Abstract
The frizzled (fz) gene in Drosophila controls two distinct signaling pathways: it directs the planar cell polarization (PCP) of epithelia and it regulates cell fate decisions through Armadillo (Arm) by acting as a receptor for the Wnt protein Wingless (Wg). With the exception of dishevelled (dsh), the genes functioning in these two pathways are distinct. We have taken a genetic approach, based on a series of new and existing fz alleles, for identifying individual amino acids required for PCP or Arm signaling. For each allele, we have attempted to quantify the strength of signaling by phenotypic measurements. For PCP signaling, the defect was measured by counting the number of cells secreting multiple hairs in the wing. We then examined each allele for its ability to participate in Arm signaling by the rescue of fz mutant embryos with maternally provided fz function. For both PCP and Arm signaling we observed a broad range of phenotypes, but for every allele there is a strong correlation between its phenotypic strength in each pathway. Therefore, even though the PCP and Arm signaling pathways are genetically distinct, the set of signaling-defective fz alleles affected both pathways to a similar extent. This suggests that fz controls these two different signaling activities by a common mechanism. In addition, this screen yielded a set of missense mutations that identify amino acids specifically required for fz signaling function.
ANIMAL development requires the interplay between cell fate decisions and morphogenetic events. It is not well understood how cell fate decisions, which are the outcome of changes in gene regulation, are coordinated with changes in morphology, such as cell movement, shape change, and polarization. The frizzled (fz) gene in Drosophila has the remarkable ability to control both cell fate decisions and the planar polarization of epithelia. What makes fz exceptional is that it interacts with two distinct signaling cascades to accomplish these functions. We are trying to understand the mechanism that regulates the switch between these two pathways. Uncovering how fz chooses between these two functions will provide insight into how morphogenesis and cell fate choices are orchestrated.
During Drosophila development, one of the first functions of fz is to pattern the embryo. Cells of the embryonic epidermis secrete a cuticle that displays a reiterative pattern of denticle belts separated by regions of smooth (naked) cuticle. Wg, the Drosophila Wnt-1 ortholog, is responsible for specifying the naked cuticle cell fate. During signaling, Wg acts through the related receptors Fz and Frizzled-2 (Fz2), which are genetically redundant (Bhat 1998; Kennerdell and Carthew 1998; Bhanot et al. 1999; Chen and Struhl 1999; Muller et al. 1999). Frizzled proteins consist of an amino-terminal domain, situated outside of the cell, called the cysteine-rich domain (CRD), followed by a seven-transmembrane signaling moiety. Several studies have shown that the CRD is necessary and sufficient for Wnt binding (Bhanot et al. 1996; Hsieh et al. 1999; Dann et al. 2001). In combination with Arrow (Arr) (Wehrli et al. 2000), another transmembrane protein, the Fz proteins transduce Wg signaling to a cytoplasmic complex among the proteins Axin (Axn), Armadillo (Arm, β-catenin), Adenomatous polyposis coli (APC), and Zw3 (Zw3), which regulates the stability of the Arm protein, normally leading to its degradation. Upon Fz-mediated activation of Dishevelled (Dsh), the degradation of cytoplasmic Arm is blocked. As Arm accumulates in the cytoplasm and nucleus, it interacts with Pangolin (Pan), a transcription factor from the T-cell factor/lymphoid enhancer factor family to regulate target gene expression (reviewed in Logan and Nusse 2004; Tolwinski and Wieschaus 2004; Bejsovec 2005). In wg, dsh, arm, and pan mutants or in fz, fz2 double mutants, naked cells are no longer specified and only denticle-producing cells remain, resulting in the characteristic “lawn of denticles” phenotype (Nusslein-Volhard and Wieschaus 1980; Wieschaus and Riggleman 1987; Perrimon et al. 1989, 1996; Brunner et al. 1997; van de Wetering et al. 1997;Chen and Struhl 1999). Because of the crucial role of Arm, we refer to this cell fate mode of fz signaling as Arm signaling, a process essential for many developmental events in Drosophila.
fz has another, genetically distinct, and nonredundant function in Drosophila. It is required to direct polarization of epithelia covering the surface of the fly (Vinson and Adler 1987). The adult cuticle contains numerous elements that are organized in a plane orthogonal to the apical-basal axis and that are oriented relative to the body axes. For example, wing hairs point toward the distal tip of the wing and hairs and bristles on the dorsal thorax point posteriorly. This type of epithelial polarity is referred to as PCP and has been reviewed recently (Adler 2002; Tree et al. 2002a; Strutt 2003; Fanto and McNeill 2004).
Genetic screens in Drosophila have uncovered a core set of genes acting with fz to polarize tissues. These are dsh (Klingensmith et al. 1994; Theisen et al. 1994), prickle (pk) (Gubb et al. 1999), van gogh/strabismus (vang) (Taylor et al. 1998; Wolff and Rubin 1998), and starry night/flamingo (stan) (Chae et al. 1999; Usui et al. 1999). Together, these genes act through the small GTPase rhoA (rhoI) and rho-kinase (rok) to regulate the cytoskeleton, leading to the polarization of cells (Strutt et al. 1997; Winter et al. 2001). While fz and dsh participate in both Arm and PCP signaling, none of the other Arm signaling components, most notably arr, zw3, and arm, have PCP phenotypes (Axelrod et al. 1998; Wehrli et al. 2000).
Little is known about the activation of the Fz protein in both PCP and Arm signaling. Furthermore, it is not understood what regulates the separation into two signaling pathways downstream of Fz, sometimes called canonical and noncanonical signaling. Are there separate pools of Fz committed to each function or does the switch between the two pathways occur downstream of a common interaction? Understanding how these two pathways are controlled will provide insight into how cell fate choices are coordinated with tissue morphogenesis. A related question is how the differences in the two fz genes in Drosophila, fz and fz2, are translated into different functions: the Fz2 protein participates only in Arm signaling while Fz can regulate both Arm and PCP signaling (Chen and Struhl 1999). Initial experiments using chimeric molecules have indicated that the unique role of the Fz2 protein in mediating signaling from Wg to Arm is due to the high affinity of the ligand-binding domain, the CRD, for Wg (Rulifson et al. 2000). The transmembrane region of Fz allows it to couple to PCP signaling (Boutros et al. 2000). Recently, it has also been suggested that different subcellular distributions between Fz and Fz2 proteins play an important role in the choice between Arm and PCP signaling (Wu et al. 2004).
In this article, we have taken a genetic approach to examine the two functions of Fz, asking whether we could isolate specific alleles that affect fz signaling in either the PCP or the Arm pathway. Several alleles of fz that affect PCP signaling are known, identifying residues in cytoplasmic loops and other domains(Adler et al. 1994; Jones et al. 1996). In addition, various site-directed mutations in fz transgenes have been made to examine consequences for Wnt signaling (Boutros et al. 2000; Rulifson et al. 2000; Umbhauer et al. 2000; Strapps and Tomlinson 2001; Chen et al. 2004; Cong et al. 2004). These mutagenesis experiments have pointed at the need for conserved amino acids in the cytoplasmic tail. However, no study has measured how these mutations control one pathway or the other. Receptors that control different pathways often do so by using separate domains to interact with different downstream effectors. Such domains can be mapped by mutations that affect one pathway vs. another pathway (Tallquist et al. 2003).
In this work, we analyze a set of molecularly characterized fz alleles for signaling activity. We have restricted our survey to endogenous fz alleles, rather than generating ectopically expressed transgenes. By taking advantage of the ability of maternal fz to function in the Drosophila embryo as a receptor for Wg, we have directly compared how fz alleles that have PCP phenotypes in adult flies behave in the embryo to control Arm signaling. Moreover, by measuring signaling functions in a semiquantitative way, we compare allelic strength in both pathways.
MATERIALS AND METHODS
Isolation of fz alleles:
Isogenic w; P{neoFRT}80B males were mutagenized with ENU using standard protocols (Ashburner 1989; Greenspan 1997). Groups of males were mated en masse to w; fzK21/TM6C Sb Tb e virgin females. Approximately 30,000 nonbalanced F1 progeny were screened for a fz phenotype. The mutagenized chromosome was tested for germline transmission and isolated by mating F1 mutants to w; fzK21/TM6c Tb Sb e and rescreening for the fz phenotype. Forty alleles showed a PCP phenotype. Of the 40 potential alleles, 12 were transmitted to the germline. The remainder either were sterile or did not transmit the mutation due to mosaicism inherent in F1 screens (Greenspan 1997). The sex ratio of germline transmission was 10 males and 2 females, in approximately equal proportion to the number originally isolated, 29 males and 11 females. Stocks were established for the mutants with germline transmission. We obtained from Paul Adler the 8 previously described missense alleles fzHE11, fzJ22, fzF31, fzR53, fzGL31, fzR54, fzHD21 and fzR52 and 3 amorphic alleles fzD21 (deletion), fzK21 (inversion), and fzP21 (frameshift causing a premature stop) (Jones et al. 1996).
Polarity signaling strength of fz alleles:
Polarity signaling strength was determined in animals trans-heterozygous for the allele to be counted and for fzK21, an amorphic allele. We counted the number of MHCs in a compartment on the ventral surface of the wing delimited in the anterior-posterior axis by the first and second wing veins and in the proximal-distal axis by the first 15 stout margin bristles on the dorsal surface of the wing margin, similar to Winter et al. (2001). Ten wings of individual animals were counted for each wing. MHCs were also counted using fzGL31 as a reference allele with similar results.
Wingless transduction strength of fz alleles:
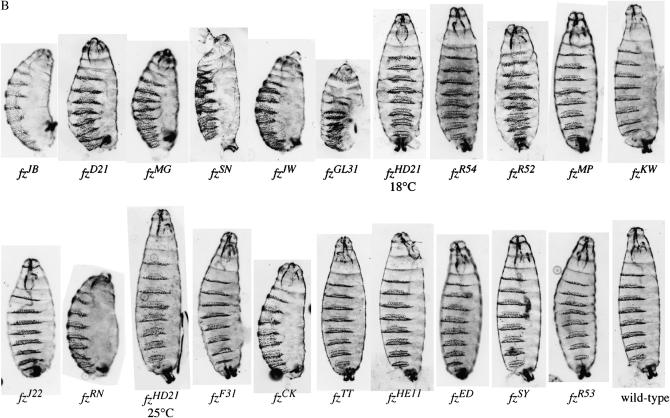
Embryos were collected from a cross of fz*/fzGL31 fz2e2 females and fzGL31 fz2e2/TM3 males. Twenty-five percent of the progeny are homozygous fzGL31 fz2e2 and receive a maternal contribution of fz*, the allele to be tested, and fzGL31 (Jones et al. 1996). For alleles displaying a strong or moderate phenotype, a representative cuticle was photographed. In these two phenotypic classes, ∼25% of the cuticles displayed a phenotype. For alleles with a weak phenotype, the number of cuticles with an observable defect was <25%. For this class we photographed cuticles showing defects.
Western blot of fz alleles:
Embryos were collected from a sibling cross of fz*/fzP21 Df(3L)fz2 flies. The only Fz in these animals will be translated from fz* since fzP21 is a protein null allele. Embryos were lysed using a Dounce homogenizer with a tight-fitting pestle in TNT buffer (150 mm NaCl, 50 mm Tris pH 7.5, and 1% Triton-X100) supplemented with protease and phosphatase inhibitors. Fz was immunoprecipitated from 300 μg of extract using 2 μl of a polyclonal antibody, NFzD, directed against amino acids 158–231 and 5 μl of a 1:1 slurry of protein-A sepharose (Pharmacia) in TNT buffer. Immunoprecipitated material was Western blotted. Fz was detected using a monoclonal antibody, clone 1C11 obtained from the Developmental Studies Hybridoma Bank (http://www.uiowa.edu/∼dshbwww/).
Molecular characterization fz alleles:
The fz genomic locus is large with the coding region distributed over 90 kb in five exons (Adler et al. 1990). We sequenced RT-PCR products produced from the 12 alleles that we isolated and fzGL31 (Jones et al. 1996). The sequenced allele was isolated either from homozygous flies or in trans with fzK21, an amorphic allele that is a euchromatic inversion breaking within a 46-kb region critical for fz function (Adler et al. 1990). Total RNA was isolated from three female flies using Trizol (GIBCO, Gaithersburg, MD). Approximately 2 μg of total RNA was primed with oligo(dT) and reversed transcribed with Thermoscript (Invitrogen, San Diego). Two microliters of the resulting cDNA was used in a 50-μl PCR reaction. Because the fzK21 allele does not contribute to the reaction, amplified products result exclusively from the mutant chromosome. The product was purified using a PCR purification column (QIAGEN) and ∼100 ng per reaction was used as a template for fluorescent dye terminator sequencing.
The RT-PCR primers used were:
sense, GATCGAGAAAAAGCCCCAAAA
antisense, GGAGTAAGGGGCGTGGACTTG.
The sequencing primers used were:
P1, GGACTAGTATGTGGCGTCAAATCCTG
P2, ATGGAGGCGAGGATTTGTG
P3, CCTTCTTGATTGACTCGTCGC
P4, TTATTCCACCTGGTTGCCTG
P5, TGGGCTGCTTGTTCTACGAG
M1, ACAACTGGAGGTCATCACTGC
M2, TTTAGTTCGTAGCCCATTCCC
M3, CAAAAACCAGGCGAATGC
M4, AAGCAGCCCAGTAATCCCAC
M5, GCTCTAGACTAGACGTACGCCTGCGC.
Isolation of fz2 alleles:
Isogenic fzGL31 th st/TM6b Tb males were mutagenized with EMS using standard protocols (Roberts 1986; Ashburner 1989). Groups of males were mated en masse to virgin females ftz e/TM6C Sb Tb. In the F1 generation, single males fzGL31 th st */TM6C Sb Tb (asterisk denotes mutation induced by EMS) were mated to fzR52 Df(3L)fz2/TM6b Tb females. The F2 generation were screened for the absence of the fzGL31 th st */fzR52 Df(3L)fz2 progeny and, if absent, the mutagenized chromosome was isolated from the fzGL31 th st */TM6b Tb e siblings. A total of 8500 chromosomes were screened and 34 lethal mutations were isolated in three complementation groups. Group I had six members that stained negatively for Fz2 protein in embryos (Muller et al. 1999) and displayed a wg phenotype when tested for the ability to pattern embryos. All but one had mutations in the fz2 ORF. Group II contained the gene reptin, a negative regulator of Wg signaling (Bauer et al. 2000). Group III remains uncharacterized.
Molecular characterization of fz2 alleles:
To characterize the mutations in fz2, we amplified the coding exon of fz2 from genomic DNA by PCR using primers 1 (sequence CCA AGG GGT TCC AAG TCA) and 2 (sequence ACC GTG CTG CTG CTC ATC). For each mutation the ORF was subcloned into pCR2.1 using the TopoTA system (Invitrogen). Each ORF was fully sequenced on both strands and a double peak indicated the presence of a mutation.
RESULTS
Screen and molecular characterization of fz alleles:
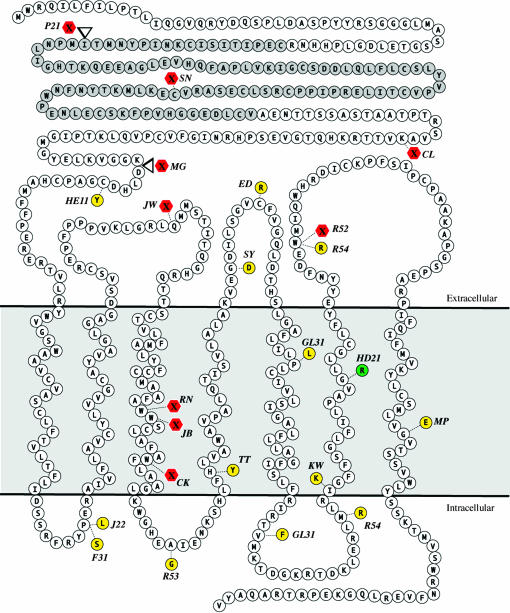
The analysis described here is based on comparing the strength of fz alleles in PCP and Arm signaling, initially by isolating and characterizing fz alleles affecting PCP. Mutations in fz are viable and result in the disruption of PCP of tissues, including the hairs on the wing and bristles on the dorsal thorax (Gubb and Garcia-Bellido 1982; Vinson and Adler 1987). By screening for a PCP phenotype on the thorax and wing due to lack of complementation of fzK21, a null inversion allele (Adler et al. 1990), we isolated 12 ENU-induced fz alleles (see materials and methods). We combined these with a set of 9 previously isolated EMS-induced fz alleles (Adler et al. 1987; Jones et al. 1996) for a total of 21. The new alleles were sequenced and the molecular lesion associated with each is presented in Table 1 and schematically in Figure 1. Of notable interest, there are 12 missense mutations, which are located throughout the transmembrane portion of the receptor (Figure 1). We found no missense mutations in the CRD, suggesting that this region of the Fz protein is dispensable for PCP signaling. Since the CRD is responsible for Wnt binding, this result is consistent with recent work suggesting that indeed Fz may not utilize a Wnt ligand in PCP signaling (Adler 2002; Lawrence et al. 2002; Amonlirdviman et al. 2005). In parallel to our screen for fz alleles, we also conducted a screen for novel alleles of fz2, the other Wg receptor in Drosophila. Fz2 is primarily involved in Arm signaling (Chen and Struhl 1999). Thus, we reasoned that screening for alleles affecting its function might generate mutations in the CRD. We recovered 5 new alleles of fz2 (Table 2); however, none have mutations within the CRD and these alleles are not considered further.
TABLE 1.
Summary of fz alleles
| Allele | Mutation | Location of mutation | Abundance of Fz protein | Apparent mass of Fz protein | PCP signaling phenotype | Arm signaling phenotype |
|---|---|---|---|---|---|---|
| fzP21 (Joneset al. 1996) | Insertion, X | CRD | None | — | ND | ND |
| fzSN | C134X | CRD | ND | ND | Strong | Strong |
| fzCL | K184X | Extracellular “hinge” | ND | ND | Strong | ND |
| fzMG | Novel splice acceptor, X | Extracellular hinge | None | — | Strong | Strong |
| fzHE11 (Joneset al. 1996) | C229Y | Extracellular hinge | Normal | Larger | Weak | Weak |
| fzJ22 (Joneset al. 1996) | P278L | First intracellular loop | Normal | Normal | Moderate | Moderate |
| fzF31 (Joneset al. 1996) | P278S | First intracellular loop | Normal | Normal | Weak | Weak |
| fzJW | Q322X | First extracellular loop | Lower | Smaller | Strong | Strong |
| fzRN | W354X | Third transmembrane domain | Lower | Smaller | Weak | Strong |
| fzJB | W355X | Third transmembrane domain | None | — | Strong | Strong |
| fzCK | W362X | Third transmembrane domain | Lower | Smaller | Weak | Strong |
| fzR53 (Joneset al. 1996) | A374G | Second intracellular loop | Normal | Normal | Weak | Weak |
| fzTT | H383Y | Fourth transmembrane domain/second intracellular loop | Normal | Normal | Weak | Weak |
| fzSY | G405D | Second extracellular loop | ND | ND | Weak | Weak |
| fzED | C412R | Second extracellular loop | Normal | Normal | Weak | Weak |
| fzGL31 (Joneset al. 1996) | P429L, V454F | Fifth transmembrane domain, third intracellular loop | Lower | Larger | Moderate | Strong |
| fzR54 (Joneset al. 1996) | M469R, W500R | Third intracellular loop, third extracellular loop | Lower | Larger | Moderate | Moderate |
| fzKW | I472K | Sixth transmembrane domain/third intracellular loop | Lower | Normal | Moderate | Moderate |
| fzHD21 (Adleret al. 1994) | G485R | Sixth transmembrane domain | Lower (18°), very low (25°) | Larger | Moderate (18°), weak (25°) | Moderate (18°), moderate (25°) |
| fzR52 (Joneset al. 1996) | W500X | Third extracellular loop | Very low | Smaller | Moderate | Moderate |
| fzMP | G545E | Seventh transmembrane domain | Lower | Normal | Moderate | Moderate |
Figure 1.
Schematic of fz alleles. Mutations found in fz alleles isolated in PCP screens are located throughout the transmembrane portion of the receptor. There are no missense mutations located in the CRD, shaded in gray. The cold-sensitive mutation encoded by the fzHD21 allele is green. With the exception of fzHD21, all other missense mutations (yellow) affect amino acids that are conserved in the frizzled family. Nonsense mutations are red. Mutations that insert amino acids are indicated with open triangles. All insertion mutations encounter a stop codon that results in the synthesis of a truncated protein.
TABLE 2.
Summary of fz2 alleles
| Allele | Mutation | Location of mutation |
|---|---|---|
| fz2e1 | Q268X | Extracellular hinge |
| fz2e2 | W285X | Extracellular hinge |
| fz2e3 | P487L | Fifth transmembrane domain |
| fz2e4 | W603X | Seventh TM/cytoplasmic |
| fz2e5 | W603X | Seventh TM/cytoplasmic |
| fz2e6 | None detected | NA |
PCP signaling function of fz alleles:
Having collected a significant set of fz alleles, we first assessed their relative signaling strength in PCP. Each cell of the wing epithelium secretes a single hair (Wong and Adler 1993). In addition to having abnormal hair polarity, fz mutations cause cells to secrete multiple hairs (Figure 2) (Adler et al. 1987). Quantifying the occurrences of multiple hair cells (MHCs) provides an informative measure of fz signaling strength (Adler et al. 1987; Krasnow and Adler 1994). By this analysis, strong hypomorphic alleles can be distinguished from null alleles in cases in which studying hair polarity alone cannot (Adler et al. 1987).
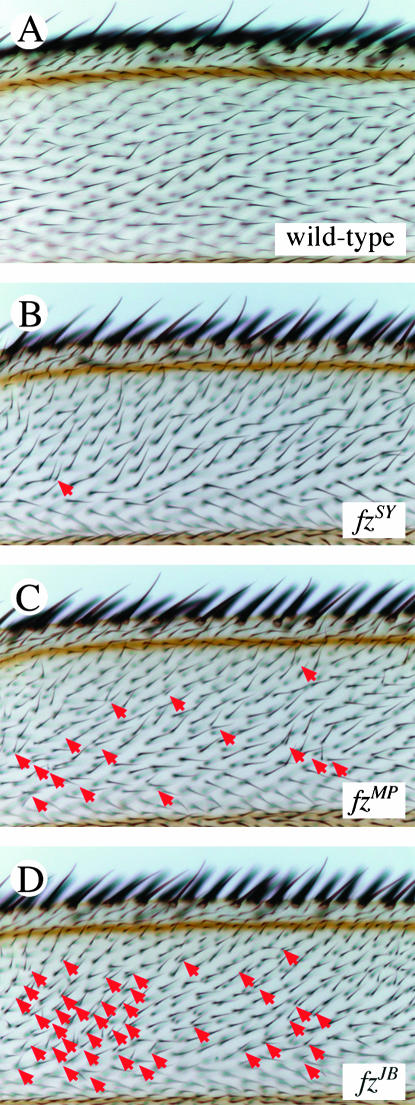
Figure 2.
The MHC phenotype of fz mutants. This portion of the wing contains the region of the anterior ventral surface used to quantify the MHCs of the fz alleles (see materials and methods). (A) Cells in a wild-type wing each secrete a single hair. (B) In a weak fz mutant, fzSY, some cells may secrete more than one hair (red arrows). The number of MHCs increases in moderate (fzMP) (C) and strong (fzJB) (D) fz mutants. The number of MHCs observed in this region of the wing is a strong predictor of the severity of the phenotype observed in other tissues, such as the bristles on the dorsal thorax.
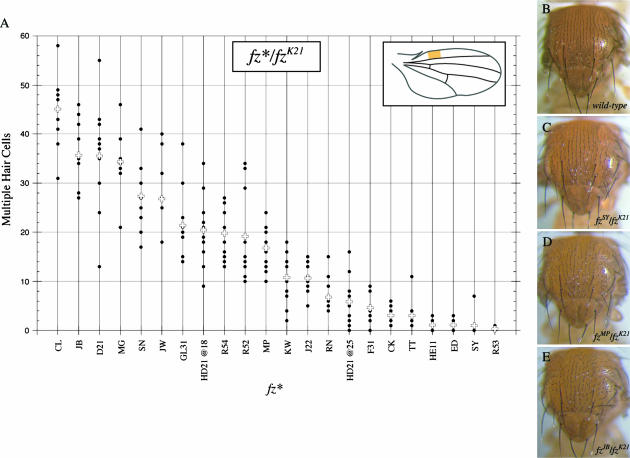
We assayed the number of MHCs for all of the fz alleles in trans over a null allele, fzK21 (Figure 3A). The alleles fall into a broad range. Significantly, there is a direct correlation between the number of MHCs and the extent of the hair polarity disruption that is observed in wing hairs (data not shown) or bristles on the dorsal thorax (Figure 3, B–E). Mutations that lead to only a few MHCs have a weak polarity phenotype (Figure 3C). Mutations that produce more MHCs lead to more pronounced disruptions of the thorax bristles (Figure 3, D and E). Similar results were obtained when we counted the number of MHCs in trans over a hypomorphic allele, fzGL31. Despite small changes in the order, the alleles fell into approximately the same range that fzK21 did and showed a good correlation between the number of MHCs and the PCP phenotype on the dorsal thorax (data not shown). Thus, the MHC phenotype is useful as a semiquantifiable measure of PCP signaling in fz mutants and allowed us to rank the fz alleles on the basis of their PCP signaling strength.
Figure 3.
PCP signaling strength of fz alleles. (A) The number of MHCs was counted in a section of the ventral wing between the first and second wing veins in a region defined by the first 15 stout margin bristles (inset). All alleles (fz*) were assayed in trans with fzK21, a null allele. The number of MHCs was plotted for each of 10 wings (solid circle) along with their average (plus symbol). Some alleles appear to have <10 data points because the dots overlap due to identical values. The number of MHCs correlates with the severity of the polarity disruptions in other tissues, such as the dorsal thorax. Alleles that display the greatest numbers of MHCs have the strongest polarity phenotypes in other tissues. fzD21 is an amorphic deletion allele (Jones et al. 1996). (B) Photomicrograph of the dorsal thorax of a wild-type fly. Note the parallel arrangement and uniform posterior polarity of the bristles. (C) Alleles with the fewest number of MHCs have near wild-type bristle polarity. Only slight deviations from the normal posterior alignment are observed in fzSY/fzK21. (D) Alleles with an intermediate number of MHCs also exhibit a mild bristle phenotype on the thorax. The majority of the lateral bristles in fzMP/fzK21 deviate from wild type by 45° or less. (E) Lateral bristles in fzJB/fzK21 that normally point posteriorly frequently deviate from their normal orientation up to 90°, pointing straight toward the middle of the thorax. When all the alleles were assayed in trans with the hypomorphic allele fzGL31, the ranking was similar with only minor differences (data not shown). In addition, when fzGL31 was used as the reference allele, the correlation remained between the number of MHCs and the polarity phenotypes observed in other tissues, such as the thorax (data not shown).
Arm signaling function of fz alleles:
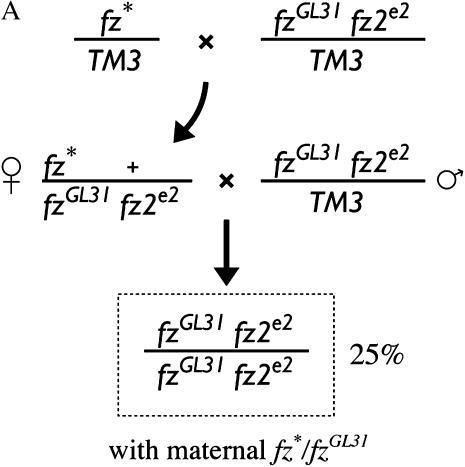
Next we asked whether the new and previously isolated fz alleles also exhibit a range of phenotypes when assayed for their Arm signaling function. In embryos, both fz and fz2 can function as a Wg receptor (Bhat 1998; Kennerdell and Carthew 1998; Bhanot et al. 1999; Chen and Struhl 1999; Muller et al. 1999). Because of its redundancy with fz2, the Arm signaling function of fz missense mutations has not been analyzed. We devised an assay to measure the Arm signaling function of the fz alleles by taking advantage of the fact that the maternal contribution of one copy of fz, but not of fz2, is sufficient to specify the normal pattern of naked cuticle in zygotic fz fz2 mutants (Bhanot et al. 1999; Chen and Struhl 1999) (Figure 4A). This assay made use of a chromosome carrying both the strong, but not null, fzGL31 allele (Figures 3 and 4B) (Jones et al. 1996) and the fz2e2 allele (Table 2), which encodes an extracellular stop codon before the first transmembrane domain. A relatively simple crossing scheme yielded informative mutant embryos that displayed a range of cuticle phenotypes (Figure 4B). The alleles broadly fall into three phenotypic classes: (1) strong alleles that cause dramatically shortened embryos and the absence of naked cuticle; (2) moderate alleles that result in modestly shortened embryos and display mild patterning defects such as denticle belt fusions or numerous denticles in regions that should be entirely composed of naked cuticle; and (3) weak alleles that do not affect the length of the embryos and have minor, if any, patterning defects. Thus, as with PCP signaling, the fz alleles also display a range of phenotypes in Arm signaling.
Figure 4.
Arm signaling strength of fz alleles. (A) Cross to test the Arm signaling strength of fz alleles where fz* denotes the allele tested. (B) The fz alleles exhibit a spectrum of Arm signaling phenotypes in embryos. Representative mutant cuticles from a cross of fz*/fzGL31 fz2e2 mothers and fzGL31 fz2e2/TM3 fathers are shown. Twenty-five percent of the progeny are homozygous for the fzGL31 fz2e2 chromosome and receive maternal fz* and fzGL31 transcripts. The order from upper left to lower right was determined by PCP signaling strength (Figure 3). Approximately 25% of the progeny from strong and moderate alleles display a cuticle similar to that shown. For the weak class of mutants, <25% of the progeny displayed a phenotype.
We then compared the phenotypic strength of each fz allele in both pathways. Interestingly, there is an overall correlation between the strength of the two phenotypes (Figures 3A and 4B). Alleles with the greatest disruption of PCP signaling clustered together and showed the strongest defect in Arm signaling. The alleles with moderate and weak PCP phenotypes likewise had moderate and weak Arm signaling phenotypes, respectively.
Two alleles seem to break the correlation between the PCP and Arm signaling phenotypes: fzRN and fzCK. Both alleles encode nonsense mutations that would truncate the Fz protein within the third transmembrane domain, likely generating a strong loss-of-function allele. These alleles indeed have a moderate-to-strong cuticle phenotype (Figure 4B); however, their PCP defect is unexpectedly weak (Figure 3A). We attribute the relatively weak PCP phenotype to translational readthrough of the stop codon, resulting in the synthesis of full-length Fz protein. Indeed, as we show below, both fzRN and fzCK produce some full-length Fz protein.
We conclude that the strength of fz signaling in PCP is related to its strength in the Arm pathway, suggesting that these two readouts of receptor activity are mechanistically similar.
Western blot analysis of Fz mutant proteins:
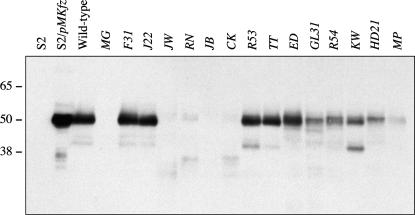
The loss of function of mutant alleles can be due to a variety of defects in the protein, ranging from misfolding to quicker turnover and lack of proper transport. We tested the properties of the various mutant proteins by examining the abundance and mobility by gel electrophoresis. The endogenous Fz protein is present at a low level (Krasnow and Adler 1994). Therefore, to determine the abundance and mobility of the mutant Fz proteins, we concentrated them from embryos by immunoprecipitation and then performed Western blot analysis.
Fz protein produced from wild-type embryos migrates with a similar mobility as Fz produced in cultured Drosophila S2 cells stably expressing a fz transgene (Figure 5). The FzTT and FzED mutant proteins are indistinguishable from wild-type Fz, as are the previously described FzJ22, FzF31, and FzR53 mutants (Figure 5) (Jones et al. 1996). We believe that another allele, fzSY, which was not assayed by Western blot, also belongs in this class, given that it is one of the weakest alleles in the collection. Thus, six alleles encode amino acid changes that do not appreciably disrupt the synthesis or processing of the Fz protein. In contrast, the FzGL31, FzR54, FzHD21, FzKW, and FzMP mutants have a markedly lower abundance than wild-type Fz (Figure 5). Each immunoprecipitation started with an equal amount of embryo extract. Therefore, the observed decrease in Fz abundance reveals that these mutations cause increased protein turnover. In addition to changes in abundance, the mobility of the FzR54, FzHD21, and FzMP mutants is reduced compared to that of wild-type Fz (Figure 5) (Adler et al. 1994; Jones et al. 1996). The truncation mutants FzMG and FzJB are not detectable (Figure 5). Truncated proteins that migrate at the predicted size are present for the nonsense mutants FzJW, FzRN, and FzCK (Figure 5). A slower mobility protein that migrates in a similar position as wild-type Fz is also present for these three alleles. We interpret the slower mobility form to be translational readthrough of the introduced stop codon and, as previously mentioned, the production of some full-length Fz protein as the underlying reason that fzCK and fzRN have a weak PCP phenotype. We analyzed Fz protein produced in embryos, which raises the question, Why does translational readthrough suppress only the PCP phenotype? A likely explanation is that there is a differential threshold for fz in PCP and Arm signaling, where PCP signaling requires less fz to function. This hypothesis is supported by previous work showing that fz can function in PCP signaling even when present at extremely low levels (Krasnow and Adler 1994). Not all of the alleles that are subject to stop suppression result in functional proteins. The full-length protein produced by readthrough of the fzJW stop codon is not functional in PCP or Arm signaling since this allele has strong phenotypes in both pathways. It is possible that the amino acid replacing the stop itself affects signaling or folding of the full-length FzJW protein. Finally, consistent with previous observations that stop suppression is context dependent, not all of the nonsense alleles produce full-length Fz protein (Chao et al. 2003). These data are summarized in Table 1.
Figure 5.
Western blot of fz alleles. Characterization of protein abundance and mobility of Fz isolated from embryos produced by a sibling cross of fz*/fzP21 Df(3L)fz2, where fz* denotes the allele tested. The only fz contribution in the resulting embryos is fz* or fzP21. Since fzP21 encodes a protein null allele (Jones et al. 1996), the only detectable Fz protein is translated from fz* transcripts. Fz protein cannot be observed in whole-embryo extracts (data not shown). Therefore, Fz protein was concentrated from total embryo extract by immunoprecipitation using an antibody that recognizes the hinge portion of the receptor, which is located between the end of the CRD and the first transmembrane domain. An equal amount of protein (300 μg) was used for each immunoprecipitation. Fz was detected in the immunoprecipitated material by probing Western blots with a monoclonal antibody that recognizes an epitope in the CRD (Park et al. 1994). Drosophila S2 cells do not express fz and were used as a negative control. Probing an extract prepared from S2 cells stably transformed with a fz transgene (pMKfz) marks the position of Fz with a relative molecular mass slightly >50 kDa. Fz protein isolated from embryos having a single copy of wild-type fz comigrates with Fz produced in S2/pMKfz cells. The fzMG and fzJB truncation alleles do not express detectable levels of protein. The nonsense mutations fzRN and fzCK produce both the predicted size truncated protein and an additional product that migrates near the position of wild-type Fz. The fzJW also has a larger protein product. However, it migrates more slowly than wild-type Fz and the comparable bands in fzRN and fzCK. Slower-mobility products result from improper processing in the endoplasmic reticulum, which is likely the result of misfolding. The missense alleles fzJ22, fzF31, fzR53, fzTT, and fzED all have an abundance and mobility similar to that of wild-type Fz. The remaining alleles all display a marked decrease in protein level with fzR54, fzHD21, and fzMP also having a reduced mobility.
DISCUSSION
In this article, we have surveyed a representative set of new and existing fz mutant alleles and asked whether the two pathways to which fz can contribute, PCP and Arm signaling, can be separated genetically. Such an approach has been taken for other receptors, including the platelet-derived growth factor receptor, where a set of site-directed mutants discriminates between the various downstream effectors (Tallquist et al. 2003). Here, we took advantage of the relative ease by which new endogenous alleles of genes can be isolated in Drosophila. This allowed us to examine the defects caused by mutant alleles without relying on gene transfer methods. Moreover, we tested for in vivo gene function rather than for cell culture, the only approach by which Fz mutations have previously been examined for Wnt signaling function.
Our main finding is that we cannot separate PCP and Arm signaling activity. While we cannot exclude that pathway-specific alleles would be uncovered in a larger series, our data support the view that the mechanism by which Fz operates is similar in both pathways. This conclusion is based on the correlation in signaling activity, in particular the finding that several alleles behave as intermediate or weak in both assays. In general, such a correlation suggests that one activity of a gene is related to another activity in a mechanistic and functional way. We restricted our analysis to measurements in vivo. Therefore, we cannot quantify the data in such a way that would allow us to state that the two functions are linearly (or otherwise) related. However, the data suggest that the phenotypes display, at least by approximation, a semilinear relationship to each other (Figures 3A and 4B). Therefore, we propose that Fz engages a component shared by both PCP and Arm signaling.
Elucidating the identity of this shared component is crucial to understanding how this receptor activates signaling. Defining amino acids in Fz specifically required for signaling could potentially provide a valuable means to probe Fz interactions. Previous attempts to generate signaling-compromised mutations in Fz have been restricted to examining only the intracellular amino acids (Umbhauer et al. 2000; Cong et al. 2004). Because we made alleles of the endogenous gene, we were able to sample the entire Fz protein. Importantly, we found signaling-specific mutations in the extracellular loops and transmembrane helices in addition to in the intracellular loops. Because these mutations are located throughout the entire protein, we argue that Fz is a dynamic molecule that changes conformation to relay its activation to a shared intracellular signal component. There are two major candidates for this shared component: Dsh and Gαo (Katanaev et al. 2005). Both proteins have been implicated in PCP and in Arm signaling and the Dsh protein has been shown to bind Fz (Chen et al. 2003; Wong et al. 2003; Cong et al. 2004). How Dsh and Gαo are coupled to Fz is not yet clear.
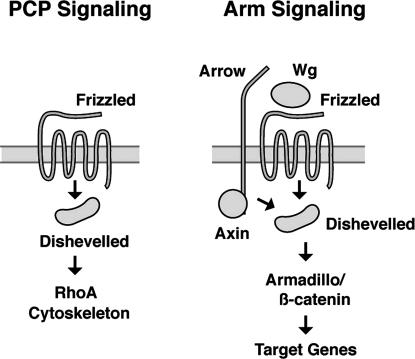
We propose the following model as the way in which the Fz protein controls two different signaling pathways (Figure 6). Fz signals in a ground state to the PCP pathway in the absence of a Wnt. Consistent with this model, we found that none of the fz missense mutations, which were all isolated on the basis of their PCP phenotype, are located in the CRD. This model is also supported by recent work suggesting that Fz does not require a Wnt ligand in PCP signaling (Lawrence et al. 2002; Amonlirdviman et al. 2005) but that its activity is regulated by interactions between neighboring cells and differential levels of the cytoplasmic mediators Pk and Dsh (Tree et al. 2002b; Amonlirdviman et al. 2005). Furthermore, experiments with chimeric fz transgenes have shown that the transmembrane portion of Fz, not the CRD, is responsible for coupling it to PCP signaling (Rulifson et al. 2000; Wu et al. 2004). In this context, it should also be mentioned that global signaling during PCP may be under the control of differential expression of molecules involved in cell adhesion (Yang et al. 2002; Ma et al. 2003; Simon 2004).
Figure 6.
Model of Fz activation in PCP and Arm signaling. In the absence of a Wnt ligand, Fz has an intrinsic ability to signal to the PCP pathway. This may occur via a direct interaction with Dsh. When Wg is present, the coreceptor Arr is brought into proximity to Fz, which results in the close apposition of Dsh and Axn. This combination of intracellular components switches the output of Fz from PCP to Arm signaling.
In our model, the presence of a Wnt ligand switches Fz to the Arm signaling pathway by recruiting the membrane protein Arr to it. It has been previously shown that arr mutants disrupt Arm signaling but do not have a PCP phenotype (Wehrli et al. 2000). While the exact mechanism of Arr-Fz-Wg interactions is not clear, a currently favored hypothesis is that Arr acts together with Fz as a coreceptor for Wg, generating a ternary complex (Tamai et al. 2000; Liu et al. 2005). On the cytoplasmic side, Arr recruits Axn to the membrane (Mao et al. 2001; Tolwinski et al. 2003; Tamai et al. 2004). We speculate that the membrane localization of Axn via Arr brings it in close proximity with Dsh, resulting in the inactivation of Axn and the stabilization of Arm. However, it is also possible that recruiting Axn to the membrane via Arr is sufficient to drive signaling (Cliffe et al. 2003; Tolwinski et al. 2003). It has also been proposed that Wg initiates signaling by recruiting Axn to the membrane via Dsh and that once at the membrane Axn is inactivated, allowing Arm to accumulate (Cliffe et al. 2003). Axn is a negative regulator of Arm signaling and, like arr, axn mutants have no polarity phenotype (Hamada et al. 1999). During PCP signaling, in the absence of Wg, a trimeric complex between Wg, Fz, and Arr would not be assembled and Fz functions independently of Arr but also of Axn.
We postulate that the presence of ligand-unbound Fz facilitates Dsh activation in PCP signaling. In contrast to arr and axn, dsh is required for both the Wg and the PCP pathway, making fz and dsh the only two genes shared between Wg and PCP signaling (Figure 6). The pathways are genetically distinct, however, in that there are three specific alleles of dsh that prevent it from participating in the PCP pathway but allow it to function normally in Arm signaling (Axelrod et al. 1998; Penton et al. 2002). These dsh alleles contain missense mutations that map to the same domain of the protein (Axelrod et al. 1998; Penton et al. 2002). Here we have shown that, unlike dsh, the two functions of fz are not separable by specific mutations. This suggests that Fz activates a common component and that the switch between the two pathways occurs downstream of fz.
There is one other series of endogenous fz mutant alleles: mutations in the human Frizzled 4 (FZD4) gene lead to familial exudative vitreoretinopathy (FEVR) (Robitaille et al. 2002; Kondo et al. 2003; Omoto et al. 2004; Toomes et al. 2004b; Xu et al. 2004). The signaling pathway used by FZD4 is not entirely clear. Some studies suggest that FZD4 signals through an alternative (Ca2+) Wnt pathway (Robitaille et al. 2002). However, the finding that the mutations in the Wnt coreceptor LRP (Jiao et al. 2004; Toomes et al. 2004a), which is implicated in Arm/β-catenin signaling and not in the Ca2+ pathway, also cause FEVR does not support this model. Moreover, FZD4 in collaboration with LRP5 can be stimulated to activate Arm/β-catenin signaling by Norrin, a non-Wnt ligand that binds to the CRD (Xu et al. 2004). Of the 12 FEVR alleles, 4 result in mutations in the CRD (Robitaille et al. 2002; Kondo et al. 2003; Omoto et al. 2004; Toomes et al. 2004b; Xu et al. 2004). Of the remaining alleles, one is located N terminal to the CRD (G36D), thus potentially interfering with the processing of the hydrophobic signal peptide (Toomes et al. 2004b). Another mutation (C181R) affects a conserved cysteine residue in the “hinge” region (Omoto et al. 2004). There is a mutation (R417Q) in a conserved arginine in the third intracellular loop and a stop codon (W319X) identical to the truncation allele fzRN described in this study (Kondo et al. 2003). Interestingly, there is a FEVR allele (G488D) that affects a conserved glycine residue in the seventh transmembrane domain that is also altered in the fz alleles fzMP (G545E, this article) and fzHC52 (G545R) (Jones et al. 1996). Three FEVR alleles, S495F and Q505X (Toomes et al. 2004b) and the in-frame deletion of MW493-4 (Robitaille et al. 2002), are located at the junction of the seventh transmembrane domain and the cytoplasmic tail near a conserved motif that has been shown to be required for Arm/β-catenin signaling function (Umbhauer et al. 2000). The existence of CRD mutations in FEVR alleles of FZD4 demonstrates that mutations in this domain can indeed have a functional consequence and highlights the role of the CRD in FZD4 function in the retina. It also supports the hypothesis that CRD mutations have not been recovered in any of the PCP screens for fz alleles because this domain is dispensable in that process.
Acknowledgments
We acknowledge Catriona Logan, Michael Gordon, and Eric Rulifson for insightful discussions and comments during the preparation of this manuscript. We thank Paul Adler for providing us with fz alleles from his existing collection. This work was supported by the Howard Hughes Medical Institute and by a grant from the National Institutes of Health (R01 GM60388).
References
- Adler, P. N., 2002. Planar signaling and morphogenesis in Drosophila. Dev. Cell 2: 525–535. [DOI] [PubMed] [Google Scholar]
- Adler, P. N., J. Charlton and C. Vinson, 1987. Allelic variation at the frizzled locus of Drosophila. Dev. Genet. 8: 99–119. [Google Scholar]
- Adler, P. N., C. Vinson, W. J. Park, S. Conover and L. Klein, 1990. Molecular structure of frizzled, a Drosophila tissue polarity gene. Genetics 126: 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler, P. N., J. Charlton, K. H. Jones and J. Liu, 1994. The cold-sensitive period for frizzled in the development of wing hair polarity ends prior to the start of hair morphogenesis. Mech. Dev. 46: 101–107. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman, K., N. A. Khare, D. R. Tree, W. S. Chen, J. D. Axelrod et al., 2005. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science 307: 423–426. [DOI] [PubMed] [Google Scholar]
- Ashburner, M., 1989. Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Axelrod, J. D., J. R. Miller, J. M. Shulman, R. T. Moon and N. Perrimon, 1998. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 12: 2610–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, A., S. Chauvet, O. Huber, F. Usseglio, U. Rothbacher et al., 2000. Pontin52 and reptin52 function as antagonistic regulators of beta-catenin signalling activity. EMBO J. 19: 6121–6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejsovec, A., 2005. Wnt pathway activation: new relations and locations. Cell 120: 11–14. [DOI] [PubMed] [Google Scholar]
- Bhanot, P., M. Brink, C. H. Samos, J. C. Hsieh, Y. Wang et al., 1996. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382: 225–230. [DOI] [PubMed] [Google Scholar]
- Bhanot, P., M. Fish, J. A. Jemison, R. Nusse, J. Nathans et al., 1999. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development 126: 4175–4186. [DOI] [PubMed] [Google Scholar]
- Bhat, K. M., 1998. frizzled and frizzled 2 play a partially redundant role in wingless signaling and have similar requirements to wingless in neurogenesis. Cell 95: 1027–1036. [DOI] [PubMed] [Google Scholar]
- Boutros, M., J. Mihaly, T. Bouwmeester and M. Mlodzik, 2000. Signaling specificity by Frizzled receptors in Drosophila. Science 288: 1825–1828. [DOI] [PubMed] [Google Scholar]
- Brunner, E., O. Peter, L. Schweizer and K. Basler, 1997. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature 385: 829–833. [DOI] [PubMed] [Google Scholar]
- Chae, J., M. J. Kim, J. H. Goo, S. Collier, D. Gubb et al., 1999. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development 126: 5421–5429. [DOI] [PubMed] [Google Scholar]
- Chao, A. T., H. A. Dierick, T. M. Addy and A. Bejsovec, 2003. Mutations in eukaryotic release factors 1 and 3 act as general nonsense suppressors in Drosophila. Genetics 165: 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. M., and G. Struhl, 1999. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development 126: 5441–5452. [DOI] [PubMed] [Google Scholar]
- Chen, C. M., W. Strapps, A. Tomlinson and G. Struhl, 2004. Evidence that the cysteine-rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless. Proc. Natl. Acad. Sci. USA 101: 15961–15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., D. Ten Berge, J. Brown, S. Ahn, L. A. Hu et al., 2003. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 301: 1391–1394. [DOI] [PubMed] [Google Scholar]
- Cliffe, A., F. Hamada and M. Bienz, 2003. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr. Biol. 13: 960–966. [DOI] [PubMed] [Google Scholar]
- Cong, F., L. Schweizer and H. Varmus, 2004. Wnt signals across the plasma membrane to activate the {beta}-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131: 5103–5115. [DOI] [PubMed] [Google Scholar]
- Dann, C. E., J. C. Hsieh, A. Rattner, D. Sharma, J. Nathans et al., 2001. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 412: 86–90. [DOI] [PubMed] [Google Scholar]
- Fanto, M., and H. Mcneill, 2004. Planar polarity from flies to vertebrates. J. Cell Sci. 117: 527–533. [DOI] [PubMed] [Google Scholar]
- Greenspan, R. J., 1997. Fly Pushing: The Theory and Practice of Drosophila Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Gubb, D., and A. Garcia-Bellido, 1982. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 68: 37–57. [PubMed] [Google Scholar]
- Gubb, D., C. Green, D. Huen, D. Coulson, G. Johnson et al., 1999. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 13: 2315–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada, F., Y. Tomoyasu, Y. Takatsu, M. Nakamura, S. Nagai et al., 1999. Negative regulation of Wingless signaling by D-axin, a Drosophila homolog of axin. Science 283: 1739–1742. [DOI] [PubMed] [Google Scholar]
- Hsieh, J. C., A. Rattner, P. M. Smallwood and J. Nathans, 1999. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc. Natl. Acad. Sci. USA 96: 3546–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, X., V. Ventruto, M. T. Trese, B. S. Shastry and J. F. Hejtmancik, 2004. Autosomal recessive familial exudative vitreoretinopathy is associated with mutations in LRP5. Am. J. Hum. Genet. 75: 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, K. H., J. Liu and P. N. Adler, 1996. Molecular analysis of EMS-induced frizzled mutations in Drosophila melanogaster. Genetics 142: 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanaev, V. L., R. Ponzielli, M. Semeriva and A. Tomlinson, 2005. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell 120: 111–122. [DOI] [PubMed] [Google Scholar]
- Kennerdell, J. R., and R. W. Carthew, 1998. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 95: 1017–1026. [DOI] [PubMed] [Google Scholar]
- Klingensmith, J., R. Nusse and N. Perrimon, 1994. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 8: 118–130. [DOI] [PubMed] [Google Scholar]
- Kondo, H., H. Hayashi, K. Oshima, T. Tahira and K. Hayashi, 2003. Frizzled 4 gene (FZD4) mutations in patients with familial exudative vitreoretinopathy with variable expressivity. Br. J. Ophthalmol. 87: 1291–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow, R. E., and P. N. Adler, 1994. A single frizzled protein has a dual function in tissue polarity. Development 120: 1883–1893. [DOI] [PubMed] [Google Scholar]
- Lawrence, P. A., J. Casal and G. Struhl, 2002. Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development 129: 2749–2760. [DOI] [PubMed] [Google Scholar]
- Liu, G., A. Bafico and S. A. Aaronson, 2005. The mechanism of endogenous receptor activation functionally distinguishes prototype canonical and noncanonical wnts. Mol. Cell. Biol. 25: 3475–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, C. Y., and R. Nusse, 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20: 781–810. [DOI] [PubMed] [Google Scholar]
- Ma, D., C. H. Yang, H. McNeill, M. A. Simon and J. D. Axelrod, 2003. Fidelity in planar cell polarity signalling. Nature 421: 543–547. [DOI] [PubMed] [Google Scholar]
- Mao, J., J. Wang, B. Liu, W. Pan, G. H. Farr, III et al., 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol, Cell 7: 801–809. [DOI] [PubMed] [Google Scholar]
- Muller, H., R. Samanta and E. Wieschaus, 1999. Wingless signaling in the Drosophila embryo: zygotic requirements and the role of the frizzled genes. Development 126: 577–586. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard, C., and E. Wieschaus, 1980. Mutations affecting segment number and polarity in Drosophila. Nature 287: 795–801. [DOI] [PubMed] [Google Scholar]
- Omoto, S., T. Hayashi, K. Kitahara, T. Takeuchi and Y. Ueoka, 2004. Autosomal dominant familial exudative vitreoretinopathy in two Japanese families with FZD4 mutations (H69Y and C181R). Ophthalmic Genet. 25: 81–90. [DOI] [PubMed] [Google Scholar]
- Park, W. J., J. Liu and P. N. Adler, 1994. The frizzled gene of Drosophila encodes a membrane protein with an odd number of transmembrane domains. Mech. Dev. 45: 127–137. [DOI] [PubMed] [Google Scholar]
- Penton, A., A. Wodarz and R. Nusse, 2002. A mutational analysis of dishevelled in Drosophila defines novel domains in the dishevelled protein as well as novel suppressing alleles of axin. Genetics 161: 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon, N., L. Engstrom and A. P. Mahowald, 1989. Zygotic lethals with specific maternal effect phenotypes in Drosophila melanogaster. I. Loci on the X chromosome. Genetics 121: 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon, N., A. Lanjuin, C. Arnold and E. Noll, 1996. Zygotic lethal mutations with maternal effect phenotypes in Drosophila melanogaster. II. Loci on the second and third chromosomes identified by P-element-induced mutations. Genetics 144: 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, D. B., 1986. Drosophila: A Practical Approach. IRL Press, Oxford/Washington, DC.
- Robitaille, J., M. L. Macdonald, A. Kaykas, L. C. Sheldahl, J. Zeisler et al., 2002. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat. Genet. 32: 326–330. [DOI] [PubMed] [Google Scholar]
- Rulifson, E. J., C. H. Wu and R. Nusse, 2000. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol. Cell 6: 117–126. [PubMed] [Google Scholar]
- Simon, M. A., 2004. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development 131: 6175–6184. [DOI] [PubMed] [Google Scholar]
- Strapps, W. R., and A. Tomlinson, 2001. Transducing properties of Drosophila Frizzled proteins. Development 128: 4829–4835. [DOI] [PubMed] [Google Scholar]
- Strutt, D., 2003. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development 130: 4501–4513. [DOI] [PubMed] [Google Scholar]
- Strutt, D. I., U. Weber and M. Mlodzik, 1997. The role of RhoA in tissue polarity and Frizzled signalling. Nature 387: 292–295. [DOI] [PubMed] [Google Scholar]
- Tallquist, M. D., W. J. French and P. Soriano, 2003. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 1: E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai, K., M. Semenov, Y. Kato, R. Spokony, C. Liu et al., 2000. LDL-receptor-related proteins in Wnt signal transduction. Nature 407: 530–535. [DOI] [PubMed] [Google Scholar]
- Tamai, K., X. Zeng, C. Liu, X. Zhang, Y. Harada et al., 2004. A mechanism for Wnt coreceptor activation. Mol. Cell 13: 149–156. [DOI] [PubMed] [Google Scholar]
- Taylor, J., N. Abramova, J. Charlton and P. N. Adler, 1998. Van Gogh: a new Drosophila tissue polarity gene. Genetics 150: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen, H., J. Purcell, M. Bennett, D. Kansagara, A. Syed et al., 1994. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development 120: 347–360. [DOI] [PubMed] [Google Scholar]
- Tolwinski, N. S., and E. Wieschaus, 2004. Rethinking WNT signaling. Trends Genet. 20: 177–181. [DOI] [PubMed] [Google Scholar]
- Tolwinski, N. S., M. Wehrli, A. Rives, N. Erdeniz, S. Dinardo et al., 2003. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev. Cell 4: 407–418. [DOI] [PubMed] [Google Scholar]
- Toomes, C., H. M. Bottomley, R. M. Jackson, K. V. Towns, S. Scott et al., 2004. a Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am. J. Hum. Genet. 74: 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomes, C., H. M. Bottomley, S. Scott, D. A. Mackey, J. E. Craig et al., 2004. b Spectrum and frequency of FZD4 mutations in familial exudative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 45: 2083–2090. [DOI] [PubMed] [Google Scholar]
- Tree, D. R., D. Ma and J. D. Axelrod, 2002. a A three-tiered mechanism for regulation of planar cell polarity. Semin. Cell Dev. Biol. 13: 217–224. [DOI] [PubMed] [Google Scholar]
- Tree, D. R., J. M. Shulman, R. Rousset, M. P. Scott, D. Gubb et al., 2002. b Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109: 371–381. [DOI] [PubMed] [Google Scholar]
- Umbhauer, M., A. Djiane, C. Goisset, A. Penzo-Mendez, J. F. Riou et al., 2000. The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. EMBO J. 19: 4944–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui, T., Y. Shima, Y. Shimada, S. Hirano, R. W. Burgess et al., 1999. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98: 585–595. [DOI] [PubMed] [Google Scholar]
- Van De Wetering, M., R. Cavallo, D. Dooijes, M. Van Beest, J. Van Es et al., 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799. [DOI] [PubMed] [Google Scholar]
- Vinson, C. R., and P. N. Adler, 1987. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329: 549–551. [DOI] [PubMed] [Google Scholar]
- Wehrli, M., S. T. Dougan, K. Caldwell, L. O'Keefe, S. Schwartz et al., 2000. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407: 527–530. [DOI] [PubMed] [Google Scholar]
- Wieschaus, E., and R. Riggleman, 1987. Autonomous requirements for the segment polarity gene armadillo during Drosophila embryogenesis. Cell 49: 177–184. [DOI] [PubMed] [Google Scholar]
- Winter, C. G., B. Wang, A. Ballew, A. Royou, R. Karess et al., 2001. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105: 81–91. [DOI] [PubMed] [Google Scholar]
- Wolff, T., and G. M. Rubin, 1998. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development 125: 1149–1159. [DOI] [PubMed] [Google Scholar]
- Wong, H. C., A. Bourdelas, A. Krauss, H. J. Lee, Y. Shao et al., 2003. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol. Cell 12: 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, L. L., and P. N. Adler, 1993. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J. Cell Biol. 123: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., T. J. Klein and M. Mlodzik, 2004. Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2: E158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q., Y. Wang, A. Dabdoub, P. M. Smallwood, J. Williams et al., 2004. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116: 883–895. [DOI] [PubMed] [Google Scholar]
- Yang, C. H., J. D. Axelrod and M. A. Simon, 2002. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell 108: 675–688. [DOI] [PubMed] [Google Scholar]