Abstract
Proper repair of DNA double-strand breaks (DSBs) is necessary for the maintenance of genomic integrity. Here, a new simple assay was used to study extrachromosomal DSB repair in Schizosaccharomyces pombe. Strikingly, DSB repair was associated with the capture of fission yeast mitochondrial DNA (mtDNA) at high frequency. Capture of mtDNA fragments required the Lig4p/Pku70p nonhomologous end-joining (NHEJ) machinery and its frequency was highly increased in fission yeast cells grown to stationary phase. The fission yeast Mre11 complex Rad32p/Rad50p/Nbs1p was also required for efficient capture of mtDNA at DSBs, supporting a role for the complex in promoting intermolecular ligation. Competition assays further revealed that microsatellite DNA from higher eukaryotes was preferentially captured at yeast DSBs. Finally, cotransformation experiments indicated that, in NHEJ-deficient cells, capture of extranuclear DNA at DSBs was observed if homologies—as short as 8 bp—were present between DNA substrate and DSB ends. Hence, whether driven by NHEJ, microhomology-mediated end-joining, or homologous recombination, DNA capture associated with DSB repair is a mutagenic process threatening genomic stability.
DNA double-strand breaks (DSBs) are genotoxic lesions, which can be caused either by external agents such as ionizing radiations or radiomimetic drugs or by physiological cellular processes such as V(D)J recombination (Roth et al. 1992) or meiosis (Sun et al. 1989; Cao et al. 1990). Replication fork collapse during DNA replication provides another source of DSBs (Michel et al. 1997). Proper repair of chromosome breaks is necessary to prevent genomic rearrangements, a hallmark of cancer cells, or cell death. Cells from all organisms have evolved several mechanisms to reseal DSBs. Homologous recombination (HR) is the main pathway for DSB repair in yeast. It is mainly error free and requires the RAD52 epistasis group of genes including Saccharomyces cerevisiae RAD51 and Schizosaccharomyces pombe rhp51+, two orthologs of bacterial RecA (Pâques and Haber 1999). Mammalian cells are thought to rely preferentially on nonhomologous end-joining (NHEJ) for DSB repair, a mechanism that seals two broken ends in the absence of extended sequence homology. In all organisms, NHEJ requires, among others, the heterodimeric DNA-binding proteins Ku70 and Ku80 (S. pombe Pku70p and Pku80p) and DNA ligase IV (S. pombe Lig4p). More recently, studies in NHEJ-deficient cells, including yeast (Boulton and Jackson 1996; Manolis et al. 2001; Ma et al. 2003; Yu and Gabriel 2003), mammalian (Feldmann et al. 2000; Zhong et al. 2002; Bentley et al. 2004; Guirouilh-Barbat et al. 2004), or plant cells (Heacock et al. 2004), revealed the existence of an alternative KU70/KU80- and RAD52-independent microhomology-mediated end-joining mechanism (MMEJ). This error-prone mechanism relies on the presence of DNA microhomologies (often imperfect) for ligation, leading to deletions during the complementary search (Feldmann et al. 2000; Manolis et al. 2001; Ma et al. 2003; Yu and Gabriel 2003; Bentley et al. 2004). In budding yeast, microhomologies at MMEJ junctions (∼11 bp) are longer than the typical overlapping sequences (<5 bp) found at NHEJ junctions but shorter than the ∼30 bp thought to be required for RAD52-dependent HR (Kramer et al. 1994; Manivasakam et al. 1995; Boulton and Jackson 1996; Ma et al. 2003; Yu and Gabriel 2003). Further evidence for in vivo MMEJ repair of DSBs has been provided in Ku-deficient mice where some V(D)J recombination still persists and is associated with extensive deletions at repair junctions (Gu et al. 1997). Although genetic and biochemical experiments, including the dissociation of NHEJ from MMEJ activity by fractionation of calf thymus cell extracts (Mason et al. 1996), have suggested that the two DNA repair mechanisms rely on distinct enzymes, the genetic requirements for MMEJ remain largely elusive. Two recent studies in S. cerevisiae and Arabidopsis (Ma et al. 2003; Heacock et al. 2004) reported a reduced frequency of MMEJ in the absence of the conserved Mre11 complex (S. cerevisiae Mre11p/Rad50p/Xrs2p, human Mre11/Rad50/Nbs1, and S. pombe Rad32p/Rad50p/Nbs1p), a complex further involved in DNA damage checkpoint signaling and HR- and NHEJ-mediated DSB repair (D'Amours and Jackson 2002). Whereas loss of the Mre11 complex strongly decreases the efficiency of DSB repair in budding yeast (Schiestl et al. 1994; Moore and Haber 1996; Boulton and Jackson 1998; Ma et al. 2003), extrachromosomal DSB repair assays have suggested that it has either little or no effect on the frequency of NHEJ-mediated repair of linearized plasmids in fission yeast (Wilson et al. 1999; Manolis et al. 2001).
From yeast to mammals, studies have reported the insertion of DNA fragments of various sources at experimentally induced DSBs, including mitochondrial DNA (mtDNA) and retrotransposons in yeast (Teng et al. 1996; Ricchetti et al. 1999; Yu and Gabriel 1999) and repetitive DNA (Sargent et al. 1997; Salomon and Puchta 1998; Lin and Waldman 2001a), microsatellite DNA (Liang et al. 1998; Lin and Waldman 2001a), or the vector encoding the I-SceI endonuclease used to create the DSB in higher eukaryotic cells (Sargent et al. 1997; Liang et al. 1998; Salomon and Puchta 1998; Lin and Waldman 2001a,b; Allen et al. 2003). Interestingly, recent studies reported the association of human genetic diseases with de novo insertions of mtDNA in the nuclear genome (Willett-Brozick et al. 2001; Borensztajn et al. 2002; Turner et al. 2003; Goldin et al. 2004). Reported cases included a patient exposed to Chernobyl radiation, suggesting that DSB repair-driven chromosomal integration of mtDNA may not occur exclusively under experimental conditions. Finally, systematic sequencing of nuclear genomes from budding yeast, human, and various plant species revealed that integration of mtDNA fragments occurred during evolution and is probably an ongoing process (Ricchetti et al. 1999, 2004; Mourier et al. 2001; Woischnik and Moraes 2002; Richly and Leister 2004). It is noteworthy that mtDNA insertion has also been detected in the coding sequence of the c-myc oncogene in HeLa cells, providing a potential mechanism for tumorigenesis (Shay and Werbin 1992). Similarly, the capture of microsatellite DNA at mammalian DSBs not only has been reported experimentally (Liang et al. 1998; Lin and Waldman 2001a) but also was detected at the breakpoints of lymphoid tumor-specific translocations (Boehm et al. 1989). Insertion of microsatellite DNA at DSBs provides a source of genomic instability as DNA repeats are prone to expansions/contractions during cellular processes like DNA replication or DSB-induced gene conversion (Richards and Sutherland 1997; Richard et al. 1999).
In this study, I investigated genetic requirements and DNA substrates for DSB repair in S. pombe using a new simple extrachromosomal (EC) DSB repair assay. In particular, I wanted to clarify the role of the fission yeast Mre11 complex in DSB repair. The assay revealed the association of EC DSB repair with NHEJ-dependent capture of fission yeast mtDNA, a process also requiring the Mre11 complex. Next, the EC DSB repair assay was used to screen for DNA sequences from higher eukaryotes that may be preferentially captured at DSBs. Finally, I compared the relative efficiencies of NHEJ, MMEJ, and HR for insertion of DNA substrates at DSBs in fission yeast.
MATERIALS AND METHODS
Fission yeast strains and methods:
The S. pombe strains used in this study are described in Table 1. The KT1a0 and KT120 strains were kindly provided by Masaru Ueno. The PN559, PN2490, and PN3773 strains were provided by Paul Nurse. Cells were cultured at 32° in rich (YE5S) or Edinburgh minimal (EMM2) media and sporulated on malt extract media as described in Moreno et al. (1991). Yeast transformations were performed using the lithium acetate method as described in Okazaki et al. (1990). Specifically, cells grown to a density of 5–20 × 106 cells/ml in YE5S were harvested, washed twice with 20 ml LiOAc 0.1 m (pH 4.9), and resuspended to a final concentration of 2 × 109 cells/ml in LiOAc 0.1 m. Aliquots of 100 μl were incubated at 25° for 1 hr before addition of DNA and another 1-hr incubation at 25°. Cells were mixed with 290 μl 50% PEG 3350/LiOAc 0.1 m and incubated at 25° for 1 hr. After heat shock at 43° for 15 min, followed by incubation at 25° for 10 min, cells were washed with water and directly plated onto selective medium. Exponentially growing cells refer to overnight yeast cultures grown to a final density of 5–20 × 106 cells/ml YE5S and cells in stationary phase were obtained by incubating exponentially growing cells (5–20 × 106 cells/ml) for another 24 hr at 32° without changing the culture medium at a final density of 50–100 × 106 nondividing cells/ml.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| PN559 | h− leu1-32 ura4-D18 ade6-M216 | P. Nurse's lab collection |
| PN2490 | h+ ura4-D18 rhp51Δ∷kanR | P. Nurse's lab collection |
| PN3773 | h+ leu1-32 ura4-D18 his3-D1 ade6-M210 pku70Δ∷ kanR | Baumann and Cech (2000) |
| KT1a0 | h+ leu1-32 ura4-D18 ade6-M210 lig4Δ∷LEU2 | Tomita et al. (2003) |
| KT120 | h+ leu1-32 ura4-D18 ade6-M210 rad50Δ∷LEU2 | Tomita et al. (2003) |
| AD459 | h− leu1-32 ura4-D18 ade6-M216 lig4Δ∷LEU2 | PN559 × KT1a0 |
| AD462 | h+ leu1-32 ura4-D18 ade6-M216 lig4Δ∷LEU2 pku70Δ∷ kanR | AD459 × PN3773 |
DNA for yeast transformation:
The 1747-bp S. pombe ura4+ gene was PCR amplified on REP4 plasmid (Maundrell 1993) with 5′-TAGCTACAAATCCCACTGGC and 5′-TTGACGAAACTTTTTGACAT and Taq polymerase (Takara, Berkeley, CA). To get the pUC18∷ura4+ plasmid, the S. pombe ura4+ gene was PCR amplified on REP4 plasmid with 5′-TTATAGATCTGTTTTATCTTGTTTGTCTACATGG and 5′-TGCATGGATCCTAAAAAAGTTTGTATAGATTATTT, digested with BglII and BamHI, and cloned into BamHI-cleaved pUC18 plasmid. I used 1 μg of EcoRI-linearized pUC18∷ura4+ plasmid for yeast transformation. In cotransformation experiments, yeasts were transformed with 2 μg ura4+ DNA and 2 μg cotransforming DNA (1:1 weight ratio) obtained as follows: the 600-bp mtDNA fragment (position 4501–5100 on the fission yeast mtDNA map) was PCR amplified on wild-type cells (PN559) with 5′-AACCGTAGTGGAAGTTGCGGTTGAACTAAT and 5′-ATAAGTATACCATGTGCTGAGATTGCAACA; the 250-bp human genomic DNA fragment flanked by 8 bp of microhomology to S. pombe ura4+ 5′ and 3′ extremities was amplified by PCR on clone 7 (described in Figure 5D) with 5′-TTCGTCAACTGTACA and 3′-ATTTGTAGATATAATATATATTTG (microhomologous nucleotides are underlined). To obtain the 856-bp DNA fragment containing long stretches of homology to ura4+, the same piece of human genomic DNA was PCR amplified on clone 7 with 5′-CACCATGCCAAAAATTACAC and 5′-TTGGTTGGTTATTGAAAAAGTCG. For cotransformation experiments with salmon or human genomic DNA, cells were transformed with 2 μg ura4+ DNA and 50 μg of either sheared salmon sperm DNA (Sigma, St. Louis), or human genomic DNA extracted from 293 human embryonic kidney cells (QIAamp kit, QIAGEN, Chatsworth, CA).
Figure 5.
Capture of DNA fragments from higher eukaryotes at fission yeast DSBs. (A) Frequency of ura4+ molecules with inserted DNA after transformation of exponentially growing wild-type (PN559), rhp51Δ (PN2490), rad50Δ (KT120), lig4Δ (AD459), and pku70Δ (PN3773) cells with 2 μg ura4+ DNA and 50 μg sheared salmon sperm DNA. The number of ura4+ molecules analyzed is shown on the bars. Three to six independent transformations were performed for each strain. (B) Frequency of fission yeast mtDNA in ura4+ inserts recovered from cells transformed with 2 μg ura4+ DNA only (−) and either 50 μg salmon sperm DNA or 50 μg human genomic DNA. (C) Inserts recovered from wild-type and rhp51Δ cells were classified as follows: fission yeast mtDNA, salmon microsatellite DNA, salmon repetitive DNA including ribosomal DNA, and other salmon DNA sequences. (D) Characteristics of human genomic DNA inserts recovered in the assay. (E) Insert of clone 4 showing the presence of (GT)17 repeats at the 3′ junction. WT, wild type.
Identification of ura4+ circular DNA junctions:
Junctions in ura4+ circles were PCR amplified on boiled yeast colonies with 5′-TTAGAGAAAGAATGCTGAGTA and 5′-TTGGTTGGTTATTGAAAAAGTCG, yielding 489-bp-long PCR products for intact junctions. More internal primer (5′-CACCATGCCAAAAATTACAC) was used to amplify truncated junctions in NHEJ-deficient cells. PCR products were purified from agarose gel and sequenced using the DYEnamic sequencing kit from Amersham Biosciences.
Observation of mitochondria in living cells:
S. pombe wild-type cells (PN559) were transformed with plasmid TA25 (kindly provided by Yasushi Hiraoka) containing a fusion between the 5′-end of the atp3+ gene, encoding a subunit of the fission yeast mitochondrial ATP synthase and GFP (Ding et al. 2000). Mitochondria fluorescence was observed in vivo with a Zeiss Axioplan microscope, using a 100×, 1.3 oil immersion lens. GFP was excited with a mercury lamp, using a HQ 450/50 filter (Chroma, Brattleboro, VT). A HQ 510/50 filter was used for fluorescence emission and images were captured with a Hamamatsu 3CCD chilled camera and processed with Adobe PhotoShop 6.0 software (Adobe Systems, San Jose, CA).
DNA sequence comparison:
Sequences of ura4+ circle inserts were compared to NCBI nonreductant database using BLAST software (http://www.ncbi.nlm.nih.gov:80/blast). Fission yeast nuclear DNA sequences of mitochondrial origin (NUMTs) were detected through BLAST search against the S. pombe nuclear genome (http://www.sanger.ac.uk/cgi-bin/blast/submitblast/s_pombe/) using mtDNA genome (accession no. X54421) as query. The selection criteria were a minimal NUMT size of 22 bp with an identity to mtDNA ≥85%.
RESULTS
Extrachromosomal DSB repair assay:
So far, study of EC DSB repair in eukaryotic cells has relied on endonuclease-cleaved plasmid as substrate. Here, I tested whether the extremities of a PCR-amplified piece of DNA may be recognized as DSB and processed by the DNA repair machinery of the cell. One advantage of the system lies in the possibility of adding selected nucleotide sequences to 5′-ends of primers to monitor the repair of various DSB end sequences.
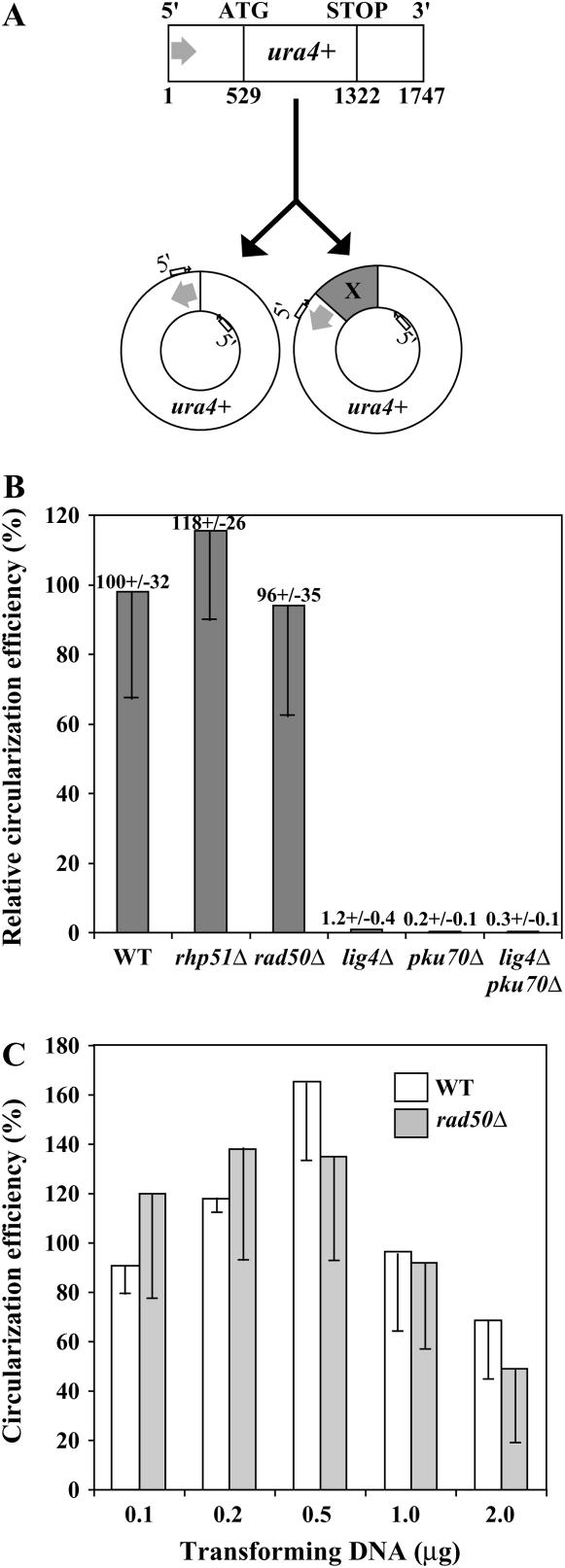
To test the feasibility of the system, exponentially growing ura4-D18 S. pombe cells were transformed with 1747-bp PCR-amplified ura4+ DNA composing the S. pombe ura4+ ORF flanked by 528 bp upstream of the ATG and 426 bp downstream of the STOP codon (Figure 1A), a fragment with no homology to the nuclear genome of ura4-D18 cells. Grimm and Kohli (1988) reported that, although devoid of a known autonomously replicating sequence, the ura4+ DNA replicates autonomously in fission yeast. Transformation of 2 × 108 wild-type cells with 2 μg ura4+ PCR fragment yielded an average of 4800 Ura+ colonies. This number was reduced to 0–100 Ura+ colonies in lig4Δ and pku70Δ NHEJ-deficient strains. The instability of most Ura+ colonies (∼90% after two replica platings onto nonselective medium) suggested that the ura4+ DNA was not integrated at high frequency into the genome. Accordingly, ∼70% (221/317) of the wild-type Ura+ colonies gave a PCR product after amplification with primers in the 5′ and 3′ regions of ura4+ designed to amplify ura4+ junctions resulting from intramolecular ligation (Figures 1A and 3A). Circularization of ura4+ DNA was confirmed by Southern blot analysis of total DNA isolated from both wild-type and rad50Δ Ura+ cells (data not shown). Therefore, it appears that the ura4+ fragment ends were indeed recognized as DSB and subjected to end-joining. The absence of PCR product in a subset of Ura+ clones may be, at least partially, due to the presence of long extranuclear DNA inserts (see below). To compare ura4+ circularization efficiency in different yeast genetic backgrounds, I calculated the ratio of repair events to transformation efficiency. This was achieved by transforming cells with either 1 μg PCR-amplified ura4+ (1.7 kb) or 1 μg uncut REP4 [ura4+] plasmid (8.5 kb) (ura4+ PCR/REP4 molar ratio of 5) as control for transformation efficiency (Figure 1B). Circularization efficiency was then calculated as the ratio of Ura+ colonies obtained with PCR-amplified ura4+/Ura+ colonies obtained with REP4. In wild-type cells, the ratio was of 96% ± 32%. The ura4+ circularization efficiency was not affected in rhp51Δ and rad50Δ cells (Figure 1B) even though the number of Ura+ colonies obtained after transformation with either ura4+ DNA or REP4 uncut plasmid was reduced compared to wild-type cells. To rule out the possibility that the presence of more than one molecule of ura4+ DNA in the nucleus may mask an intramolecular ligation deficiency of rad50Δ cells, wild-type and rad50Δ cells were transformed with decreasing amounts of ura4+ and REP4 DNA (down to 0.1 μg). Strikingly, circularization efficiencies were similar in both strains at all DNA concentrations tested (Figure 1C).
Figure 1.
A new extrachromosomal DSB repair assay in S. pombe. (A) A 1747-bp PCR-amplified DNA fragment containing the S. pombe ura4+ gene was used as substrate to monitor EC DSB repair in fission yeast. Circularization of ura4+ DNA occurred with or without insertion of DNA fragments (X). (B) The number of yeast Ura+ colonies obtained after transformation of wild-type (PN559), rhp51Δ (PN2490), rad50Δ (KT120), lig4Δ (AD459), pku70Δ (PN3773), and lig4Δpku70Δ (AD462) cells with 1 μg of either ura4+ PCR product or REP4[ura4+] uncut plasmid was monitored in at least four independent transformations. Circularization efficiencies were calculated as the ratio of Ura+ colonies obtained with both transforming DNA species (ura4+ PCR product/REP4). Values were compared to the wild-type efficiency (WT = 100%). (C) Ura4+ circularization efficiency was measured at different concentrations of transforming DNA (ura4+ PCR product or REP4[ura4+]) in wild-type (PN559) and rad50Δ (KT120) cells. WT, wild type.
Figure 3.
mtDNA capture at EC DSBs. PCR analysis of ura4+ repair junctions in Ura+ colonies obtained either after transformation of exponentially growing wild-type (PN559), rhp51Δ (PN2490), rad50Δ (KT120), lig4Δ (AD459), pku70Δ (PN3773), and lig4Δpku70Δ (AD462) cells with 2 μg PCR-amplified ura4+ DNA (A) or after transformation of wild-type cells with 1 μg EcoRI-linearized pUC18∷ura4+ plasmid (B). WT, wild type.
In lig4Δ and pku70Δ cells, however, ura4+ circularization efficiency was reduced to 1.2% and 0.2% of the wild-type value, respectively (Figure 1B), in agreement with the pku70+ and lig4+ requirement for efficient end-joining activity. The lig4Δpku70Δ double mutant did not show reduced circularization efficiency as compared to pku70Δ cells (Figure 1B). The drastic reduction in the number of Ura+ colonies obtained in NHEJ-deficient strains further argues against a high frequency of ura4+ DNA integration into the yeast genome.
Next, repair mechanisms involved in ura4+ circularization (NHEJ or MMEJ) were classified according to the extent of microhomology at ura4+ junctions. Overlapping sequences ≥5 bp and composing at least 4 bp of perfect microhomology were classified as MMEJ-mediated intramolecular ligations of ura4+ and overlap <5 bp as NHEJ-dependent ligations. Following these criteria, 93% (43/46) of ura4+ circularizations were mediated through NHEJ in wild-type cells (Figure 2A), and nucleotide loss at junctions was detected in 83% of the repair events (Figure 2, A and B). Deletion of the rad50+ gene did not affect significantly the extent of nucleotide deletion at repair junctions and NHEJ produced 81% (57/70) of the ura4+ circles (Figure 2, A and B). In pku70Δ cells, nucleotide deletion was observed at all junctions and MMEJ accounted for 92% (24/26) of the repair events. MMEJ frequency was reduced to 71% (10/14) in lig4Δ cells, suggesting that another ligase may be involved in pku70+-dependent NHEJ in S. pombe. The situation observed in lig4Δ pku70Δ cells was similar to the one observed in pku70Δ cells in agreement with the involvement of pku70+ and lig4+ genes in the same NHEJ pathway (Figure 2, A and B). Hence, PCR-amplified ura4+ DNA seems to be a good substrate to monitor EC DSB repair in fission yeast, allowing the study of both NHEJ and MMEJ repair pathways.
Figure 2.
Repair junctions in ura4+ circles. (A) Nucleotide sequence of ura4+ repair junctions. Overlapping residues at junctions are shaded. The accumulated nucleotide loss (5′ + 3′) is given under “Δ.” (B) Distribution of accumulated nucleotide loss at repair junctions.
High frequency of mtDNA insertion at extrachromosomal DSBs:
A striking feature of the ura4+ EC DSB repair assay was the presence of DNA inserts in 28% (61/221) and 11% (7/61) of the circular ura4+ molecules isolated from wild-type and rhp51Δ cells, respectively (Figures 1A and 3A). All DNA inserts consisted of fission yeast mtDNA exclusively and included 19% (7/36) and 28% (2/7) of multiple mtDNA insertions in wild-type and rhp51Δ cells, respectively (Table 2). mtDNA capture at EC DSBs was not dependent upon DSB end sequence as similar capture frequency was observed with ura4+ DNA flanked by 20-bp-long random sequences (data not shown). mtDNA capture also was not restricted to PCR-amplified repair substrate as a similar mtDNA insertion frequency (24%) was observed at repair junctions of yeast cells transformed with EcoRI-linearized pUC18∷ura4+ plasmid (Figure 3B). mtDNA insert size ranged from 83 to 4004 bp (Table 2). However, longer mtDNA inserts may not have been detected (1 min 30 sec elongation time for PCR). In both strains, homologies of 0–3 bp were detected at the mtDNA-ura4+ junctions, a hallmark of NHEJ-mediated ligations. Accordingly, mtDNA was never found at repair junctions in pku70Δ (0/229), lig4Δ (0/372), and lig4Δ pku70Δ (0/158) NHEJ-deficient strains (Figure 3A). Interestingly, mtDNA also was not detected in the 215 repair events analyzed in the rad50Δ background (Figure 3A), reflecting either intermolecular ligation deficiency of the strain or the absence/reduction of mtDNA fragments in the nucleus of rad50Δ cells.
TABLE 2.
Examples of mitochondrial DNA inserts
| Insert length (bp) | Position on mitochondrial genome |
|---|---|
| WT exponential | |
| 4004 | 9082–13085 |
| 3148 | 15308–12161 |
| 2811 | 14128–16938 |
| 2465 | 10006–7542 |
| 2333 | 16207–18539 |
| 1840 | 8856–7017 |
| 1322 | 7606–6285 |
| 1182 | 7402–8583 |
| 994 | 18752–404 |
| 878 | 5665–6542 |
| 792 | 5976–5185 |
| 767 | 5665–6431 |
| 760 | 6515–5756 |
| 732 | 10018–10749 |
| 718 | 7075–7792 |
| 687 | 5214–4528 |
| 669 | 7870–7202 |
| 620 | 5879–6498 |
| 559 | 15533–14975 |
| 537 | 5980–6516 |
| 521 | 14925–14425 |
| 435 | 6015–5581 |
| 429 | 6887–7315 |
| 400 | 2869–2470 |
| 400 | 2470–2869 |
| 355 | 6217–6571 |
| 348 | 7947–7600 |
| 320 | 15081–14762 |
| 159 | 14041–13883 |
| 936, 111 | 13896–14831, 314–424 |
| 217, 457 | 18678–18462, 11770–12226 |
| 543, 285 | 16658–17200, 19020–19304 |
| 189, 167 | 15503–15315, 6329–6163 |
| 92, 213 | 4675–4766, 12198–12410 |
| 126, 207, 87 | 5834–5959, 15912–16118, 15781–15867 |
| 115, 102, 160, 83 | 5579–5465, 17505–17404, 14264–14105, 17754–17836 |
| rhp51Δ exponential | |
| 868 | 17612–18479 |
| 529 | 14606–15134 |
| 296 | 16306–16011 |
| 244 | 19337–239 |
| 178 | 4965–4788 |
| 773, 806 | 3698–4470, 14301–15106 |
| 295, 298 | 13088–12794, 13607–13904 |
| WT stationary | |
| 1045 | 13728–14772 |
| 917 | 14599–15515 |
| 879 | 16060–16937 |
| 485 | 13083–12599 |
| 361 | 5661–6021 |
| 359 | 7683–8041 |
| 322 | 14587–14908 |
| 183, 451 | 16224–16041, 5649–6099 |
| 84, 81 | 1057–1140, 6199–6279 |
| 112, 80, 210 | 4757–4646, 4718–4639, 8988–8779 |
| 219, 124, 117 | 4757–4539, 18245–18368, 18992–18876 |
| 201, 215, 815 | 3865–3665, 1855–2069, 7277–8041 |
| 216, 178, 115, 140 | 12321–12106, 4951–4774, 13987–14101, 5440–5579 |
| 152, 156, 106, 108, 114 | 6546–6697, 7710–7865, 18723–18828, 6654–6761, 8800–8687 |
| 176, 86, 117, 409, 173, 437 | 5417–5242, 7683–7598, 8451–8567, 3945–4353, 6157–6329, 5625–6061 |
| 421, 138, 98, 367, 87, 95, 83 | 8634–9054, 15071–14934, 5885–5788, 17138–16772, 10774–10688, 9475–9381, 1027–945 |
| rad50Δ stationary | |
| 598 | 14642–15239 |
| 424 | 7592–8015 |
| 406 | 16525–16126 |
| 371 | 1777–2147 |
| 331 | 6524–6194 |
| 297 | 6119–5823 |
| 164 | 11401–11564 |
| 60 | 16522–16463 |
| 78, 248 | 7626–7703, 6569–6322 |
Intermolecular ligation deficiency of rad50Δ cells:
To investigate the ability of rad50Δ cells to ligate two pieces of DNA together (intermolecular ligation), I next measured the efficiency of mtDNA capture at EC DSBs in cotransformation experiments (Figure 4A). Exponentially growing rad50Δ cells were cotransformed with 2 μg ura4+ DNA and 2 μg 600-bp PCR-amplified S. pombe mtDNA fragment (1:3 molar ratio). Although the mtDNA fragment was detected in 53% (9/17) of the ura4+ circles produced in wild-type cells, the frequency of mtDNA ligation to ura4+ was decreased by fourfold, down to 13% (5/38), in rad50Δ cells, suggesting that Rad50p is indeed required for efficient intermolecular ligation. However, loss of Lig4p had a more drastic effect on intermolecular ligation since mtDNA was absent from the 23 ura4+ circles analyzed in lig4Δ cells. These data suggest that the fission yeast Rad32p/Rad50p/Nbs1p complex is required for efficient intermolecular ligation.
Figure 4.
Intermolecular ligation deficiency of rad50Δ cells. (A) mtDNA insertion frequencies after cotransformation of exponentially growing wild-type (PN559), rad50Δ (KT120), and lig4Δ (AD459) cells with PCR-amplified ura4+ and mtDNA fragments (1:3 molar ratio). The number of ura4+ molecules analyzed is shown in parentheses. Data are the result of at least two independent yeast transformations. (B) Live observation of mitochondria morphology using the atp3-GFP fusion in wild-type cells grown either exponentially or to stationary phase in glucose medium. (C) Insertion frequency of mtDNA fragments in ura4+ circles recovered from wild-type and rad50Δ cells grown to either exponential or stationary phase. The number of ura4+ circular molecules analyzed is given at the top of each bar. (D) Number of mtDNA pieces ligated to a single ura4+ molecule in wild-type and rad50Δ cells grown under both conditions. WT, wild type.
Increased capture of mtDNA fragments in stationary phase:
Next, to test whether endogenously produced mtDNA fragments may be inserted at EC DSBs in rad50Δ cells, I searched for growth conditions that would increase mtDNA capture frequency, thereby circumventing the intermolecular ligation defect. Because of increased superoxide production in yeast cells grown to stationary phase (after the diauxic shift) and because of the proximity of mtDNA to superoxide production sites, the lack of histones, and the reduced repair activity, mtDNA may be damaged, and possibly more fragmented, in such cells (Longo et al. 1999). Moreover, stationary phase may increase the rate of mtDNA transfer to the nucleus through increased mitochondria degradation (Takeshige et al. 1992). Therefore, I tested whether capture of endogenously produced mtDNA fragments may be detected at EC DSBs of rad50Δ cells grown to stationary phase.
Accordingly, I found that transformation of stationary-phase wild-type cells with 2 μg ura4+ PCR product increased the frequency of mtDNA insertions in ura4+ circles to 73% (16/22), compared to 28% (61/221) in exponentially grown wild-type cells and multiple insertions amounted to 56% (9/16) of the events, with up to seven pieces of mtDNA ligated to a single ura4+ molecule (Figure 4, C and D, and Table 2). Under these conditions, mtDNA insertions were observed in 31% (9/29) of the ura4+ circles isolated from rad50Δ cells, thereby demonstrating that, at least in stationary phase, mtDNA fragments are available for ligation to ura4+ in rad50Δ cells (Figure 4C and Table 2). However, multiple insertions of mtDNA pieces were much less abundant than in wild-type cells (Figure 4D), further supporting the intermolecular ligation deficiency of rad50Δ cells. Mitochondria-targeted GFP fluorescence revealed that the transition to stationary phase had caused drastic changes, shifting the pattern from tubular networks to punctate mitochondrial structures (Figure 4B), as reported in budding yeast (Visser et al. 1995). Strikingly, mitochondria from glucose-starved cells were enriched at the cell periphery and around the nucleus, raising the interesting hypothesis that this may help the transfer of mtDNA fragments into the nucleus.
Screen for higher eukaryotic DNA sequences captured at DSBs:
Although capture of mtDNA at experimentally induced DSBs had been previously reported only in S. cerevisiae, a few studies in plant and mammalian cells have reported the insertion of genomic DNA (Gorbunova and Levy 1997; Sargent et al. 1997; Salomon and Puchta 1998; Little and Chartrand 2004), including microsatellite sequences (Liang et al. 1998; Lin and Waldman 2001a). Using the fission yeast EC DSB repair assay, I screened for higher eukaryotic DNA sequences that may be preferentially captured at DSBs. This was achieved by cotransforming 2 × 108 fission yeast cells with 2 μg ura4+ DNA and 50 μg genomic DNA from either salmon sperm or 293 human embryonic kidney cells, knowing that 50 μg human genomic DNA represent ∼107 nuclear genomes (and presumably a similar amount of salmon nuclear genomes). Cotransformation of wild-type cells with ura4+ PCR product and sheared salmon sperm DNA yielded ∼30% (42/141) ura4+ circles with inserted DNA (Figure 5A). Frequency of yeast mtDNA in the inserts was reduced to 13% (Figure 5B). Interestingly, salmon microsatellite DNA fragments (15–∼500 bp), mainly (GT)n dinucleotide repeats, amounted to 21% of the ura4+ circle inserts and, in two cases, two tracts of dinucleotide repeats were present within the same circular molecule (Figure 5C and supplemental data I at http://www.genetics.org/supplemental/). Since (GT)n dinucleotide repeats of >38 bp long are absent from the S. pombe genome, the long microsatellite DNA inserts must come from the cotransforming salmon DNA. Nonmicrosatellite repetitive DNA fragments of ∼100–∼350 bp, including ribosomal DNA, represented another 39% of the inserts in wild-type cells (Figure 5C). Similar distribution of microsatellite and nonmicrosatellite salmon repetitive DNA was observed in rhp51Δ inserts (Figure 5C). However, no capture of salmon sperm DNA had occurred at the 96 repair junctions analyzed in rad50Δ cells (Figure 5A). This may be related to the intermolecular deficiency of the strain and/or to the inability of rad50Δ cells to excise a piece of DNA from the bulk of salmon genomic DNA to fill in EC DSBs. Similarly, none of the ura4+ circular molecules analyzed in either lig4Δ (0/74) or pku70Δ (0/71) cells had captured salmon DNA (Figure 5A). In a second experiment, wild-type fission yeast cells were cotransformed with 2 μg ura4+ PCR product and 50 μg human genomic DNA. Similarly, human genomic DNA competed with endogenous yeast mtDNA fragments for ligation to the ura4+ gene, reducing the frequency of mtDNA to ∼11% (1/9) of the inserts (Figure 5B). Here also (GT)n microsatellite DNA was recovered at the insert-ura4+ junction in one of the clones analyzed (Figure 5, D and E). The remaining inserts comprised other kinds of human repetitive DNA (Figure 5D), which, because of their abundancy in the human genome (repetitive DNA accounts for at least 50% of the total genomic content according to Lander et al. 2001), may not represent preferred substrates for NHEJ.
MMEJ-mediated intermolecular ligation in NHEJ-deficient cells:
It is well established that NHEJ machinery is required for efficient ligation of two pieces of DNA. Here, I tested whether MMEJ may also drive the insertion of a DNA fragment into ura4+ circles.
Two micrograms of a 267-bp piece of PCR-amplified human genomic DNA (hDNA) flanked by stretches of 8 bp of homology to ura4+ 5′- and 3′-ends was introduced into yeast together with 2 μg of ura4+ DNA (6:1 molar ratio) (Figure 6A). Microhomology at the 5′-end of hDNA corresponded to the very last 8 bp of the ura4+ 3′-end while the microhomologous region between the hDNA 3′-end and ura4+ lay 3 bp away from the 5′-end of ura4+, thereby mimicking a MMEJ substrate. In wild-type cells, the hDNA fragment was inserted in 68% (32/47) of ura4+ circles and the insertion frequency was reduced to 28% (14/50) in the rad50Δ mutant (Figure 6C). In cells lacking either lig4+ or pku70+ or both genes, although the number of Ura+ colonies recovered after cotransformation was low (Figure 6B), the frequency of hDNA insertion was high, amounting to, respectively, 70% (23/33), 55% (18/33), and 74% (14/19) of the circular molecules (Figure 6C). Hence, it appears that, under the assay conditions, the relative cellular efficiency of intermolecular ligation (hDNA insertion into ura4+) vs. intramolecular ligation (ura4+ circularization without hDNA insertion) was affected by the loss of the Rad32p/Rad50p/Nbs1p complex but was not dependent on the Lig4p/Pku70p NHEJ machinery.
Figure 6.
MMEJ-mediated intermolecular ligation in NHEJ-deficient cells. (A) Exponentially growing cells were cotransformed with ura4+ DNA and hDNA flanked by either microhomologous (8 bp) or homologous (205 and 401 bp) sequences to 5′- and 3′-ends of ura4+ DNA. (B) Number of Ura+ colonies obtained after independent transformations of wild-type (PN559), rhp51Δ (PN2490), rad50Δ (KT120), lig4Δ (AD459), pku70Δ (PN3773), and lig4Δpku70Δ (AD462) cells with 2 μg ura4+ DNA and either 2 μg microhomologous hDNA or 2 μg homologous hDNA. (C) Insertion frequency of both hDNA types. (D) Repair pathway for hDNA insertion in ura4+ circles based on overlapping sequences at junctions. For cotransformation with microhomologous hDNA, insertions were classified under MMEJ if the 8 bp overlapped at junctions. The number of sequenced inserts is shown in parentheses. WT, wild type.
The mechanism involved in hDNA insertion was identified after sequencing of the junctions between hDNA and ura4+. The absence of an 8-bp overlap at circle junctions in both wild-type (0/32) and rhp51Δ (0/7) cells suggests that NHEJ, and not MMEJ was involved in 100% of the insertions (Figure 6D). However, in lig4Δ, pku70Δ, and lig4Δpku70Δ cells, insertion of hDNA was probably exclusively mediated through MMEJ as 8-bp overlaps were detected at all junctions (23/23, 18/18, and 14/14, respectively) (Figure 6D). Interestingly, MMEJ-mediated insertions of hDNA were also detected in 29% (4/14) of insertional events in rad50Δ cells. Taking into account the frequency of hDNA insertion and the number of Ura+ colonies obtained after transformation of rad50Δ and lig4Δ cells, data suggest that, under the experimental conditions, the number of MMEJ-mediated intermolecular ligations may be similar in rad50Δ and lig4Δ backgrounds, amounting to an average of 70 events/yeast transformation, which represents a frequency of ∼3.5 × 10−7/2 μg of each transforming DNA.
As expected, the efficiency of hDNA insertion mediated through long stretches of homology was very high in all yeast backgrounds tested (Figure 6, A–D). Indeed, cotransformation of yeast with 2 μg ura4+ DNA and 2 μg hDNA flanked by 205 bp (5′) and 401 bp (3′) of homology to ura4+ (1:2 molar ratio) yielded a high number of Ura+ colonies, and the hDNA insert was present in 100% of the ura4+ circles formed (Figure 6, A–D). The presence of the hDNA insert in all ura4+ molecules isolated from rhp51Δ cells suggests that yeast relied on the RAD51-independent single-strand-annealing (SSA) mechanism of HR and not on RAD51-dependent gene conversion for insertion of hDNA with long stretches of homology. In the lig4Δ background, the frequency of SSA-driven insertions of hDNA was 5 × 10−4, representing a 1500-fold increase in frequency compared to MMEJ (5 × 10−4/3.5 × 10−7). In wild-type cells, SSA-dependent insertion of hDNA was ∼30-fold more frequent than NHEJ under the assay conditions (5 × 10−4/1.5 × 10−5).
All together, the PCR assay provided evidence that MMEJ-dependent intermolecular ligation is feasible, but not very efficient, in fission yeast. However, in the presence of both the Rad32p/Rad50p/Nbs1p complex and the Lig4p/Pku70p NHEJ machinery, cells relied exclusively on the more efficient NHEJ pathway for ligation of hDNA to ura4+. Both MMEJ and NHEJ pathways of DNA insertion were less efficient than SSA.
DISCUSSION
New assay to monitor extrachromosomal DSB repair in S. pombe:
This work validated the use of PCR-amplified ura4+ DNA as substrate for the study of EC DSB repair in fission yeast as the extremities of ura4+ linear DNA were recognized as DSBs and subjected to either NHEJ or MMEJ to produce circular ura4+ molecules. In wild-type cells, nucleotide loss was observed at 83% of repair junctions in agreement with previous studies in fission yeast reporting the formation of 15–40% accurate junctions during NHEJ-mediated repair of linearized plasmids (Wilson et al. 1999; Manolis et al. 2001). Deletion of the rhp51+ gene did not impair EC DSB repair efficiency. However, ura4+ circularization efficiency was drastically reduced in lig4Δ and pku70Δ cells. No accurate junction was detected in the latter strains since ligation had mainly occurred at microhomologous regions that were buried within the ura4+ DNA. Sequence overlaps were often imperfect and had a mean length of 8.8 bp (5–12 bp), a value close to the mean length of 11.2 bp (5–20 bp) reported at budding yeast overlapping MMEJ junctions (Kramer et al. 1994; Manivasakam et al. 1995; Boulton and Jackson 1996; Yu and Gabriel 1999; Ma et al. 2003) and similar to the 8-bp overlap found at NHEJ-independent repair junctions in human bladder cancer cell-free extracts (Bentley et al. 2004). Under the assay conditions, absence of the functional fission yeast Mre11 complex affected neither ura4+ intramolecular ligation efficiency nor the extent of nucleotide loss at repair junctions in agreement with Manolis et al. (2001). However, the budding yeast Mre11 complex is required for efficient end-joining of DSBs (Schiestl et al. 1994; Moore and Haber 1996; Boulton and Jackson 1998; Ma et al. 2003).
On the other hand, this study confirmed that fission yeast NHEJ efficiently repairs EC DSBs with noncohesive ends, unlike budding yeast for which Boulton and Jackson (1996) reported a 50-fold decrease in the efficiency of YKU70-dependent repair of blunt ends.
Insertion of mtDNA at EC DSBs:
mtDNA insertions were detected in 28% of the ura4+ circles recovered from exponentially growing wild-type cells and included 19% of multiple insertions (two to four pieces). mtDNA insertions were also detected in rhp51Δ cells, but not in NHEJ-deficient lig4Δ or pku70Δ strains. mtDNA also was not recovered from rad50Δ ura4+ circles probably because, in the absence of the Mre11 complex end-bridging activity (Anderson et al. 2001; Chen et al. 2001), the local concentration of ura4+ and mtDNA molecules is not high enough, reducing intermolecular ligation efficiency. Cotransformation of yeast with ura4+ DNA and a PCR-amplified mtDNA fragment in a 1:3 molar ratio probably increased the intracellular mtDNA fragment concentration in rad50Δ cells, resulting in a capture frequency of 13%. However, capture of a cotransformed mtDNA fragment was four times more efficient in wild-type cells compared to rad50Δ cells, providing direct evidence for reduced intermolecular efficiency in Mre11-deficient cells. Hence, rad50Δ cells may not be able to capture endogenous mtDNA fragments if their cellular concentration is below a threshold value required for intermolecular ligation.
Growing cells to stationary phase provided more evidence in favor of reduced intermolecular ligation efficiency in rad50Δ cells. Indeed, although 73% of ura4+ circles had captured endogenous mtDNA in stationary-phase wild-type cells, the capture frequency was reduced to 31% in rad50Δ cells and multiple insertional events were much less frequent than in wild-type cells. Southern blot analysis did not reveal significant differences in the amount of mtDNA in wild-type and rad50Δ postdiauxic vs. exponentially growing cells (data not shown). However, the higher production of superoxide in mitochondria from stationary-phase yeast (Longo et al. 1999) may increase mtDNA fragmentation, given the lack of histones and the reduced repair activity in mitochondria. In addition, mitochondria-containing vacuolar autophagic bodies accumulate in budding yeast stationary phase (Takeshige et al. 1992), providing a mechanism for increased release of mtDNA molecules in the cytoplasm (Campbell and Thorsness 1998). Alternatively, increased mtDNA capture frequency may be due to enhanced NHEJ efficiency in glucose-starved cells as suggested by studies of budding yeast (Karathanasis and Wilson 2002; Heidenreich et al. 2003). Finally, it should be emphasized that yeast cells grown to stationary phase resemble most of the cells from multicellular organisms since (1) most energy comes from mitochondrial respiration and (2) cells have exited from the cell cycle; i.e., they have entered the G0 phase.
Capture of mtDNA fragments at EC DSBs has been previously reported in S. cerevisiae and amounted to ∼10% of the repair events (Schiestl et al. 1993). One hypothesis to explain the surprisingly high frequency of mtDNA insertion at EC DSBs in either budding yeast or fission yeast cells may be that the transformation procedure facilitates mtDNA fragment transfer to the nucleus as previously suggested (Schiestl et al. 1993). Alternatively, the ligation of mtDNA to ura4+ DNA may take place in the cytoplasm prior to transfer into the nucleus as different studies in higher eukaryotic cells reported a cytoplasmic localization of both the Mre11 complex and the Ku proteins under certain circumstances (Zhu et al. 2001; Koike 2002; Seno and Dynlacht 2004). However, translocation of these DNA repair proteins to the cytoplasm is rather viewed as a regulatory mechanism that inhibits nuclear NHEJ and it remains to be established whether cytoplasmic DNA repair exists. Nevertheless, mtDNA capture at chromosomal DSBs has been clearly established in budding yeast experimental models, supporting the existence of mtDNA transfer to the nucleus (Ricchetti et al. 1999; Yu and Gabriel 1999, 2003). Consistent with this view, the de novo chromosomal integration of mtDNA associated with Pallister-Hall syndrome in a patient exposed to Chernobyl radiations may be the consequence of NHEJ-mediated repair of radiation-induced DSBs (Turner et al. 2003). Human mtDNA insertion has also been detected at the breakpoint junction of a reciprocal constitutive translocation (Willett-Brozick et al. 2001). Genome sequence analysis of budding yeast (Ricchetti et al. 1999), human (Mourier et al. 2001; Tourmen et al. 2002; Woischnik and Moraes 2002; Ricchetti et al. 2004), and various plant species (Richly and Leister 2004) provided more evidence in favor of nuclear genome colonization by mtDNA. Depending on the threshold value used for the BLAST search, 211–612 human NUMTs (up to 14,654 bp long) and 34 budding yeast NUMTs (22–230 bp long) were detected. S. pombe nuclear genome analysis revealed the presence of 33 NUMTs (22–358 bp long), mainly in intergenic regions and spread over three chromosomes (Figure 7 and supplemental data II at http://www.genetics.org/supplemental/). The presence of two to three NUMTs at some genomic loci suggests that multiple insertions of mtDNA fragments also occurred during S. pombe genome evolution. There was no obvious bias for the nature of colonizing mtDNA although three NUMTs and two identical 400-bp inserts recovered in independent yeast transformations are within the 3′-end region of the rRNA large subunit gene (Figure 7). On the other hand, the ura4+ circle mtDNA inserts were enriched in DNA from cox1+ gene introns (Figure 7) in agreement with the presence of COX1 intronic DNA in four of nine mtDNA inserts recovered at budding yeast experimental chromosomal DSBs (Ricchetti et al. 1999).
Figure 7.
S. pombe NUMTs. Comparison of fission yeast mitochondrial and nuclear genomes revealed the presence of 33 putative NUMTs (black lines). The position of NUMTs on the S. pombe mitochondrial genomic map is compared to the position of mtDNA fragments recovered in ura4+ circles in this study (gray lines).
This study revealed that NHEJ-mediated mtDNA insertion at EC DSBs occurs in fission yeast and provides a tool for understanding the mechanisms of production and/or transfer of mtDNA fragments in the nucleus.
Microsatellite DNA is a good substrate for NHEJ in fission yeast:
Microsatellite DNA from higher eukaryotes was preferentially captured at EC DSBs in wild-type and rhp51Δ fission yeast cells. Dinucleotide repeats (15–∼500 bp long) were found in 21% of the ura4+ inserts recovered after cotransformation with salmon DNA and the (CCG)8 trinucleotide repeat was detected at one of the repair junctions. Because dinucleotide repeats amount to only ∼0.25% of the total genomic DNA in vertebrates (Tóth et al. 2000), their capture at DSBs may be as much as 100-fold more frequent than expected. (GT)n repeats, the most abundant dinucleotide repeats in vertebrates (60% of them) (Tóth et al. 2000), accounted for 67% of the microsatellite DNA inserts. In mammalian cells, two previous studies have reported the insertion of microsatellite DNA at DSBs (Liang et al. 1998; Lin and Waldman 2001a). Moreover, Boehm et al. (1989) reported the presence of (GT)n tracts (62–∼800 bp long) at the breakpoint of three different human lymphoid tumor-specific translocations, one of them involving the T-cell receptor β-gene. Hence, it appears that the preferential patching of DSBs with microsatellite DNA may be conserved in eukaryotic cells, validating yeast as a model for understanding the molecular basis of that preference. As previously suggested (Liang et al. 1998), DSB repair may provide a mechanism for the spreading of microsatellite DNA in the genome. Hence, DSB repair may possibly be associated with an increased risk of repeat-DNA expansion-associated genetic diseases, such as Huntington's disease [(AGC)n], Friedreich's ataxia [(AAG)n] (Richards and Sutherland 1997), or (AT)n expansions associated with autoimmune disorders (Huang et al. 2000).
MMEJ-dependent intermolecular ligation:
Although the NHEJ machinery has been shown to drive insertion of DNA fragments at budding yeast DSBs (Yu and Gabriel 2003), MMEJ-mediated insertions have not been reported so far. This study provided evidence that, in the absence of Pku70p/Lig4p, MMEJ is able to mediate the insertion of microhomologous DNA substrates at EC DSBs, albeit with low efficiency. However, in wild-type cells, NHEJ was exclusively responsible for microhomologous DNA substrate insertion. In rad50Δ cells, the DNA substrate capture frequency was decreased in agreement with the intermolecular ligation deficiency, and MMEJ-mediated insertions accounted for nearly 30% of the insertional events. Therefore, in the absence of the Rad32p/Rad50p/Nbs1p complex, the processing of DSBs required for annealing of complementary nucleotides in MMEJ may have more time to proceed, changing the competition rules between NHEJ and MMEJ. Finally, the data suggested that SSA-driven insertions of DNA with long stretches of homology have an increased efficiency of 30- and 1500-fold compared to NHEJ- and MMEJ-dependent insertions, respectively.
In summary, a new simple assay for EC DSB repair, based on PCR-amplified DNA substrate, was developed in fission yeast. The flexibility in the design of primer sequences offers a tool to investigate the repair of various DSB end sequences. The assay demonstrated a role for the fission yeast Rad32p/Rad50p/Nbs1p complex in promoting intermolecular ligation and unraveled the capture of fission yeast mtDNA and microsatellite DNA from higher eukaryotes at EC DSBs in S. pombe. The conservation of the NHEJ machinery between S. pombe and mammalian cells suggests that fission yeast may be a good model to investigate these processes, which probably represent new mechanisms of human inherited diseases.
Acknowledgments
I thank M. Ueno, P. Nurse, and Y. Hiraoka for generous gifts of strains and plasmid. I am grateful to T. Boon. I also thank M. Dutreix for helpful discussion and F. Foury, F. d'Adda di Fagagna, D. Hermand, B. Llorente, S. Marcand, and A. Goffeau for extremely useful comments on the manuscript. A.D. is supported by the Fonds National de la Recherche Scientifique.
References
- Allen, C., C. A. Miller and J. A. Nickoloff, 2003. The mutagenic potential of a single double-strand break in a mammalian chromosome is not influenced by transcription. DNA Repair 2: 1147–1156. [DOI] [PubMed] [Google Scholar]
- Anderson, D. E., K. M. Trujillo, P. Sung and H. P. Erickson, 2001. Structure of the Rad50·Mre11 DNA repair complex from Saccharomyces cerevisiae by electron microscopy. J. Biol. Chem. 276: 37027–37033. [DOI] [PubMed] [Google Scholar]
- Baumann, P., and T. R. Cech, 2000. Protection of telomeres by the Ku protein in fission yeast. Mol. Biol. Cell 11: 3265–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley, J., C. P. Diggle, P. Harnden, M. A. Knowles and A. E. Kiltie, 2004. DNA double strand break repair in human bladder cancer is error prone and involves microhomology-associated end-joining. Nucleic Acids Res. 32: 5249–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm, T., L. Mengle-Gaw, U. R. Kees, N. Spurr, I. Lavenir et al., 1989. Alternating purine-pyrimidine tracts may promote chromosomal translocations seen in a variety of human lymphoid tumours. EMBO J. 8: 2621–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borensztajn, K., O. Chafa, M. Alhenc-Gelas, S. Salha, A. Reghis et al., 2002. Characterization of two novel splice mutations in human factor VII gene causing severe plasma factor VII deficiency and bleeding diathesis. Br. J. Haematol. 117: 168–171. [DOI] [PubMed] [Google Scholar]
- Boulton, S. J., and S. P. Jackson, 1996. Saccharomyces cerevisiae Ku70 potentiates illegitimate DNA double-strand break repair and serves as a barrier to error-prone DNA repair pathways. EMBO J. 15: 5093–5103. [PMC free article] [PubMed] [Google Scholar]
- Boulton, S. J., and S. P. Jackson, 1998. Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 17: 1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, C. L., and P. E. Thorsness, 1998. Escape of mitochondrial DNA to the nucleus in yme1 yeast is mediated by vacuolar-dependent turnover of abnormal mitochondrial compartments. J. Cell Sci. 111: 2455–2464. [DOI] [PubMed] [Google Scholar]
- Cao, L., E. Alani and N. Kleckner, 1990. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell 61: 1089–1101. [DOI] [PubMed] [Google Scholar]
- Chen, L., K. Trujillo, W. Ramos, P. Sung and A. E. Tomkinson, 2001. Promotion of Dnl4-catalyzed DNA end-joining by the Rad50/Mre11/Xrs2 and Hdf1/Hdf2 complexes. Mol. Cell 8: 1105–1115. [DOI] [PubMed] [Google Scholar]
- D'Amours, D., and S. P. Jackson, 2002. The MRE11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat. Rev. Mol. Cell Biol. 3: 317–327. [DOI] [PubMed] [Google Scholar]
- Ding, D. Q., Y. Tomita, A. Yamamoto, Y. Chikashige, T. Haraguchi et al., 2000. Large-scale screening of intracellular protein localization in living fission yeast cells by the use of a GFP-fusion genomic DNA library. Genes Cells 5: 169–190. [DOI] [PubMed] [Google Scholar]
- Feldmann, E. X., V. X. Schmiemann, W. X. Goedecke, S. Reichenberger and P. Pfeiffer, 2000. DNA double-strand break repair in cell-free extracts from Ku80-deficient cells: implications for Ku serving as an alignment factor in non-homologous DNA end joining. Nucleic Acids Res. 28: 2585–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin, E., S. Stahl, A. M. Cooney, C. R. Kaneski, S. Gupta et al., 2004. Transfer of a mitochondrial DNA fragment to MCOLN1 causes an inherited case of mucolipidosis IV. Hum. Mutat. 24: 460–465. [DOI] [PubMed] [Google Scholar]
- Gorbunova, V., and A. A. Levy, 1997. Non-homologous DNA end joining in plant cells is associated with deletions and filler DNA insertions. Nucleic Acids Res. 25: 4650–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, C., and J. Kohli, 1988. Observations on integrative transformation in Schizosaccharomyces pombe. Mol. Gen. Genet. 215: 87–93. [DOI] [PubMed] [Google Scholar]
- Gu, Y., S. Jin, Y. Gao, D. T. Weaver and F. W. Alt, 1997. Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA end-binding activity, and inability to support V(D)J recombination. Proc. Natl. Acad. Sci. USA 94: 8076–8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirouilh-Barbat, J., S. Huck, P. Bertrand, L. Pirzio, C. Desmaze et al., 2004. Impact of the Ku80 pathway on NHEJ-induced genome rearrangements in mammalian cells. Mol. Cell 14: 611–623. [DOI] [PubMed] [Google Scholar]
- Heacock, M., E. Spangler, K. Riha, J. Puizina and D. E. Shippen, 2004. Molecular analysis of telomere fusions in Arabidospis: multiple pathways for chromosome end-joining. EMBO J. 23: 2304–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich, E., R. Novotny, B. Kneidinger, V. Holzmann and U. Wintersberger, 2003. Non-homologous end-joining as an important mutagenic process in cell cycle-arrested cells. EMBO J. 22: 2274–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, D., R. Giscombe, Y. Zhou, R. Pirskanen and A. K. Lefvert, 2000. Dinucleotide repeat expansion in the CTLA-4 gene leads to T cell hyper-activity via the CD28 pathway in myasthenia gravis. J. Neuroimmunol. 105: 69–77. [DOI] [PubMed] [Google Scholar]
- Karathanasis, E., and T. E. Wilson, 2002. Enhancement of Saccharomyces cerevisiae end-joining efficiency by cell growth stage but not by impairment of recombination. Genetics 161: 1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike, M., 2002. Dimerization, translocation and localization of Ku70 and Ku80 proteins. J. Radiat. Res. 43: 223–236. [DOI] [PubMed] [Google Scholar]
- Kramer, K. M., J. A. Brock, K. Bloom, J. K. Moor and J. E. Haber, 1994. Two different types of double-strand breaks in Saccharomyces cerevisiae are repaired by similar RAD52-independent, nonhomologous recombination events. Mol. Cell. Biol. 14: 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander, E. S., L. M. Linton, B. Birren, C. Nusbaum, M. C. Zody et al., 2001. Initial sequencing and analysis of the human genome. Nature 409: 860–921. [DOI] [PubMed] [Google Scholar]
- Liang, F., M. Han, P. J. Romanienko and M. Jasin, 1998. Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc. Natl. Acad. Sci. USA 95: 5172–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., and A. S. Waldman, 2001. a Capture of DNA sequences at double-strand breaks in mammalian chromosomes. Genetics 158: 1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y., and A. S. Waldman, 2001. b Promiscuous patching of broken chromosomes in mammalian cells with extrachromosomal DNA. Nucleic Acids Res. 29: 3975–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, K. C. E., and P. Chartrand, 2004. Genomic DNA is captured and amplified during double-strand break (DSB) repair in human cells. Oncogene 23: 4166–4172. [DOI] [PubMed] [Google Scholar]
- Longo, V. D., L. L. Liou, J. S. Valentine and E. B. Gralla, 1999. Mitochondrial superoxide decreases yeast survival in stationary phase. Arch. Biochem. Biophys. 365: 131–142. [DOI] [PubMed] [Google Scholar]
- Ma, J.-L., E. M. Kim, J. E. Haber and S. E. Lee, 2003. Yeast Mre11 and Rad1 proteins define a Ku-independent mechanism to repair double-strand breaks lacking overlapping end sequences. Mol. Cell. Biol. 23: 8820–8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manivasakam, P., S. C. Weber, J. McElver and R. H. Schiestl, 1995. Micro-homology mediated PCR targeting in Saccharomyces cerevisiae. Nucleic Acids Res. 23: 2799–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolis, K. G., E. R. Nimmo, E. Hartsuiker, A. M. Carr, P. A. Jeggo et al., 2001. Novel functional requirements for non-homologous DNA end joining in Schizosaccharomyces pombe. EMBO J. 20: 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, R. M., J. Thacker and M. P. Fairman, 1996. The joining of non-complementary DNA double-strand breaks by mammalian extracts. Nucleic Acids Res. 24: 4946–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell, K., 1993. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123: 127–130. [DOI] [PubMed] [Google Scholar]
- Michel, B., S. D. Erlich and M. Uzest, 1997. DNA double-strand breaks caused by replication arrest. EMBO J. 16: 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J. K., and J. E. Haber, 1996. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 2164–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., A. Klar and P. Nurse, 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 795–823. [DOI] [PubMed] [Google Scholar]
- Mourier, T., A. J. Hansen, E. Willerslev and P. Arctander, 2001. The human genome project reveals a continuous transfer of large mitochondrial fragments to the nucleus. Mol. Biol. Evol. 18: 1833–1837. [DOI] [PubMed] [Google Scholar]
- Okazaki, K., N. Okazaki, K. Kume, S. Jinno, K. Tanaka et al., 1990. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 18: 6485–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pâques, F., and J. E. Haber, 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63: 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricchetti, M., C. Fairhead and B. Dujon, 1999. Mitochondrial DNA repairs double-strand breaks in yeast chromosomes. Nature 402: 96–100. [DOI] [PubMed] [Google Scholar]
- Ricchetti, M., F. Tekaia and B. Dujon, 2004. Continued colonization of the human genome by mitochondrial DNA. PLoS Biol. 2(9): e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, G.-F., B. Dujon and J. E. Haber, 1999. Double-strand break repair can lead to high frequencies of deletions within short CAG/CTG trinucleotide repeats. Mol. Gen. Genet. 261: 871–882. [DOI] [PubMed] [Google Scholar]
- Richards, R. I., and G. R. Sutherland, 1997. Dynamic mutation: possible mechanisms and significance in human disease. Trends Biochem. Sci. 22: 432–436. [DOI] [PubMed] [Google Scholar]
- Richly, E., and D. Leister, 2004. NUPTs in sequenced eukaryotes and their genomic organization in relation to NUMTs. Mol. Biol. Evol. 21: 1972–1980. [DOI] [PubMed] [Google Scholar]
- Roth, D. B., P. B. Nakajima, J. P. Menetski, M. J. Bosma and M. Gellert, 1992. V(D)J recombination in mouse thymocytes: double-strand breaks near T cell receptor delta rearrangement signals. Cell 69: 41–53. [DOI] [PubMed] [Google Scholar]
- Salomon, S., and H. Puchta, 1998. Capture of genomic and T-DNA sequences during double-strand break repair in somatic plant cells. EMBO J. 17: 6086–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent, R. G., M. A. Brenneman and J. H. Wilson, 1997. Repair of site-specific double-strand breaks in a mammalian chromosome by homologous and illegitimate recombination. Mol. Cell. Biol. 17: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R. H., M. Dominska and T. D. Petes, 1993. Transformation of Saccharomyces cerevisiae with nonhomologous DNA: illegitimate integration of transforming DNA into yeast chromosomes and in vivo ligation of transforming DNA to mitochondrial DNA sequences. Mol. Cell. Biol. 13: 2697–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R. H., J. Zhu and T. D. Petes, 1994. Effect of mutations in genes affecting homologous recombination on restriction enzyme mediated and illegitimate recombination in Saccharomyces cerevisiae. Mol. Cell. Biol. 14: 4493–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno, J. D., and J. R. Dynlacht, 2004. Intracellular redistribution and modification of proteins of the Mre11/Rad50/Nbs1 DNA repair complex following irradiation and heat-shock. J. Cell. Physiol. 199: 157–170. [DOI] [PubMed] [Google Scholar]
- Shay, J. H., and H. Werbin, 1992. New evidence for the insertion of mitochondrial DNA into the human genome: significance for cancer and aging. Mutat. Res. 275: 227–235. [DOI] [PubMed] [Google Scholar]
- Sun, H., D. Treco, N. P. Schultes and J. W. Szostak, 1989. Double-strand breaks at an initiation site for meiotic gene conversion. Nature 338: 87–90. [DOI] [PubMed] [Google Scholar]
- Takeshige, K., M. Baba, S. Tsuboi, T. Noda and Y. Ohsumi, 1992. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 119: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, S. C., B. Kim and A. Gabriel, 1996. Retrotransposon reverse-transcriptase-mediated repair of chromosomal breaks. Nature 383: 641–644. [DOI] [PubMed] [Google Scholar]
- Tomita, K., A. Matsuura, T. Caspari, A. M. Carr, Y. Akamatsu et al., 2003. Competition between the Rad50 complex and the Ku heterodimer reveals a role for Exo1 in processing double-strand breaks but not telomeres. Mol. Cell. Biol. 23: 5186–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth, G., Z. Gáspári and J. Jurka, 2000. Microsatellites in different eukaryotic genomes: survey and analysis. Genome Res. 10: 967–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourmen, Y., O. Baris, P. Dessen, C. Jacques, Y. Malthiery et al., 2002. Structure and chromosomal distribution of human mitochondrial pseudogenes. Genomics 80: 71–77. [DOI] [PubMed] [Google Scholar]
- Turner, C., C. Killoran, N. S. T. Thomas, M. Rosenberg, N. A. Chuzhanova et al., 2003. Human genetic disease caused by de novo mitochondrial-nuclear DNA transfer. Hum. Genet. 112: 303–309. [DOI] [PubMed] [Google Scholar]
- Visser, W., E. A. van Spronsen, N. Nanninga, J. T. Pronk, J. Gijs Kuenen et al., 1995. Effects of growth conditions on mitochondrial morphology in Saccharomyces cerevisiae. Antonie Van Leeuwenhoek 67: 243–253. [DOI] [PubMed] [Google Scholar]
- Willett-Brozick, J. E., S. A. Savul, L. E. Richey and B. E. Baysal, 2001. Germ line insertion of mtDNA at the breakpoint junction of a reciprocal constitutional translocation. Hum. Genet. 109: 216–223. [DOI] [PubMed] [Google Scholar]
- Wilson, S., N. Warr, D. L. Taylor and F. Z. Watts, 1999. The role of Schizosaccharomyces pombe Rad32, the Mre11 homologue, and other DNA damage response proteins in non-homologous end joining and telomere length maintenance. Nucleic Acids Res. 27: 2655–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woischnik, M., and C. T. Moraes, 2002. Pattern of organization of human mitochondrial pseudogenes in the nuclear genome. Genome Res. 12: 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X., and A. Gabriel, 1999. Patching broken chromosomes with extranuclear cellular DNA. Mol. Cell 4: 873–881. [DOI] [PubMed] [Google Scholar]
- Yu, X., and A. Gabriel, 2003. Ku-dependent and Ku-independent end-joining pathways lead to chromosomal rearrangements during double-strand break repair in Saccharomyces cerevisiae. Genetics 163: 843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, Q., C.-F. Chen, P.-L. Chen and W.-H. Lee, 2002. BRCA1 facilitates microhomology-mediated end joining of DNA double strand breaks. J. Biol. Chem. 277: 28641–28647. [DOI] [PubMed] [Google Scholar]
- Zhu, W. G., J. D. Seno, B. D. Beck and J. R. Dynlacht, 2001. Translocation of MRE11 from the nucleus to the cytoplasm as a mechanism of radiosensitization by heat. Radiat. Res. 156: 95–102. [DOI] [PubMed] [Google Scholar]