Abstract
Spo11 or a homologous protein appears to be essential for meiotic DNA double-strand break (DSB) formation and recombination in all organisms tested. We report here the first example of an alternative, mutationally activated pathway for meiotic recombination in the absence of Rec12, the Spo11 homolog of Schizosaccharomyces pombe. Rad2, a FEN-1 flap endonuclease homolog, is involved in processing Okazaki fragments. In its absence, meiotic recombination and proper segregation of chromosomes were restored in rec12Δ mutants to nearly wild-type levels. Although readily detectable in wild-type strains, meiosis-specific DSBs were undetectable in recombination-proficient rad2Δ rec12Δ strains. On the basis of the biochemical properties of Rad2, we propose that meiotic recombination by this alternative (Rec*) pathway can be initiated by non-DSB lesions, such as nicks and gaps, which accumulate during premeiotic DNA replication in the absence of Okazaki fragment processing. We compare the Rec* pathway to alternative pathways of homologous recombination in other organisms.
MEIOSIS, the hallmark of sexual reproduction, is characterized in most organisms by high levels of homologous recombination during prophase I (Roeder 1997). Meiotic recombination serves at least two purposes. First, it generates crossovers between homologous chromosomes (homologs) that mature into visible chiasmata. The chiasmata connect homologs and allow them to segregate from each other during the unique first division of meiosis. Second, homologous recombination generates new combinations of alleles among gametes, an important factor in the evolution of species.
In the well-studied budding yeast Saccharomyces cerevisiae and fission yeast Schizosaccharomyces pombe meiotic recombination is initiated by the formation of DNA double-strand breaks (DSBs) at recombination hotspots (Sun et al. 1989; Steiner et al. 2002). In S. cerevisiae the initiating protein Spo11 is meiosis specific and introduces DSBs by a topoisomerase-like mechanism, in which the protein attaches covalently to the 5′ end at the break (Bergerat et al. 1997; Keeney et al. 1997). The widespread presence of Spo11 orthologs from unicellular fungi to mammals indicates that the mechanism of meiotic recombination initiation is conserved among eukaryotes (Lichten 2001). The S. pombe Spo11 ortholog, Rec12, is essential in generating meiotic recombinants and meiosis-specific DSBs (DeVeaux et al. 1992; Cervantes et al. 2000). In the absence of Rec12, meiotic recombination is reduced to the level observed during vegetative growth and segregation of homologs is defective (Farah et al. 2002, 2005; Sharif et al. 2002; Davis and Smith 2003).
DSB formation during meiosis is preceded by DNA replication (Borde et al. 2000; Cervantes et al. 2000). Because of the antiparallel nature of DNA and the specificity of the replication machinery, synthesis of one strand, the lagging strand, proceeds by steps, whereby short DNA fragments, Okazaki fragments, are synthesized from RNA primers, processed by nucleases, and ligated into long intact DNA. An important enzyme in this processing is the FEN-1 flap endonuclease (Lieber 1997). In one model, RNAse H removes all but one ribonucleotide from the 5′ end of an Okazaki fragment. Subsequently, FEN-1, through its 5′ → 3′ exonuclease activity, removes the last 5′ ribonucleotide, allowing the Okazaki fragment to be ligated to the adjacent upstream fragment. In another model, through its endonuclease activity, FEN-1 tracks along and cleaves 5′ flaps generated by synthesis-dependent displacement of a downstream Okazaki fragment (Kao et al. 2004). Although essential in mammals, FEN-1 is not essential in yeasts (Murray et al. 1994; Tishkoff et al. 1997b; Kucherlapati et al. 2002). Perhaps, in the absence of FEN-1, other nucleases such as ExoI or the 3′ → 5′ exonuclease activity of DNA polymerase δ lead to ligatable nicks between adjacent Okazaki fragments and hence preserve the integrity of the genome (Tishkoff et al. 1997a; Jin et al. 2001).
In S. cerevisiae, the absence of Rad27, a FEN-1 ortholog, confers multiple vegetative phenotypes, such as high mutation rates, destabilization of mini- and microsatellites, and destabilization of telomeres (Tishkoff et al. 1997b; Kokoska et al. 1998; Parenteau and Wellinger 1999). These phenotypes seem to result directly from the deficiency in Okazaki fragment processing in the absence of Rad27. Several observations indicate that Rad27 also plays a role in genome stability independent of its role in replication. For instance, Rad27 limits recombination between short homologous sequences, perhaps by aborting recombination intermediates (Negritto et al. 2001), and it inhibits Ty1 mobility by destabilizing the unincorporated Ty1 cDNA intermediate (Sundararajan et al. 2003). No meiotic phenotype of a FEN-1 mutant has been reported to date. In S. pombe, a mutation in rad2, encoding a FEN-1 homolog, increased mitotic recombination rates but had no significant effect on meiotic intragenic recombination (gene conversion) (Grossenbacher-Grunder 1985).
Although Rec12 is normally essential for generating meiotic recombinants and for efficient segregation of homologs, we report here that the absence of Rad2 restored high levels of both gene conversion and crossing over as well as efficient segregation of homologs during rec12 mutant meiosis. Our results reveal an alternative, Rec12-independent pathway of meiotic recombination. In the intervals tested, DSB levels were undetectable in the absence of both Rad2 and Rec12. We speculate that single-strand lesions at unprocessed Okazaki fragments promote recombination by this alternative pathway, whose properties are similar to those of an alternative pathway of homologous recombination in bacteria.
MATERIALS AND METHODS
Yeast strains and genetic techniques:
Supplemental Table 1S (at http://www.genetics.org/supplemental/) lists the S. pombe strains, their genotypes, and the crosses in which they were used. The rec8-176∷kanMX6 allele was constructed by replacing the entire rec8 ORF with a 3HA-6His-kanMX6 disruption cassette (abbreviated kanMX6) amplified from plasmid pFA6a-3HA-6His-kanMX6 (Davis and Smith 2003) using oligonucleotides rec8kanD and rec8kanR (supplemental Table 2S at http://www.genetics.org/supplemental/ and Bahler et al. 1998).
Meiotic crosses were conducted and analyzed essentially as described by DeVeaux et al. (1992). In this standard S. pombe method (Moreno et al. 1991) mitotic recombination is essentially absent, since haploid cells mate and immediately undergo meiosis. Ade+ recombinants were selected on yeast extract agar (YEA 4S) supplemented with guanine (100 μg/ml), which inhibits the uptake of adenine; 4S is uracil, histidine, lysine, and leucine, each at 100 μg/ml. Auxotrophies were scored on appropriately supplemented nitrogen-base minimal agar (NBA). tps16 was scored on YEA 4S plus phloxin B (20 μg/ml; Moreno et al. 1991) at 37°. In crosses involving the rad2∷ura4+ allele, the ura4+-aim allele was scored by PCR using oligos OL1073, OL1074, and OL1075 (see supplemental Table 2S at http://www.genetics.org/supplemental/), which amplify different size fragments depending on the ura4 allele.
To measure the mitotic association of crossovers with gene conversion at ade6 (Table 3B), diploid strains were selected on NBA plus adenine (Ade; 100 μg/ml) by complementation of his4-239 and lys4-95 (closely linked on chromosome 2), purified, and cultured at 25°. A diploid colony was picked to nitrogen-base minimal liquid medium (NBL) plus Ade and grown to saturation. Cells were plated on NBA plus Ade, and after 4 days individual colonies were gridded onto NBA plus Ade. After 2 days the plate was replicated onto NBA (without Ade). Ade+ recombinants from the edge of 60 patches were streaked onto NBA to isolate Ade+ single colonies, which were gridded onto NBA. After 2 days the plate was replicated onto sporulation agar (SPA) 6S (4S plus adenine and arginine), and after 3 days patches containing asci were harvested separately and treated with glusulase and ethanol (DeVeaux et al. 1992). These spore suspensions were streaked onto YEA 4S plus guanine plates to give single Ade+ colonies, and four colonies were gridded onto NBA 4S plus guanine and scored for tps16 and ura4+-aim as described above. The assignment of a particular genotype was based on the most frequent phenotype among the four colonies. This procedure ensured that independent mitotic recombination events were analyzed. The frequency of aneuploid meiotic products was determined by random spore analysis as described by Davis and Smith (2003).
TABLE 3.
Meiotic recombination in the absence of both Rad2 and Rec12 has a high frequency of crossovers associated with gene conversion
| % among Ade+ sporesb
|
|||||||
|---|---|---|---|---|---|---|---|
| Parental genotypea
|
COc
|
NCOc
|
|||||
| Cross | rad2 | rec12 | R1 | R2 | P1 | P2 | Total Ade+ tested |
| A. Meiotic association of crossovers and conversion | |||||||
| 17 | + | + | 60 | 2 | 29 | 8 | 1140 |
| 18 | Δ | Δ152 | 39 | 6 | 29 | 27 | 374 |
| 19 | Δ | + | 42 | 6 | 22 | 30 | 346 |
| % among Ade+ recombinantsb
| |||||||
|---|---|---|---|---|---|---|---|
| Parental genotypea
|
COc
|
NCOc
|
|||||
| Diploid series | rad2 | rec12 | R1 | R2 | P1 | P2 | Total Ade+ tested |
| B. Mitotic association of crossovers and conversion | |||||||
| 20 | + | + | 2 | 2 | 56 | 40 | 60 |
| 21 | Δ | Δ152 | 11 | 7 | 58 | 22 | 46 |
Crosses (A) or diploids (B) were homozygous for the indicated rad2 and rec12 alleles and heteroallelic for ade6-3034 and ade6-52. Complete genotypes are in Table 1S(see supplemental data at http://www.genetics.org/supplemental/).
Selected Ade+ spore colonies (convertants) were classified according to configuration of the flanking markers ura4+-aim and tps16 (Figure 1C). The parental (NCO) configurations were P1 (ura4-(0) tps16+) and P2 (ura4+-aim tps16-23). P1 results from conversion of ade6-3034 to ade6+ and P2 from conversion of ade6-52. The recombinant (CO) configurations were R1 (ura4+-aim tps16+) and R2 (ura4-(0) tps16-23). R1 results from a single exchange and R2 from a triple exchange.
CO, crossover; NCO, noncrossover.
Meiotic inductions and DNA break analysis:
Meiotic inductions, flow cytometry, and analysis of DNA were performed as described by Young et al. (2002); the radioactive probe for Figure 2 spanned nucleotides 524–1354 on cosmid c1921 (GenBank accession no. AL122033). Blots were quantitated using a Typhoon 8600 phosphorimager (Amersham Biosciences, Piscataway, NJ).
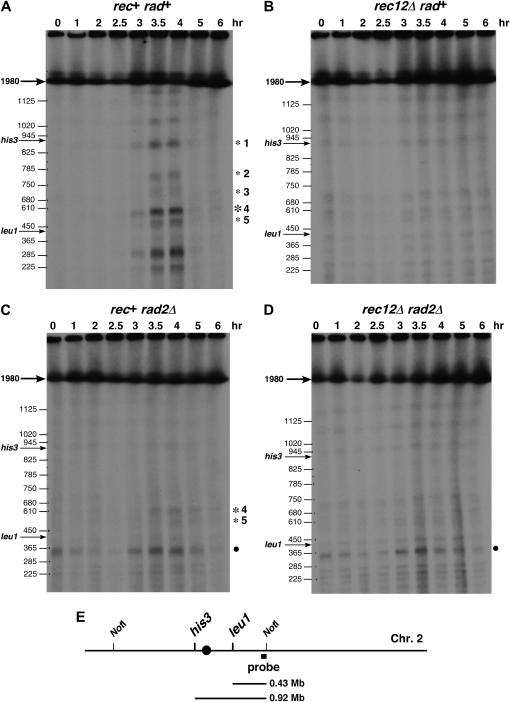
Figure 2.
Meiosis-specific DSBs are undetectable in the absence of both Rad2 and Rec12. DNA was prepared at the indicated times after thermal induction of pat1-114 meiosis, digested with NotI, separated by pulsed-field gel electrophoresis, blotted, and probed as indicated in E. The locations of meiosis-specific DSBs in the his3–leu1 interval are indicated by asterisks. A solid dot indicates a nonmeiosis-specific break that appeared only in rad2Δ strains. (A) Strain GP4207 (rad+ rec+). (B) Strain GP4899 (rec12Δ). (C) Strain GP4901 (rad2Δ). (D) Strain GP4903 (rec12Δ rad2Δ). (E) Diagram of chromosome 2 with NotI fragment B and the probe used.
RESULTS
Meiotic recombination at ade6 in the absence of both Rad2 and Rec12:
Although meiotic recombination is normally dependent on DSBs made by Rec12 (Cervantes et al. 2000; Steiner et al. 2002), we recently found a special situation involving a 160-bp DNA palindrome in which there is substantial recombination in the absence of Rec12 (Farah et al. 2005). DNA elements that can adopt secondary structures, such as palindromes, are destabilized in the absence of S. cerevisiae Rad27 (Freudenreich et al. 1998; Kokoska et al. 1998). We therefore determined the effect of a rad2Δ mutation on S. pombe meiotic recombination involving the palindrome (our unpublished observations). Surprisingly, we found that recombination of the control allele, containing an insertion of one arm of the palindrome, was dramatically affected by the rad2Δ mutation. Recombination between this allele, ade6-3034, and a missense mutation, ade6-52, was strongly reduced in the absence of Rec12 (Figure 1A and Table 1, series 1 and 2 crosses). In the absence of both Rad2 and Rec12, the Ade+ recombinant frequency was increased nearly 100-fold relative to that in the absence of Rec12 alone (series 2 and 3 crosses), to a level approximately double that in wild type (rad2+ rec12+). This result indicates that a rad2Δ mutation fully suppressed the recombination-deficient phenotype associated with a rec12Δ mutation.
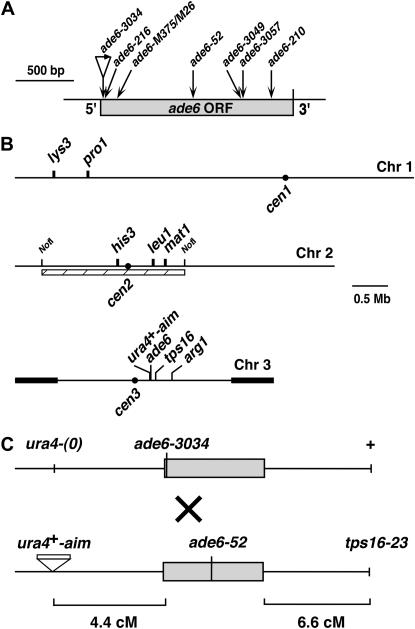
Figure 1.
Genetic markers used in this study. (A) ade6 alleles. ade6-3034 is an 80-bp insertion, not drawn to scale (Farah et al. 2002). ade6-52 (G1670A) is a missense mutation (M. E. Fox, unpublished observations). ade6-3049 (C2088A) creates an M26 heptamer with ∼10 times more hotspot activity than the original M26 hotspot allele (G1010T; nonsense; Steiner and Smith 2005). ade6-3057 (T2097G) is a nonhotspot control for ade6-3049. ade6-M375 (G1007T) is a control for M26 and creates a nonsense codon (Steiner and Smith 2005). (B) Diagram of genetic markers used. Thick bars at both ends of chromosome 3 represent rDNA; dots, centromeres; and hatched bar, the NotI fragment analyzed in Figure 2. (C) Markers flanking ade6 for crosses in Table 3. ura4+-aim is an insertion of a 1.8-kb ura4+ cassette ∼15 kb from ade6 (Grimm et al. 1994). tps16-23 (Ts) is in ags1, 56.1 kb centromere-distal to ade6 (Hochstenbach et al. 1998). The (0) indicates the absence of an insertion. The diagram is not drawn to scale.
TABLE 1.
rad2Δ restores meiosis-specific ade6 gene conversion in the absence of Rec12
| Ade+ recombinants per 106 viable sporesb
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Parental genotypea
|
Insertion alleles
|
Point mutation alleles
|
Viable spore yield (% of wild type)c
|
|||||
| Cross series | rad2d | rec12d | dmc1d | 3034 × 52 | M375 × 3057 | M375 × 3049 | M375 × 3057 | M375 × 3049 |
| 1 | + | + | + | 150 ± 20 | 510 ± 170 | 14,000 ± 3,400 | 100 | 100 |
| 2 | + | Δ152 | + | 4 ± 1 | 14 ± 1 | 34 ± 4 | 18 ± 3 | 13 ± 1.5 |
| 3 | Δ | Δ152 | + | 350 ± 40 | 720 ± 95 | 1,060 ± 100 | 37 ± 9 | 28 ± 5 |
| 4 | Δ | + | + | 360 ± 63 | 660 ± 100 | 4,300 ± 620 | 49 ± 11 | 33 ± 1.2 |
| 5 | + | + | Δ | NDe | 165 ± 25 | ND | ND | ND |
| 6 | + | Δ152 | Δ | ND | 24 ± 2 | ND | ND | ND |
| 7 | Δ | Δ152 | Δ | ND | 880 ± 130 | ND | ND | ND |
| 8 | Δ | + | Δ | ND | 735 ± 190 | ND | ND | ND |
Strains with the ade6-3034 (80-bp insertion) allele were crossed with an ade6-52 rad2Δ rec12Δ strain. Strains with either the ade6-3057 (control) allele or the ade6-3049 (hotspot) allele were crossed with an ade6-M375 rad2Δrec12Δ dmc1Δ strain. Crosses were homozygous for the indicated mutations or heterozygous for the indicated wild-type (+) alleles.
Values are the means of three independent experiments ± SEM.
The number of viable spores from each cross was divided by the number of the lesser viable parent cells in the cross. Values are the mean ± SEM from three experiments and are expressed relative to the value of the wild type (0.81 ± 0.03 with the M375 × 3049 crosses and 0.78 ± 0.21 with the M375 × 3057 crosses).
The rad2∷ura4+ (Δ) mutation deletes the entire rad2 coding region (Murray et al. 1994). The rec12-152∷LEU2 allele (Δ152) deletes 144 codons (of 346) from the rec12 ORF (Lin and Smith 1994). The dmc1∷ura4+ (Δ) mutation is a ura4+ insertion into the BamHI site of the dmc1 ORF (Fukushima et al. 2000).
Not determined.
We next tested whether suppression of rec12Δ by rad2Δ was also true with single-base-pair (point) mutations in ade6 (Figure 1A). One mutation, ade6-3049, creates an intense M26-like recombination hotspot, whereas its nearby control, ade6-3057, does not (Steiner et al. 2002; Steiner and Smith 2005); each was crossed with the nonhotspot mutation ade6-M375. In the absence of Rec12, recombination was strongly reduced with both ade6 alleles (Table 1, series 2 crosses). Recombination was partially restored in the absence of both Rad2 and Rec12 with the ade6-3049 hotspot but restored to a level slightly above that of the wild type with the ade6-3057 control allele (series 3 crosses). In all three cases the Ade+ recombinant frequency in rec12Δ mutants was increased by a factor of 30–100 by the rad2Δ mutation. Hence, with both insertion and point mutations in ade6, the absence of Rad2 suppressed the Ade+ recombination deficiency observed in the absence of Rec12. This suppression was complete except in the presence of the hotspot ade6-3049, which is almost completely dependent on Rec12 (Steiner and Smith 2005; see Table 1 and discussion).
Meiotic crossovers occur at high level in the absence of both Rad2 and Rec12:
Intragenic recombination at ade6, as studied above, is mostly gene conversion (>95% nonreciprocal recombination; Gutz 1971). We tested whether crossing over (reciprocal recombination) was also restored in the absence of both Rad2 and Rec12. Because gene conversion is infrequent for nonhotspot alleles in S. pombe (mean <0.1%/spore; P. Munz, cited in Young et al. 2002), intergenic recombination occurs almost exclusively by crossing over. Expressed as genetic distances, the recombinant frequencies in six intergenic intervals on the three chromosomes of S. pombe were reduced by factors of 40–300 in the absence of Rec12 (Table 2A, series 9 and 10 crosses; Figure 1B). In the absence of both Rad2 and Rec12, these genetic distances were increased, relative to those in rec12Δ strains, by factors ranging from ∼3.5 in the lys3–pro1 interval on chromosome 1 to 70 in the ade6–arg1 interval on chromosome 3 (series 10 and 11 crosses). These genetic distances were restored nearly to the levels observed in the absence of only Rad2. Since crossing over was reduced by a factor of ∼3 by rad2Δ (series 9 and 12 crosses), the suppression of rec12Δ by rad2Δ was complete, as we do not expect deletion of rec12 to increase recombination. Hence, as for nonhotspot intragenic recombination at ade6, the absence of Rad2 fully suppressed the intergenic recombination-deficiency of rec12Δ mutants (see discussion for a more complete explanation).
TABLE 2.
rad2Δ restores crossing-over in the absence of Rec12
| Centimorgans in genetic intervala
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Parental genotype
|
Chr. 1 | Chr. 2
|
Chr. 3
|
|||||
| Cross | rad2 | rec12 | lys3–pro1 | mat1–his3 | his3–leu1 | ade6–arg1 | ura4-aim–tps16 | tps16–arg1 |
| A. Random spore analysis | ||||||||
| 9 | + | + | 42 | 110b | 78b | 48b | 16 | 61 |
| 10 | + | Δ152 | <1 | 0.6 | 1 | 0.2 | NDc | ND |
| 11 | Δ | Δ152 | 3.4 | 15 | 16 | 14 | 6 | 13 |
| 12 | Δ | + | 28 | 18 | 31 | 19 | 4 | 19 |
| nd | 349–360 | 349–356 | 95–202 | 398–473 | 168–179 | 176–180 | ||
| Parental genotype
|
No. of tetradsf
|
||||||
|---|---|---|---|---|---|---|---|
| Cross | rad2 | rec12c | PD | NPD | TT | cMa | Spore viabilityg (%) |
| B. Tetrad analysis | |||||||
| 13 | + | + | 5 | 3 | 18 | 78b | 92 (34) |
| 14 | + | Δ169 | ND | ND | ND | ND | ND |
| 15 | Δ | Δ169 | 7 | 1 | 1 | 20 | 52 (50) |
| 16 | Δ | + | 2 | 0 | 2 | 35 | 36 (43) |
Genetic distances were calculated using Haldane's formula, cM = −50 ln(1 − 2R), where R is the recombinant frequency among spores.
Nominal genetic distance, calculated from the genome average of 0.16 cM/kb (Young et al. 2002).
ND, not determined.
Range of the number of colonies tested for each genetic interval in each series.
The rec12-169∷3HA6His-kanMX6 (Δ169) mutation deletes the entire coding region of the rec12 gene (Davis and Smith 2003).
Types of tetrads with respect to the his3 and leu1 alleles. PD, parental ditype; NPD, nonparental ditype; TT, tetratype. No aberrant segregants (gene convertants) were observed.
Spore viability is the fraction of viable spores among the number of dissected tetrads in parentheses.
To confirm the reciprocality of the intergenic recombinants, we dissected tetrads from crosses involving the his3 and leu1 markers (Table 2B). Because spore viability was not fully restored, we obtained only a few four-spore-viable asci in the absence of both Rad2 and Rec12. Nevertheless, all recombinants detected were reciprocal (i.e., there were no gene convertants among them; cross 15), and the genetic distances determined from the tetratype and nonparental ditype asci were close to the genetic distances determined from random spore analysis (Table 2A). Furthermore, among nine three-spore-viable asci in the rad2Δ rec12Δ crosses, there were no 3:0 or 0:3 segregation patterns of either the his3 or the leu1 markers, indicative of gene conversion. Hence, in the absence of both Rad2 and Rec12, both gene conversion and crossing over were restored at multiple loci and perhaps genome-wide.
High frequency of crossing over associated with gene conversion in the absence of both Rad2 and Rec12:
One hallmark of meiotic recombination, as opposed to mitotic recombination, is the high frequency of crossing over of flanking markers among gene convertants at a given locus. In the absence of both Rad2 and Rec12, we observed among gene convertants at ade6 a high association of crossing over of the flanking markers ura4+-aim and tps16 (45%, Table 3A, cross 18; Figure 1C), although not quite as high as that observed in the wild-type cross (62%, cross 17). The association of crossing over with gene conversion in the absence of both Rad2 and Rec12 was not significantly different from that observed in the absence of only Rad2 (48%, cross 19; contingency χ2 = 0.56, 1 d.f., P > 0.3). As with crossing over discussed above, the suppression was complete, as we do not expect deletion of rec12 to increase the association of crossing over with conversion. Hence, in the absence of Rec12, meiotic recombination restored by the absence of Rad2 displayed a high association of crossing over of the flanking markers among ade6+ gene convertants.
Is the association of crossing over among gene convertants during meiosis higher than that during mitosis in the absence of both Rad2 and Rec12, as is the case in wild type (Virgin et al. 2001)? Using diploid strains, we determined the association of mitotic gene conversion at ade6 with crossovers between the same flanking markers, ura4+-aim and tps16. Approximately 18% of the mitotic Ade+ convertants had an associated crossover of the flanking markers in the rad2Δ rec12Δ strain (Table 3B, cross 21). Although this association was higher than that observed in wild-type cells (4%, cross 20), it was much lower than that observed during meiosis in the absence of both Rad2 and Rec12 (45%, Table 3A, cross 18; contingency χ2 = 11.7, 1 d.f., P < 0.001). These results indicate that the high association of crossing over with gene conversion during meiotic recombination in the absence of both Rad2 and Rec12 was meiosis specific and not just a recapitulation of the mitotic recombination characteristic of a rad2Δ rec12Δ strain.
Proper segregation of homologs is restored in the absence of both Rad2 and Rec12:
In the absence of Rec12, the reductional division of meiosis is defective because the homologs are not connected to each other by chiasmata, the sites of crossover, and hence homologs do not segregate properly (Molnar et al. 1995; Sharif et al. 2002; Davis and Smith 2003). Using two different assays, we tested whether the crossovers formed during meiosis in the absence of both Rad2 and Rec12 were functional in promoting chromosome segregation. In the first assay, we measured homolog missegregation by determining the frequency of disome spores, using the complementing ade6-216 and ade6-210 markers on chromosome 3 (Figure 1A; Niwa and Yanagida 1985). In the absence of Rec12, the frequency of meiotic disome spores was ∼21% (Table 4, series 23 crosses), not far from that expected if segregation of homologs were random (∼30%, Davis and Smith 2003). In the absence of both Rad2 and Rec12 the disome frequency was reduced by a factor of ∼100 (series 24 crosses), to a level similar to that in the absence of Rad2 alone (series 25 crosses) and not far from that of wild type (series 22 crosses). More than 99% of the missegregation events in the absence of Rec12 was suppressed by rad2Δ. We conclude that crossovers in the absence of both Rad2 and Rec12 were functional in properly segregating chromosome 3 and, as shown next, in the other two chromosomes.
TABLE 4.
rad2Δ restores faithful chromosome segregation in the absence of Rec12
| Parental genotypea
|
Chr. 3 disomes (% of viable spores)b
|
Heterozygous diploids (% of viable spores)
|
||
|---|---|---|---|---|
| Cross series | rad2 | rec12 | ||
| 22 | + | + | 0.030 ± 0.005 | <0.2c |
| 23 | + | Δ169 | 21 ± 4 | 1.3 ± 0.2b |
| 24 | Δ | Δ169 | 0.18 ± 0.04 | 0.2c |
| 25 | Δ | + | 0.21 ± 0.03 | 0.2c |
Strains with the complementing alleles ade6-216 and ade6-210 were crossed. Crosses were homozygous for the indicated rec12 and rad2 alleles. Complete genotypes are in Table 1S (see supplementary data at http://www.genetics.org/supplemental/).
Values are the means of five independent experiments ± SEM.
For series 24 and 25 crosses, the total number of heterozygous diploids was divided by the total viable spores from the five experiments. For series 22 crosses, no heterozygous diploids were observed; the upper 95% confidence limit was calculated on the basis of the Poisson distribution. For each cross in each experiment 147–442 colonies were counted.
In the second assay for meiotic homolog missegregation, we determined the frequency of diploid spores heterozygous for the h+ and h− alleles on chromosome 2. Such spores grow into colonies that can sporulate, unlike haploids or homozygous diploids; since chromosome 2 disomes are inviable, only complete diploids are assayed (Niwa and Yanagida 1985). In the absence of Rec12, the frequency of heterozygous h+/h− diploids was increased >6.5-fold relative to that of wild type (Table 4, series 22 and 23 crosses). This missegregation defect was reduced by a factor of ∼6–7 in the absence of both Rad2 and Rec12 (series 24 crosses), to the level observed in the absence of Rad2 alone (series 25 crosses) or in wild type (series 22 crosses). Hence, two separate assays show that the absence of Rad2 nearly completely suppressed the chromosome missegregation phenotype associated with the absence of recombination.
In accord with the restoration of chromosome segregation, the rad2Δ mutation also increased the viable spore yield in rec12Δ strains. Relative to wild type, the viable spore yield was ∼16% in rec12Δ strains but was increased in rad2Δ rec12Δ strains to ∼33% (P < 0.01, Student's paired t-test). The level in the rad2Δ rec12Δ strains was less than that in the rad2Δ strains (∼41%, P = 0.02, Student's paired t-test; Table 1).
Meiotic recombination defect, but not the segregation defect, associated with the absence of Rec8 cohesin is suppressed by the absence of Rad2:
The Rec8 protein is a meiosis-specific cohesin that maintains sister chromatid cohesion during both meiotic divisions and stabilizes chiasmata (Watanabe and Nurse 1999; Nasmyth 2001). In the absence of Rec8, meiotic recombination is reduced by factors of up to 800 in some genome regions such as ade6 (Table 5, series 27 crosses; DeVeaux and Smith 1994; Molnar et al. 1995). This is expected, since little if any meiosis-specific DNA breakage is observed in rec8Δ mutants (Ellermeier and Smith 2005). This result contrasts with that in S. cerevisiae, in which meiotic DSBs are formed in a rec8Δ mutant, albeit at a slightly reduced level (Klein et al. 1999). The Ade+ recombinant frequency was increased ∼70-fold in the absence of both Rad2 and Rec8 (series 27 and 28 crosses), to a level similar to that observed in the absence of Rad2 alone (series 29 crosses). Similarly, the ade6–arg1 intergenic recombination proficiency was partially restored in the absence of both Rad2 and Rec8, to ∼30% of that in the absence of Rad2 alone (series 27–29 crosses). Hence, the absence of Rad2 strongly suppressed the meiotic recombination defect observed in crosses deficient in meiosis-specific DSB formation, i.e., in the absence of either Rec12 or Rec8.
TABLE 5.
rad2Δ restores meiotic recombination, but not chromosome segregation, in the absence of Rec8
| Parental genotypea
|
Ade+ recombinants/ 106 viable sporesb
|
Centimorgans in genetic intervalc
|
Heterozygous diploids (% of viable spores)
|
||
|---|---|---|---|---|---|
| Cross series | rad2 | rec8d | M26 × 210 | ade6–arg1 | |
| 26 | + | + | 6500 ± 400 | 48e | 1.5 ± 0.6 |
| 27 | + | Δ176 | 8.0 ± 3.8 | 0.3 | 3.8 ± 0.3 |
| 28 | Δ | Δ176 | 560 ± 40 | 8 | 24 ± 1.4 |
| 29 | Δ | + | 1200 ± 100 | 36 | 0.9 ± 0.2 |
Strains with the ade6-M26 hotspot allele were crossed with strains with either ade6-210 (for intragenic recombination) or ade6-52 (for intergenic recombination). Crosses were homozygous for the indicated rad2 and rec8 alleles.
Values are the means of four independent experiments ± SEM.
Genetic distances were calculated using Haldane's formula, on the basis of data pooled from two experiments. For each cross in each experiment 114–290 colonies were tested.
The rec8-176∷kanMX6 allele deletes the entire rec8 ORF.
Nominal genetic distance (see Table 2, footnote b).
In the absence of Rec8, meiosis I division becomes equational because sister kinetochores are no longer oriented toward the same pole (Watanabe and Nurse 1999). We expected that the absence of Rad2 would not restore proper reductional division in the absence of Rec8, because Rad2 is not expected to affect kinetochore orientation. In the absence of both Rad2 and Rec8, the frequency of heterozygous h+/h− diploid spore formation was not suppressed but instead was drastically increased compared to that in the absence of either Rec8 or Rad2 alone (Table 5, series 28 crosses compared with series 27 and 29 crosses). The reason for this synergistic effect is unclear. Nevertheless, these results indicate that the segregation defect of rec8Δ was not suppressed by the restoration of meiotic recombination in the absence of Rad2.
Recombination in rad2Δ rec12Δ strains does not require the meiosis-specific Dmc1 protein:
Since Rec12-dependent meiotic recombination is dependent on Dmc1, a meiosis-specific RecA homolog (Fukushima et al. 2000), we determined whether recombination in the absence of Rad2 and Rec12 also depends on Dmc1. As expected, the absence of Dmc1 reduced by a factor of 3 the recombinant frequency between ade6-3057 and ade6-M375 in otherwise wild-type strains (Table 1, series 1 and 5 crosses). Surprisingly, however, recombination in the absence of Rad2, with or without Rec12, was not affected by the absence of Dmc1 (series 3, 4, 7, and 8 crosses). The Dmc1 independence of recombination in the absence of Rad2 may stem from initiating lesions different from those in wild-type cells (see discussion).
Meiotic DNA replication and nuclear division I proceed normally in the absence of Rad2 and Rec12:
To determine if the absence of Rad2 and Rec12 altered the timing of DNA replication or the timing and frequency of the first nuclear division, we monitored these parameters in synchronously induced cultures. DNA replication, assayed by flow cytometry, occurred between 2 and 3 hr after induction in all four strains analyzed (rec12+ rad2+, rec12Δ rad2+, rec12+ rad2Δ, and rec12Δ rad2Δ); at 2.5 hr after induction ∼50% of the cells had completed replication in each case (see supplemental Figure 1S at http://www.genetics.org/supplemental/). The first nuclear division, assayed by microscopy of Hoechst 33342-stained cells, occurred at ∼5 hr after induction in each case, although there was a slight delay of ∼20 min in the rad2Δ strains (see supplemental Figure 2S at http://www.genetics.org/supplemental/). Thus, these aspects of meiosis appeared to proceed nearly normally in all cases examined.
Meiosis-specific DSBs are undetectable in the absence of both Rad2 and Rec12:
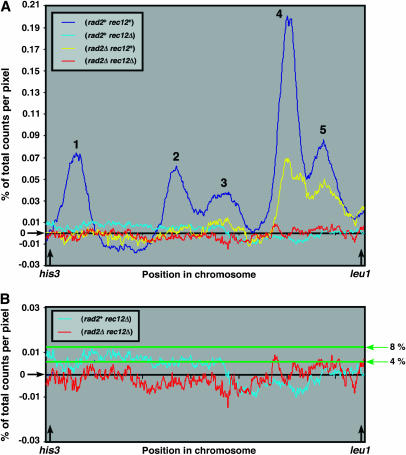
In the absence of Rec12 both meiotic recombination and meiosis-specific DSB formation are abolished (DeVeaux et al. 1992; Cervantes et al. 2000; Young et al. 2002; Davis and Smith 2003). Since recombination was restored to rec12 mutants by the absence of Rad2, we determined whether meiosis-specific DSBs were also restored. Using synchronously induced cultures, we analyzed the 1.98-Mb NotI fragment B on chromosome 2 by Southern blot hybridization (Figure 2E). This fragment encompasses the 0.49-Mb his3–leu1 interval, which contains one prominent and four weaker clusters of Rec12-dependent meiosis-specific DSBs (Figure 2A and B). In the absence of only Rad2, the prominent DSB cluster was visible, although reduced relative to that in wild type (Figure 2C). However, DSBs were undetectable in the absence of Rec12 or both Rad2 and Rec12 (Figure 2B and D). Similar results were obtained when the ade6 locus, harboring ade6-3049, as well as the intervals ade6–arg1 (chromosome 3) and lys3–pro1 (chromosome 1) were analyzed: no DSBs were visible in the absence of both Rad2 and Rec12, while they were readily visible in the wild type (our unpublished observations).
Quantitation by PhosphorImage analysis (Figure 3A) showed that 23% of the DNA was broken in the his3–leu1 interval in the wild type and 6.4% in the rad2Δ mutant. The values in the rec12Δ and rad2Δ rec12Δ strains were 1.3 and −1.3%, respectively, indicating that DSBs were near the background level in both strains. We calculated an expected value of DSBs in the rad2Δ rec12Δ strain in two ways: (1) since the genetic distance for the his3–leu1 interval in the rad2Δ rec12Δ strain was about one-fifth of that in the wild-type strain (Table 2), one might expect about 5% of the DNA to be broken in the his3 – leu1 interval in the rad2Δ rec12Δ strain, and (2) in most models of recombination (e.g., Szostak et al. 1983) one DSB produces one crossover chromosome (if the DSB always interacts with a homolog and resolution is half the time to the crossover configuration) or two crossover chromosomes (if resolution is always to the crossover configuration). Since we observed 16 cM in the his3–leu1 interval in the rad2Δ rec12Δ strain (Table 2), one might expect, respectively, ∼16 or 8% DSBs in this interval. In rad50S strains, DSBs accumulate to twice the level observed in rad50+ strains, as used here (Young et al. 2002, 2004; R. Hyppa and G. R. Smith, unpublished observations; the inviability of rad2Δ rad50S strains precluded their study here). Thus, 8 or 4% DSBs would be expected at the time of maximal DSBs. These levels would have been detectable (Figure 3B). As discussed below, non-DSB lesions may account for meiotic recombination detected in the absence of both Rad2 and Rec12.
Figure 3.
Quantitation of DSBs in the his3–leu1 interval. For each strain in Figure 2 the amount of radioactive hybridization in each lane was normalized to the same level; the normalized value in the his3–leu1 interval, between the arrows in Figure 2, at 1 hr was subtracted from that at 4 hr and expressed as the percentage of total hybridization in the lane at and below the intact NotI fragment. The amount of breakage in the interval was 23% (GP4207, rad2+ rec12+), 1.3% (GP4899, rad2+ rec12Δ), 6.4% (GP4901, rad2Δ rec12+), and −1.3% (GP4903, rad2Δ rec12Δ). B is an expanded version of a portion of A. The horizontal lines in B indicate the levels of uniformly distributed DSBs in the rad2Δ rec12Δ strain expected under different assumptions (see discussion).
DISCUSSION
In all eukaryotes tested to date Spo11 or its ortholog, Rec12 in S. pombe, is essential for generating meiotic recombinants in the absence of exogenous DNA damage (Keeney 2001). Restoration of recombination in the absence of Spo11 was previously observed in both S. cerevisiae and the worm Caenorhabditis elegans after ionizing irradiation of the corresponding mutants (Thorne and Byers 1993; Dernburg et al. 1998). We report here the first example, to our knowledge, of genetic suppression of the absence of a Spo11 homolog: deletion of S. pombe rad2, which encodes a DNA flap endonuclease, suppressed the recombination deficiency and chromosome missegregation phenotypes of rec12Δ mutants (Tables 1–4). The absence of checkpoint proteins increases the spore viability of a rec12Δ mutant from ∼25 to ∼50%, but recombination between homologs was not measured (Pankratz and Forsburg 2005). In the following discussion, meiotic recombination in the suppressed rec12Δ rad2Δ strains is referred to as the Rec* pathway. Unless specified, the term FEN-1 refers to flap endonucleases in general.
An alternative pathway of meiotic recombination in the absence of Rec12:
The FEN-1 protein processes Okazaki fragments to complete DNA replication on the lagging strand (Kao et al. 2004). In organisms in which mutations eliminating the FEN-1 homolog are not lethal, such as S. cerevisiae and S. pombe, the corresponding deletion mutants are envisioned to accumulate single-strand (ss) nicks, gaps, and flaps in the newly synthesized DNA (Henneke et al. 2003). We propose that in the absence of Rad2 in S. pombe such lesions initiate recombination by the Rec* pathway. The Rec* pathway has many of the characteristics of meiotic recombination by the wild-type pathway: (1) high levels of both gene conversion and crossing over were observed (Tables 1 and 2), (2) in contrast to mitotic recombination, crossing over was highly associated with selected gene convertants (Table 3), and (3) homolog segregation was faithful (Table 4). Hence, the Rec* pathway is not just a recapitulation of mitotic-type recombination occurring during meiosis but is bona fide meiotic recombination initiated by Rec12-independent lesions. The Rec* pathway does differ, however, from the wild-type pathway: (1) the Rec* pathway was not dependent on the meiosis-specific Dmc1 protein (Table 1), and (2) no DSBs that could account for recombination by the Rec* pathway were detectable (Figures 2 and 3). Hence, the Rec* pathway also has novel properties. Since rad2Δ suppressed the recombination deficiency of both rec12Δ and rec8Δ (Tables 1–2 and 5), the Rec* pathway bypasses at least two steps of the wild-type pathway.
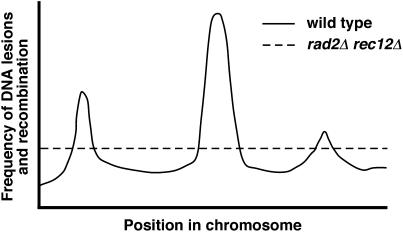
The extent of suppression by rad2Δ of the recombination deficiency of rec12Δ depended on the interval examined. We used a variety of alleles in ade6 and other genes to test the generality of the suppression. In some cases, such as intragenic recombination not involving an ade6 hotspot marker (e.g., ade6-3034 × ade6-52; Table 1), recombinant frequencies were greater than those in wild type. In other cases, such as intergenic recombination (Table 2) and intragenic recombination involving the hotspot marker ade6-3049 (Table 1), the suppression was substantial but not up to the wild-type level. ade6 hotspot recombination and intergenic recombination are associated with strong DSB formation that is entirely Rec12 dependent (Steiner et al. 2002; Young et al. 2002; Cromie et al. 2005). We suppose that the lesions promoting recombination in rad2Δ rec12Δ strains are more uniformly distributed than the Rec12-dependent DSBs (Figure 4). If this is the case, then recombination by the Rec* pathway, compared to that by the wild-type pathway, would be more frequent in intervals far from Rec12-dependent DSBs but less frequent in intervals close to or containing Rec12-dependent hotspots, as observed. We have seen no evidence of hotspots of recombination by the Rec* pathway. We note, however, that robust suppression was observed in all intervals tested. In rec12+ strains the rad2Δ mutation reduced recombination in some intervals but not in others (Tables 1 and 2). We suppose that the rad2Δ mutation alters replication and, indirectly, chromatin structure and Rec12 action more in some intervals than in others (Figure 2C). For example, DNA lesions left in the absence of Rad2 may inhibit the action of Rec12, but incompletely at Rec12 hotspots.
Figure 4.
Proposed distribution of recombination-initiating lesions. In wild-type (rec12+ rad2+) strains Rec12-dependent DSBs (continuous line) occur at hotspots and produce recombination intensities greater than that of the genome average. In suppressed (rad2Δ rec12Δ) strains with the Rec* pathway the initiating lesions may be more uniformly distributed (dashed line). Recombination far from Rec12-dependent DSBs would be more intense in Rec* strains, whereas recombination close to Rec12-dependent DSBs (hotspots) would be more intense in wild-type strains.
We expect that mutations other than rad2 that interfere with Okazaki fragment processing might also suppress a rec12 mutation. Processing of Okazaki fragments is a complex process involving several proteins including FEN-1, Dna2, and perhaps ExoI. Dna2 is a DNA helicase and endonuclease that cooperates with FEN-1 during Okazaki fragment processing, and ExoI is a 5′→ 3′ exonuclease involved in DNA mismatch repair (Szankasi and Smith 1995; Tishkoff et al. 1997a; Kao et al. 2004). In S. cerevisiae, Rad27 is a FEN-1 homolog (Lieber 1997); overexpression of ExoI suppresses some of the rad27Δ phenotypes, suggesting that the two enzymes are partially redundant (Tishkoff et al. 1997a). Likewise, in S. pombe, overexpression of Rad2 partially suppresses the inviability of a dna2 mutant (Kang et al. 2000). However, the absence of ExoI in S. pombe did not activate the Rec* pathway and partial inactivation of a thermosensitive Dna2 protein displayed only a slight activation of the Rec* pathway (J. Farah, unpublished observations). Perhaps ExoI is not involved in Okazaki fragment processing when Rad2 is present, or the Dna2 ts mutant protein processes Okazaki fragments at the semirestrictive temperature but is deficient for other functions such as DNA repair (Kang et al. 2000).
Comparison of the S. pombe Rec* pathway and the E. coli RecF suppressor pathway:
There is a similarity between the Rec* pathway of S. pombe and the RecF suppressor pathway of E. coli (Smith 1989). In both cases, elimination of nucleases activates a potent alternative recombination pathway that appears to be minor in wild-type cells for some types of recombination. In S. pombe, the major meiotic recombination pathway requires Rec12, the active-site protein that generates DSBs (Cervantes et al. 2000). In its absence recombination can be restored by inactivation of Rad2, an endo- and exonuclease (Tables 1–3). Likewise, in E. coli the major DSB-dependent recombination pathway, the RecBCD pathway, requires RecBCD enzyme (a helicase and nuclease; Smith 2001). In the absence of RecBCD enzyme, recombination can be restored by the RecF pathway when SbcCD (an endo- and exonuclease) and ExoI (a 3′ → 5′ ss exonuclease) are inactivated (Amundsen and Smith 2003). In the Rec* pathway the absence of Rad2 presumably allows the accumulation of recombinogenic lesions that substitute for the Rec12-dependent DSBs. In the RecF pathway, the absence of RecBCD enzyme, ExoI, and SbcCD presumably allows the persistence of DNA substrates for recombination by the RecF, RecO, RecR, and other proteins. One indication that the RecF pathway can use non-DSB lesions such as nicks is that, in wild-type cells, spontaneous plasmid recombination is almost exclusively dependent on the RecF pathway (Smith 1989). Hence, the analogy between the RecF and the Rec* pathways may include both the nature of the lesions that initiate recombination, as discussed below, and the enzymes that affect that recombination.
The sbcBCD suppressor mutations that uncovered the RecF pathway revealed a pathway that is minor for conjugational recombination but is major for plasmid recombination (Smith 1989). The Rec* pathway may similarly be a major pathway for some types of meiotic recombination not yet recognized.
Lack of visible meiotic DSBs in the absence of both Rad2 and Rec12:
We suppose that, in the absence of Rad2, nicks, gaps, and flaps accumulate in the genome. Evidence for accumulation of such lesions comes from the detection in S. cerevisiae of short ss DNA fragments (∼500 nucleotides) in the absence of Rad27, likely representing unligated Okazaki fragments (Merrill and Holm 1998). We observed no meiosis-specific DSBs, in the absence of both Rad2 and Rec12, in genomic intervals spanning >2.5 Mb that contain several meiosis-specific DSBs in wild-type meiosis (Figures 2 and 3; our unpublished observations). There are several possible explanations for this observation. First, DSBs might be formed by an unknown enzyme at low levels undetectable by Southern blot hybridization. Second, DNA nicks could be left from the previous (mitotic) replication and be converted into DSBs during meiotic replication. These DSBs might also be difficult to detect because of their likely random distribution in the genome, as speculated above, because of a half-life shorter than that of Rec12-dependent DSBs or because they occur on replication intermediates, such as branched DNA molecules, which do not enter the agarose gel during electrophoresis. Third, DNA nicks could be formed and be recombinogenic on their own without prior conversion to DSBs. At this point we cannot rigorously exclude any of these possibilities. However, we do not favor the first possibility because we do not expect activation of a DSB-generating enzyme in the absence of Rad2 nuclease, and our quantitative analysis of DSBs (Figure 3) argues against the second possibility.
A model for the alternative Rec* recombination pathway initiated by nicks in the absence of Rec12:
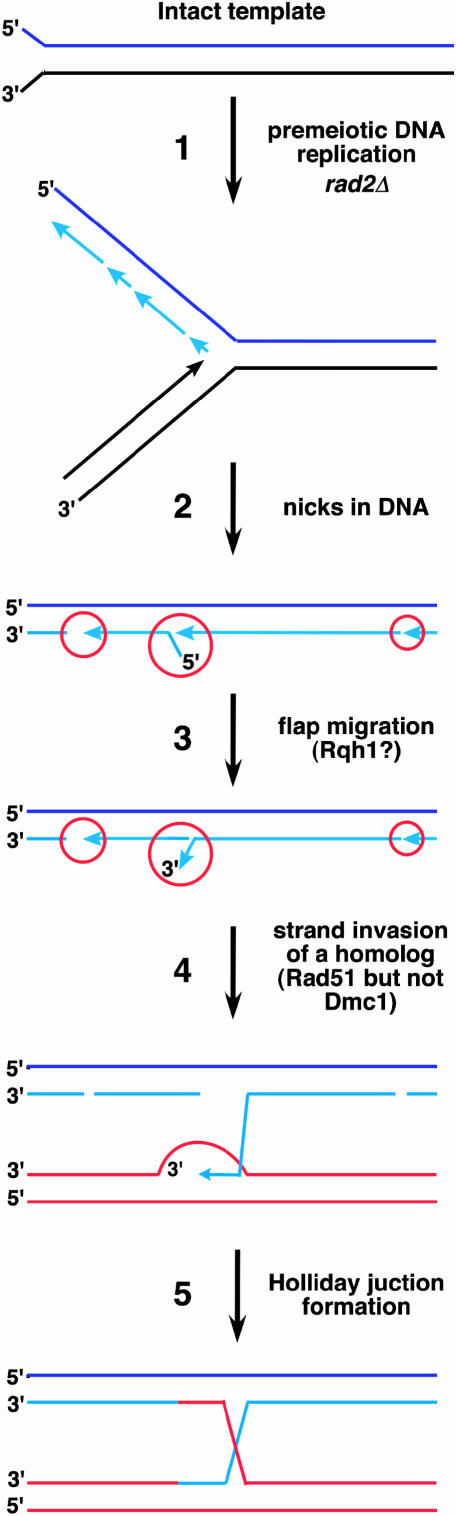
Although, as suggested above, ss discontinuities in the DNA could be converted to DSBs upon passage of a replication fork, we would like to entertain the idea that these discontinuities are recombinogenic on their own without being converted to DSBs. In the model illustrated in Figure 5, discontinuities that accumulate in the genome during vegetative lagging strand synthesis in the absence of FEN-1 are eventually repaired by alternative nucleases, such as ExoI or Dna2, or by recombination. Hence, cells enter meiosis with an intact genome and start premeiotic DNA replication during which discontinuities again accumulate on the lagging strand. Flaps with a 5′ end could be converted to 3′ flaps by a mechanism similar to branch migration, perhaps involving a helicase such as a RecQ homolog (Rqh1 in S. pombe). These 3′ flaps could then be used by the strand exchange protein Rad51, to form a joint molecule with a homolog. The Dmc1 protein was not required for this pathway (Table 1) and might be active only in the wild-type pathway. Alternatively, 5′ flaps could be used directly for strand exchange, as S. cerevisiae Rad51 has both 3′ → 5′ and 5′ → 3′ strand exchange activities (Namsaraev and Berg 1997). This model is similar to that proposed by Meselson and Radding (1975).
Figure 5.
Model for meiotic recombination initiated by non-DSB lesions. During premeiotic DNA replication (step 1), the lagging strand accumulates discontinuities such as nicks, gaps, and flaps (red circles; step 2). In this example, a DNA helicase such as Rqh1 converts a 5′ flap into a 3′ flap (step 3) that serves as an invading strand to initiate strand exchange (step 4) without formation of DSBs and to generate a Holliday junction (step 5). Alternatively, a 5′ flap can be directly used for strand exchange by Rad51 (not shown).
Extensive studies in S. cerevisiae support the notion that most of its meiotic recombination can be accounted for by the detected meiosis-specific Spo11-dependent DSBs (Baudat and Nicolas 1997). This notion was challenged by the finding in S. pombe of a discrepancy between crossover distribution and DSB distribution: in some intervals too few DSBs are observed to account for the observed crossovers, suggesting that lesions other than DSBs can initiate recombination (Young et al. 2002; Figures 2 and 3, Table 2; R. Hyppa and G. R. Smith, unpublished observations). Prior to the DSB-repair model of Resnick (1976), all models to our knowledge proposed that DNA nicks or gaps initiate recombination (e.g., Holliday 1964; Meselson and Radding 1975; see Smith 2004 for a recent review). These models lost favor after the publication of another DSB repair model by Szostak et al. (1983) and the observation of meiosis-specific DSBs at a recombination hotspot in S. cerevisiae (Sun et al. 1989).
Several studies have suggested that nicks are recombinogenic in mitotic cells and could initiate a subset of meiotic recombination events as well. In S. cerevisiae, rad52 mutations confer high sensitivity to ionizing radiation, which is thought to make primarily DSBs, and are lethal in combination with HO-induced DSBs at the mat locus (Malone and Esposito 1980), indicating that Rad52 is required for DSB repair. However, some separation-of-function rad52 mutant alleles are only marginally sensitive to ionizing radiation, yet these rad52 mutants are highly deficient in mitotic recombination (Mortensen et al. 2002). This phenotype suggests that Rad52 has two separable activities, one involved in the repair of DSBs (generated by ionizing irradiation) and one involved in spontaneous mitotic recombination initiated by non-DSB lesions such as nicks or gaps.
Three previous studies more directly implicate ss lesions as initiators of recombination. First, high-frequency meiotic gene conversion at the mat1 locus of S. pombe depends on the presence of a DNA lesion at mat1, first thought to be a DSB but later found to be a ss nick (Klar and Miglio 1986; Arcangioli 1998). Second, a DNA-nicking enzyme, the product of gene II from bacteriophage f1, expressed in S. cerevisiae, strongly stimulates mitotic recombination (Strathern et al. 1991). In each of these two cases the ss lesions might be converted into DSBs that lead to recombination. In a third case, however, this seems less likely: the RAG-1 protein is normally involved in generating DSBs at a specific signal sequence during V(D)J recombination in lymphocytes. But Lee et al. (2004) showed that certain nonnull mutant RAG-1 proteins that form nicks but no detectable DSBs in vitro strongly stimulate homologous recombination in cultured mammalian cells without forming detectable DSBs at the signal sequence. The authors proposed that nicks are the recombinogenic lesions. These precedents are concordant with the possibility that ss lesions initiate recombination by the Rec* pathway during meiosis in S. pombe.
Conclusion:
We have found evidence for two meiotic recombination pathways in S. pombe. The major pathway active in wild-type cells is initiated by Rec12-dependent DSBs at discrete locations (hotspots) in the genome and seems to be conserved in all organisms displaying meiotic recombination. A second pathway is activated in the absence of Rad2, a flap endonuclease homolog. This pathway, perhaps minor in wild-type cells, may be initiated by ss nicks randomly distributed in the genome. By repressing this pathway, Rad2 may safeguard the genome by restricting non-DSB lesions from promoting untimely recombination during meiosis.
Acknowledgments
We thank Eric Foss, Andrew Taylor, and Meng-Chao Yao for critical reading of the manuscript and Hideo Shinagawa for the rad2Δ strain. G.C. was supported by a long-term European Molecular Biology Organization fellowship (ALTF 123-2001), and W.W.S. by a Leukemia and Lymphoma Society special fellowship (no. 3230-05). This work was supported by research grant GM32194 from the National Institutes of Health to G.R.S.
References
- Amundsen, S. K., and G. R. Smith, 2003. Interchangeable parts of the Escherichia coli recombination machinery. Cell 112: 741–744. [DOI] [PubMed] [Google Scholar]
- Arcangioli, B., 1998. A site- and strand-specific DNA break confers asymmetric switching potential in fission yeast. EMBO J. 17: 4503–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie, III et al., 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951. [DOI] [PubMed] [Google Scholar]
- Baudat, F., and A. Nicolas, 1997. Clustering of meiotic double-strand breaks on yeast chromosome III. Proc. Natl. Acad. Sci. USA 94: 5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerat, A., B. de Massy, D. Gadelle, P.-C. Varoutas, A. Nicolas et al., 1997. An atypical topoisomerase II from archaea with implications for meiotic recombination. Nature 386: 414–417. [DOI] [PubMed] [Google Scholar]
- Borde, V., A. S. H. Goldman and M. Lichten, 2000. Direct coupling between meiotic DNA replication and recombination initiation. Science 290: 806–809. [DOI] [PubMed] [Google Scholar]
- Cervantes, M. D., J. A. Farah and G. R. Smith, 2000. Meiotic DNA breaks associated with recombination in S. pombe. Mol. Cell 5: 883–888. [DOI] [PubMed] [Google Scholar]
- Cromie, G. A., C. A. Rubio, R. W. Hyppa and G. R. Smith, 2005. A natural meiotic DNA break site in Schizosaccharomyces pombe is a hotspot of gene conversion, highly associated with crossing over. Genetics 169: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, L., and G. R. Smith, 2003. Nonrandom homolog segregation at meiosis I in Schizosaccharomyces pombe mutants lacking recombination. Genetics 163: 857–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg, A. F., K. McDonald, G. Moulder, R. Barstead, M. Dresser et al., 1998. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94: 387–398. [DOI] [PubMed] [Google Scholar]
- DeVeaux, L. C., and G. R. Smith, 1994. Region-specific activators of meiotic recombination in Schizosaccharomyces pombe. Genes Dev. 8: 203–210. [DOI] [PubMed] [Google Scholar]
- DeVeaux, L. C., N. A. Hoagland and G. R. Smith, 1992. Seventeen complementation groups of mutations decreasing meiotic recombination in Schizosaccharomyces pombe. Genetics 130: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier, C., and G. R. Smith, 2005. Cohesins are required for meiotic DNA breakage and recombination in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 102: 10952–10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah, J. A., E. Hartsuiker, K. Mizuno, K. Ohta and G. R. Smith, 2002. A 160-bp palindrome is a Rad50·Rad32-dependent mitotic recombination hotspot in Schizosaccharomyces pombe. Genetics 161: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah, J. A., G. Cromie, W. W. Steiner and G. R. Smith, 2005. A novel recombination pathway initiated by the Mre11/Rad50/Nbs1 complex eliminates palindromes during meiosis in Schizosaccharomyces pombe. Genetics 169: 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenreich, C. H., S. M. Kantrow and V. A. Zakian, 1998. Expansion and length-dependent fragility of CTG repeats in yeast. Science 279: 853–856. [DOI] [PubMed] [Google Scholar]
- Fukushima, K., T. Yoshimi, K. Nabeshima, T. Yoneki, T. Tougan et al., 2000. Dmc1 of Schizosaccharomyces pombe plays a role in meiotic recombination. Nucleic Acids Res. 28: 2709–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, C., J. Bähler and J. Kohli, 1994. M26 recombinational hotspot and physical conversion tract analysis in the ade6 gene of Schizosaccharomyces pombe. Genetics 135: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossenbacher-Grunder, A.-M., 1985. Spontaneous mitotic recombination in Schizosaccharomyces pombe. Curr. Genet. 10: 95–101. [DOI] [PubMed] [Google Scholar]
- Gutz, H., 1971. Site-specific induction of gene conversion in Schizosaccharomyces pombe. Genetics 69: 317–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneke, G., E. Friedrich-Heineken and U. Hubscher, 2003. Flap endonuclease 1: a novel tumour suppresser protein. Trends Biochem. Sci. 28: 384–390. [DOI] [PubMed] [Google Scholar]
- Hochstenbach, F., F. M. Klis, H. van den Ende, E. van Donselaar, P. J. Peters et al., 1998. Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc. Natl. Acad. Sci. USA 95: 9161–9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday, R., 1964. A mechanism for gene conversion in fungi. Genet. Res. 5: 282–304. [DOI] [PubMed] [Google Scholar]
- Jin, Y. H., R. Obert, P. M. Burgers, T. A. Kunkel, M. A. Resnick et al., 2001. The 3′→5′ exonuclease of DNA polymerase delta can substitute for the 5′ flap endonuclease Rad27/Fen1 in processing Okazaki fragments and preventing genome instability. Proc. Natl. Acad. Sci. USA 98: 5122–5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, H. Y., E. Choi, S. H. Bae, K. H. Lee, B. S. Gim et al., 2000. Genetic analyses of Schizosaccharomyces pombe dna2+ reveal that Dna2 plays an essential role in Okazaki fragment metabolism. Genetics 155: 1055–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, H. I., J. Veeraraghavan, P. Polaczek, J. L. Campbell and R. A. Bambara, 2004. On the roles of Saccharomyces cerevisiae Dna2p and flap endonuclease 1 in Okazaki fragment processing. J. Biol. Chem. 279: 15014–15024. [DOI] [PubMed] [Google Scholar]
- Keeney, S., 2001. Mechanism and control of meiotic recombination initiation. Current Topic Dev. Biol. 52: 1–53. [DOI] [PubMed] [Google Scholar]
- Keeney, S., C. N. Giroux and N. Kleckner, 1997. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88: 375–384. [DOI] [PubMed] [Google Scholar]
- Klar, A. J. S., and L. M. Miglio, 1986. Initiation of meiotic recombination by double-strand DNA breaks in S. pombe. Cell 46: 725–731. [DOI] [PubMed] [Google Scholar]
- Klein, F., P. Mahr, M. Galova, S. B. C. Buonomo, C. Michaelis et al., 1999. A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 98: 91–103. [DOI] [PubMed] [Google Scholar]
- Kokoska, R. J., L. Stefanovic, H. T. Tran, M. A. Resnick, D. A. Gordenin et al., 1998. Destabilization of yeast micro- and minisatellite DNA sequences by mutations affecting a nuclease involved in Okazaki fragment processing (rad27) and DNA polymerase delta (pol3-t). Mol. Cell. Biol. 18: 2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucherlapati, M., K. Yang, M. Kuraguchi, J. Zhao, M. Lia et al., 2002. Haploinsufficiency of flap endonuclease (Fen1) leads to rapid tumor progression. Proc. Natl. Acad. Sci. USA 99: 9924–9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, G. S., M. B. Neiditch, S. S. Salus and D. B. Roth, 2004. RAG proteins shepherd double-strand breaks to a specific pathway, suppressing error-prone repair, but RAG nicking initiates homologous recombination. Cell 117: 171–184. [DOI] [PubMed] [Google Scholar]
- Lichten, M., 2001. Meiotic recombination: breaking the genome to save it. Curr. Biol. 11: R253–R256. [DOI] [PubMed] [Google Scholar]
- Lieber, M. R., 1997. The FEN-1 family of structure-specific nucleases in eukaryotic DNA replication, recombination and repair. BioEssays 19: 233–240. [DOI] [PubMed] [Google Scholar]
- Lin, Y., and G. R. Smith, 1994. Transient meiosis-induced expression of the rec6 and rec12 genes of Schizosaccharomyces pombe. Genetics 136: 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone, R. E., and R. E. Esposito, 1980. The RAD52 gene is required for homothallic interconversion of mating types and spontaneous mitotic recombination in yeast. Proc. Natl. Acad. Sci. USA 77: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, B. J., and C. Holm, 1998. The RAD52 recombinational repair pathway is essential in pol30 (PCNA) mutants that accumulate small single-stranded DNA fragments during DNA synthesis. Genetics 148: 611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson, M., and C. Radding, 1975. A general model for genetic recombination. Proc. Natl. Acad. Sci. USA 72: 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar, M., J. Bahler, M. Sipiczki and J. Kohli, 1995. The rec8 gene of Schizosaccaromyces pombe is involved in linear element formation, chromosome pairing and sister-chromatid cohesion during meiosis. Genetics 141: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., A. J. S. Klar and P. Nurse, 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 795–823. [DOI] [PubMed] [Google Scholar]
- Mortensen, U. H., N. Erdeniz, Q. Feng and R. Rothstein, 2002. A molecular genetic dissection of the evolutionarily conserved N terminus of yeast Rad52. Genetics 161: 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, J. M., M. Tavassoli, R. al-Harithy, K. S. Sheldrick, A. R. Lehmann et al., 1994. Structural and functional conservation of the human homolog of the Schizosaccharomyces pombe rad2 gene, which is required for chromosome segregation and recovery from DNA damage. Mol. Cell. Biol. 14: 4878–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namsaraev, E., and P. Berg, 1997. Characterization of strand exchange activity of yeast Rad51 protein. Mol. Cell. Biol. 17: 5359–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth, K., 2001. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 35: 673–745. [DOI] [PubMed] [Google Scholar]
- Negritto, M. C., J. Qiu, D. O. Ratay, B. Shen and A. M. Bailis, 2001. Novel function of Rad27 (FEN-1) in restricting short-sequence recombination. Mol. Cell. Biol. 21: 2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa, O., and M. Yanagida, 1985. Triploid meiosis and aneuploidy in Schizosaccharomyces pombe: an unstable aneuploid disomic for chromosome III. Curr. Genet. 9: 463–470. [Google Scholar]
- Pankratz, D. G., and S. L. Forsburg, 2005. Meiotic S-phase damage activates recombination without checkpoint arrest. Mol. Biol. Cell. 16: 1651–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenteau, J., and R. J. Wellinger, 1999. Accumulation of single-stranded DNA and destabilization of telomeric repeats in yeast mutant strains carrying a deletion of RAD27. Mol. Cell. Biol. 19: 4143–4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick, M. A., 1976. The repair of double-strand breaks in DNA: a model involving recombination. J. Theor. Biol. 59: 97–106. [DOI] [PubMed] [Google Scholar]
- Roeder, G. S., 1997. Meiotic chromosomes: it takes two to tango. Genes Dev. 11: 2600–2621. [DOI] [PubMed] [Google Scholar]
- Sharif, W. D., G. G. Glick, M. K. Davidson and W. P. Wahls, 2002. Distinct functions of S. pombe Rec12 (Spo11) protein and Rec12-dependent crossover recombination (chiasmata) in meiosis I; and a requirement for Rec12 in meiosis II. Cell Chromosome 1: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. R., 1989. Homologous recombination in E. coli: multiple pathways for multiple reasons. Cell 58: 807–809. [DOI] [PubMed] [Google Scholar]
- Smith, G. R., 2001. Homologous recombination near and far from DNA breaks: alternative roles and contrasting views. Ann. Rev. Genet. 35: 243–274. [DOI] [PubMed] [Google Scholar]
- Smith, G. R., 2004. How homologous recombination is initiated: unexpected evidence for single-strand nicks from V(D)J site-specific recombination. Cell 117: 146–148. [DOI] [PubMed] [Google Scholar]
- Steiner, W. W., and G. R. Smith, 2005. Optimizing the nucleotide sequence of a meiotic recombination hotspot in Schizosaccharomyces pombe. Genetics 169: 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, W. W., R. W. Schreckhise and G. R. Smith, 2002. Meiotic DNA breaks at the S. pombe recombination hotspot M26. Mol. Cell 9: 847–855. [DOI] [PubMed] [Google Scholar]
- Strathern, J. N., K. G. Weinstock, D. R. Higgins and C. B. McGill, 1991. A novel recombinator in yeast based on gene II protein from bacteriophage f1. Genetics 127: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H., D. Treco, N. P. Schultes and J. W. Szostak, 1989. Double-strand breaks at an initiation site for meiotic gene conversion. Nature 338: 87–90. [DOI] [PubMed] [Google Scholar]
- Sundararajan, A., B. S. Lee and D. J. Garfinkel, 2003. The Rad27 (Fen-1) nuclease inhibits Ty1 mobility in Saccharomyces cerevisiae. Genetics 163: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szankasi, P., and G. R. Smith, 1995. A role for exonuclease I from S. pombe in mutation avoidance and mismatch correction. Science 267: 1166–1169. [DOI] [PubMed] [Google Scholar]
- Szostak, J. W., T. L. Orr-Weaver, R. J. Rothstein and F. W. Stahl, 1983. The double-strand-break repair model for recombination. Cell 33: 25–35. [DOI] [PubMed] [Google Scholar]
- Thorne, L. W., and B. Byers, 1993. Stage-specific effects of X-irradiation on yeast meiosis. Genetics 134: 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff, D. X., A. L. Boerger, P. Bertrand, N. Filosi, G. M. Gaida et al., 1997. a Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc. Natl. Acad. Sci. USA 94: 7487–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff, D. X., N. Filosi, G. M. Gaida and R. D. Kolodner, 1997. b A novel mutation avoidance mechanism dependent on S. cerevisiae RAD27 is distinct from DNA mismatch repair. Cell 88: 253–263. [DOI] [PubMed] [Google Scholar]
- Virgin, J. B., J. P. Bailey, F. Hasteh, J. Neville, A. Cole et al., 2001. Crossing over is rarely associated with mitotic intragenic recombination in Schizosaccharomyces pombe. Genetics 157: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, Y., and P. Nurse, 1999. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 400: 461–464. [DOI] [PubMed] [Google Scholar]
- Young, J. A., R. W. Schreckhise, W. W. Steiner and G. R. Smith, 2002. Meiotic recombination remote from prominent DNA break sites in S. pombe. Mol. Cell 9: 253–263. [DOI] [PubMed] [Google Scholar]
- Young, J. A., R. W. Hyppa and G. R. Smith, 2004. Conserved and nonconserved proteins for meiotic DNA breakage and repair in yeasts. Genetics 167: 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]