Abstract
The widespread occurrence of sexual organisms despite the high costs of sex has long intrigued biologists. The best-known costs are the twofold cost of producing males and the cost associated with producing traits to attract mates and to interact with mating partners, such as exaggerated sexual behaviors and morphological modifications. These costs have been inferred from studies of plants and animals but are thought to be absent in facultative sexual microbes. Here, using the facultative sexual fungus Cryptococcus neoformans, I provide experimental evidence showing that: (i) interactions with active sexual partners can be costly for vegetative fitness in a facultative sexual microbe; (ii) this cost is positively correlated to mating ability; (iii) this cost is composed of at least two distinct components, the cost of producing mating signals that exert effects on mating partners and that associated with responding to active mating partners; and (iv) extended asexual reproduction can reduce both components of the cost. This cost must have been compensated for by the production of zygotes and sexual spores to allow the initial evolution and spread of sexual reproduction in eukaryotes.
SEX and sexual reproduction are widespread among eukaryotes (Bell 1982). Mating—the fusion of genetically differentiated gametes—is an essential feature of all sexual reproductive cycles. Evolutionary studies indicate that eukaryotic sexual reproduction has an ancient origin: it likely evolved among the first eukaryotic microbes (Michod and Levin 1988; Dacks and Roger 1999). The ubiquitous distribution of sex and sexual reproduction among all eukaryotic phyla suggests an essential role of sexual reproduction in the diversification and evolution of eukaryotes. However, theoretical and empirical investigations of sexually dimorphic eukaryotes such as insects, birds, and mammals suggest that sex and sexual reproduction can be costly (Michod and Levin 1988), the most discussed being the twofold cost of producing males and the cost associated with producing exaggerated sexual behaviors and morphological modifications (Bell and Koufopanou 1986; Michod and Levin 1988). However, these costs are thought to be absent in facultative sexual microbes.
Unlike obligate sexual organisms such as the majority of higher plants and animals, sex and reproduction can be easily separated in facultative sexual microbes (Xu 2004a). One major component of sexual fitness in all sexual eukaryotes is mating success rate (Bell and Koufopanou 1986; Xu 1995, 2002; Xu et al. 1996). In many microbial eukaryotes, mating between compatible partners occurs only under certain stressful conditions. Under these conditions, both mating (i.e., the formation of zygotes) and vegetative growth of individual mating partners may occur. Using appropriately marked strains, all three cell types (i.e., the two parental types and the zygotes) in such a mating mixture can be directly counted using selective media. From these cell counts, the mating success rate and the relative vegetative fitness of the parental strains can be estimated. The vegetative fitness of individual parental strains in the presence of an active mating partner may change in comparison to that in the absence of such a partner. If the vegetative fitness were lower in the presence of an active mating partner, the reduction would constitute one type of cost of sex, i.e., the cost of interacting with a mating partner. Surprisingly, such a cost has never been evaluated or documented for any facultative sexual organisms.
Here, I used genetically marked strains of the facultative sexual fungus Cryptococcus neoformans to examine whether such a cost exists. C. neoformans is an encapsulated basidiomycetous yeast (Casadevall and Perfect 1998) and is an emerging model organism for studying fungal molecular and evolutionary biology (e.g., Lengeler et al. 2000; Hull and Heitman 2002; Xu 2002, 2004b; Xu and Mitchell 2003). It has a well-established mating and genetic manipulation system. The mating system in C. neoformans is controlled by one large locus with two alternative alleles, a and α (Kwon-Chung 1976; Lengeler et al. 2002). When parental cells of opposite mating types are mixed on a nitrogen-limiting media and incubated at a temperature <30°, each parental yeast cell may take one of the following two reproductive paths.
In the first path, the parental yeast cells of opposite mating types may fuse to form zygotes. In C. neoformans, both the MATa and the MATα cells can secrete sex pheromones and release them into the surrounding medium (Hull and Heitman 2002). In response to pheromones from the opposite mating types, the MATα cells may make conjugation tubes and the MATa cells may expand in size (Hull and Heitman 2002; McClelland et al. 2004). These morphologically differentiated MATa and MATα cells then may fuse and form diploid or dikaryotic zygotes. The zygotes can be detected after ∼12 hr of incubation and are abundant at ∼20–24 hr (Xu 2002; Yan et al. 2004). At this time (i.e., after ∼20–24 hr of co-incubation), the zygotes may produce hyphal filaments that gradually intertwine among each other to form microscopic mycelial mats. After a few more days of incubation, the ends of some mycelia may enlarge and form basidia within which nuclear fusion and meiosis may occur. Four chains of basidiospores, the sexual spores of C. neoformans, are then produced from each basidium. Each basidiospore contains a recombinant haploid nucleus, the product of meiosis. Because after ∼20–24 hr the zygotes form hyphal filaments that can intertwine with each other and are difficult to separate for counting, an accurate estimate of zygote number is often difficult to obtain beyond this time point. Therefore, mating success rate is typically estimated within 24 hr of incubation (Xu 2002). The mating process in C. neoformans is different from that of the baker's yeast Saccharomyces cerevisiae where hyphal formation is not part of the sexual reproduction process (Guthrie and Fink 1991).
In the second path, the parental cell does not fuse with that of opposite mating types but instead reproduces asexually through mitosis. However, because these cells are exposed to their mating partners, their rates of asexual reproduction may change. The change could be brought about by the simple presence of their mating partners (either inactive or active) in the competition for resources or by potential chemicals such as pheromones that are secreted by their partners. In C. neoformans, the nitrogen-limiting conditions are known to induce pheromone production and secretion. In response to pheromones from cells of opposite mating types, cells may slow their rates of asexual reproduction. Whether this is true has not been examined in C. neoformans or in any other facultative sexual microbes. Because genetically marked strains with isogenic backgrounds are available and both mating success rate and vegetative growth rates for both parents can be quantified in C. neoformans, this species is therefore an excellent candidate organism for examining the potential cost of interacting with mating partners in a facultative sexual microbe.
This study was designed to address the following questions. First, does interacting with sexual partners incur a cost in C. neoformans? Second, if a cost is found, is this cost correlated to mating success rate? My hypothesis is that if a cost of interacting with sexual partners exists, the extent of the cost should be positively correlated to mating success rate. Third, since sexual mating in C. neoformans (and other eukaryotes) is a complex and highly interactive process, can the cost of interacting with mating partners be partitioned into specific components that are involved in either producing mating signals or responding to partner's mating signals? And finally, do strains of the two mating types show similar responses in the interactions with mating partners?
MATERIALS AND METHODS
Strains:
Two strains, JEC50 (MATα ade2) and MCC3 (MATa cna ura5), were used as parental strains to assay the potential cost of sex. These two strains belong to C. neoformans var. neoformans (serotype D) and were haploid and isogenic except for the indicated loci (Kwon-Chung et al. 1992; Sia et al. 2000). Two other isogenic strains, JXC4 (MATα cna ura5) and JEC61 (MATa ade2) were used as controls in mating experiments. These four strains are used to determine the potential cost of interacting with sexual partners under three medium conditions.
To determine the relationship between the potential cost of interacting with sexual partners and mating success rate, 16 mutation-accumulation (MA) lines of JEC50 and MCC3 (8 for each strain) obtained previously (Xu 2002) were examined. These MA lines went through 30 rounds of population bottlenecks and a total of ∼600 asexual mitotic generations with minimum selection. Cultures from the 5th, 10th, 20th, and 30th bottlenecks were stored and they represented cells from ∼100, 200, 400, and 600 mitotic generations, respectively. Compared to the original parental strains, these 64 MA clones have shown variable degrees of reduced mating abilities (Xu 2002). All cells were stored in a −70° freezer prior to use.
Media and pairing for assaying the potential cost of interacting with mating partners:
To determine the potential cost of interacting with mating partners and how media conditions might influence such a potential cost, I used three media: one nitrogen limiting and two nitrogen rich. The nitrogen-limiting medium is SSΔN agar [0.17% Difco (Detroit) yeast nitrogen base without amino acids and ammonium sulfate, 2% sucrose, 2% Bacto-agar, and 0.02 g/liter of uracil and adenine] (Xu 2002). The two nitrogen-rich media are SS (0.17% Difco yeast nitrogen base with amino acids and ammonium sulfate, 2% sucrose, 2% Bacto-agar, and 0.02 g/liter of uracil and adenine) (Xu 2002) and the common laboratory yeast growth medium YEPD (2% yeast extract, 1% peptone, 2% dextrose, 2% agar). The mating-conducive nitrogen-limiting medium SSΔN agar for C. neoformans is similar but not identical to the common sporulating medium used for the baker's yeast S. cerevisiae (Guthrie and Fink 1991). In contrast, the two nitrogen-rich media are also known as nonsporulating media for both the baker's yeast and C. neoformans. While no mating is expected for C. neoformans on the nitrogen-rich media, whether a cost of interacting with sexual partners exists on such media is not known.
To assess the mating success rate and vegetative fitness of the mating pair JEC50 and MCC3 on the three above media, cells were first grown on a synthetic medium, SD (0.17% Difco yeast nitrogen base with ammonium sulfate and amino acids, 2% dextrose, and 2% Bacto-agar). After 4 days of growth on SD, cells were scraped off the agar and resuspended in 200 μl of sterile distilled H2O. Cell density for each culture was determined using a hemocytometer and microscopy and adjusted to 5 × 104 cells/μl. For each mating, 8 μl from each of the JEC50 and MCC3 cultures were mixed by repeated pipetting. Four microliters of the mating mixture were plated on each of the three media. Four microliters of each parental suspension were plated as a negative control on each medium. Eight repeats were done for each mating and for the negative controls.
Because strains with auxotrophic markers were used in the above assay, these markers might have contributed to our estimations of vegetative fitness. To test and control for this potential effect, an additional set of controls was used. In this set, strains with auxotrophic markers identical to the JEC50 (MATα ade2) and MCC3 (MATa cna ura5) pair but with the same mating type were mixed. Specifically, the MATα JEC50 cells were mixed with another MATα strain, JXC4 (MATα cna ura5), and the MATa MCC3 cells were mixed with another MATa strain, JEC61 (MATa ade2). The procedures for this set of controls were the same as that between JEC50 and MCC3 and eight repeats were done for each pairing.
Mating success rate and vegetative fitness determination:
After incubating the mating mixtures at 25° for 24 hr, each mixture was cut out of the agar medium, placed in 1 ml of sterile distilled H2O, washed into the H2O by vigorous vortexing, and diluted (10, 100, and 1000 times). Aliquots of the original and diluted cells were spread plated onto three different media: YEPD agar that allows all viable cells to grow, SDΔN agar (0.17% of Difco yeast nitrogen base without amino acids and ammonium sulfate, 2% dextrose, and 2% agar) that allows only mated cells to grow, and SD agar supplemented with 5-fluoroorotic acid (5-FOA; 0.2 g/liter) that allows only cells with the ura5 mutations to grow (i.e., MCC3 and JXC4 in this study). Cells were incubated at 25° for 3 days before visible colonies were counted.
In total, 72 pairing mixtures (3 strain pairs × 3 media × 8 repeats each) and 96 negative controls of single parental strains (4 strains × 3 media × 8 repeats each) were set up to examine the potential cost of interacting with mating partners. A total of 2016 screens (168 × 4 dilutions × 3 plating media for counting cells) were performed for quality control and for determining cell numbers of all three types of cells in each mating mixture for all three media environments.
Relationship between the cost of sex and mating success rate:
For each of the 64 MA clones, tests for mating ability and vegetative fitness in the presence and absence of active mating partners were determined using a protocol identical to that described above. However, among the three media mentioned above, only SSΔN agar showed a cost of sex (see below); therefore, only medium SSΔN was used for the 64 MA clones. The 32 MA clones from strain JEC50 were paired with the original clone of MCC3 while those from strain MCC3 were paired with the original clone of JEC50. A total of 64 pairing treatments were made and three replicates were performed for each pairing. Coupled to these 64 pairings were 64 negative controls with each containing a single MA clone. A total of 4608 screens (128 treatments × 3 repeats × 4 dilutions × 3 plating media) were performed for control and for determining cell numbers of all three types of cells in each treatment.
Data analysis:
Cell counts from each of the three media, YEPD, SDΔN, and SD + 5-FOA, were determined for each repeat of each treatment. The counts of MCC3 (and JXC4) and mated zygotes were obtained directly from the cell counts on SD + 5-FOA medium and SDΔN medium, respectively. The counts of JEC50 and JEC61 were obtained as the colony numbers from YEPD medium minus the numbers from those on SD + 5-FOA and SDΔN media.
The vegetative growth rates (generation time) of individual strains on respective media were estimated on the basis of cell number increases over the 24-hr period, using the logistic growth model. Mating ability was calculated as the ratio between mated cells (obtained on SDΔN medium) and the total number of cells (obtained on YEPD medium) in the mating mixture. The cost of sex was calculated as the percentage of reduction in vegetative fitness (i.e., the percentage of increase in generation time) between cultures in the presence of active mating partners and those in the absence of active mating partners. In our case,
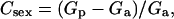 |
where Csex refers to the cost of sex, Gp is the generation time in the presence of active mating partners, and Ga is the generation time in the absence of active mating partners.
The reduction in the cost of sex among MA clones was calculated as the percentage of decrease in the cost over the original clones of strains JEC50 and MCC3. In all calculations, the zygotes were counted toward the parental cell counts of both parents for each mating.
Statistical significances of vegetative fitness changes and the reductions in the cost of sex were determined using Student's t-test (Sokal and Rohlf 1981). The relationships among phenotypic traits were assessed using programs implemented in Microsoft Excel.
RESULTS
A cost of interacting with mating partners in the nitrogen-limiting medium, SSΔN agar:
In mating mixtures on a nitrogen-limiting medium, SSΔN agar, the mean generation times of strains JEC50 and MCC3 increased by >10.1 and 8.9%, respectively, relative to those in the absence of compatible mating partners (Table 1). While both reductions were statistically significant (P < 0.01), there was no difference in the amount of reduction between the two strains with different mating types (P > 0.9). As expected, we observed no difference between the two negative control groups. The pairing involving strains with different auxotrophic markers but identical mating type exhibited no reduction in vegetative fitness compared to those by themselves individually (data not shown).
TABLE 1.
A cost of sex in Cryptococcus neoformans
| Traits | JEC50 (MATα ade) | MCC3 (MATacna ura) |
|---|---|---|
| Mating ability | 6.111 ± 0.508% | 6.111 ± 0.508% |
| Vegetative fitness (generation time, hours) | ||
| Without active mating partnersa | 4.112 ± 0.092 | 6.834 ± 0.124 |
| With active mating partners | 4.527 ± 0.108 | 7.442 ± 0.126 |
| Cost of sexb | 0.101 ± 0.006 | 0.089 ± 0.011 |
Only data from SSΔN medium are shown. All values represent the means ± standard deviations of eight repeats.
There was no difference between the two negative treatment groups: one group with only one strain and the other group with a strain of the same mating type but different auxotrophic markers (see materials and methods for details of the two treatment groups).
The cost of sex is calculated as (Gp − Ga)/Ga, where Gp is the generation time in the presence of active mating partners and Ga is the generation time in the absence of active mating partners (see materials and methods for details).
This ∼10% reduction in vegetative fitness of the two original clones in the presence of compatible mating partners was medium specific. On nitrogen-rich agar media such as SS and YEPD, no mating was observed and there was no difference for either parent in vegetative growth rates between those with and those without compatible mating partners (data not shown).
Correlation between the cost of sex and mating success rate:
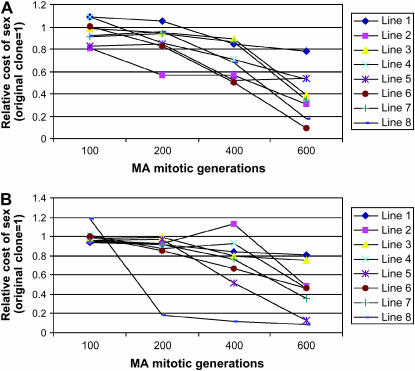
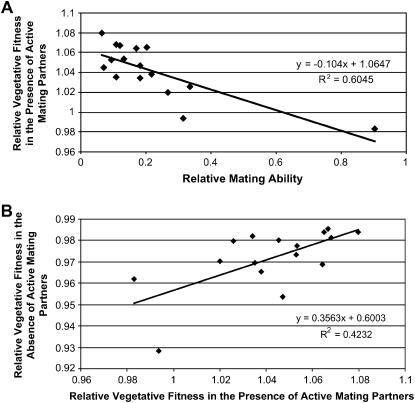
The above results suggested that the reduction of vegetative fitness was due to the presence of active mating partners. Therefore, we proceeded to test the hypothesis that this cost should be reduced for strains with reduced mating abilities. Indeed, when the MA clones with reduced mating abilities were mixed with the original clones of opposite mating types on SSΔN agar, their cost of sex was reduced relative to those of the original clones. Figure 1 shows the changes in the cost of sex for each of the 16 MA lines. A summary result of the 16 MA clones from the 30th transfer (i.e., after 600 mitotic generations of mutation accumulation) is presented in Table 2. As expected, among these MA clones, there was a negative correlation between mating success rate and the relative vegetative fitness in the presence of active mating partners (Pearson correlation coefficient r = −0.777, P < 0.0001; Figure 2a). This result is consistent with the hypothesis that the cost of sex is greater with increasing mating ability.
Figure 1.
The reduction in the cost of sex among MA clones over time. (A) MA lines from strain JEC50. (B) MA lines from strain MCC3. In both charts, the x-axis represents the number of mitotic generations the MA clones had gone through. The y-axis represents the relative cost of sex of the MA clones relative to that of the starting clones. Only the reduction in the cost of responding to their mating partner is shown here. Each data point represents the mean of three repeats.
TABLE 2.
Reductions in the cost of sex in asexually evolved lines in Cryptococcus neoformans
| MA lines of progenitor
|
||
|---|---|---|
| Traits | JEC50 (MATα ade) | MCC3 (MATacna ura) |
| Mating ability | 1.656 ± 1.643%*** | 1.002 ± 0.507%*** |
| Vegetative fitness on SSΔN | ||
| Without mating partners | 4.251 ± 0.179* | 7.009 ± 0.181* |
| With mating partners | 4.422 ± 0.253** | 7.285 ± 0.194** |
| Cost of sexa | 0.040 ± 0.022*** | 0.039 ± 0.023*** |
| Relative cost of sex on SSΔNb | ||
| Cost in response to mating partners | 0.398 ± 0.219*** | 0.442 ± 0.257*** |
| Cost exerted on mating partners | 0.715 ± 0.179*** | 0.775 ± 0.148*** |
Only data from generation 600 (30th transfers) are shown. The means and standard deviations for each strain background are from eight independent MA lines after ∼600 mitotic generations of mutation accumulation.
For calculations, see footnote b in Table 1.
These relative costs represent the ratios between those of the MA clones over those of the original clones, with those of the original clones scaled to 1.
Significant differences between the MA clones and their respective progenitor clones are shown at *P < 0.05, **P < 0.01, and ***P < 0.001, respectively. Although the two parental strains differ significantly in their intrinsic fitness, there was no significant difference in the changes of vegetative fitness and relative costs of sex after 600 MA mitotic generations.
Figure 2.
Correlations between traits among MA clones of C. neoformans. Because clones within each MA line are not independent, only data from generation 600 were used. (a) A negative correlation between mating ability (x-axis) and the relative vegetative fitness in the presence of active mating partners (y-axis) (r = −0.777; P < 0.0001). (b) A positive correlation between the relative vegetative fitness in the presence (x-axis) and absence (y-axis) of active mating partners (r = 0.651; P = 0.006). There was a negative correlation between mating ability and relative vegetative fitness in the absence of active mating partners (r = −0.357); however, similar to that reported in an earlier study on YEPD medium (Xu 2002), this correlation was statistically not significant (P = 0.175).
The reduction in the cost of interacting with mating partners among the MA clones was not due to greater intrinsic vegetative fitness of these clones. In fact, compared to the original clones, after 600 generations of mutation accumulations, the vegetative fitness of all MA clones decreased slightly in the absence of mating partners on SSΔN agar (Table 2). Among the MA clones, these two vegetative fitness components were positively correlated (r = 0.651, P = 0.006; Figure 2b), indicating that at least some of the accumulated mutations have deleterious effects on vegetative fitness in both testing environments (i.e., the presence and absence of mating partners on SSΔN agar). However, there was no significant correlation between vegetative fitness in the absence of active mating partners and the relative mating ability.
Two types of cost of interacting with mating partners:
Sexual mating in eukaryotes is a highly complex and interactive process. Current evidence suggests that in C. neoformans, pheromone production, secretion, and reception; conjugation tube formation; cell size change; cell-cell recognition; and fusion are controlled by multiple signal transduction pathways (Lengeler et al. 2000; Hull and Heitman 2002; McClelland et al. 2004). When active mating partners are incubated together, the cost of interaction on vegetative fitness may contain three components: the cost of producing mating signals that will exert its effect on its mating partners, the cost associated with responding to mating signals from its mating partners, and the mating partner-specific interaction effects.
In a typical mating, the individual influences of these three components cannot be determined because only the total effect is measurable and expressed in a change of relative vegetative fitness. However, when MA clones are compared to their original clones, the reductions in two of the three costs may be obtained, assuming that the interaction effect is negligible (however, see the discussion). One reduction refers the effect of mutations that decreases mating signal production, secretion, and other associated traits that exert effects on mating partners. Assuming that the original clone had the same capacity to respond to different mating partners, this reduction could be estimated by comparing the vegetative fitness of the original clone in two environments: (i) in the presence of the MA clones of the opposite mating type and (ii) in the presence of the original clone of the opposite mating type (i.e., the progenitor of the MA clone). Indeed, a significant reduction in this cost was observed among the MA clones (Table 2). Compared to the original clones, the MA clones from generation 600 imposed an average cost of 74.525% (SD ± 17.173%, N = 16) of their original ancestral clones to their mating partner (Table 2). MA clones from the two mating-type backgrounds showed a similar rate of reduction in this cost (Table 2).
The second type of reduction was that associated with responding to active mating partners. To estimate this reduction, we assumed that the two original clones maintained the same capacity for exerting costs on mating partners. Therefore, in mating mixtures between the MA clones of one mating type and the original clone of the opposite mating type, the relative change in the cost of sex of the MA clones (in relation to their progenitor clone) would be the result of altered response to the same mating partners. After ∼600 mitotic generations, these MA clones showed an average of 41.974% (±23.186%) of the cost of their progenitor clones (Table 2). Similar to the reduction in cost exerted on mating partners, there was no significant difference between the MA lines from JEC50 and MCC3 in the reductions of cost associated with responding to mating partners. Overall, this rate of reduction was significantly greater than that for effects exerted on mating partners (Student's t = 4.606, P < 0.001). These two reductions of costs were not correlated (r = 0.276, P = 0.301).
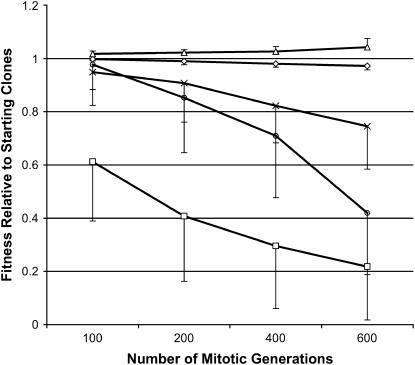
Figure 3 summarizes the patterns of change in relative vegetative fitness in the presence and absence of active mating partners, in mating ability, and in the reduction of the two types of cost associated with interacting with mating partners among the MA clones on SSΔN medium. Because of a lack of statistically significant difference between MA clones from JEC50 and those from MCC3 in the patterns of changes, the data from all 16 MA lines were combined in Figure 3.
Figure 3.
Changes of relative phenotypic values of five traits in 16 asexually evolved populations of C. neoformans. The x-axis represents the number of asexual generations and the y-axis represents phenotypic values relative to those of the starting clones (1 for all traits). Triangles represent relative vegetative growth rate in the presence of an active mating partner. Diamonds represent relative vegetative growth rate in the absence of an active mating partner. X's represent the relative cost of the asexually evolved clones exerted on their mating partners of the opposite mating types. Circles represent the relative cost of the asexually evolved clones responding to their mating partners of the opposite mating types. Boxes represent relative mating ability. Standard deviations are shown for all data points and they represent those estimated from the 16 independent mutation-accumulation lines.
DISCUSSION
This study unambiguously demonstrated that interacting with active mating partners in a facultative sexual microbe can incur a cost. Among clones derived from a laboratory mutation-accumulation experiment, this cost of sex was positively correlated to mating ability—the greater the mating ability, the greater the reduction in vegetative fitness in the presence of a mating partner. Compared to the original clones, all 16 MA lines at generation 600 showed reduced mating abilities and had lower costs of interacting with mating partners. In addition, assuming the absence of a mating partner-specific interaction effect on the cost of sex, the reductions in the cost of sex observed here among the MA clones were partitioned into that responding to mating partners and that exerting on mating partners.
The assumption for the absence of a mating partner-specific interaction effect in this study is highly tentative and may not be applicable to natural strains in C. neoformans. As in other eukaryotes, sexual mating in C. neoformans is a highly interactive and complex process and many of the genes and their interactions involved in this process remain to be identified and quantified. However, for several reasons, the analyses conducted here are informative. First, although the relative amounts are unknown, under nitrogen-limiting conditions, strains of both MATa and MATα may produce and secrete pheromones on their own, in the absence of a mating partner. Therefore, at least parts of the mating and sexual reproductive pathway in this fungus may be independent of mating partners. Indeed, given sufficient time and suitable conditions, MATα strains may undergo haploid fruiting (Wickes et al. 1996). Second, the MA clones used here were all isogenic to their progenitors except at the loci where new mutations have accumulated. Because each progenitor and its MA-derived clones were all mated to the same original clone of opposite mating type (also isogenic), it is therefore reasonable to assume that changes in both sexual and asexual fitness of the MA clones and their mating partners were due to the accumulated mutations. It is highly possible and likely that using totally different mating partners would result in different estimates of mating abilities and costs of such interactions. Unfortunately, as far as I know, no natural strain with suitable genetic markers is available in C. neoformans var. neoformans to allow comparable analyses and test the possibility of mating partner-specific interaction effect on this cost of sex. In a preliminary screening using JXC4 (to replace the original JEC50 clone) and JEC61 (to replace the original MCC3 clone) as mating partners for 8 of the 16 MA clones from generation 600, we found no significant difference in any of the estimates and no mating partner-specific interaction effect (data not shown). This is not surprising given that JXC4, JEC50, JEC61, and MCC3 are all isogenic except at the indicated loci.
Similar to results from a previous analysis using YEPD medium on the same MA clones (Xu 2002), we found no significant correlation between vegetative fitness in the absence of an active mating partner and mating ability on SSΔN agar. This was expected because these MA clones were derived in the absence of selection and mutations for different fitness components were allowed to accumulate freely and independently. However, in nature, selective forces might have favored either vegetative fitness or mating ability and a negative correlation between these two traits might exist; therefore, the result observed here might not be found in natural populations of C. neoformans. At present, the lack of suitable genetic markers among natural strains prohibits such an investigation. Indeed, investigations of the green alga Chlamydomonas reinhardtii, of higher plants, and of animals have identified that sexual and asexual fitness often showed negative correlations (Bell and Koufopanou 1986; Scheiner et al. 1989; Da Silva and Bell 1992).
In contrast to the lack of correlation between vegetative fitness in the absence of active mating partners and mating ability, a negative correlation was observed between vegetative fitness in the presence of active mating partners and mating ability among the MA clones (Figure 2a). There are two hypotheses for this observed pattern. First, the mutations accumulated here have independent effects on mating ability and vegetative fitness in the presence of active mating partners. The negative correlation observed between these two traits is simply a byproduct of the positive correlation between vegetative fitness in the absence of active mating partners and that in the presence of active mating partners. However, because we observed no significant negative correlation between mating ability and vegetative fitness in the absence of active mating partners, some of the accumulated mutations affecting mating ability must have antagonistically pleiotropic effects on vegetative fitness in the presence of active mating partners and vice versa (hypothesis II). In C. neoformans, when cells of opposite mating types are mixed on a nitrogen-limiting medium, both the MATa and the MATα cells can secrete sex pheromones and release them into the surrounding medium. Because pheromone production, secretion, and response are essential for sexual mating in C. neoformans (and other sexual eukaryotic microbes), in their absence, sexual reproductive life cycles cannot proceed in these organisms. However, pheromone production, secretion, and response are only parts of the mating process and mutations in other genes (e.g., downstream of the pheromone response pathway) can decrease mating ability or completely block the mating process (Hull and Heitman 2002). In extreme cases where no actual mating occurs (i.e., no zygotes are formed), a cost might still exist as a result of the effects exerted by the initial steps of pheromone responses in a mating environment—that is, a cost of sex without the ability to mate could still exist. This could be simply due to being a member of a sexual species and having sexually reproducing ancestors. The greater reduction in mating ability than in the cost of sex among MA clones (Figure 3) is also consistent with the hypothesis: not all mutations affecting mating ability have an effect on the cost of sex. For these reasons, while mating ability and the cost of sex are positively correlated among MA lines investigated here, these two traits cannot be directly scaled to each other. However, as shown here, the cost of sex is context dependent and, as a result, it is essential to present other relevant data (e.g., mating ability or other sexual fitness traits as well as mating partners and mating conditions) when discussing the cost of sex.
The patterns of reduction in the cost of sex within individual MA lines (Figure 1) suggested that multiple genes likely contributed to this phenotype, consistent with what we know about the processes and mechanisms of sexual mating in C. neoformans (Hull and Heitman 2002). Because the progenitor strains and the MA clones are isogenic except at the indicated marker loci, we cannot use typical genetic crosses among these strains to identify the specific regions where the mutations are located. Analyses using whole-genome microarrays could contribute to the identification of such mutations.
The results here clearly demonstrated that interactions with active mating partners in C. neoformans can generate a significant cost. Such a cost might exist in other facultative sexual microbes as well. Indeed, comparable morphological, cellular, and molecular mechanisms for mating have been found among diverse groups of sexual eukaryotic microbes such as the baker's yeast S. cerevisiae (e.g., Bardwell 2004), the unicellular alga C. reinhardtii (Harris 1989), and the slime mold Dictyostelium discoideum (Urushihara 1996). Because natural environments likely have only a limited supply of essential nutrients such as nitrogen during the growths of most microorganisms (including C. neoformans), the conditions for mating might be prevalent. As a result, while this cost of sex may be small initially, it might have been prevalent and must have been compensated for by the production of sexual progeny to allow the origin, persistence, and spread of sexual reproduction in facultative sexual microbes and subsequently to obligate sexual eukaryotes.
Acknowledgments
I thank Jasmine Samra and Maria Memaris for technical assistance and Jim Quinn and Heather Yoell for comments on an earlier version of the manuscript. I greatly appreciate the comments from two anonymous reviewers and from the associate editor, Marcy Uyenoyama. This project was supported by funding from McMaster University, the Natural Science and Engineering Research Council of Canada, the Premier's Research Excellence Award, the Canadian Foundation for Innovation, and the Ontario Innovation Trust.
References
- Bardwell, L., 2004. A walk-through of the yeast mating pheromone response pathway. Peptides 25: 1465–1476. [DOI] [PubMed] [Google Scholar]
- Bell, G., 1982. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. University of California Press, Berkeley, CA.
- Bell, G., and V. Koufopanou, 1986. The cost of reproduction. Oxf. Surv. Evol. Biol. 3: 83–131. [Google Scholar]
- Casadevall, A., and J. R. Perfect, 1998. Cryptococcus neoformans. ASM Press, Washington, DC.
- Dacks, J., and A. J. Roger, 1999. The first sexual lineage and the relevance of facultative sex. J. Mol. Evol. 48: 779–783. [DOI] [PubMed] [Google Scholar]
- Da Silva, J., and G. Bell, 1992. The ecology and genetics of fitness in Chlamydomonas VI. Antagonism between natural selection and sexual selection. Proc. R. Soc. Lond. Ser. B 249: 227–233. [Google Scholar]
- Guthrie, C., and G. R. Fink, 1991. Guide to Yeast Genetics and Molecular Biology. Academic Press, San Diego.
- Harris, E. H., 1989. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego. [DOI] [PubMed]
- Hull, C. M., and J. Heitman, 2002. Genetics of Cryptococcus neoformans. Annu. Rev. Genet. 36: 557–615. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung, K. J., 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 67: 821–833. [PubMed] [Google Scholar]
- Kwon-Chung, K. J., J. C. Edman and B. L. Wickes, 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60: 602–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler, K. B., R. B. Davison, C. D'Souza, T. Harashima, W.-C. Shen et al., 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64: 746–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler, K. B., D. S. Fox, J. A. Fraser, A. Allen, K. Forrester et al., 2002. Mating type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot. Cell 1: 704–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland, C. M., Y. C. Chang, A. Varma and K. J. Kwon-Chung, 2004. Uniqueness of the mating system in Cryptococcus neoformans. Trends Microbiol. 12: 208–212. [DOI] [PubMed] [Google Scholar]
- Michod, R. E., and B. R. Levin, 1988. The Evolution of Sex: An Examination of Current Ideas. Sinauer Associates, Sunderland, MA.
- Scheiner, S. M., R. L. Caplan and R. F. Lyman, 1989. A search for trade-offs among life history traits in Drosophila melanogaster. Evol. Ecol. 3: 51–63. [Google Scholar]
- Sia, R. A., K. B. Lengeler and J. Heitman, 2000. Diploid strains of the pathogenic basidiomycete Cryptococcus neoformans are thermally dimorphic. Fungal Genet. Biol. 29: 153–163. [DOI] [PubMed] [Google Scholar]
- Sokal, R. R., and F. J. Rohlf, 1981. Biometry: The Principles and Practices of Statistics in Biological Research, Ed. 2. W. H. Freeman, New York.
- Urushihara, H., 1996. Choice of partners: sexual cell interactions in Dictyostelium discoideum. Cell Struct. Funct. 21: 231–236. [DOI] [PubMed] [Google Scholar]
- Wickes, B. L., M. E. Mayorga, U. Edman and J. C. Edman, 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type. Proc. Natl. Acad. Sci. USA 93: 7327–7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., 1995. Analysis of inbreeding depression in Agaricus bisporus. Genetics 141: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., 2002. Estimating the spontaneous mutation rate of loss of sex in the human pathogenic fungus Cryptococcus neoformans. Genetics 162: 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., 2004. a The prevalence and evolution of sex in microorganisms. Genome 47: 775–780. [DOI] [PubMed] [Google Scholar]
- Xu, J., 2004. b Genotype-environment interactions of spontaneous mutations affecting vegetative fitness in the human pathogenic fungus Cryptococcus neoformans. Genetics 168: 1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., and T. G. Mitchell, 2003. Comparative gene genealogical analyses of strains of serotype AD identify recombination in populations of serotypes A and D in the human pathogenic yeast Cryptococcus neoformans. Microbiology 149: 2147–2154. [DOI] [PubMed] [Google Scholar]
- Xu, J., P. A. Horgen and J. B. Anderson, 1996. Variation in mating interactions in Agaricus bisporus. Cultivated Mushroom Res. Newsl. 3: 23–30. [Google Scholar]
- Yan, Z., C. M. Hull, J. Heitman, S. Sun and J. Xu, 2004. SXI1α controls uniparental mitochondrial inheritance in Cryptococcus neoformans. Curr. Biol. 14: R743–R744. [DOI] [PubMed] [Google Scholar]