the dendrites of CA1 neurones. In many cases (including the CA3–CA1 synapses), changes in synaptic strength are initiated by calcium entry through synaptic NMDA receptors. Precisely how calcium signals trigger alterations in synaptic strength is unclear, although it is thought that calcium/calmodulin-dependent protein kinases (Lisman et al. 2002) and the phosphorylation and trafficking of AMPA-type glutamate receptors play a central role (Malenka & Nicoll, 1997; Malinow et al. 2000; Luscher et al. 2000; Hering & Sheng, 2001; Malinow & Malenka, 2002; Song & Huganir, 2002; Malenka, 2003; Henley, 2003). In addition to local signal processing at the synapse, calcium transients evoked by electrical activity and NMDA receptor activation can also stimulate signalling pathways to the cell nucleus leading to the induction of gene expression (Bading et al. 1993; Bito et al. 1996, 1997; Bading, 2000; West et al. 2002). Experiments using pharmacological blockers of transcription indicate that LTP induction can occur in the absence of ongoing transcription. However, for changes in synaptic efficacy to sustain beyond 2–3 h, gene transcription taking place in a critical time window of 2 h after induction is critical (Nguyen et al. 1994; Milner et al. 1998).
The propagation of synaptic activity-induced signals to the nucleus takes place via two major routes. One is the ERK1/2 signalling pathway (Bading & Greenberg, 1991; Ginty et al. 1993; Wu et al. 2001; Sweatt, 2001; Thiels & Klann, 2001). Upon their activation by a submembranous, near-NMDA receptor calcium transient (Hardingham et al. 2001b), ERK1/2 translocate to the nucleus leading to gene expression mediated for example by the transcription factor TCF-Elk1 (Cruzalegui & Bading, 2000; Valjent et al. 2001). The second route is a propagating calcium signal to the nucleus (Hardingham et al. 2001a). Increases in the nuclear calcium concentration stimulate the transcription factor cAMP response element binding protein (CREB) and its coactivator CREB binding protein (CBP) (Hardingham et al. 1997, 1999, 2001b; Chawla et al. 1998). Both CREB and CBP have been implicated in learning and memory (Milner et al. 1998; Bading, 2000; Alarcon et al. 2004; Korzus et al. 2004).
Although the principles of synapse-to-nucleus communication are now well understood, it remains unknown which calcium-regulated target genes are critical for neuronal plasticity and learning-related events. Gene knock-out studies in mice have revealed some insight; however, the large number of genes with apparent critical importance for plasticity has confused rather than clarified the issues (Sanes & Lichtman, 1999). Research into the role of gene expression in neuronal plasticity would greatly benefit from a simple experimental system that can be tightly controlled and manipulated, and for which a comprehensive transcriptional analysis is feasible. In addition, such a system should allow plasticity to be assessed ideally by a non-invasive method that does not interfere with the health of the preparation and therefore allows analyses over long periods of time (hours to days). This is a critical issue as gene expression programs are only relevant for the late phase of plasticity, which is difficult to assess with traditional invasive techniques such as brain slice electrophysiology.
In this study we established a novel, very simple experimental system that fulfils the above mentioned criteria. Multi-site electrical recordings with MEA chips were used to monitor, long-term and non-invasively, the firing patterns of synaptically interconnected hippocampal neurones. We reasoned that signal-induced changes in synaptic strength affect the network firing pattern, which could serve as a read-out for changes in connectivity. Indeed, we found dramatic, signal-induced changes in network firing patterns that were long-lasting and dependent on gene transcription. The method described here may prove useful for the identification of activity-regulated genes with a role in neuronal plasticity.
Methods
Hippocampal networks and microelectrode array recordings
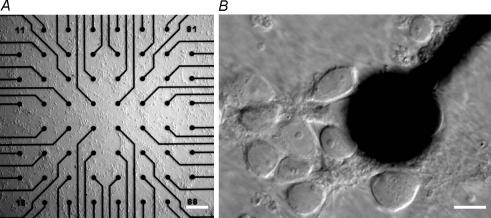
Hippocampal neurones from new-born Long-Evans or Sprague-Dawley rats were prepared as described (Bading & Greenberg, 1991) except that growth media was supplemented with B27 (Gibco/BRL). Neurones were plated onto MEA dishes containing a grid of 60 planar electrodes (Multi Channel Systems, Reutlingen, Germany) and on 12 mm glass coverslips at a density of about 400 cells (neurones and glia) per mm2 (low density) to about 600 cells per mm2 (high density). Optimal final cell density was assessed relative to cell quality and spontaneous activity (see Results and Fig. 1). A photomicrograph of cells at a typical plating density is shown in Fig. 3A and B. The distance between the electrodes on the MEA dishes was 200 μm and the electrode diameter was 30 μm (Fig. 3A and B). Recordings were acquired with an MEA-60 amplifier board (10Hz–35 kHz, gain 1200, sampling frequency 20 kHz, Multi Channel Systems). Stimulations and recordings were done after a culturing period of 10–14 days. Before stimulation, network activity was recorded for 3 min; cultures with spontaneous bursting activity (see Fig. 1) were excluded. Recurrent synchronous network bursting was induced by treatment of the neurones with 50 μm bicuculline. Bicuculline was dissolved in DMSO. The final concentration of DMSO did not exceed 0.05% which caused no independent effects for the incubation periods used (maximum 15 min). After another 3 min of recording, bicuculline was washed away by changing the medium three times. Cultures were put back into the incubator and 3 min recordings were repeatedly performed at different time points following the washout of bicuculline. Actinomycin D (10 μg ml−1), anisomycin (10 μg ml−1), MK-801 (10 μm) and nifedipine (20 μm) were pre-incubated for 20 min; PD 98059 (50 μm) was pre-incubated for 1 h. These compounds were also co-applied during bicuculline treatment and washout. Care was taken to minimize the exposure of nifedipine to light with all incubations being performed in a dark incubator for all compounds. In some experiments, actinomycin D was added 2 h after bicuculline washout. 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX), nifedipine and anisomycin were obtained from Sigma (St Louis, MO, USA); MK-801 was obtained from Tocris Cookson Ltd (Bristol, UK); PD 98059 and actinomycin D were obtained from Calbiochem (Bad Soden, Germany). Spikes were detected with the integrated spike detector of the MC Rack software (Multi Channel Systems). Burst analysis was done with Neuroexplorer (NEX Technologies, http://www.neuroexplorer.com). All results are given as mean ±s.e.m. Statistical tests were done by comparing data sets from treated cultures to controls for each time point separately by an independent samples t test.
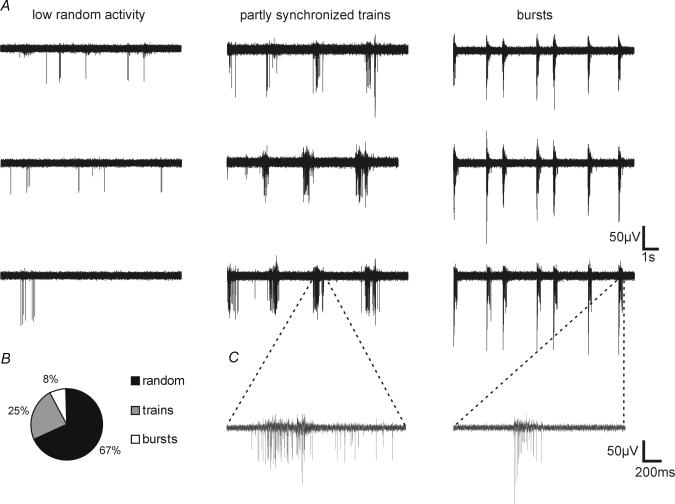
Figure 1. MEA recordings of spontaneous activity in cultured hippocampal networks.
A, for each type of spontaneous network firing (i.e. low random activity, partly synchronized trains, and bursts) one typical example with MEA recordings from 3 separate electrodes is shown. B, distribution of the observed type of spontaneous activity in 197 cultured hippocampal networks. C, expanded traces illustrate examples of a single spike train and a burst.
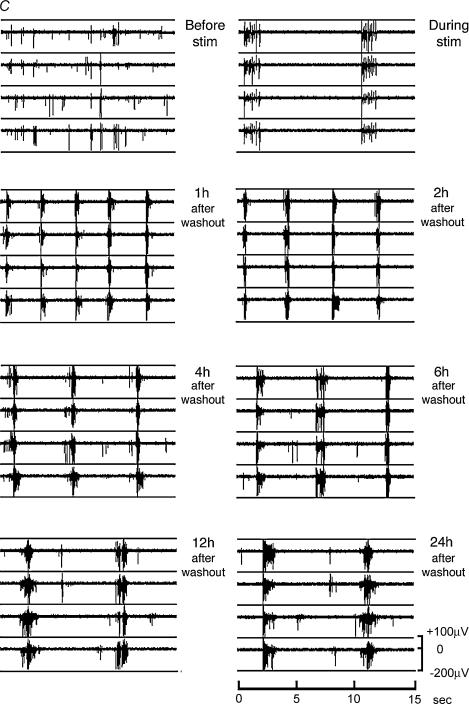
Figure 3. Induction of recurrent synchronous bursting in cultured hippocampal networks.
A, hippocampal cells cultured on a microelectrode array. Inter-electrode distance is 200 μm, electrode diameter is 30 μm (bar: 200 μm). B, a single electrode at higher magnification (bar: 10 μm). C, a typical example of an MEA recording from a hippocampal network before, during and after exposure to bicuculline (50 μm) to trigger trains of action potentials. Simultaneous recordings from 4 separate electrodes are shown in each panel. The trains of action potentials observed during the bicuculline exposure are about 1.5 s long and recur regularly with a frequency of about 6 trains per minute. After washout of bicuculline the network shows a highly organized, synchronous and periodic burst pattern; at 1 h after washout, the length of the bursts is 0.55 s and their frequency is 20 per minute, gradually changing to a duration of 0.8 s and a frequency of 8 per minute at 24 h after washout.
Calcium imaging and Western blot analysis
Fluo-3 calcium imaging was done using confocal laser scanning microscopy as described previously (Hardingham et al. 1999, 2001a). Calcium concentrations were expressed as a function of the fluo-3 fluorescence [(F−Fmin)/(Fmax−F)]. Activation of ERK1/2 phosphorylation was measured by immunoblotting using antibodies to the phosphorylated form of ERK1/2 as described previously (Hardingham et al. 1999, 2001a,b). Phosphorylation of ERK1/2 is indicative of stimulation of their enzymatic activities. The phospho-ERK1/2-specific antibodies were obtained from New England Biolabs (Beverly, MA, USA). Immunoblot analysis of calmodulin expression was used to control for protein loading; the antibody to calmodulin was obtained from Upstate Biotechnology (Lake Placid, NY, USA).
Patch clamp analysis
Whole-cell patch clamp recordings were made from cultured hippocampal neurones plated on coverslips after a culturing period of 10–14 days. Coverslips were placed in a recording chamber (PM-1, Warner Instruments, Hamden, CT, USA) mounted on a fixed-stage upright microscope (BX51WI, Olympus, Hamburg, Germany). Differential interference contrast optics, infrared illumination and a CCD camera (Photometrics Coolsnap HQ, Visitron Systems, Puchheim, Germany) were used to view neurones on a computer monitor using a software interface (Metamorph, Universal Imaging Systems, Downington, PA, USA). Patch electrodes (3–4 MΩ) were made from borosilicate glass (1.5 mm, WPI, Sarasota, FL, USA) and filled with a potassium gluconate-based solution (containing (mm): potassium gluconate 155, MgCl2 2, Hepes 10, K2-phosphocreatine 10, Mg2-ATP 2, Na3-GTP 0.3; pH 7.35 with KOH). The extracellular solution was a salt–glucose–glycine solution containing (mm) NaCl 140, KCl 5.3, MgCl2 1, CaCl2 2, Hepes 10, glycine 1, glucose 30 and sodium pyruvate 0.5. Recordings were made with a Multiclamp 700A amplifier, digitized through a Digidata 1322A A/D converter, acquired and analysed using pCLAMP software (Axon Instruments, Union City, CA, USA). Miniature excitatory postsynaptic currents (mEPSCs) were recorded in the presence of tetrodotoxin (1 μm, Alomone, Jerusalem, Israel) or with magnesium replacing calcium in the extracellular solution (i.e. [Mg2+] increased from 1 to 3 mm and the nominal [Ca2+] was decreased from 2 to 0 mm). As no difference between mEPSC frequency, amplitude or decay was detectable and no difference in the qualitative effects of bicuculline-induced bursting on mEPSC amplitude and frequency were seen, results obtained with these two solutions were pooled. All voltage clamp recordings were performed at a holding potential of −80 mV (junction potential corrected) at which GABAA-mediated inhibitory postsynaptic currents (IPSCs) were outward and slower EPSCs indicative of NMDA receptor-mediated EPSCs were not apparent. All remaining mEPSCs were AMPA receptor mediated as confirmed by their complete blockade by 6,7-dinitroquinoxaline (DNQX, 20 μm, Tocris) in 12 cells. mEPSCs events were detected using Mini-analysis (Synaptosoft, Decatur, GA, USA) with a 5 pA amplitude threshold and all mEPSCs were verified visually. Events occurring less than 10 ms after the previous event were included in frequency but not amplitude analyses. Access (range: 10–28 MΩ) and membrane resistance (range: 150–650 MΩ) were monitored regularly during voltage clamp recordings and data was rejected if changes greater than 30% occurred. Cumulative probability histograms, generated from at least 300 mEPSCs, were examined for each recording and compared using the Kolmogorov-Smirnov test. Mean values for mEPSC parameters from cell groups were compared using independent (between cells comparisons) or paired (within cells comparisons) t tests. Where t test results are given, the t value is expressed as t (degrees of freedom). EPSCs were also evoked with single 40–100 μs constant current pulses (50–200 μA) from an A365 stimulus isolator using a tungsten stereotrode with a 30 μm tip separation (WPI) positioned within 100 μm of the recorded cell. All results are presented as mean ±s.e.m. In a subset of experiments, AMPA (Tocris) was bath applied through a perfusion system in the presence of tetrodotoxin to prevent the contribution of AMPA currents due to the excitation of presynaptic cells.
Results
Assessment of spontaneous network firing using MEA
Hippocampal neurones, derived from newborn rats, were grown on MEA dishes. Over a time course of 10–14 days, the neurones develop a rich network of processes, express functional NMDA-type and AMPA/kainate-type glutamate receptors, and form numerous functional synaptic contacts (Bading et al. 1995; Hardingham et al. 2001a). MEA recordings revealed that depending on the plating density of cells, the degree of spontaneous electrical activity of the network varies (Fig. 1). Networks with high cell densities often show a spontaneous, partly synchronized, activity pattern. Synchronized short spike trains with few single spikes occurring between trains can be recorded throughout the network (Fig. 1A, middle; 49 of 197 cultures). In some cases (16 of 197 cultures), highly synchronized bursts on all active electrodes could be detected (Fig. 1A, right). These bursts resembled the periodic and synchronous burst pattern observed after stimulation with bicuculline (Fig. 2A). Networks with lower cell density display a random activity pattern with single spikes and without obvious synchronization between different electrodes (Fig. 1A, left; 132 of 197 cultures). For all experiments on signal-induced changes in network behaviour (see below) hippocampal networks were used that display little or no spontaneous bursting activity. This density-dependent mode of network firing is consistent with predictions from theoretical models recently suggested by Giugliano et al. (2004).
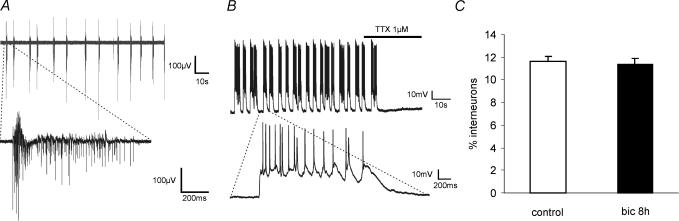
Figure 2. MEA and current clamp recordings of bicuculline-induced bursting.
A, a typical 2 min MEA recording from a hippocampal network during exposure to bicuculline (50 μm) and an expanded trace of an individual burst are shown. B, a current clamp recording from a hippocampal culture exposed to bicuculline (50 μm) reveals a strong depolarization accompanying each burst of action potentials. This depolarizing shift was blocked by tetrodotoxin (1 μm). The expanded trace (beneath) shows that individual cells typically fired 10–20 action potentials per burst. C, induction of sustained recurrent activity by synaptic NMDA receptors leaves the percentage of interneurones in the network unchanged (P= 0.569, n= 3 for each group, independent samples t test). The percentage of GABAergic interneurones in control hippocampal networks (control) and in networks exposed for 8 h to 50 μm bicuculline (bic 8 h). Inhibitory interneurones were identified by GABA immunostaining and expressed as a percentage of the total number of cells identified by Hoechst staining. Bicuculline treatment does not lead to an increase in the number of apoptotic or necrotic neurones (Hardingham et al. 2002).
Signal-induced increases in synaptic efficacy in cultured hippocampal neurones
Previous studies have demonstrated that upon bath application of glutamate hippocampal neurones in culture can undergo lasting changes in synaptic efficacy (Antonova et al. 2001); in hippocampal slice preparations, the potentiation of synaptic transmission can be induced by bursting activity (Buzsaki et al. 1987; Ben-Ari & Gho, 1988; Bains et al. 1999). We produced increased synaptic efficacy by exposing hippocampal cultures for 15 min to the GABAA receptor antagonist bicuculline. GABAergic interneurones, which represent about 11% of the neurone population (see Fig. 2C), impose a tonic inhibition onto the network. Removal of GABAergic inhibition with bicuculline led to action potential bursting with an intraburst frequency of 4.1 ± 0.4 Hz accompanied by a strong depolarizing shift in membrane potential (29.5 ± 3.7 mV) (Fig. 2A and B). The rhythmic depolarizations and bursts were mediated by network activity as they were abolished by the application of tetrodotoxin (1 μm, n= 20, Fig. 2B). Such bursting triggers calcium entry into the neurones through synaptic NMDA receptors (Hardingham et al. 2001a). Although the in vivo application of bicuculline leads to seizure activity and neuronal degeneration (Ben-Ari, 2001), we did not observe any cell death even after prolonged (several hours) exposure of the neurones to bicuculline (Hardingham et al. 2002; data not shown) and found that the number of GABAergic interneurones remained constant (Fig. 2C). Moreover, the bicuculline stimulation protocol induces nuclear calcium signalling, increases expression of CREB-regulated genes including brain-derived neurotrophic factor (BDNF), and activates pro-survival programs (Hardingham et al. 2001a, 2002).
Signal-induced switch in network activity pattern
We expected that, after bicuculline washout, the network bursting would stop and the firing would return to its pre-stimulation patterns. Surprisingly, MEA and current clamp recordings revealed that the network activity was transformed into a new state. It displayed highly regular short periodic bursts with an interburst frequency around 0.2 Hz, an intraburst frequency of individual cells of 4.1 ± 0.3 Hz and a depolarizing shift of 8.0 ± 1.2 mV during bursts. Comparisons of the timing of the bursts recorded from different electrodes on the same MEA revealed that the network fired in synchrony (Fig. 3C). Thus, an activity-induced increase in synaptic efficacy in a network of cultured hippocampal neurones causes a ‘macroscopically visible’ shift towards slow rhythm recurrent bursting activity. To describe and quantify this stimulus-induced change in network behaviour, we analysed the following parameters including: (i) bursts per minute; (ii) percentage of spikes within the bursts; (iii) burst duration; (iv) number of spikes per burst; (v) mean and peak frequency of spikes in the bursts; (vi) spikes per minute. The data for the parameters mentioned in (i) and (ii) are shown in Figs 5, 6, 7 and 8.
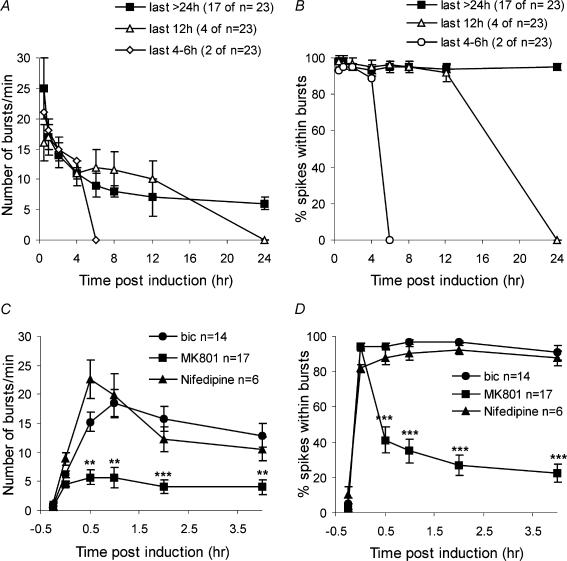
Figure 5. Calcium flux through synaptic NMDA receptors initiates recurrent synchronous bursting.
A and B, quantitative analysis of the number of bursts per minute and the percentage of spikes within bursts from 23 experiments. The behaviour of the networks was grouped into three categories based on the duration of the maintenance phase of oscillatory synchrony (more than 24 h; 12 h; 4–6 h). The frequency of the bursts gradually declined from 25.2 ± 4.9 min−1 at 30 min (or 16.9 ± 1.9 min−1 at 1 h) after bicuculline washout to 7.6 ± 0.6 min−1 at 24 h after bicuculline washout. The percentage of spikes within bursts ranged from 98.9 ± 0.6% at 1 h after washout to 96.5 ± 0.8% at 24 h after washout. The duration of the bursts ranged from 0.57 ± 0.05 s at 1 h after washout to 0.79 ± 0.08 s at 24 h after washout; the number of spikes per burst ranged from 67.4 ± 6.3 at 30 min after washout to 91.8 ± 13.1 at 24 h after washout; the spike frequency within bursts ranged from 132.1 ± 11.2 Hz at 1 h after washout to 119.2 ± 8.9 Hz at 24 h after washout; the peak frequency of spikes within bursts was 459.6 ± 6.7 Hz at 1 h after washout and 459.6 ± 6.2 Hz at 24 h after washout; the number of spikes per minute expressed as a percentage of pre-induction levels was 258% at 1 h after washout and 143% at 24 h after washout. At the end of each experiment, the hippocampal networks were stimulated again with 50 μm bicuculline; this stimulation gave rise to recurrent synchronous bursting that was virtually identical to that obtained following the initial stimulation with 50 μm bicuculline (data not shown). C and D, the NMDA receptor antagonist MK-801 (10 μm) did not affect spontaneous electrical activity or activity during bicuculline (bic) exposure (Hardingham et al. 2002), but inhibited the induction of sustained recurrent activity by the 15 min long exposure to bicuculline (50 μm). Analysis of 17 cultures revealed that the number of spikes per minute and the percentage of spikes within bursts during bicuculline application did not differ between control cultures and cultures treated with MK-801. However, 4 h after bicuculline washout the number of bursts per minute decreased from 12.9 ± 2.0 (control) to 4.0 ± 1.3 (MK-801). The percentage of spikes within bursts decreased from 90.9 ± 4.1% (control) to 22.7 ± 5.2% (MK-801). Induction and maintenance of recurrent synchronous bursting was little affected by the blockade of L-type voltage-gated calcium channels with nifedipine (20 μm). For each time point, cultures treated with MK-801 or nifedipine were compared with controls by an independent samples t test. *P < 0.01, **P < 0.001, ***P < 0.0001. At the end of each experiment, the hippocampal networks were again stimulated with 50 μm bicuculline; this stimulation gave rise to recurrent synchronous bursting that was virtually identical to that obtained during the initial stimulation with 50 μm bicuculline (data not shown).
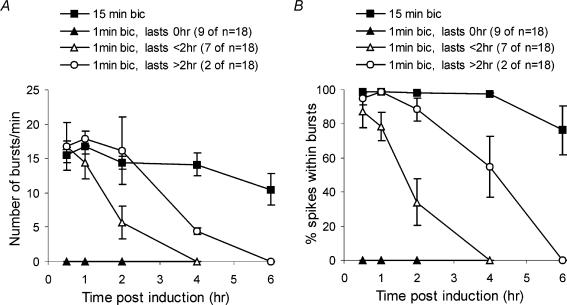
Figure 6. Induction of recurrent synchronous bursting has a stimulus-duration threshold.
Quantitative analysis of the number of bursts per minute (A) and the percentage of spikes within bursts (B) revealed that stimulation of hippocampal networks for 1 min with 50 μm bicuculline led to either no induction of sustained recurrent activity (9 out of 18 experiments), or induced it for 1–2 h (7 out of 18 experiments), or for 2–4 h (2 out of 18 experiments). Analysis of the experiments in which a temporary burst pattern was induced showed that the duration of these bursts, the number of spikes per burst, and the mean and peak spike frequencies within bursts were similar to the values obtained in hippocampal networks stimulated for 15 min with 50 μm bicuculline; the percentage of spikes within bursts and the percentage increase in the number of spikes per minute was reduced compared with the 15 min stimulation protocol (data not shown). At the end of the experiment, the hippocampal networks, previously stimulated with bicuculline for 1 min, were stimulated again with 50 μm bicuculline for 15 min; this gave rise to recurrent synchronous bursting that was virtually identical to that obtained in naive networks stimulated with 50 μm bicuculline for 15 min (data not shown).
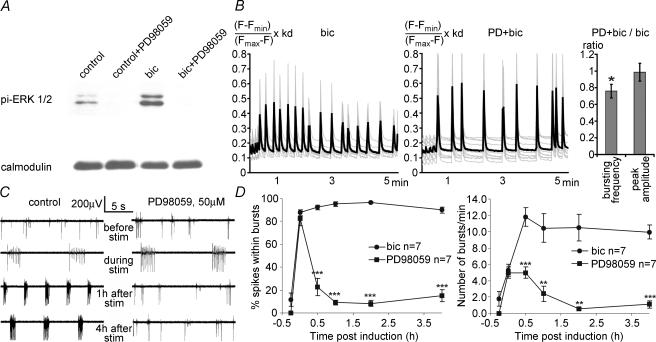
Figure 7. ERK1/2 signalling controls induction of recurrent synchronous bursting.
A, Western blot analysis of ERK1/2 phosphorylation in hippocampal neurones stimulated for 7 min with bicuculline (50 μm) in the presence or absence of 100 μm PD 98059. PD 98059 treatment started 1 h before bicuculline stimulation. Calmodulin expression was used as the loading control. B, typical example of results from an imaging experiment showing global calcium transients in hippocampal neurones during a 240 s exposure to bicuculline (bic, 50 μm) in the presence (middle panel) or absence (left panel) of the MEK inhibitor PD 98059 (PD, 50 μm). The ratio of the peak size and frequency of the calcium signals obtained with bicuculline treatment versus bicuculline treatment in the presence of PD 98059 is given (right panel, n= 26). Statistically significant differences (P < 0.05, two-tailed, independent samples t test) between PD 98059 + bicuculline and bicuculline are indicated with an asterisk. C, typical examples of MEA recordings from hippocampal networks before, during and after exposure to bicuculline (50 μm) in the presence or absence of 50 μm PD 98059. PD 98059 treatment started 1 h before bicuculline stimulation. D, quantitative analysis of the number of bursts per minute and the percentage of spikes within bursts. Thirty minutes after washout of bicuculline, the percentage of spikes within bursts decreased from 92.3 ± 2.0% (control) to 22.7 ± 7.4% (PD 98059). The number of bursts per minute decreased from 11.8 ± 1.1 (control) to 5.0 ± 0.7 (PD 98059). For each time point, cultures treated with PD 98059 were compared with controls with an independent samples t test. *P < 0.01, **P < 0.001, ***P < 0.0001.
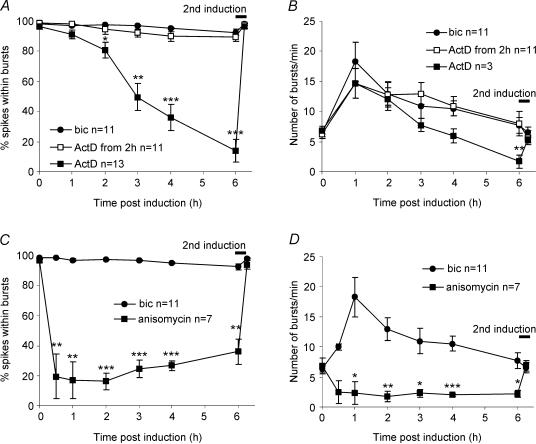
Figure 8. The maintenance of recurrent synchronous bursting is dependent on gene transcription and on-going protein synthesis.
A and B, the switch in hippocampal network behaviour was analysed in the presence of 10 μg ml−1 actinomycin D (ActD), an inhibitor of gene transcription. The effects of adding actinomycin D 15 min before and 2 h after bicuculline stimulation on indices of network bursting activity (i.e. number of bursts per minute and the percentage of spikes within bursts) were compared (n=13). The activity pattern observed during bicuculline stimulation was unaffected by actinomycin D (data not shown). In networks treated with actinomycin D before bicuculline exposure, recurrent synchronous bursting was inducible and persisted during the first 2 h in a manner similar to that of control networks. Thereafter, the bursting activity of actinomycin D-pre-treated networks decayed rapidly. Indices of bursting activity in hippocampal networks treated with actinomycin D 2 h after the bicuculline exposure were not statistically different from those of control networks not treated with actinomycin D (n=11). In an additional series of experiments hippocampal networks treated with actinomycin D 2 h after the bicuculline exposure were analysed for a period of 8 h following bicuculline stimulation; no statistical differences in the indices of bursting activity were found when compared with bicuculline-treated control networks (n= 9) (data not shown). C and D, analysis of the effects of the protein synthesis inhibitor anisomycin (10 μg ml−1) on hippocampal network plasticity. The percentage of spikes within bursts (C) and the number of bursts per minute (D) are shown. Anisomycin was added to the cultures 15 min prior to stimulation with bicuculline (n= 7). Compared with actinomycin D treatment, blockade of protein synthesis caused a much more rapid decay of recurrent synchronous bursting. The network returned to a pre-stimulation activity pattern within 30 min of bicuculline washout. To rule out the possibility that toxic effects of actinomycin D and anisomycin interfere with the ability of the network to generate bursts, 50 μm bicuculline was added a second time at the end of each experiment (indicated as 2nd induction); this gave rise to synchronous bursting that was virtually identical to that obtained during the first stimulation. For each time point, cultures treated with actinomycin D or anisomycin, respectively, were compared with controls with an independent samples t test. *P < 0.01, **P < 0.001, ***P < 0.0001.
Analysis of miniature EPSCs and AMPA responses
To assess potential changes in synaptic transmission following bicuculline treatment we compared AMPA receptor-mediated mEPSCs from control (vehicle) and bicuculline (50 μm in vehicle)-treated cells from the same culture preparations. A 15 min incubation followed by a 30 min period after washout was selected due to the robust maintenance of bursting shown for this time point with MEA recordings. The mean inter-event interval and amplitude of mEPSCs in each cell in both groups were calculated. The inter-event interval of mEPSCs was shorter in the bicuculline group but the amplitude of mEPSCs did not differ (inter-event interval: t(29) = 2.417, P= 0.022; amplitude: t(29) = 0.077, P= 0.938; t tests for independent samples; n= 15 for vehicle and n= 16 for bicuculline-treated cells; Fig. 4A). However, a large variability in both parameters was observed partly accounted for by the greater amplitude and frequency of mEPSCs in cells displaying bursting activity in control conditions. It was not possible to assess the presence of bursting before bicuculline treatment making it impossible to remove this source of variability from the data.
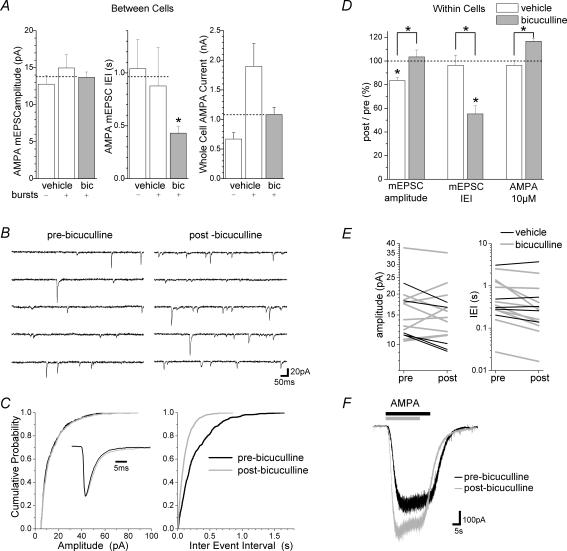
Figure 4. Changes in mEPSCs and whole-cell AMPA currents following bicuculline-induced bursting.
A, histograms show the group mean ±s.e.m. from mean values in each cell for mEPSC amplitude, inter-event interval (IEI) and the amplitude of responses to bath-applied AMPA (10 μm) in the presence of tetrodotoxin (1 μm). Prior to recordings, networks had been treated with either bicuculline (bic) or vehicle for 15 min followed by a 30 min washout period. Vehicle-treated cells were divided into those exhibiting or not exhibiting bursting activity (bursts) during cell-attached and current clamp recordings. All bicuculline-treated cells showed bursting activity. Dashed lines represent the mean of the combined vehicle treated groups which differed from the bicuculline-treated group with respect to mEPSC inter-event interval but not mEPSC amplitude or AMPA response amplitude (P= 0.022, P= 0.94 and P= 0.98, respectively; independent t tests; n= 6–8 for non-bursting vehicle-treated, n= 7–10 for bursting vehicle-treated, n= 16–20 for bicuculline-treated cells). B, consecutive sweeps show mEPSCs recorded from the same cell before (pre-bicuculline) and after (post-bicuculline) the bicuculline treatment protocol (i.e. 15 min bicuculline followed by 30 min washout in current clamp recording conditions). C, cumulative probability histograms generated from the cell shown in B indicate a reduced inter-event interval (IEI) but no change in amplitude following bicuculline treatment (IEI: P < 0.0001, amplitude: P= 0.27; Kolmogorov-Smirnov tests). The inset shows a scaled superimposed average of scaled mEPSCs recorded in both conditions. D and E, histograms (D) show the group mean ±s.e.m. of normalized (post-treatment/pre-treatment) mean values for amplitude and inter-event interval of AMPA receptor-mediated mEPSCs recorded from each cell before and after bicuculline (n= 10) or vehicle (n= 5) treatment protocols. Line series plots (E) show the individual means for amplitude (left) and inter-event interval (IEI, right) in each cell before (pre) and after (post) bicuculline (grey lines) or vehicle (black lines) treatments. Each line connects mean values from the same cell. No consistent change in mEPSC amplitude occurred following bicuculline treatment (increased amplitude in 3 cells, P < 0.0001; decreased amplitude in 3 cells, P < 0.001; no change in 4 cells, P > 0.15 Kolmogorov-Smirnov tests; P= 0.84 group mean comparison of pre- and post-values in all bicuculline-treated cells with paired t test). Bicuculline treatment reduced the mEPSC inter-event interval (P < 0.0001 in 9 cells, P= 0.24 in 1 cell Kolmogorov-Smirnov tests; P= 0.024 group mean comparison of pre- and post-values in all bicuculline-treated cells with paired t test). Vehicle treatment caused a decrease in mEPSC amplitude in all cells (n= 5, P < 0.02 for all cells with Kolmogorov-Smirnov tests, P= 0.024 group mean comparison of pre- and post-values in all vehicle-treated cells with paired t test). No change in mEPSC inter-event interval was apparent following vehicle treatment (no change in 3 cells, P > 0.1; decreased IEI in 1 cell, P < 0.01; increased IEI in 1 cell, P < 0.02 Kolmogorov-Smirnov tests; P= 0.39 group mean comparison of pre- and post-values in all vehicle-treated cells with paired t test). The differences between bicuculline- and vehicle-treatment groups were significant for mEPSC amplitude and inter-event interval (see asterisks above the dashed line in D; amplitude: P= 0.036; IEI: P= 0.004; comparison of normalized mean values from all bicuculline-treated cells with those from all vehicle-treated cells using independent samples t tests). F, overlaid current responses to bath application of AMPA (10 μm) from the same cell before and after the bicuculline protocol. Peak responses were measured in cells before and after the bicuculline- or the vehicle-treatment protocols and the group mean ±s.e.m. of normalized responses is shown in the histogram in D. Although AMPA response size did not differ from its pre-treatment measurement in either bicuculline- or vehicle-treatment groups (bicuculline group: P= 0.12, n= 4; vehicle group: P= 0.63, n= 4; comparison between pre- and post-treatment response values from all cells using paired t tests), the difference between bicuculline- and vehicle-treatment groups was significant (P= 0.008, comparison of normalized responses from all bicuculline-treated cells with those of vehicle-treated cells using an independent samples t test; see asterisk at top right of D).
To exclude such variability, we selected a further 10 neurones not showing regular bursting activity (i.e. low random activity category; see Fig. 1) in control conditions and compared mEPSCs recorded from the same cell before and after 15 min bicuculline treatment plus a period of 30 min after bicuculline washout (Fig. 4B). Once again, mEPSCs showed a strong, robust increase in frequency but no consistent change in amplitude (Fig. 4C–E). Comparisons of cumulative probability histograms generated from mEPSC recordings before and after this bicuculline treatment protocol showed no consistent change in mEPSC amplitude (increased amplitude in 3 cells, P < 0.0001; decreased amplitude in 3 cells, P < 0.001; no change in 4 cells, P > 0.15 Kolmogorov-Smirnov tests; t(9) = 2.695, P= 0.837 group mean comparison with paired t test). Bicuculline treatment, however, reduced the inter-event interval (P < 0.0001 in 9 cells, P= 0.24 in 1 cell Kolmogorov-Smirnov tests; t(9) = 2.695, P= 0.024, n= 10, group mean comparison with a paired t test). Similar effects of bicuculline were found in cells where mEPSCs were recorded in either tetrodotoxin or in solutions with magnesium replacing calcium (data not shown). In summary, both between and within cells analyses indicated an approximately 2-fold increase in mEPSC frequency with varied effects on mEPSC amplitude.
To detect any overall change in surface expression of AMPA receptors, whole-cell responses to bath application of 10 μm AMPA were recorded in the presence of tetrodotoxin. Although non-bursting cells (i.e. low random activity category; see Fig. 1) showed smaller responses to AMPA than cells with regular bursting (i.e. other categories), as a whole, the control group responses were not different from those of the bicuculline-treated group (t(34) =−0.021, P= 0.98; n= 15 for control, n= 21 for bicuculline-treated cells; independent t test; Fig. 4A). In a within-cells analysis from non-bursting cells, bicuculline-induced bursting caused a 17 ± 2% increase in AMPA currents relative to responses measured before bicuculline treatment; however, this failed to reach statistical significance (t(3) =−2.142, P= 0.121, n= 4, paired t test; Fig. 4D and F). Note that bath-applied AMPA would not detect any modulation of kainate receptors or the rapidly desensitizing component of AMPA receptor-mediated responses by bicuculline-induced changes in synaptic efficacy.
To assess potential changes in mEPSCs and AMPA responses due to the prolonged recording protocol including tetrodotoxin and AMPA applications, control experiments were also performed using the same protocol without bicuculline (i.e. ‘vehicle’). No change in mean mEPSC frequency was apparent in 80% of cells although a slight decrease in mEPSC amplitude occurred in all vehicle-treated cells and no change occurred in AMPA responses (inter-event interval: no change in 3 cells, P > 0.1; decrease in 1 cell, P < 0.01; increase in 1 cell, P < 0.02; Kolmogorov-Smirnov tests; t(4) = 0.527, P= 0.395, n= 5, group mean comparison with paired t test; amplitude: P < 0.02 for 5 cells with Kolmogorov-Smirnov tests, t(4) = 3.533, P= 0.024, n= 5, group mean comparison with paired t test; AMPA response: t(3) = 0.527, P= 0.634, n= 4, paired t test; Fig. 4D and E). These effects were possibly due to increases in access resistance of 20 ± 4% during the vehicle-treatment protocol. A comparable increase of 25 ± 3% in access resistance also occurred between mEPSCs recordings in bicuculline-treated cells. This is probably responsible for the slightly slower mEPSC kinetics in many cells (Fig. 4C inset) and may have masked a small increase in mEPSC amplitude and an even greater increase in frequency due to the filtering of subthreshold events. Indeed the normalized (pre-treatment/post-treatment) mEPSC amplitude, inter-event interval and the response to AMPA following bicuculline treatment were significantly different from those following vehicle treatment (mEPSC amplitude: t(13) = 2.329, P= 0.036; mEPSC inter-event interval: t(13) =−3.480, P= 0.004; AMPA response: t(6) = 3.891, P= 0.008; independent t tests; Fig. 4D). This comparison with vehicle-treated cells confirms that bicuculline increases mEPSC frequency and provides weak evidence for an increased mEPSC amplitude and AMPA response. Similar results have been reported following overnight treatment with bicuculline in organotypic hippocampal cultures (Abegg et al. 2003) where a between cells analysis revealed a strong increase in frequency and a weaker increase in the amplitude of mEPSCs.
Paired pulse experiments were attempted to further elucidate the mechanisms of bicuculline-induced changes in synaptic transmission; however, analysis of evoked EPSCs in bicuculline-treated cells was not possible. Pre-synaptic stimuli in bicuculline-treated cells evoked a wave of recurring spontaneous EPSCs, indicating that a presynaptic burst had been triggered. This problem and the presence of regular bursts of spontaneous EPSCs affected evoked EPSC amplitudes in an uncontrollable manner.
Changes in network behaviour are long-lasting
A striking feature of the stimulus-induced recurrent activity is its persistence. In the example shown in Fig. 3C it lasted for 24 h. Analysis of a large number of experiments (n= 23) revealed that the maintenance phase of this network state falls into one of three categories (Fig. 5A and B): maintenance for at least 24 h, maintenance for about 12 h, and maintenance for about 4 h. The majority of the networks analysed fell into the first category (17 out of 23; see Fig. 5A and B). The parameter that best illustrates the organized nature of recurrent activity is the ‘percentage of spikes within bursts’ (Fig. 5B). During recurrent synchronous bursting virtually all spikes are within the bursts; with time the percentage of spikes within bursts progressively declined reflecting a decay of the recurrent activity pattern.
NMDA receptors and ERK1/2 control induction of recurrent synchronous bursting
Recurrent synchronous bursting was triggered by NMDA receptor activation and was greatly reduced when the bicuculline exposure was done in the presence of the NMDA receptor antagonist MK-801. The percentage of spikes within bursts and the number of spikes per minute were both reduced by MK-801 (10 μm) at all time points after bicuculline treatment (P < 0.001 to P < 0.0001, independent t tests, Fig. 5C and D). MK-801 did not affect action potential firing that occurs during the bicuculline exposure (Hardingham et al. 2002). The NMDA receptor-mediated switch of the network to recurrent synchronous bursting has a stimulus-duration threshold: networks exposed to bicuculline for only 1 min did not acquire recurrent activity at all (9 out of 18 experiments) or did so for only short periods of time (maintenance for 1–2 h, 7 out of 18 experiments; maintenance for 2–4 h, 2 out of 18 experiments) (Fig. 6).
As bicuculline-induced bursting can also lead to calcium entry through L-type voltage-gated calcium channels (data not shown), we investigated their role in this model of network plasticity. We found that blockade of L-type voltage-gated calcium channels with nifedipine (20 μm) did not affect the percentage of spikes within bursts 30 min after bicuculline washout and caused a transient increase in burst frequency (Fig. 5C and D). This suggests that L-type voltage-gated calcium channels are not required for the induction or maintenance of sustained recurrent activity.
The NMDA receptor is a key regulator of activity-dependent changes in neurones (Bliss & Collingridge, 1993; Milner et al. 1998). One of the intracellular consequences of calcium entry through NMDA receptors is the activation of the extracellular signal-regulated kinase (ERK)1/2 (Bading & Greenberg, 1991; Hardingham et al. 2001b). ERK1/2 signalling plays a role in several plasticity-related processes in the nervous system (Thiels & Klann, 2001; Sweatt, 2001). We therefore tested its involvement in the induction of recurrent synchronous bursting. We found that treatment with the MAP kinase/ERK kinase (MEK) 1 inhibitor PD90859 blocked bicuculline-induced ERK1/2 activation (measured by Western blot analysis; Fig. 7A) but left bicuculline-induced calcium transients largely unaffected causing no reduction in peak size although the peak frequency was significantly reduced (P < 0.05, t test for independent samples, Fig. 7B). However, the induction of recurrent synchronous bursting was blocked; we observed significant reductions in the number of spikes within bursts and the number of bursts per minute at all time points following bicuculline treatment (P < 0.001 to P < 0.0001, t tests for independent samples, Fig. 7C and D), indicating that the NMDA receptor-induced switch in network activity pattern was ERK1/2 dependent.
Maintenance of recurrent synchronous bursting requires gene transcription and protein synthesis
Plasticity-related events require electrical activity-induced gene expression (Nguyen et al. 1994; Linden, 1996; Milner et al. 1998; Bading, 2000). We therefore investigated the possibility that NMDA receptor-induced recurrent synchronous bursting is dependent on transcription. We found that compared with control cultures, recurrent synchronous bursting decayed rapidly under conditions where gene transcription was blocked with actinomycin D at the time of stimulation (Fig. 8A and B). This is not simply due to a general requirement of on-going gene transcription: actinomycin D, added 2 h after the stimulation, did not compromise the maintenance phase of recurrent bursting activity even at 8 h post-stimulation (Fig. 8A and B). The percentage of spikes within bursts and the number of spikes per minute were both reduced by actinomycin D (10 μg ml−1) at all time points 3 h or more after bicuculline treatment (P < 0.01 to P < 0.0001, independent t test) but had no effect when added 2 h later (P > 0.3 for all time points, independent t test). This narrow time window indicates that gene transcription responses, possibly triggered by calcium flux through synaptic NMDA receptors, are critical for the neuronal network to sustain recurrent synchronous bursting. The genes involved in this process remain to be identified; however, it is possible that the genomic response is mediated by the transcription factor CREB that is potently activated by synaptic NMDA receptors and nuclear calcium signals (Hardingham et al. 2001a, 2002).
We found that induction of recurrent synchronous bursting was also blocked by the protein synthesis inhibitor anisomycin (Fig. 8C and D), indicating the requirement for protein synthesis. Compared with actinomycin D treatment, blockade of protein synthesis caused a much more rapid decay of recurrent synchronous bursting; indeed in most experiments the network returned to pre-stimulation activity patterns within 30 min of bicuculline washout. This suggests that translation of pre-exiting mRNA, possibly localized to dendritic areas, is an early requirement for the establishment of recurrent synchronous bursting.
Discussion
Although plasticity at the level of individual synapses has been extensively studied, network properties that emerge from the dynamics of individual contact points are not well understood. With the development of extracellular MEA recordings it became possible to monitor network activity patterns over long periods of time and to study their possible modulation by signalling pathways. The use of 60 electrodes simultaneously also made it much easier to investigate synchronous network activity. Our results show that simple hippocampal networks can be induced to generate periodic and synchronous bursting that is sustained far beyond the duration of the stimulus. Rhythmic firing has been observed previously in other cell culture systems (Murphy et al. 1992; Kamioka et al. 1996; Bacci et al. 1999; Streit et al. 2001; Tscherter et al. 2001; Opitz et al. 2002); it has some resemblance with activity patterns detected in vivo during anaesthesia, certain forms of sleep (Steriade et al. 1993a,b,c; Metherate & Ashe, 1993; Contreras et al. 1996; Stern et al. 1997; Buzsaki, 1998; Lampl et al. 1999), and in the developing visual and auditory systems, as well as in the developing hippocampus and neocortex (Galli & Maffei, 1988; Meister et al. 1991; Lippe, 1994; Wong et al. 1995; Weliky & Katz, 1999; Leinekugel et al. 2002). Moreover, in CA3 hippocampal neurones in slice preparations, network-mediated bursts similar to those reported here, can be induced by bicuculline, kainic acid or high extracellular potassium concentrations and lead to NMDA receptor-dependent potentiation of synaptic transmission at recurrent collateral connections (Buszaki et al. 1987; Ben-Ari & Gho, 1988; Bains et al. 1999). In the developing brain, self-sustaining (i.e. input-independent) recurrent network activity may have a role in network maturation (Buzsaki, 1989); in the adult it may contribute to information storage (Wang, 2001), but could also promote pathological conditions such as epilepsy (McCormick, 2002).
New tool for studying transcription-dependent plasticity
Irrespective of any resemblance of activity patterns generated in vitro versus in vivo and their possible implications for brain function, the value of our study is the establishment of a simple and robust experimental system as a tool to study the cell biology of neuronal plasticity. The non-invasive nature of the recording technique facilitates in particular the analysis of the late phases of changes in synaptic efficacy that are difficult to assess using standard slice electrophysiology methods. As is the case for numerous other forms of adaptive changes in the developing and adult nervous system, the alterations in the hippocampal network described here (i.e. the shift of firing pattern towards recurrent synchronous bursting) are initiated by calcium flux through synaptic NMDA receptors. The subsequent activation of ERK1/2 by a submembranous, near-NMDA receptor calcium transient (Hardingham et al. 2001b) appears to control the early phase of plasticity. Precisely how ERK1/2 modulate the network activity pattern is unclear, however; similar to LTP, the regulation of AMPA receptor trafficking may be involved. Through a process known as ‘unsilencing’, AMPA receptors can be inserted into the plasma membrane of silent (i.e. NMDA receptor-only containing) synapses, which allows neurones to increase the strength of their excitatory synaptic contacts (Malenka & Nicoll, 1997; Malinow et al. 2000; Luscher et al. 2000; Hering & Sheng, 2001; Song & Huganir, 2002; Malenka, 2003; Henley, 2003). AMPA receptor exocytosis appears to be regulated by the Ras-ERK1/2 cascade (Zhu et al. 2002). This raises the possibility that an ERK1/2-mediated increase in AMPA receptor surface expression contributes to the observed changes in network firing patterns. Alternatively, the unsilencing of synapses may be mediated by increases in release probability (Emptage et al. 2003; Reid et al. 2004) or by factors which raise the glutamate concentration in the cleft sufficiently to activate AMPA receptors which have a lower affinity for glutamate than NMDA receptors (Choi et al. 2000; Voronin & Cherubini, 2004). Thus, an increased frequency of mEPSCs and a possible increase in mEPSC amplitude and whole cell AMPA currents following bicuculline induced bursting could be accounted for by the unsilencing of synapses through either pre or postsynaptic mechanisms or both.
Nuclear signalling and gene transcription
For recurrent synchronous bursting to be maintained transcription is needed in a critical time window of about 120 min following NMDA receptor stimulation. Transcription dependency is common to the late phases of many forms of neuronal plasticity and learning (Nguyen et al. 1994; Linden, 1996; Taha & Stryker, 2002). Although the critical activity-regulated genes remain to be identified, the key transcription-regulating machinery may involve the CREB/CBP complex (Milner et al. 1998; Bading, 2000; Alarcon et al. 2004; Korzus et al. 2004). CREB/CBP-mediated gene expression is controlled by one of two signals: increased levels of cAMP (Mayr & Montminy, 2001) or increases in nuclear calcium (Hardingham et al. 1997; Chawla et al. 1998). The stimulation paradigms used here to trigger changes in network behaviour do not lead to global increases in the levels of cAMP (Pokorska et al. 2003). However, they do induce robust nuclear calcium transients (Hardingham et al. 2001a,b). We therefore propose that nuclear calcium controls transcription-dependent network plasticity. A transcriptional analysis using Affymetrix gene chips has revealed a comprehensive picture of genes regulated under the condition used to induce network plasticity (Zhang and Bading, unpublished). The simplicity of our experimental system will help determine the functional significance of nuclear calcium and putative nuclear calcium-regulated genes for the maintenance phase of plasticity.
Requirement for protein synthesis
Besides the importance of nuclear signalling and gene transcription, the maintenance of recurrent synchronous bursting is also critically dependent on on-going protein synthesis. The decay of recurrent synchronous bursting is even faster in the presence of a protein synthesis blocker than it is after blockade of transcription. This indicates that translation of pre-existing mRNAs is important. It is conceivable (and consistent with our findings) that local protein synthesis of AMPA receptors contributes to the early phase of plasticity, as has been suggested recently (Ju et al. 2004).
Conclusion
This study established a novel, very simple and experimentally accessible system for studying activity-induced changes in network behaviour. The results obtained illustrate that both local, near-NMDA receptor signalling (activating ERK1/2) and the dialogue between the synapse and the nucleus are important to induce and maintain a particular activity pattern. Non-invasive multisite recordings will facilitate in particular studies of the transcription-dependency of certain network behaviours and may prove useful for testing the hypothesis that nuclear calcium-regulated gene expression is important for late phase synaptic plasticity.
Acknowledgments
We thank Bill Wisden for discussion and comments on the manuscript. This work was supported by the Alexander von Humboldt-Foundation (Wolfgang Paul Prize to H.B) and the Sonderforschungsbereich 488 of the Deutsche Forschungsgemeinschaft, and by the MRC, the European Community, the Ethel Househam Fellowship in Neurosurgery, the Dunhill Medical Trust, The Royal College of Surgeons of England, the Sackler Medical Research Centre, and the Boehringer Ingelheim Fonds.
References
- Abegg MH, Savic N, Ehrengruber R, McKinney RA, Gähwiler BH. Epileptiform activity in rat hippocampus strengthens excitatory synapses. J Physiol. 2003;554:439–448. doi: 10.1113/jphysiol.2003.052662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/– mice: a model for the cognitive deficit in Rubinstein–Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Antonova I, Arancio O, Trillat AC, Wang HG, Zablow L, Udo H, Kandel ER, Hawkins RD. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science. 2001;294:1547–1550. doi: 10.1126/science.1066273. [DOI] [PubMed] [Google Scholar]
- Bacci A, Verderio C, Pravettoni E, Matteoli M. Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurons in culture. Eur J Neurosci. 1999;11:389–397. doi: 10.1046/j.1460-9568.1999.00440.x. [DOI] [PubMed] [Google Scholar]
- Bading H. Transcription-dependent neuronal plasticity: The nuclear calcium hypothesis. Eur J Biochem. 2000;267:5280–5283. doi: 10.1046/j.1432-1327.2000.01565.x. [DOI] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Bading H, Segal MM, Sucher NJ, Dudek H, Lipton SA, Greenberg ME. N-methyl-D-aspartate receptors are critical for mediating the effects of glutamate on intracellular calcium concentration and immediate early gene expression in cultured hippocampal neurons. Neuroscience. 1995;64:653–664. doi: 10.1016/0306-4522(94)00462-e. [DOI] [PubMed] [Google Scholar]
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci. 1999;2:720–726. doi: 10.1038/11184. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Cell death and synaptic reorganizations produced by seizures. Epilepsia. 2001;42(suppl. 3):5–7. doi: 10.1046/j.1528-1157.2001.042suppl.3005.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gho M. Long-lasting modification of the synaptic properties of rat CA3 hippocampal neurones induced by kainic acid. J Physiol. 1988;404:365–384. doi: 10.1113/jphysiol.1988.sp017294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. Ca2+-dependent regulation in neuronal gene expression. Curr Opin Neurobiol. 1997;7:419–429. doi: 10.1016/s0959-4388(97)80072-4. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7:17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Haas HL, Anderson EG. Long-term potentiation induced by physiologically relevant stimulus patterns. Brain Res. 1987;435:331–333. doi: 10.1016/0006-8993(87)91618-0. [DOI] [PubMed] [Google Scholar]
- Chawla S, Hardingham GE, Quinn DR, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- Choi S, Klingauf J, Tsien RW. Postfusional regulation of cleft glutamate concentration during LTP at ‘silent synapses’. Nat Neurosci. 2000;3:330–336. doi: 10.1038/73895. [DOI] [PubMed] [Google Scholar]
- Contreras D, Timofeev I, Steriade M. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol. 1996;494:251–264. doi: 10.1113/jphysiol.1996.sp021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzalegui FH, Bading H. Calcium-regulated protein kinase cascades and their transcription factor targets. Cell Mol Life Sci. 2000;57:402–410. doi: 10.1007/PL00000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A, Bliss TV. Optical quantal analysis reveals a presynaptic component of LTP at hippocampal Schaffer associational synapses. Neuron. 2003;38:797–804. doi: 10.1016/s0896-6273(03)00325-8. [DOI] [PubMed] [Google Scholar]
- Galli L, Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988;242:90–91. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Giugliano M, Darbon P, Arsiero M, Luscher HR, Streit J. Single-neuron discharge properties and network activity in dissociated cultures of neocortex. J Neurophysiol. 2004;92:977–996. doi: 10.1152/jn.00067.2004. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJL, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001a;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJL, Bading H. A calcium microdomain near NMDA receptors: on-switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001b;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Cruzalegui FH, Bading H. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron. 1999;22:789–798. doi: 10.1016/s0896-6273(00)80737-0. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Henley JM. Proteins interactions implicated in AMPA receptor trafficking: a clear destination and an improving route map. Neurosci Res. 2003;45:243–254. doi: 10.1016/s0168-0102(02)00229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Kamioka H, Maeda E, Jimbo Y, Robinson HP, Kawana A. Spontaneous periodic synchronized bursting during formation of mature patterns of connections in cortical cultures. Neurosci Lett. 1996;206:109–112. doi: 10.1016/s0304-3940(96)12448-4. [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl I, Reichova I, Ferster D. Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron. 1999;22:361–374. doi: 10.1016/s0896-6273(00)81096-x. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsaki G. Correlated bursts of activity in the neonatal hippocampus in vivo. Science. 2002;296:2049–2052. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- Linden DJ. A protein synthesis-dependent late phase of cerebellar long-term depression. Neuron. 1996;17:483–490. doi: 10.1016/s0896-6273(00)80180-4. [DOI] [PubMed] [Google Scholar]
- Lippe WR. Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci. 1994;14:1486–1495. doi: 10.1523/JNEUROSCI.14-03-01486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Cortical and subcortical generators of normal and abnormal rhythmicity. Int Rev Neurobiol. 2002;49:99–114. doi: 10.1016/s0074-7742(02)49009-5. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Silent synapses speak up. Neuron. 1997;19:473–476. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Ionic flux contributions to neocortical slow waves and nucleus basalis-mediated activation: whole-cell recordings in vivo. J Neurosci. 1993;13:5312–5323. doi: 10.1523/JNEUROSCI.13-12-05312.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Blatter LA, Wier WG, Baraban JM. Spontaneous synchronous synaptic calcium transients in cultured cortical neurons. J Neurosci. 1992;12:4834–4845. doi: 10.1523/JNEUROSCI.12-12-04834.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Opitz T, De Lima AD, Voigt T. Spontaneous development of synchronous oscillatory activity during maturation of cortical networks in vitro. J Neurophysiol. 2002;88:2196–2206. doi: 10.1152/jn.00316.2002. [DOI] [PubMed] [Google Scholar]
- Pokorska A, Vanhoutte P, Arnold FJL, Silvagno F, Hardingham GE, Bading H. Synaptic activity induces signalling to CREB without increasing global levels of cAMP in hippocampal neurons. J Neurochem. 2003;84:447–452. doi: 10.1046/j.1471-4159.2003.01504.x. [DOI] [PubMed] [Google Scholar]
- Reid CA, Dixon DB, Takahashi M, Bliss TV, Fine A. Optical quantal analysis indicates that long-term potentiation at single hippocampal mossy fiber synapses is expressed through increased release probability, recruitment of new release sites, and activation of silent synapses. J Neurosci. 2004;24:3618–3626. doi: 10.1523/JNEUROSCI.3567-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Can molecules explain long-term potentiation? Nat Neurosci. 1999;2:597–604. doi: 10.1038/10154. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nunez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993c;13:3284–3299. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993a;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993b;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol. 1997;77:1697–1715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Sullivan J. Synaptic plasticity. Curr Biol. 1998;8:R151–153. doi: 10.1016/s0960-9822(98)70097-1. [DOI] [PubMed] [Google Scholar]
- Streit J, Tscherter A, Heuschkel MO, Renaud P. The generation of rhythmic activity in dissociated cultures of rat spinal cord. Eur J Neurosci. 2001;14:191–202. doi: 10.1046/j.0953-816x.2001.01636.x. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Taha S, Stryker MP. Rapid ocular dominance plasticity requires cortical but not geniculate protein synthesis. Neuron. 2002;34:425–436. doi: 10.1016/s0896-6273(02)00673-6. [DOI] [PubMed] [Google Scholar]
- Thiels E, Klann E. Extracellular signal-regulated kinase, synaptic plasticity, and memory. Rev Neurosci. 2001;12:327–345. doi: 10.1515/revneuro.2001.12.4.327. [DOI] [PubMed] [Google Scholar]
- Tscherter A, Heuschkel MO, Renaud P, Streit J. Spatiotemporal characterization of rhythmic activity in rat spinal cord slice cultures. Eur J Neurosci. 2001;14:179–190. doi: 10.1046/j.0953-816x.2001.01635.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Caboche J, Vanhoutte P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: a molecular substrate for learning and memory? Mol Neurobiol. 2001;23:83–99. doi: 10.1385/MN:23:2-3:083. [DOI] [PubMed] [Google Scholar]
- Voronin LL, Cherubini E. ‘Deaf, mute and whispering’ silent synapses: their role in synaptic plasticity. J Physiol. 2004;557:3–12. doi: 10.1113/jphysiol.2003.058966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285:599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Wong RO, Chernjavsky A, Smith SJ, Shatz CJ. Early functional neural networks in the developing retina. Nature. 1995;374:716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]