Abstract
One of the best examples of a natural behavioral syndrome is the pollen-hoarding syndrome in honeybees that ties together multiple behavioral phenotypes, ranging from foraging behavior to behavioral ontogeny and learning performance. A central behavioral factor is the bees' responsiveness to sucrose, measured as their proboscis extension reflex. This study examines the genetics of this trait in diploid worker and haploid male honeybees (drones) to learn more about the genetic architecture of the overall behavioral syndrome, using original strains selected for pollen-hoarding behavior. We show that a significant proportion of the phenotypic variability is determined by genotype in males and workers. Second, our data present overwhelming evidence for pleiotropic effects of previously identified quantitative trait loci for foraging behavior (pln-QTL) and epistatic interactions among them. Furthermore, we report on three genomic QTL scans (two reciprocal worker backcrosses and one drone hybrid population) derived from our selection strains. We present at least one significant and two putative new QTL directly affecting the sucrose response of honeybees. Thus, this study demonstrates the modular genetic architecture of behavioral syndromes in general, and elucidates the genetic architecture of the pollen-hoarding behavioral syndrome in particular. Understanding this behavioral syndrome is important for understanding the division of labor in social insects and social evolution itself.
STUDIES of protein–protein interactions suggest that cooperative networks at the molecular level are a universal feature of biological systems (Walhout et al. 2000; Giot et al. 2003). Likewise, interactive networks have been demonstrated at the genetic level as epistasis (Wolf and Brodie 2000) and polygenic inheritance (Lynch and Walsh 1998; Mackay 2001). The degree of genetic complexity varies among organisms, phenotypes, cell types, and classes of molecules (Walhout et al. 2000; Giot et al. 2003). High complexity is expected for components of second messenger cascades because they not only transmit a high number of different extracellular signals but also induce numerous intracellular responses in a variety of contexts (Antoni 2000). Cellular signaling is particularly prominent in the central nervous system and thus the control of behavior. Consequently, from a molecular perspective, extensive correlations among behavioral phenotypes and a high percentage of conditional phenotypes due to epistasis are expected (Gibson 1996), particularly when studying natural genetic variation of behavioral phenotypes (Anholt 2004).
Various components of second messenger cascades influence behavior (Page and Erber 2002) and some are known particularly for their pleiotropic effects (Sokolowski 2001; Scheiner et al. 2004b). Manipulations of secondary messenger cascades may lead to profound, concerted changes in behavior (Corbet 1991; Barron et al. 2002), reminiscent of different human personalities, or behavioral syndromes (Sih et al. 2004). Heritable personality traits have been widely recognized in human behavioral sciences and in animal husbandry (Gosling 2001), but only recently is it recognized that such traits are probably ubiquitous in animals and behavioral correlations are important for understanding animal behavior (Faller 2003; Flint 2003; Sih et al. 2004). As many behavioral traits have a considerable genetic basis, it is important to study how far these behavioral syndromes are caused by direct genetic correlation, i.e., whether natural variation in a suite of behavioral phenotypes can be traced back to variability in the same genes (Faller 2003; Flint 2003; Sih et al. 2004).
The foraging behavior of honeybee workers seems to comprise such a suite of correlated behavioral phenotypes (Pankiw 2003; Pankiw et al. 2004). After initiation of foraging in their second to third week of life, workers make several decisions on each foraging trip by integrating their own behavioral and physiological status, social colony stimuli, and the external environment (Seeley 1995). Thus, foraging behavior is a complex social trait involving a number of variables and decisions. Correlations among those different components of foraging are to be expected because they coordinate overall foraging behavior.
These predicted correlations have been found in honeybee strains that were selected bidirectionally for their pollen-hoarding behavior (stored pollen in the colony) for several generations (Page and Fondrk 1995). Selection for high pollen hoarding coincided with workers specializing more in pollen collection (Page et al. 1995), workers collecting heavier pollen loads (Page et al. 1995), performing more recruitment for pollen resources (Waddington et al. 1998), and collecting nectar of lower concentration (Page et al. 1998). However, a suite of seemingly unrelated behavioral differences between the selection strains has also been demonstrated: high pollen-hoarding bees mature faster to become foragers (Pankiw and Page 2001; Rueppell et al. 2004), they have higher locomotory activity upon emergence (Humphries et al. 2005), and they perform better at various learning tasks (Scheiner et al. 2001a,b). The latter differences in learning behavior between the two selected strains are presumably caused by differences in reward perception due to differential sucrose perception (Page et al. 1998; Pankiw and Page 1999; Scheiner et al. 1999, 2001b, 2004a).
Sucrose perception of honeybees can be readily assessed by the proboscis extension reflex that they exhibit when their antennae come in contact with a sucrose solution that meets or exceeds their response threshold (Bitterman et al. 1983). Sucrose responsiveness is associated with learning (Scheiner et al. 2001a,b, 2004a), foraging behavior (Pankiw and Page 1999, 2000), and maturation rate (Page and Erber 2002), and thus seems to be a central factor (or a measure of a central factor) of the pollen-hoarding behavioral syndrome in honeybees.
Several of these correlations have been reconfirmed in nonselected bees. Proboscis extension response (PER) scores directly after emergence predict the foraging choice of “wild-type” honeybee workers (Pankiw and Page 2000). In two independent studies, PER scores were predictive of foraging choice and the rate of behavioral ontogeny (measured as the age at foraging initiation) in European and Africanized honeybees (Pankiw and Page 2000; Pankiw 2003). Additionally, sucrose responsiveness was found directly correlated in wild-type bees to locomotion rate (Humphries et al. 2005). The generality of these behavioral associations is further reflected in racial differences in honeybees. Like high pollen-hoarding bees, Africanized honeybees have lower response threshold to sucrose (Pankiw and Rubink 2002; Pankiw 2003), mature faster into foragers (Winston and Katz 1982; Pankiw 2003), and are more likely to forage for pollen (Pankiw 2003).
Theoretically, the correlations among individual phenotypes should be reflected in pleiotropic genes or tightly linked gene clusters, and several studies have demonstrated pleiotropy for quantitative trait loci (QTL) that were initially identified for their effect on colony-level pollen hoarding. Initially, two such QTL (pln1 and pln2) were identified and verified studying individual-level foraging traits (Hunt et al. 1995). Subsequently, a third QTL (pln3) was identified and confirmed (Page et al. 2000). Two (pln2 and pln3) influence the nectar concentration collected by foragers (Page et al. 2000), and pln1 affects directly the age at foraging initiation (Rueppell et al. 2004), and it interacts with pln2 and pln3, affecting various foraging decisions of workers (Rüppell et al. 2004). An additional QTL (pln4) was found to directly influence the pollen proportion collected by foragers, and interactively with pln1, pln2, and pln3 various other foraging variables (Rüppell et al. 2004).
The behavioral breadth of the pollen-hoarding syndrome makes studies of its genetic architecture interesting in its own right. Genomic resources and genetic tools that have been developing for the honeybee (Page et al. 2002) put the study of its complex social behavior at the forefront of behavioral genetics. However, the genetic architecture of the pollen-hoarding syndrome has also profound implications for the understanding of social evolution (Amdam et al. 2004). From a wider perspective, this system represents a promising model for the emerging study of naturally occurring behavioral syndromes (Sih et al. 2004), which have not been studied in any depth in invertebrates (Gosling 2001).
For these reasons, we studied the genetic architecture of sucrose responsiveness in honeybees as the most central component of the pollen-hoarding syndrome (Page and Erber 2002). To our knowledge, it is also the first study of the genetic architecture of a behavioral reflex, generally believed to be a hard-wired (little influenced by the environment) behavioral trait. We used the original selected pollen-hoarding strains (Page and Fondrk 1995) to set up crosses [hybrid and backcross: (Rueppell et al. 2004)] for quantifying the degree of genetic determination, evaluation of previously identified QTL and genomic scans for new QTL in workers and drones.
MATERIALS AND METHODS
Crosses:
The high and low pollen-hoarding strains (consisting of several lines) of Page and Fondrk (1995) were used as genetic sources for the experiment. These strains were derived from commercial stocks of honeybees (Apis mellifera L.) in 1990 and have been maintained under continuous, bidirectional selection for pollen-hoarding behavior. To avoid inbreeding depression the strains were bred by circular inbreeding among five lines and three out-crossings to commercial hives of similar (high pollen-hoarding or low pollen-hoarding) phenotype. At the beginning of the experiment, the strains were in the 18th generation of selection and the inbred high and low pollen-hoarding lines used in these experiments differed dramatically in the amount of stored pollen (812 cm2 and 97 cm2, respectively; F(1,55) = 56.06, P < 0.001).
We generated hybrid super-sister queens (Page and Laidlaw 1988) by instrumentally inseminating a virgin queen from a low inbred line (“C”) with semen of a single drone from a high inbred line (“Q1”). Eggs of this queen were grafted into queen cups to produce hybrid queen offspring. Subsequently, the hybrid queens were instrumentally inseminated with sperm of either a high-line male or a low-line male. Two of these queens were selected to generate the high and low backcross populations (HBC and LBC) and worker offspring from the C line and Q1 line represented the low and high bees, respectively. Male offspring of one of the hybrid queens were used as a source for the hybrid drones and high and low drones were derived from queens of the inbred lines.
Experiments:
All four experiments (two with workers, two with males) were conducted in the following manner (Pankiw and Page 1999): Empty combs were introduced into the source hives to induce simultaneous egg laying by the queens. Prior to emergence, these combs were transferred to a temperature (34°) and humidity (50%) controlled incubator from which bees were collected within 30 min of emergence. Individuals were marked with paint (Testors) and placed into a common, unrelated foster hive. After 4–10 days, these individuals were collected from the colonies, and then taken indoors where they were tested for their responsiveness to water and sucrose solutions.
Individuals were mounted in small brass tubes that restrained body movement but allowed free movement of their antennae and mouthparts (Bitterman et al. 1983). They were allowed to recover for ∼1 hr before beginning the assay. Bees were tested for their responses to water and sucrose by presenting them with sucrose solutions that increased in concentration in a log10 series of −1.0, −0.5, 0.0, 0.5, 1.0, and 1.5 corresponding to sucrose concentrations of 0.1, 0.3, 1, 3, 10, and 30% (w/v). Sucrose solutions were prepared using distilled and Millipore filtered water as the solvent for Sigma sucrose (99.5% minimum purity). A droplet of water was touched to each antenna before the application of 0.1% sucrose and before each subsequent sucrose application (i.e., water, 0.1%; water, 0.3%; water, 1%; water, 3%; water, 10%; water, 30%). The intertrial interval between presentations of water or sucrose solutions was ∼2 min.
Responses were scored as a fully extended proboscis in response to the stimulus, the proboscis extension response (Kuwabara 1957). The total number of times an individual extended the proboscis to sucrose solution was reported (sucrose response score). Individuals usually continue to respond to all higher concentrations of sucrose once their response thresholds have been exceeded in the ascending series presented. Immediately after the PER assay individuals were individually placed in labeled Eppendorf tubes and stored at −80° prior to DNA extraction.
In the first experiment, we measured the sucrose response score for workers from all four genetic groups to establish the differentiation among them and thus the minimum proportion of phenotypic variation that is attributable to genetic factors (sample sizes: Figure 1A). A second set of only HBC (n = 359) and LBC (n = 354) workers was scored for their sucrose response score and frozen at −80° for subsequent genetic analyses. Additionally, we measured two sets of drones: the first consisted of high, hybrid, and low drones for determining the genetic differentiation among them (sample sizes: Figure 1B), and the second, larger set of only hybrid drones (n = 1007) was scored in two PER testing rounds (different days, drones were 3 days older during the second round) and frozen for genetic mapping and evaluation of the pln-QTL as the second set of workers.
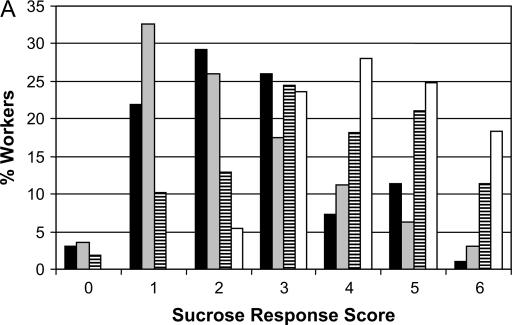
Figure 1.
Phenotypic distribution of the sucrose response scores of the workers (A) and males (B) used for the quantification of the degree of genetic determination. The differences between worker groups suggest some dominance effects because the high backcross (HBC) is similar to the high pollen-hoarding strain, and low backcross (LBC) is similar to the low strain. The male data fit a general additive model. (A) ( ) Low (n = 96), (
) Low (n = 96), ( ) LBC (n = 258), (
) LBC (n = 258), ( ) HBC (n = 265), (□) high (n = 93). (B) (
) HBC (n = 265), (□) high (n = 93). (B) ( ) Low (n = 81), (
) Low (n = 81), ( ) hybrid (n = 158), (□) high (n = 34).
) hybrid (n = 158), (□) high (n = 34).
Genetic analysis:
DNA from whole bees was extracted using the CTAB-lysis method (Hunt and Page 1995) and the DNA concentration was adjusted to 100 ng/μl. To evaluate the effects of the pln-QTL (Hunt et al. 1995; Page et al. 2000) we amplified sequence tagged site (sts) markers (specific, polymorphic DNA sequences) linked to the QTL by polymerase chain reaction (PCR). Polymorphisms were scored on agarose (0.6%)/Synergel (1%) gels directly after either PCR or a subsequent digest with a specific restriction endonuclease (Hunt and Page 1995; Rueppell et al. 2004). Gels were run at 3–5 V/cm and stained with ethidium bromide to visualize the presence/absence or length polymorphism.
For the genomic mapping, we used selective genotyping, choosing individuals with extreme phenotypes to increase the probability to detect QTL (Lander and Botstein 1989). In both worker maps all PER scores were represented, while the drone mapping population consisted only of individuals with the two highest and two lowest PER scores. We used 191 (out of 1007 total phenotyped), 96 (of 359), and 95 (of 354) individuals for the drone, HBC, and LBC maps, respectively. We generated amplified fragment length polymorphism (AFLP) markers from HBC and LBC workers and hybrid drones. These markers are anonymous, but have practical advantages and are believed to be reliable and relatively evenly distributed across genomes (Mueller and Wolfenbarger 1999). The AFLP core reagent kit (Invitrogen Life Technologies) was used according to the manufacturer's recommendations (with reaction volumes reduced to 50%) for the double restriction (EcoRI and MseI) and ligation of the adaptors. The preamplification involved 24 cycles of denaturation (94°/60 sec), annealing (56°/60 sec), and elongation (72°/60 sec). The product was diluted 1:50 to serve as template for a second, selective PCR. Selective primers contained two specific nucleotides and their sequences are given in the appendix. The EcoRI-primers were labeled with [γ-33P]ATP and used at a final concentration of 85 ng/ml. The unmodified MseI primers were used at 625 ng/ml.
The products of the selective amplifications were run out on denaturing, 6% polyacrylamide gels (35 × 45 × 0.4 mm), along with radioactively labeled Sequamark size marker (Research Genetics). Gels were dried on filter paper and exposed for 24–96 hr to Biomax MR film (Kodak). Polymorphic bands were identified visually and sized and scored for all individuals. Finally, all films were rescored once to check for scoring errors. The final drone, HBC, and LBC data sets contained 417, 405, and 471 markers, respectively. This resulted in an average marker spacing of more than one marker per 10 cM or 500 kb (Hunt and Page 1995).
Statistical analysis:
Effects of the sts-marker genotypes were evaluated by multiway ANOVAs. Although the data did not conform to parametric assumptions, ANOVAs are robust given our relatively large sample sizes (Kallenberg 1997). Furthermore, the simultaneous quantification of the effects of multiple genotypic factors and their interactions with nonparametric tests is problematic. We tested simultaneously the effect of all genotypic markers in the three test populations (drones, worker HBC, and worker LBC) with full-factorial ANOVAs. Following Rueppell et al. (2004), we performed additional two-way ANOVAs on the worker data (single marker × backcross) to increase statistical power for detection of overall marker effects and to study possible effects of genetic background.
Linkage groups were identified with Mapmaker 3.0b (Lander and Botstein 1989). We used Kosambi's mapping function and 37.5 cM/LOD 3 as linkage criteria between markers (Hunt and Page 1995). Linkage groups identified by two-point analysis were subjected to multipoint analyses and exhaustive tests of local map permutations to find the correct marker order within linkage groups (Lincoln et al. 1993). An ordered data set was generated and the data checked for potential PCR artifacts indicated by double-crossovers (Hunt and Page 1995). These linkage groups provided the basis for QTL mapping with the software program MapQTL 4.0 (Van Ooijen et al. 2002). We established single marker effects with Kruskal-Wallis tests and used interval mapping in the main analysis. We could not perform more refined QTL analyses, such as multiple QTL mapping (Jansen 1993) or composite interval mapping (Zeng 1994) because our selectively genotyped data sets violate the assumptions of these methods (van Ooijen et al. 2002). A genome-wide significance threshold of LOD = 3.0 was adopted from previous studies (Hunt et al. 1995; Page et al. 2000; Rueppell et al. 2004; see also Lander and Botstein 1989). Additionally, we determined the significance thresholds empirically by bootstrapping (Churchill and Doerge 1994) using QTL Cartographer (Basten et al. 1994, 2002). Furthermore, we report all suggestive QTL with a LOD score >2.0.
RESULTS
Sucrose response scores ranged in all worker and drone groups (except for high workers) from zero (the minimal value) to six (the maximum) and the phenotypic distributions are shown for the worker and drone groups in Figure 1, A and B, respectively. In workers, we calculated the proportion of variation that is attributable to genotype to be 18.1% among all groups and 32.6% between the two parental strains (high and low) only. This indicated a highly significant genetic effect on the phenotype in both cases (F(1,710) = 156.6, P < 0.001 and F(1,187) = 90.4, P < 0.001, respectively). High workers had a median sucrose response score of 4 [3 (25% quartile) – 4.75 (75% quartile)], HBC scores = 4 (3–5), LBC scores = 2 (1–3.5), and low scores = 2 (1–3). In drones the proportion of genetic variation was lower: 8.6% when all three groups were taken into consideration, and 17.9% between the two parental strains. High drones had a median sucrose response score of 4 (2.75–5.25), hybrid drones were intermediate [score = 3 (2–4)], and low drones exhibited the lowest response [score = 2 (1–3)]. Water and sucrose response scores in the drones were highly correlated (r = 0.64, n = 192, P < 0.001) and thus we report only sucrose response results.
pln-QTL effects:
In the worker HBC, the full factorial ANOVA involving all four pln-QTL markers revealed a significant effect of pln4 (F(1,130) = 5.84, P = 0.017) and significant interaction effects of pln2 × pln3 (F(1,130) = 6.26, P = 0.014) and pln3 × pln4 (F(1,130) = 4.20, P = 0.043). An additional effect by pln1 was suggestive but not significant (F(1,130) = 3.18, P = 0.077). In both pln1 and pln4, the overall effect of the high strain allele relative to a low strain allele was a reduction in sucrose response score (by 0.33 and 0.42 units, respectively). However, the magnitude of the effect of pln4 depended on the genotype at pln3: in combination with a high strain allele at pln3, the effect of the high pln4 allele was much stronger (0.96 units) than in combination with a low allele at pln3 (0.11 units). On the other hand, pln3 interacted with pln2. Relative to a low strain allele, the high strain allele at pln3 increased the responsiveness of workers in the presence of a low strain allele at pln2 (0.67 units) but decreased responsiveness in the presence of a high strain allele at pln2 (0.28 units).
In the LBC, the full factorial ANOVA revealed only one significant effect: the interaction between pln3 and pln4 (F(1,88) = 4.86, P = 0.030). This was due to the increase of sucrose responsiveness by the high strain allele relative to the low strain allele at pln3 in the presence of a high strain allele at pln4 (by 0.88 units) but a relative decrease (0.53 units) in the presence of a low strain allele at pln4. All two-way ANOVAs on both worker groups (HBC and LBC) confirmed the strong effect of genotype (Table 1). Additionally, these analyses demonstrated an overall effect of pln1 (F(1,360) = 7.64, P = 0.006), but no other marker or interaction between single marker genotypes and genetic background were significant (Table 1).
TABLE 1.
Two-factorial ANOVA results for pln-QTL genotype × overall genotype effects in workers
| Test | QTL effecta | Backcross effecta | Interaction effect |
|---|---|---|---|
| pln1 × backcross | F(1,360) = 7.64, P = 0.006 | F(1,360) = 189.95, P < 0.001 | F(1,360) = 0.02, P = 0.888 |
| Pln2 × backcross | F(1,297) = 0.79, P = 0.374 | F(1,297) = 136.12, P < 0.001 | F(1,297) = 0.37, P = 0.546 |
| Pln3 × backcross | F(1,331) = 0.02, P = 0.888 | F(1,331) = 170.29, P < 0.001 | F(1,331) = 0.00, P = 0.971 |
| Pln4 × backcross | F(1,340) = 2.39, P = 0.123 | F(1,340) = 176.56, P < 0.001 | F(1,340) = 0.06, P = 0.803 |
Significant results are in italics.
In males, the effect of pln4 could not be evaluated because of the lack of a suitable variable marker but instead testing round was incorporated as a fourth factor into the model. An overall significant effect of pln1 was indicated (F(1,171) = 8.87, P = 0.003) but the pln1 effect significantly interacted with testing round (F(1,171) = 4.98, P = 0.027). In both rounds the high allele of the pln1 marker increased the drones' responsiveness to sucrose, but the effect was much stronger in the second testing round (1.3 units) than in the first (0.32). No other single marker or interaction effects were significant.
QTL mapping:
The three genetic maps generated (HBC, LBC, and hybrid drones) differed in the numbers of linkage groups (45, 40, 31, respectively), but the recombinational size estimates (5336 cM, 5141 cM, 5310 cM, respectively) were relatively similar. Thus, we are confident that these genetic maps cover most of the honeybee genome. On the basis of the 16 chromosomes that make up the honeybee genome (Sanderson and Hall 1948), our linkage maps have 29, 24, and 15 gaps, respectively. Efforts to determine the homology of linkage groups among those maps based on the AFLP markers (Rueppell et al. 2004) failed. Thus, no overall comparison of the results could be performed among the three maps, nor could they be combined for a combined QTL analysis. Only the sts markers provided some anchors to compare the linkage groups on which the pln-QTL are located between the HBC and LBC maps. The markers for pln1 and pln4 grouped together in one linkage group in both maps, but this group was 284 cM in the HBC and only 112 cM in the LBC. Conversely, the pln2 marker was located on a 109-cM linkage group in the HBC and a 393-cM group in the LBC. The pln4 marker proved to be unlinked in the HBC but mapped to a linkage group in the LBC (86 cM).
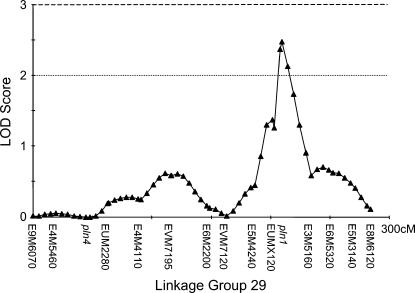
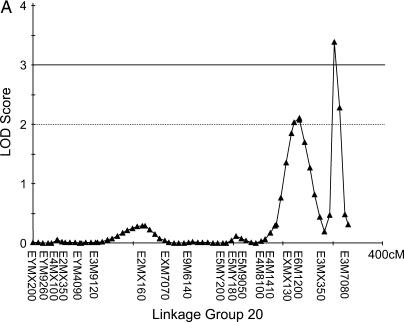
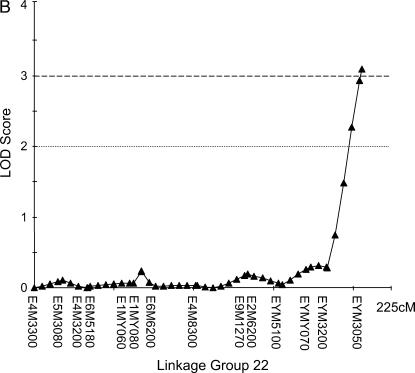
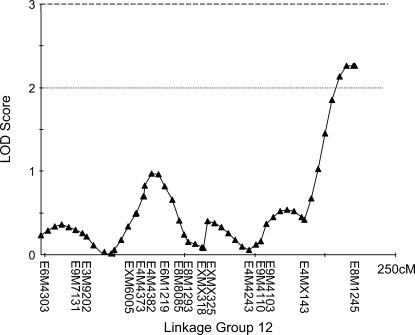
The three markers with the single most effects in each backcross (Table 2) were in three different linkage groups in each of the genomic maps (HBC, LBC, and drone). All these markers are represented in our linkage groups and each of the QTL reported below is partially caused by one of the markers in Table 2. The interval mapping revealed one suggestive QTL in the HBC, which is identical to the previously mapped pln1-QTL (Figure 2) with LOD score of 2.48. Markers at the other pln-QTL had no significant effect on the sucrose responsiveness: pln2 LOD = 0.14, pln3 LOD = 0.07, pln4 LOD = 0.00. In the LBC, two QTL could be detected on two independent linkage groups with LOD scores of 3.39 and 3.09 (Figure 3). According to the theoretically determined significance threshold of LOD = 3.0 (Hunt et al. 1995) both QTL are significant. However, bootstrapping suggested a significance threshold of LOD = 3.25, which would make the second (Figure 3B) a suggestive QTL. No additional, suggestive QTL were detected in the LBC and all markers at previously mapped QTL locations for foraging behavior exhibited no significant effects on the PER scores: pln1 LOD = 0.23, pln2 LOD = 0.54, pln3 LOD = 0.08, pln4 LOD = 0.04. One suggestive QTL could be detected in the drone population (Figure 4) but no significant QTL. No sts-markers were scored in the drone mapping population.
TABLE 2.
The three markers with the strongest independent effects in each of the worker backcross and drone populations
| High backcross | Low backcross | Hybrid drones | |||
|---|---|---|---|---|---|
| D8 (pln1) | K(57,38) = 13.0, P < 0.0005 | EYM3050 | K(62,32) = 13.7, P < 0.0005 | E8M1245 | K(111,78) = 10.9, P < 0.001 |
| EUM7050 | K(45,43) = 7.4, P < 0.01 | EXMX130 | K(54,40) = 8.9, P < 0.005 | EXM5135 | K(102,88) = 9.7, P < 0.005 |
| E5M7110 | K(50,42) = 6.6, P < 0.05 | E4M7080 | K(54,38) = 5.9, P < 0.05 | E9M2313 | K(101,88) = 6.6, P < 0.05 |
Kruskal-Wallis' K is given with the significance value before correcting for multiple independent tests. The number of multiple independent tests performed is between the number of linkage groups and the number of markers tested (see text).
Figure 2.
One putative quantitative trait locus was detected in the high backcross with a likelihood odds ratio (LOD) score of 2.5, explaining 11.7% of the phenotypic variance. It coincided with the previously mapped pln1-QTL. A priori LOD thresholds for significant and suggestive QTL are indicated by a broken and a dotted horizontal line, respectively.
Figure 3.
Two new QTL were detected on two different linkage groups in the low backcross. The first (A) had a LOD score of 3.4 and explained 69.3% of the variance, and the second (B) has a LOD score of 3.1 and explained 14.0% of the overall phenotypic variance.
Figure 4.
One suggestive QTL was detected in the male (drone) mapping population with a LOD score of 2.3, explaining 7.9% of the total variance.
DISCUSSION
Our results support the view of a complex genetic architecture of the pollen-hoarding syndrome with interactive genetic factors (epistasis) and partial genetic overlap among the individual behavioral traits involved (pleiotropy). The investigated pollen-hoarding QTL also affect the honeybees' sucrose response scores (sucrose sensitivity). The pln1 marker showed an effect in the drones and workers, an epistatic effect between the markers for pln3 and pln4 was detected in both worker backcrosses, and pln2 interacted with pln3 in the HBC. This confirms at the genetic level the hypothesis that sucrose responsiveness is a central component (or a direct measure of a central component) of the pollen-hoarding behavioral syndrome (Pankiw and Page 2000; Scheiner et al. 2004a). Furthermore, we report some new QTL that may be part of the genetic network underlying the pollen-hoarding syndrome by influencing the sensitivity of honeybees to sucrose. Our findings reveal a modular structure of the genetic architecture of the pollen-hoarding behavioral syndrome with partially overlapping genetic ensembles (Anholt 2004).
The bidirectional selection for high and low pollen hoarding has generated strains (Page and Fondrk 1995) that show numerous behavioral differences (Page and Erber 2002) with a relatively high degree of genetic determination (Hunt et al. 1995; Page et al. 1995; Rueppell et al. 2004; Humphries et al. 2005). The degree of genetic determination found for the PER scores in this study is relatively high (as can be expected for a reflex) in workers but not in drones. Our r2-values are equivalent to heritability estimates (h2) among our experimental groups and its lower value in drones could indicate that drone bees are more susceptible to subtle variations under the chosen experimental conditions. However, the data structure and the overall similarity between worker and drone response scores suggest that the tested sucrose concentrations were appropriate for both groups.
The lower degree of genetic determination of sucrose responsiveness of drones under the experimental conditions may have contributed to the small number of genetic effects found (only one pln-QTL effect and one putative new QTL) in the male population. The low mapping success was particularly surprising, given that the drone mapping population was twice as large as each worker mapping population. Possible other explanations include sex-limited expression of the detected worker effects (Rhen 2000) or interactions with the genetic background. However, we can also not exclude that separate parts of the genome have been missed in the three mapping populations, generating differing results. Finally, the experimental conditions may have differed slightly among the different experiments (e.g., seasonal effects, age of individuals), which may influence the measurement of sucrose sensitivity (Pankiw and Page 2003).
Both behavioral and physiological studies suggest that sensory tuning plays a major role in the differentiation of the high and low pollen-hoarding strains (Humphries et al. 2003; Amdam et al. 2004; Schulz et al. 2004), and more generally in the division of labor in honeybees (Amdam et al. 2004). The response to sucrose is a convenient probe into the general sensory tuning of insects because it is easily measured with the proboscis extension reflex (Bitterman et al. 1983). The correlation between water and sucrose responses corroborates the view that sucrose response scores are a general expression of sensitivity to (probably many) stimuli, but are not an indication of a specific preference toward sucrose. As such, sucrose responsiveness is beginning to be used in other insect systems to probe the proximate effects of behavioral mutations, for example the for mutation in Drosophila (Scheiner et al. 2004b).
The honeybee homolog (Amfor) of the underlying cGMP-dependent protein kinase gene (PKG) is in the immediate vicinity of our pln4 marker (Rüppell et al. 2004). Our results suggest that PKG could be also involved in the sensory tuning of honeybees because we found a direct effect of pln4 in the HBC and interaction effects with pln3 in both worker backcrosses. The involvement of PKG is corroborated by a direct gene expression study (Ben-Shahar et al. 2002) linking PKG to the initiation of foraging in honeybee workers, which is directly tied to sucrose responsiveness (Pankiw 2003). PKG, an important element of cellular signal transduction, is a prime candidate for widespread pleiotropy (Anholt 2004) underlying coordinated behavioral syndromes and for our pln4-QTL.
The most noteworthy of the pln-QTL in this study is pln1, which exhibits an effect on both worker and drone sucrose responsiveness. We regard its LOD score of 2.48 as a significant confirmation of a QTL that influences the pollen-hoarding syndrome, since we had an a priori expectation. These results tie the female-male correlation of sucrose responsiveness between the selection lines (Pankiw and Page 1999) to an individual genomic region. However, the direction of the effect of pln1 alleles varies among worker and drone populations. Thus, genetic background and sex extensively modulate the effect of pln1, which illustrates that a partial overlap in genetic architecture does not necessarily lead to evolutionary constraints (Anholt 2004). Pln1 is also the QTL with the most wide-ranging behavioral effects. Originally mapped for its colony-level effect on pollen hoarding (Hunt et al. 1995), it has been repeatedly found to affect different aspects of individual foraging behavior (Hunt et al. 1995; Page et al. 2000; Rüppell et al. 2004), as well as the rate of behavioral ontogeny in worker honeybees (Rueppell et al. 2004). This QTL proves to be robust through multiple generations of selection with several rounds of outbreeding, and across separate behavioral measures. We will pursue the study of this genomic region and the imminent publication of the honeybee genome sequence will greatly aid us in identifying potential candidates for this remarkable behavioral QTL.
One characteristic of pln1 in this and other studies (Hunt et al. 1995; Page et al. 2000; Rueppell et al. 2004) is that the allele inherited from the high strain confers a low strain phenotype and vice versa. QTL alleles from selected lines exhibit relatively often effects that are opposite to the phenotype of the selection goal (Burke et al. 2002) because epistatic interactions make their phenotypic effect dependent on the genetic background (Anholt 2004). We identified some of these epistatic interactions between candidate genes in workers of the HBC and LBC. While the pln2 × pln3 interaction was found to be significant only in the HBC, the other interaction (pln3 × pln4) was found in both worker populations. However, the directionality of the allelic effects depended on the genetic background, which demonstrates directly that in our system epistasis plays a major role in shaping the selection response (Wolf and Brodie 2000) and masks the phenotypic effect of certain alleles. This explains why alleles from the high pollen-hoarding line may confer a low pollen-hoarding phenotype in experimental backcrosses between the two.
The results presented here also expand the genetic correlations underlying the pollen-hoarding syndrome. The responsiveness to sucrose has been put forward as a central behavioral trait of the pollen-hoarding syndrome (Page and Erber 2002; Scheiner et al. 2004a) and this study suggests pleiotropic effects of all foraging (pln) QTL on the honeybees' sucrose response score. We cannot rule out at this point that the pleiotropic effects of pln1 are due to a cluster of tightly linked genes, instead of pleiotropy of a single gene. However, the relatively high recombination rate of the honeybee genome (Gadau et al. 2000) and the repeated genetic correlation in different crosses from different genetic sources (Page et al. 2000; Rüppell et al. 2004) make this hypothesis less likely.
We extend the understanding of the genetic architecture of the pollen-hoarding behavioral syndrome (Pankiw 2003) further by reporting two significant new QTL for the proboscis extension response in honeybees (the first suggestive QTL detected in the HBC corresponds to pln1, and the second suggestive QTL, detected in the drones, is only putatively new because we lack the genetic markers to evaluate whether it is overlapping with any of the pln-QTL). The new QTL lie in different regions of the genome than the investigated pln-QTL and preliminary sequence analysis supports the view that they actually represent independent QTL. Due to the extensive behavioral correlations of PER scores with other traits of the pollen-hoarding syndrome in our selected strains and wild-type honeybees (Pankiw and Page 2000; Pankiw 2003; Scheiner et al. 2004a), these QTL constitute candidate regions that need to be investigated in more detail with respect to the other behavioral traits, such as the onset of foraging, or foraging choices. Only through these detailed, mutually informative studies will we gain a comprehensive understanding of the genetic architecture of the pollen-hoarding syndrome. With the aid of the annotated honeybee genome sequence we will then be able to formulate more specific hypotheses and candidate genes to decipher its molecular architecture. Our system provides one of the best-studied cases of a natural behavioral syndrome (Anholt 2004; Sih et al. 2004). It has important implications for understanding the division of labor in social insect societies (Beshers and Fewell 2001) and social evolution itself (Amdam et al. 2004).
Acknowledgments
We thank Jennifer Tsuruda for assistance with the laboratory procedures. This research was funded by the National Science Foundation (NSF IBN 0090482 and NSF IBN 0076811). The work described complied with all applicable ethical guidelines and laws in the United States of America.
References
- Amdam, G. V., K. Norberg, M. K. Fondrk and R. E. Page, 2004. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc. Natl. Acad. Sci. USA 101: 11350–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt, R. R. H., 2004. Genetic modules and networks for behavior: lessons from Drosophila. BioEssays 26: 1299–1306. [DOI] [PubMed] [Google Scholar]
- Antoni, F. A., 2000. Molecular diversity of cyclic AMP signaling. Front. Neuroendocrinol. 21: 103–132. [DOI] [PubMed] [Google Scholar]
- Barron, A. B., D. J. Schulz and G. E. Robinson, 2002. Octopamine modulates responsiveness to foraging-related stimuli in honey bees (Apis mellifera). J. Comp. Physiol. A 188: 603–610. [DOI] [PubMed] [Google Scholar]
- Basten, C. J., B. S. Weir and Z-B. Zeng, 1994. Zmap-a QTL cartographer, pp. 56–66 in Proceedings of the 5th World Congress on Genetics Applied to Livestock Production: Computing Strategies and Software, Vol. 22, edited by C. Smith, J. S. Gavora, B. Benkel, J. Chesnais, W. Fairfull, J. P. Gibson, B. W. Kennedy and E. B. Burnside. Guelph, Ontario, Canada.
- Basten, C. J., B. S. Weir and Z-B. Zeng, 2002. QTL Cartographer, Version 1.16: A Reference Manual and Tutorial for QTL Mapping. Department of Statistics, North Carolina State University, Raleigh, NC.
- Ben-Shahar, Y., A. Robichon, M. B. Sokolowski and G. E. Robinson, 2002. Influence of gene action across different time scales on behavior. Science 296: 741–744. [DOI] [PubMed] [Google Scholar]
- Beshers, S. N., and J. H. Fewell, 2001. Models of division of labor in social insects. Annu. Rev. Entomol. 46: 413–440. [DOI] [PubMed] [Google Scholar]
- Bitterman, M. E., R. Menzel, A. Fietz and S. Schaefer, 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp.Physiol. A 97: 107–119. [PubMed] [Google Scholar]
- Burke, J. M., S. Tang, S. J. Knapp and L. H. Rieseberg, 2002. Genetic analysis of sunflower domestication. Genetics 161: 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill, G. A., and R. W. Doerge, 1994. Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet, S. A., 1991. A fresh look at the arousal syndrome of insects. Adv. Insect Physiol. 23: 81–116. [Google Scholar]
- Faller, H., 2003. The contribution of behavioral genetics to the understanding of the development of personality traits and psychological disorders. Psychotherapeut. 48: 80–92. [Google Scholar]
- Flint, J., 2003. Analysis of quantitative trait loci that influence animal behavior. J. Neurobiol. 54: 46–77. [DOI] [PubMed] [Google Scholar]
- Gadau, J., R. E. Page, J. H. Werren and P. Schmid-Hempel, 2000. Genome organization and social evolution in hymenoptera. Naturwissenschaften 87: 87–89. [DOI] [PubMed] [Google Scholar]
- Gibson, G., 1996. Epistasis and pleiotropy as natural properties of transcriptional regulation. Theor. Popul. Biol. 49: 58–89. [DOI] [PubMed] [Google Scholar]
- Giot, L., J. S. Bader, C. Brouwer, A. Chaudhuri, B. Kuang et al., 2003. A protein interaction map of Drosophila melanogaster. Science 302: 1727–1736. [DOI] [PubMed] [Google Scholar]
- Gosling, S., 2001. From mice to men: what can we learn about personality from animal research? Psychol. Bull. 127: 45–86. [DOI] [PubMed] [Google Scholar]
- Humphries, M. A., U. Mueller, M. K. Fondrk and R. E. Page, 2003. PKA and PKC content in the honey bee central brain differs in genotypic strains with distinct foraging behavior. J. Comp. Physiol. A 189: 555–562. [DOI] [PubMed] [Google Scholar]
- Humphries, M. A., M. K. Fondrk and R. E. Page, 2005. Locomotion and the pollen hoarding behavioral syndrome of the honey bee (Apis mellifera L.). J. Comp. Physiol. A 191: 669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, G. J., and R. E. Page, 1995. Linkage map of the honey bee, Apis mellifera, based on RAPD markers. Genetics 139: 1371–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, G. J., R. E. Page, M. K. Fondrk and C. J. Dullum, 1995. Major quantitative trait loci affecting honey bee foraging behavior. Genetics 141: 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, R. C., 1993. Interval mapping of multiple quantitative trait loci. Genetics 135: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenberg, O., 1997. Foundations of Modern Probability. Springer, New York.
- Kuwabara, M., 1957. Bildung des bedingten Reflexes vom Pavlov Typus bei der Honigbiene (Apis mellifica). J. Fac. Sci. Hokkaido Univ. Zool. 13: 458–464. [Google Scholar]
- Lander, E. S., and D. Botstein, 1989. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121: 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln, S. E., M. J. Daly and E. S. Lander, 1993. Constructing genetic linkage maps with MAPMAKER/EXP version 3.0: a tutorial and reference manual. Whitehead Institute for Biomedical Research Technical Report. Whitehead Institute, Cambridge, MA.
- Lynch, M., and B. Walsh, 1998. Genetics and Analysis of Quantitative Traits. Sinauer, Sunderland, MA.
- Mackay, T. F. C., 2001. The genetic architecture of quantitative traits. Ann. Rev. Genet. 35: 303–339. [DOI] [PubMed] [Google Scholar]
- Mueller, U. G., and L. L. Wolfenbarger, 1999. AFLP genotyping and fingerprinting. Trends Ecol. Evol. 14: 389–394. [DOI] [PubMed] [Google Scholar]
- Page, R. E., and J. Erber, 2002. Levels of behavioral organization and the evolution of division of labor. Naturwissenschaften 89: 91–106. [DOI] [PubMed] [Google Scholar]
- Page, R. E., and M. K. Fondrk, 1995. The effects of colony level selection on the social organization of honey bee (Apis mellifera L) colonies—colony level components of pollen hoarding. Behav. Ecol. Sociobiol. 36: 135–144. [Google Scholar]
- Page, R. E., and H. H. Laidlaw, 1988. Full sisters and super sisters: a terminological paradigm. Anim. Behav. 36: 944–945. [Google Scholar]
- Page, R. E., K. D. Waddington, G. J. Hunt and M. K. Fondrk, 1995. Genetic determinants of honeybee foraging behavior. Anim. Behav. 50: 1617–1625. [Google Scholar]
- Page, R. E., J. Erber and M. K. Fondrk, 1998. The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J. Comp. Physiol. A 182: 489–500. [DOI] [PubMed] [Google Scholar]
- Page, R. E., M. K. Fondrk, G. J. Hunt, E. Guzman-Novoa, M. A. Humphries et al., 2000. Genetic dissection of honeybee (Apis mellifera L.) foraging behavior. J. Hered. 91: 474–479. [DOI] [PubMed] [Google Scholar]
- Page, R. E., J. Gadau and M. Beye, 2002. The emergence of hymenopteran genetics. Genetics 160: 375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiw, T., 2003. Directional change in a suite of foraging behaviors in tropical and temperate evolved honey bees (Apis mellifera L.). Behav. Ecol. Sociobiol. 54: 458–464. [Google Scholar]
- Pankiw, T., and R. E. Page, 1999. The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J. Comp. Physiol. A 185: 207–213. [DOI] [PubMed] [Google Scholar]
- Pankiw, T., and R. E. Page, 2000. Response thresholds to sucrose predict foraging division of labor in honeybees. Behav. Ecol. Sociobiol. 47: 265–267. [Google Scholar]
- Pankiw, T., and R. E. Page, 2001. Genotype and colony environment affect honeybee (Apis mellifera L.) development and foraging behavior. Behav. Ecol. Sociobiol. 51: 87–94. [Google Scholar]
- Pankiw, T., and R. E. Page, 2003. Effect of pheromones, hormones, and handling on sucrose response thresholds of honey bees (Apis mellifera L.). J. Comp. Physiol. A 189: 675–684. [DOI] [PubMed] [Google Scholar]
- Pankiw, T., and W. L. Rubink, 2002. Pollen foraging response to brood pheromone by Africanized and European honey bees (Apis mellifera L.). Ann. Entomol. Soc. Am. 95: 761–767. [Google Scholar]
- Pankiw, T., R. Roman, R. R. Sagili and K. Zhu-Salzman, 2004. Pheromone-modulated behavioral suites influence colony growth in the honey bee (Apis mellifera). Naturwissenschaften 91: 575–578. [DOI] [PubMed] [Google Scholar]
- Rhen, T., 2000. Sex-limited mutations and the evolution of sexual dimorphism. Evolution 54: 37–43. [DOI] [PubMed] [Google Scholar]
- Rueppell, O., T. Pankiw, D. I. Nielson, M. K. Fondrk, M. Beye et al., 2004. The genetic architecture of the behavioral ontogeny of foraging in honey bee workers. Genetics 167: 1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüppell, O., T. Pankiw and R. E. Page, 2004. Pleiotropy, epistasis and new QTL: the genetic architecture of honey bee foraging behavior. J. Hered. 95: 481–491. [DOI] [PubMed] [Google Scholar]
- Sanderson, A. R., and D. W. Hall, 1948. The cytology of the honey bee, Apis mellifica L. Nature 162: 34–35. [DOI] [PubMed] [Google Scholar]
- Scheiner, R., J. Erber and R. E. Page, 1999. Tactile learning and the individual evalution of the reward in honey bees (Apis mellifera L.). J. Comp. Physiol. A 185: 1–10. [DOI] [PubMed] [Google Scholar]
- Scheiner, R., R. E. Page and J. Erber, 2001. a The effects of genotype, foraging role, and sucrose responsiveness on the tactile learning performance of honey bees (Apis mellifera L.). Neurobiol. Learn. Mem. 76: 138–150. [DOI] [PubMed] [Google Scholar]
- Scheiner, R., R. E. Page and J. Erber, 2001. b Responsiveness to sucrose affects tactile and olfactory learning in preforaging honey bees of two genetic strains. Behav. Brain Res. 120: 67–73. [DOI] [PubMed] [Google Scholar]
- Scheiner, R., R. E. Page and J. Erber, 2004. a Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera). Apidologie 35: 133–142. [Google Scholar]
- Scheiner, R., M. B. Sokolowski and J. Erber, 2004. b Activity of cGMP-dependent protein kinase (PKG) affects sucrose responsiveness and habituation in Drosophila melanogaster. Learn. Mem. 11: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, D. J., T. Pankiw, M. K. Fondrk, G. E. Robinson and R. E. Page, 2004. Comparisons of juvenile hormone hemolymph and octopamine brain titers in honey bees (Hymenoptera: Apidae) selected for high and low pollen hoarding. Ann. Entomol. Soc. Am. 97: 1313–1319. [Google Scholar]
- Seeley, T. D., 1995. The Wisdom of the Hive. Harvard University Press, Cambridge, MA.
- Sih, A., A. Bell and J. C. Johnson, 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19: 372–378. [DOI] [PubMed] [Google Scholar]
- Sokolowski, M. B., 2001. Drosophila: genetics meets behavior. Nat. Rev. Genet. 2: 879–892. [DOI] [PubMed] [Google Scholar]
- Van Ooijen, J. W., M. P. Boer, R. C. Jansen and C. Maliepaard, 2002. MapQTL 4.0, Software for the Calculation of QTL Positions on Genetic Maps. Plant Research International, Wageningen, The Netherlands.
- Waddington, K. D., M. Nelson and R. E. Page, 1998. Effects of pollen quality and genotype on the dance of foraging honey bees. Anim. Behav. 56: 35–39. [DOI] [PubMed] [Google Scholar]
- Walhout, A. J. M., R. Sordella, X. Lu, J. L. Hartley, G. F. Temple et al., 2000. Protein interaction mapping in C. elegans using proteins involved in vulval development. Science 287: 116–122. [DOI] [PubMed] [Google Scholar]
- Winston, M. L., and S. J. Katz, 1982. Foraging differences between cross-fostered honeybee workers (Apis mellifera) of European and Africanized races. Behav. Ecol. Sociobiol. 1: 125–129. [Google Scholar]
- Wolf, J. B., and E. D. Brodie, III, 2000. Epistasis and the Evolutionary Process. Oxford University Press, New York.
- Zeng, Z-B., 1994. Precision mapping of quantitative trait loci. Genetics 136: 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]