Abstract
In Saccharomyces cerevisiae, local repression is promoter specific and localized to a small region on the DNA, while silencing is promoter nonspecific, encompasses large domains of chromatin, and is stably inherited for multiple generations. Sum1p is a local repressor protein that mediates repression of meiosis-specific genes in mitotic cells while the Sir proteins are long-range repressors that stably silence genes at HML, HMR, and telomeres. The SUM1-1 mutation is a dominant neomorphic mutation that enables the mutant protein to be recruited to the HMR locus and repress genes, even in the absence of the Sir proteins. In this study we show that the mutation in Sum1-1p enabled it to spread, and the native HMR barrier blocked it from spreading. Thus, like the Sir proteins, Sum1-1p was a long-range repressor, but unlike the Sir proteins, Sum1-1p-mediated repression was more promoter specific, repressing certain genes better than others. Furthermore, repression mediated by Sum1-1p was not stably maintained or inherited and we therefore propose that Sum1-1p-mediated long-range repression is related but distinct from silencing.
IN Saccharomyces cerevisiae, mating-type information is present at three locations on chromosome III. The transcriptionally active MAT locus determines the mating type of a cell and encodes either MATa or MATα genes in a or α cells, respectively. Cells of opposite mating type mate to produce a/α diploids. Additional copies of the mating-type genes are present on the distal arms of chromosome III at the HML and HMR loci. In most yeast strains, HML contains an unexpressed but intact copy of the MATα allele whereas HMR contains an unexpressed intact copy of the MATa allele. Expression of HML and HMR is stably repressed by a mechanism called silencing, which requires the Sir proteins and specific flanking DNA sequences called silencers (reviewed in Rusche et al. 2003). The silencers and the Sir proteins function together to inactivate most genes in a distance- and orientation-independent manner. The silencers contain binding sites for the origin replication complex (ORC), Rap1p, Abf1p, and Sum1p (Laurenson and Rine 1992; Loo and Rine 1995; Irlbacher et al. 2005) and these silencer-associated proteins initiate the assembly of the silenced chromatin by recruiting the Sir proteins Sir1p, Sir2p, Sir3p, and Sir4p (reviewed in Moazed 2001). Sir2p is an NAD-dependent protein deacetylase, which deacetylates the N-terminal tails of histones, and Sir3p and Sir4p interact with the hypoacetylated histone tails, resulting in silencing.
SUM1 encodes a sequence-specific DNA-binding repressor protein that binds to the operator (middle sporulation elements, or MSEs) of middle-sporulation genes (Xie et al. 1999) and to the HML-E silencer (Irlbacher et al. 2005) in mitotic cells. While Sum1p binding to the MSE element represses the genes (Pierce et al. 2003), binding to the silencer is necessary for Sir-mediated silencing at HML (Irlbacher et al. 2005). Sum1p is present in a protein complex with the Sir2p homolog, Hst1p, which is required to repress the middle sporulation genes (Xie et al. 1999; Rusche and Rine 2001; Sutton et al. 2001; McCord et al. 2003), and this Hst1p-mediated repression is gene specific and highly localized (Xie et al. 1999).
SUM1-1 (suppressor of mar) was identified in a screen of extragenic suppressors of the silencing defect in sir2Δ cells (Klar et al. 1985). SUM1-1 is a dominant mutation that restores silencing at HMR in sir1Δ, sir2Δ, sir3Δ, or sir4Δ mutants as well as mutations in the HMR-E silencer (Livi et al. 1990; Laurenson and Rine 1991; Chi and Shore 1996). The mutation in SUM1-1 is neomorphic since neither a null mutation in, nor overexpression of, SUM1 gives rise to the SUM1-1 phenotype (Chi and Shore 1996). Although Sum1p normally does not associate with HMR, it was likely that ORC recruited Sum1-1p to the silenced loci (Rusche and Rine 2001; Sutton et al. 2001). While Sum1-1p can repress in the absence of the Sir proteins, repression is dependent on Hst1p (Rusche and Rine 2001; Sutton et al. 2001; Bedalov et al. 2003). Following recruitment to the HMR silencers, Sum1-1p and Hst1p spread across the HMR domain, deacetylating the histones and thereby mediating repression (Lynch et al. 2005).
To gain further insight into Sum1-1p-mediated repression, we analyzed how this neomorph repressed genes at the HML and HMR loci. We determined if Sum1-1p was a long-range repressor that could silence a variety of genes and whether this repression was stable. We show that Sum1-1p is a long-range repressor and that the chromatin domain generated by Sum1-1p can repress genes independently of their position and orientation within this domain. However, Sum1-1p can repress certain genes better than others and this repression was not stably sustained. Together, these data suggest that the long-range repression mediated by Sum1-1p was distinct from Sir-mediated silencing.
MATERIALS AND METHODS
Yeast growth, transformations, and integrations:
Standard yeast media and genetic methods were used as described (Sherman 1991). PCR-based integrations were performed with oligonucleotides whose sequences are available upon request. PCR-based integrations and transformation with plasmids followed the standard lithium acetate procedures (Ito et al. 1983). All fragments of DNA were PCR amplified with Expand High Fidelity DNA polymerase, and 5–10 μl of PCR reaction containing 0.5 μg of product were used for a single transformation. Integrations were PCR amplified and their sequences were confirmed.
Plasmids:
pGBD-SUM1 (pRO706) was obtained from the yeast GBD fusion protein collection (Ito et al. 2001). In this plasmid the GAL4 DNA-binding domain (1–147 aa) was fused in frame to the N terminus of SUM1. The ADH1 promoter drove transcription of the chimera in the pGBK-RC-TRP1 plasmid. pGBD-SUM1-1 (pRO707) was constructed by replacing the SpeI–PstI C-terminal fragment of SUM1 in pGBD-SUM1 with that of the SUM1-1 allele. This fragment was amplified by PCR using genomic DNA from the SUM1-1 strain ROY1971 (derived from strain YMC89; Chi and Shore 1996) as template.
To overexpress SUM1 and SUM1-1, the PstI–BamHI fragments encompassing the Sum1p coding region from pGBD-SUM1 (pRO706) and pGBD-SUM1-1 (pRO707) were cloned into the PstI–BamHI site of pRS425 (Sikorski and Hieter 1989). The SUM1 promoter region (725 bp upstream of ATG) was PCR amplified from yeast genomic DNA and cloned into the SacI-BamHI sites of pRS425 to create the plasmids pSUM1 (pRO709) and pSUM1-1 (pRO711). pHST1 in pRS423 (pRO713) was constructed by cloning a BamHI–SalI fragment from pRO575 (gift of Masaya Oki) and contains HST1 with 300 bp of upstream and downstream regulatory sequences. SIR2 was cloned with its promoter in pRS315 (pRO46).
Serial dilutions:
A single colony of yeast cells was used to inoculate 5 ml of liquid YP or YM medium with glucose or galactose as the carbon source and the appropriate supplements to allow maintenance of the plasmids. The cells were grown overnight at 30°. All cells were diluted to an initial concentration of 1.0 A600/ml in YM medium and serially diluted fivefold. Approximately 3 μl of each serial dilution was spotted onto appropriately supplemented plates using a cell spotter. For mating assays, supplemented YMD plates were spread with 1.0 A600 of mating lawn (strains JRY19a, MATa his4, or MATα his4) diluted in 300 ml of YPD. Where necessary, selection for plasmids was maintained throughout this analysis. Cells were allowed to grow at 30° for 48 hr prior to photography.
Patch mating:
Patches of the appropriate strains were grown on YMD plates for 1–2 days at 30°. The mating potential of the cells was monitored by replica plating the patches onto selective YMD plates previously spread with a mating lawn, maintaining the selection for the plasmids prior to and following mating.
α-Factor arrest:
Cells grown in YPAD liquid media (Sherman 1991) were collected by centrifugation and diluted to 1 A600/ml with fresh YPAD media. Five milliliters of the culture was transferred to a flask and Na-succinate, pH 3.5, was added to a final concentration of 25 mm. Cells were allowed to grow for 15 min (30°, 200 rpm) prior to addition of α-factor. α-Factor (stock concentration 1 mm in 0.1 n HCl; Sigma, St. Louis) was added to the cultures to a final concentration of 0.012 mm. After 3 hr (30°, 200 rpm), the cells were transferred onto a YPAD plate. Five microliters of 0.1 mm α-factor solution was spotted at different locations on the plate. Cells that had arrested in α-factor (Shmoo) were moved to the regions on the plate that lacked or contained α-factor, using a dissecting microscope. Plates were placed at 30° and allowed to grow. Every 3 hr, over a period of 15 hr, the plates were removed and photographed with a digital camera. Arrested and dividing cells from two separate experiments were quantified.
RNA isolation and RT-PCR:
Strains (ROY4026, ROY4027, ROY 4028, JRY4563, JRY4013, and ROY1924) were grown overnight in YPD and were used to inoculate 80 ml of fresh YPAD. Cells were grown to an A600∼1.0, harvested, and washed with DEPC-treated water, and total RNA was isolated as described (Schmitt et al. 1990). cDNA was prepared using a RT-PCR kit from Invitrogen (San Diego) with random primers and Superscript III reverse transcriptase and the Platinum Taq DNA polymerase. Quantitative analyses of the expression of the genes at HMR were performed using multiplex PCR with primers specific to URA3 or MATa1 and ACT1. The sequences of the PCR primers specific for MATa1, URA3, and ACT1 are available upon request. Reaction volumes were typically 50 μl and contained 2 μl of cDNA in 20 mm Tris-HCl, pH 8.4, 50 mm KCl, 1.5 mm MgCl2, 0.2 mm of each dNTP, 0.2 μm of each primer and 0.034 μm of [α32P]dATP and [α32P]CTP, and 1 unit of platinum Taq DNA polymerase (Invitrogen). Templates were amplified in 25 cycles and 3 μl of the reaction was resolved on a 5%-polyacrylamide-TBE gel. The gel was dried, and the radioactive bands were analyzed on a Typhoon PhosphorImager using ImageQuant software (Amersham-Pharmacia).
Micrococcal nuclease analysis:
The HMR locus was analyzed by micrococcal nuclease digestion and indirect end labeling (Avendano et al. 2005). Briefly, strains were grown in 200 ml of YPD to 1.0 A600/ml and treated with lyticase to produce spheroplasts. Chromatin in the nystatin-permeabilized spheroplasts was digested with different amounts of micrococcal nuclease (0, 1, 2, 3, and 4 units) for 20 min at 37°. Naked DNA was prepared by phenol/chloroform extraction of spheroplasts and this was digested with 0.03 and 0.06 units of MNase for 5 min at 37°. MNase reactions were stopped with 1% SDS and 5 mm EDTA (final concentration) and proteinase K treatment, and DNA was purified by three phenol/chloroform extractions and treated with RNAse. After digestion with BglII (100 units/sample), DNA samples were run on a 1.5% agarose-Tris-borate-EDTA gel, transferred to Hybond N+ nylon membrane (Amersham, Buckinghamshire, UK), and analyzed by end labeling using a 138-bp radioactive probe that recognizes the BglII region at the C terminus of HMRa1. A pair of oligos (forward: CCAAGGAAAAAGAAGAAGTTGC and reverse: AGATCTCATACGTTTATTTATGAAC) was used to label this probe with [α-32P]dCTP by PCR amplification. A GIBCO (Gaithersburg, MD) 50-bp ladder radioactively labeled was included in the gel as a molecular size reference. Chromatin blots were scanned and analyzed with the program ImageQuant 5.2 (Molecular Dynamics, Sunnyvale, CA).
Strain construction:
SIR2, PPR1, and SUM1 genes were deleted from the start to the stop codon and replaced with HIS3, TRP1, or kanMX markers by homologous recombination to produce sir2Δ∷HIS3, sir2Δ∷TRP1, ppr1Δ∷kanMX, and sum1Δ∷kanMX strains. The SUM1-1 allele used in this study is derived from strain YMC89 (Chi and Shore 1996). The genotypes of the yeast strains used in this study are presented in Table 1.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| MATahis4 | J. Rine | |
| MATα his4 | J. Rine | |
| JRY19a | MATaura3 leu2 trp1 his4 | J. Rine |
| ROY3790 | MATα HMR∷TRP1 SIR2 SUM1 | |
| ROY3792 | MATα HMR∷TRP1 sir2Δ∷HIS3 SUM1 | |
| ROY3839 | MATα HMR∷TRP1 sir2Δ∷HIS3 SUM1-1 | |
| ROY3774 | MATα HMR∷ADE2 SIR2 SUM1 | |
| ROY3770 | MATα HMR∷ADE2 sir2Δ∷HIS3 SUM1 | |
| ROY3818 | MATaHMR∷ADE2 sir2Δ∷TRP1 SUM1-1 | |
| ROY3787 | MATα HMR∷URA3 ppr1Δ∷kanMX SUM1 SIR2 | |
| ROY3797 | MATaHMR∷URA3 ppr1Δ∷kanMX SUM1 sir2Δ∷ TRP1 | |
| ROY2677 | MATα HMR∷URA3 ppr1Δ∷kanMX SUM1-1 sir2Δ∷TRP1 | |
| ROY4027 | MATα HMR∷URA3 ppr1Δ∷kanMX SUM1 SIR2 ura3Δ∷HIS3 | |
| ROY4028 | MATaHMR∷URA3 ppr1Δ∷kanMX SUM1 sir2Δ∷ TRP1 ura3Δ∷HIS3 | |
| ROY4026 | MATα HMR∷URA3 ppr1Δ∷kanMX SUM1-1 sir2Δ∷ TRP1 ura3Δ∷HIS3 | |
| JRY4013 | MATα HMRaSUM1 SIR2 | J. Rine |
| JRY4563 | MATα HMRaSUM1 sir2Δ∷ TRP1 | J. Rine |
| ROY1924 | MATα HMRaSUM1-1 sir2Δ∷ TRP1 | |
| ROY3786 | MATaHMLα hmrΔ∷URA3 SUM1-1 sir2Δ∷HIS3 | |
| ROY3753 | MATα HMLahmrΔ∷URA3 SUM1-1 sir2Δ∷HIS3 | |
| ROY3754 | MATα HMLahmrΔ∷URA3 SUM1 SIR2 | |
| ROY3755 | MATα HMLahmrΔ∷URA3 SUM1 sir2Δ∷HIS3 | |
| ROY3785 | MATα hmlΔ∷TRP1 HMRaSUM1-1 sir2Δ∷HIS3 | |
| ROY3725 | MATahmlΔ∷TRP1 HMRα SUM1 sir2Δ∷HIS3 | |
| ROY3726 | MATahmlΔ∷TRP1 HMRα SUM1 SIR2 | |
| ROY3743 | MATahmlΔ∷TRP1 HMRα SUM1-1 sir2Δ∷HIS3 | |
| ROY3861 | MATahmlΔ∷TRP1 hmrΔ∷HMLα SUM1 SIR2 | |
| ROY3859 | MATahmlΔ∷TRP1 hmrΔ∷HMLα SUM1 sir2Δ∷HIS3 | |
| ROY3863 | MATahmlΔ∷TRP1 hmrΔ∷HMLα SUM1-1 sir2Δ∷HIS3 | |
| ROY4030 | MATahmlΔ∷TRP1 hmrΔ∷[HMR-E-α-HML-I] SUM1 sir2Δ∷HIS3 | |
| ROY4031 | MATahmlΔ∷TRP1 hmrΔ∷[HMR-E-α-HML-I] SUM1 SIR2 | |
| ROY4032 | MATahmlΔ∷TRP1 hmrΔ∷[HMR-E-α-HML-I] SUM1-1 sir2Δ∷HIS3 | |
| ROY4053 | MATahmlΔ∷TRP1 hmrΔ∷[HML-E-α-HMR-I] SIR2 SUM1 | |
| ROY4045 | MATahmlΔ∷TRP1 hmrΔ∷[HML-E-α-HMR-I] sir2Δ∷HIS3 SUM1 | |
| ROY4048 | MATahmlΔ∷TRP1 hmrΔ∷[HML-E-α-HMR-I] sir2Δ∷HIS3 SUM1-1 | |
| ROY2863 | MATα sum1Δ∷kanMX sir2Δ∷TRP1 HMR-[E+a2∷prma1-a1cds+a1Δp+I] | |
| ROY3306 | MATα sum1Δ∷kanMX sir2Δ∷TRP1 HMR-[E+a2∷prma1-URA3cds+a1Δp+I] | |
| ROY3723 | MATα sum1Δ∷kanMX sir2Δ∷TRP1 HMR-[E+a2∷prmURA3-a1cds+a1Δp+I] ppr1Δ∷kanMX | |
| ROY3769 | MATα sum1Δ∷kanMX sir2Δ∷TRP1 HMR-[E+a2∷prmURA3-URA3cds+a1Δp+I] ppr1Δ∷kanMX | |
| ROY3322 | MATα sir2Δ∷TRP1 SUM1-1 HMRa1Δp∷a1 | |
| ROY3324 | MATα sir2Δ∷TRP1 SUM1-1 HMRa1Δp - a1- tRNA | |
| ROY3259 | MATα sir2Δ∷TRP1 SUM1-1 HMRa1Δp tRNA barrier - a1 | |
| ROY4029 | MATα sir2Δ∷TRP1 SUM1-1 HMRa1Δp tRNA barrierΔ - a1 | |
| ROY4043 | MATα HMR-prma1+URA3cds-hmraΔp SIR2 SUM1 | |
| ROY4042 | MATα HMR-prma1+URA3cds-hmraΔp sir2Δ∷TRP1 SUM1 | |
| ROY3364 | MATα HMR-prma1+URA3cds-hmraΔp sir2Δ∷TRP1 SUM1-1 | |
| ROY2666 | MATα HMR sir2Δ∷HIS3 sum1Δ∷kanMX | |
| ROY4038 | MATα HMRss(1xGAL4-RAP1-ABF1)+I sir2Δ∷HIS3 sum1Δ∷kanMX | |
| ROY4039 | MATα HMRss(1xGAL4-RAP1-ABF1) Δ I sir2Δ∷HIS3 sum1Δ∷kanMX | |
| ROY4040 | MATα HMRss(3xGAL4-RAP1-ABF1) Δ I sir2Δ∷HIS3 sum1Δ∷kanMX | |
| ROY4041 | MATα HMRss(5xGAL4-RAP1-ABF1) Δ I sir2Δ∷HIS3 sum1Δ∷kanMX |
SUM1-1 sir2Δ strains with TRP1, ADE2, and URA3 reporter genes placed at HMR were obtained as follows. HMR∷TRP1 strains ROY3790, ROY3792, and ROY3839 were obtained by crossing YLS195 (HMR∷TRP1; Buck and Shore 1995) with strains containing sir2Δ sum1Δ and sir2Δ SUM1-1 alleles. HMR∷ADE2 strain YLS409 (Sussel et al. 1993) was crossed with sum1Δ sir2Δ and sir2Δ SUM1-1 strains to generate ROY3770, ROY3774, and ROY3818. In strain ROY2584 (MATa HMR∷URA3 sir2Δ), the URA3 gene with the promoter of URA3 proximal to HMR-E replaced sequences in the HMRa2 coding region [Saccharomyces Genome Database (SGD) coordinates 293212–293410]. The HMR∷URA3 strains ROY3787, ROY3797, and ROY2677 are derivatives of ROY2584 crossed with ppr1Δ and ppr1Δ SUM1-1 strains.
Strains containing HMRα or HMLa were derived from XW652 [ho MATa HMLα HMRα-Bura3 ade1 ade3∷GAL-HO (His-) leu2 trp∷hisG ura3-52 LYS2] from J. Haber (Wu and Haber 1996) or from K1107 (MATa HMLa HO∷lacZ46 can1-100 ade2-1 leu2-3,112 trp1-1 his3 ura3) from K. Nasmyth (Cvrckova and Nasmyth 1993). These strains were initially backcrossed into W-303 (JRY5078 and JRY3024 from J. Rine) four and three times, respectively. The strains were finally crossed with a sir2Δ∷HIS3, sum1Δ∷kanMX, or SUM1-1 strain to obtain the requisite genotype.
Strains ROY3859, ROY3861, and ROY3863 were constructed by integrating HMLα (sequences 415 bp upstream of the ARS301 to 511 bp downstream of the ARS302 element) at the HMR locus. HMLα was amplified using genomic DNA from ROY175 (W-303 strain) as template and integrated by homologous recombination at the SpeI–PstI sites that flank hmrΔ∷URA3 (SGD coordinates 292388–295324) in strain ROY3573 (MATa hmlΔ∷TRP1 hmrΔ∷URA sir2Δ sum1Δ). Following transformation, 5-FOA-resistant colonies were selected to obtain ROY3825 (MATa hmlΔ∷TRP1 hmrΔ∷HMLα sir2Δ sum1Δ). This strain was crossed with strains carrying SIR2 SUM1 and sir2Δ SUM1-1 alleles.
Silencer swap strains:
The hmrΔ∷HMLα strain ROY3825 described above was used to replace HML-E (from 415 bp upstream of the ARS301 element to the stop codon of HMLα2) with the URA3 cassette. The URA3 gene was then replaced by the HMR-E silencer (SGD coordinate 292388 upstream of HMR to the stop codon of HMRa2) to generate ROY4036 (MATa hmlΔ∷TRP1 hmrΔ∷HMR-E-αHML-I sir2Δ sum1Δ). ROY3825 was also used to replace HML-I (the region of HMLα from the stop codon of HMLα1 to 511 bp downstream of the ARS302 element) with URA3, which was then replaced with the HMR-I silencer (from the stop codon of HMRa1 to SGD coordinate 295324) to give ROY4037 (MATa hmlΔ∷TRP1 hmrΔ∷HML-E-αHMR-I sir2Δ sum1Δ). Replacement of the URA3 cassette was done by selection on 5-FOA, and ROY4036 and ROY4037 were crossed with SIR2 SUM1 and sir2Δ SUM1-1 strains to obtain ROY4030, ROY4031, ROY4032, ROY4045, ROY4048, and ROY4053.
To obtain strains in which transcription of the URA3 and MATa1 genes is driven by the URA3 or MATa1 promoter at the HMR locus, initially URA3 was integrated at the HMRa2 coding region (SGD coordinates 293212–293410) with transcription of URA3 going toward the HMR-E silencer (ROY2795 MATα HMR∷URA3 sir2Δ sum1Δ). In this strain the MATa1 gene at HMR was made nonfunctional. For this, the HMR locus (hmraΔp) was PCR amplified from plasmid pDR126 (gift of S. Loo). In this plasmid the promoter and the first 17 bp of the coding sequence of HMRa1 were deleted (hmraΔp). This PCR fragment was used to transform ROY2795. Transformants were selected on the basis of the recovery of the mating capacity of ROY2795. After transformation, the MATα cells were allowed to grow for 4 hr in liquid YPAD (30°, 250 rpm), mixed with wild-type MATa W-303 cells, and plated onto YPAD plates and incubated at 30° overnight. Cells were then scrapped off the YPAD plate, washed with YM, and replated on YMD plates selecting for diploids. The HMR/hmraΔp diploids were analyzed by PCR and dissected to give the strain ROY2800 (MATα HMR-URA3-hmraΔp sir2Δ sum1Δ).
The entire URA3 cassette in ROY2800 was replaced with the MATa1 gene (SGD coordinates 293512–294505) to obtain ROY2863. In the same strain (ROY2800), the URA3 coding sequence was replaced with the MATa1 coding sequence to generate strain ROY3469 (MATα HMR-prmURA3+a1cds-hmraΔp sir2Δ sum1Δ). ROY3469 was then crossed with a ppr1Δ strain to obtain ROY3723. The MATa1 coding region in strain ROY2863 was replaced with the URA3 coding region to generate ROY3306. Finally, ROY3769 was obtained by crossing ROY2800 with a ppr1Δ strain. Transcription of all the reporter genes in these strains runs toward the HMR-E silencer.
Integration of MATa1 along the HMR locus was achieved as follows. First, URA3 was integrated between HMR-I and the tRNA (SGD coordinates 295070–295281), or beyond the tRNA (SGD coordinates 296382–296482), and then the promoter of the resident HMRa1 gene was deleted as described for ROY2800 to generate ROY2798 (HMR-E-hmraΔp-HMR-I-URA3-tRNA sir2Δ sum1Δ) and ROY3141 (HMR-E-hmraΔp-HMR-I-tRNA-URA3 sir2Δ sum1Δ). The barrier element present to the right of HMR (111–1301 bp downstream of ARS318) was replaced in strain ROY2798 with sequences derived from pUC18 (1200 bp), and URA3 was reintegrated beyond the pUC18 sequences as in ROY3141 to make strain ROY4034 (HMR-E-hmraΔp-HMR-I-barrierΔ∷pUC18-URA3 sir2Δ sum1Δ). The MATa1 gene (SGD coordinates 293512–294505) was PCR amplified and used to replace the URA3 cassettes in ROY2798, ROY3141, and ROY4034. These strains along with ROY2863 were crossed with a sir2Δ SUM1-1 strain to obtain the final sir2Δ SUM1-1 genotype in strains ROY3322, ROY3324, ROY3259, and ROY4029.
Strains ROY4038, ROY4039, ROY4040, and ROY4041 with a synthetic silencer in place of HMR-E were obtained as follows. Strains bearing a synthetic silencer at the HMR locus (Jasper Rine) in which the ARS element was substituted with one GAL4-binding site and the HMR-I silencer was left intact (JRY4529 1xGEB+HMR-I)—or in which the ARS element was substituted with one (JRY4531 1xGEB+hmrIΔ; Ehrenhofer-Murray et al. 1999), three (JRY4804 3xGEB+hmrIΔ), or five (JRY4806 5xGEB+hmrIΔ; Fox et al. 1997) GAL4-binding sites and the HMR-I silencer was deleted—were transformed with a plasmid expressing GBD-SIR1 (pCF117) for mating competence and crossed with a hmrΔ∷URA3 sir2Δ sum1Δ strain, followed by selection of diploids and tetrad analysis.
RESULTS
Promoter specificity in Sum1-1p-mediated repression:
SUM1-1-mediated silencing at HMR shares several aspects of SIR-mediated silencing: SUM1-1 is recruited to the HMR silencers, is found at several sites within the HMR locus, and also generates a hypoacetylated domain that is transcriptionally repressed (Rusche and Rine 2001; Sutton et al. 2001). However, it is not clear whether SUM1-1-generated transcriptional repression fulfills other silencing criteria that include gene nonspecificity and repression that is stably inherited.
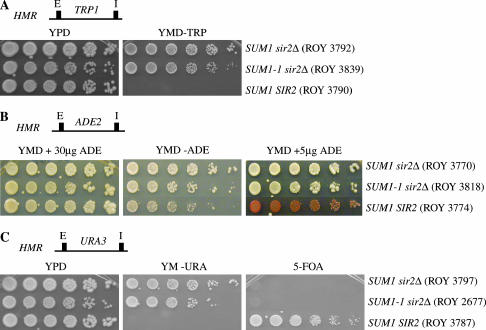
To address whether Sum1-1p could repress different promoters in the absence of Sir2p, we examined the repression of ADE2 (Sussel et al. 1993), URA3 (Donze et al. 1999), and TRP1 (Buck and Shore 1995) at the HMR locus in SUM1-1 sir2Δ strains (Figure 1). Our results showed that in the absence of Sir2p, the TRP1 gene at HMR (Figure 1A) was not repressed in SUM1-1 cells, since the sir2Δ SUM1-1 HMR∷TRP1 strain grew as robustly as the sir2Δ SUM1 HMR∷TRP1 strain on medium lacking tryptophan. The smaller colony size of the SUM1-1 strain was most likely caused by a growth defect associated with SUM1-1 since this phenotype was also observed in rich medium.
Figure 1.
Promoter-specific repression at HMR by SUM1-1. (A) The TRP1 gene was integrated at the HMR locus, and expression of the gene was monitored by growth on YMD plates lacking tryptophan. (B) The ADE2 gene was integrated at the HMR locus, and expression of the gene was monitored by growth on YMD plates lacking adenine or containing limiting amounts of adenine. (C) The URA3 gene was integrated at the HMR locus, and expression of the gene was monitored by growth on YMD plates lacking uracil or containing 5-FOA. Approximately 3 μl of fivefold serial dilutions of overnight cultures was spotted on the different plates. Cells were allowed to grow at 30° for 2 days before the plates were photographed. The plates containing the HMR∷ADE2 strains were left at 4° for an additional day to allow for accumulation of the red pigment prior to photography. (D) mRNA levels of MATa1 and URA3 present at HMR. MATa1 and URA3 gene expression at HMR was quantitated by reverse transcribing total RNA from asynchronously growing cells followed by multiplex PCR. The levels of ACT1 mRNA along with either MATa1 or URA3 primers were quantitated. The average of the ratio of the intensities of the MATa1 or URA3 signal to the ACT1 signal was determined and is shown below.
The URA3 and ADE2 genes are commonly used as reporters for Sir-mediated silencing (Gottschling et al. 1990). Silencing of ADE2, which is stable over several generations, gives rise to red colonies, while colonies where the gene is active, are white. Similarly, stably inherited repression of URA3 over several generations allows cells to form colonies on medium containing 5-FOA and is a hallmark of the silenced state. Consistent with previously published data we find that the TRP1, ADE2, and URA3 reporter genes were fully repressed by the Sir proteins.
Similar to the results obtained with the TRP1 gene, the ADE2 and URA3 reporters were not susceptible to Sum1-1p-mediated repression when located at HMR (Figure 1, B and C). The sir2Δ SUM1-1 HMR∷ADE2 strain formed white colonies when grown under limiting amounts of adenine, suggesting that the ADE2 gene was not repressed. Similarly, the sir2Δ SUM1-1 HMR∷URA3 strain did not grow on 5-FOA-containing plates, suggesting that this gene also was not repressed. Consistent with these observations, we find that sir2Δ SUM1-1 cells were able to grow robustly on medium lacking uracil or adenine although there was a subtle difference between sir2Δ SUM1 and sir2Δ SUM1-1 strains. Whether this difference was due to partial repression of these promoters by SUM1-1 or due to the slower growth of SUM1-1 strains was difficult to determine by these assays.
To confirm the phenotypic analysis, we also measured transcript levels. We measured changes in transcription of two different reporter genes at HMR-MATa1 and URA3 in wild-type, sir2Δ SUM1, and sir2Δ SUM1-1 cells. The amount of specific transcript was determined by multiplex RT-PCR with primers specific for the MATa1 or URA3 genes at HMR and the ACT1 gene (Figure 1D). The level of the URA3 and MATa1 transcript was normalized to ACT1 to compensate for any differences in handling. Our data showed that the MATa1 gene at HMR was repressed in a sir2Δ SUM1-1 strain nearly to the same extent as in the wild-type strain (SIR2 SUM1). The URA3 gene at HMR was significantly active in a sir2Δ SUM1-1 strain compared to the wild-type strain although we did observe a slight reduction in the expression levels in comparison to a sir2Δ SUM1 strain. These data are consistent with our phenotypic results showing that SUM1-1 repressed MATa1 more than URA3 did.
Specificity in Sum1-1p-mediated repression at HML and HMR:
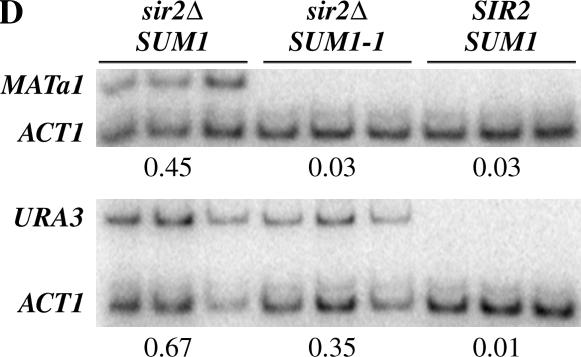
Previous analysis had shown that Sum1-1p restored repression more efficiently at HMRa than at HMLα (Laurenson and Rine 1991; Chi and Shore 1996). It was possible that as in the case of ADE2 and URA3, Sum1-1p was not able to repress MATα genes at the silenced loci. We therefore reexamined the ability of Sum1-1p to repress MATa and MATα genes at HML and HMR using a mating assay. A haploid yeast strain of a particular mating type will mate with cells of the opposite mating type to form diploids, which can then be selected for on appropriate selection plates. Derepression of the silent HML and HMR cassettes results in an inability of the haploids to mate and form diploid colonies.
Consistent with previous data (Laurenson and Rine 1991; Chi and Shore 1996), we found that the MATa genes at HMR were silenced by Sum1-1p in a MATα hmlΔ HMRa sir2Δ SUM1-1 strain (Figure 2A). This silencing was dependent on Sum1-1p since a sir2Δ strain expressing wild-type Sum1p was not able to silence MATa1 at HMR (data not shown). We also analyzed the ability of Sum1-1p to silence MATα genes located at HML in a MATa hmrΔ HMLα sir2Δ SUM1-1 strain and, again consistent with previous data (Laurenson and Rine 1991), the MATα genes at HML were not silenced by Sum1-1p (Figure 2B).
Figure 2.
SUM1-1-mediated repression of MAT genes at HMR and HML. (A–E) Strains containing the MATa genes at the HMR or HML loci (HMRa and HMLa, respectively), the MATα genes at the HMR or HML loci (HMLα and HMRα, respectively), or HMLα at HMR were generated and patched onto YPD plates. Mating assays with the appropriate mating-type tester strains were used to monitor expression of these genes and diploid colonies were allowed to grow on YMD plates for 2 days prior to documentation. The genotypes of the strains at HML, MAT, and HMR are shown schematically.
To determine whether the observed differences were due to the promoters or the silencers, we initially analyzed Sum1-1p-mediated repression of MATa genes located at the HML locus (Figure 2C). The MATa genes were efficiently repressed by Sum1-1p at HML. One possibility is that Sum1-1p was a promoter-specific repressor of the MATa genes or, alternatively, repression by Sum1-1p was effective only at weak promoters.
To distinguish between the two possibilities, we examined whether the MATα genes, which were not repressed at HML, could be repressed when present at HMR (Figure 2D). Our analysis showed that HMRα was repressed in a MATa hmlΔ HMRα sir2Δ SUM1-1 strain. This repression was Sum1-1p dependent since a MATa HMRα hmlΔ sir2Δ SUM1 strain was a nonmater. These results would argue that SUM1-1 could repress promoters of varying strengths and that the difference between HML and HMR may be due to differences in the silencers of the two loci or due to the chromosomal positions occupied by these two loci.
HML and HMR are located on opposite ends of chromosome III and it was possible that sequences adjacent to HMR cooperated with the silencers for Sum1-1p repression. We therefore replaced the HMRa locus on the right arm of chromosome III with the HMLα locus and monitored Sum1-1p-mediated repression at this locus (Figure 2E). The results showed that the MATα genes were not repressed in the MATa hmrΔ∷HMLα sir2Δ SUM1-1 strain. As a control, we monitored Sir-mediated silencing of this locus in a SIR2 SUM1 background and found that the MATα were fully repressed, indicating that the lack of repression by Sum1-1p was not an inherent property of having the HMLα locus at HMR. The results indicate that the chromosomal location of HMLα did not affect the extent of silencing by Sum1-1p and suggest that differences in the silencers may affect the outcome of Sum1-1p repression.
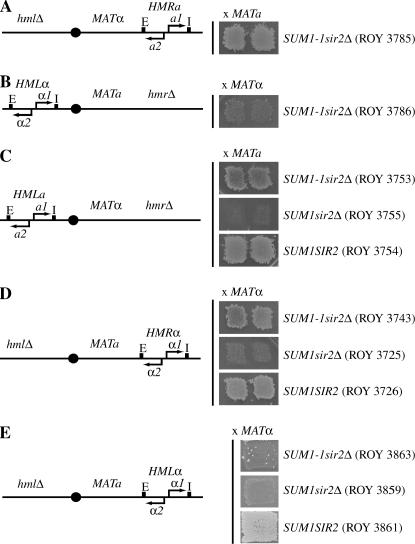
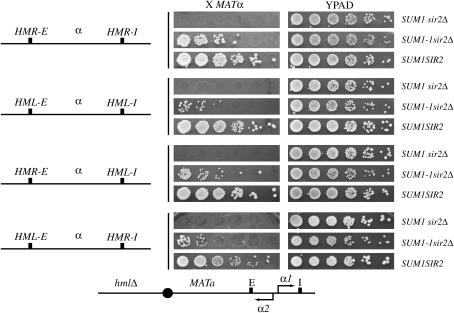
Silencer specificity in SUM1-1-mediated repression:
To determine the role of the individual silencers in repressing MATα genes, we generated four strains. All four MATa strains lacked HML and contained MATα genes at HMR. In one strain, the HMR-E and HMR-I silencers flanked the MATα genes, while in the second strain, the MATα genes were flanked by HML-E and HML-I. In the third strain, these genes were flanked by HMR-E and HML-I, while in the fourth strain these genes were flanked by HML-E and HMR-I. We monitored repression of the MATα genes in wild-type, SUM1 sir2Δ, and SUM1-1 sir2Δ backgrounds (Figure 3). Our data indicate that in a sir2Δ SUM1-1 background the MATα genes were silenced to a greater extent in constructs with HMR-E than with HML-E. As controls we also monitored repression of the genes in SIR2 SUM1 or sir2Δ SUM1 strains. In the former case the genes were silenced in all four silencer combinations while in the latter case we did not observe silencing in any of the constructs.
Figure 3.
Silencer specificity in Sum1-1p-mediated repression. MATa hmlΔ strains with MATα genes at HMR were constructed. In strains ROY3725 (SUM1 sir2Δ), ROY3743 (SUM1-1 sir2Δ) and ROY3726 (SUM1 SIR2), the HMR-E and HMR-I silencers flanked the MATα genes. In strains ROY3859 (SUM1 sir2Δ), ROY3863 (SUM1-1 sir2Δ), and ROY3861 (SUM1 SIR2), the HML-E and HML-I silencers flanked the MATα genes. In strains ROY4030 (SUM1 sir2Δ), ROY4032 (SUM1-1 sir2Δ), and ROY4031 (SUM1 SIR2), the HMR-E and HML-I silencers flanked the MATα genes. In strains ROY4045 (SUM1 sir2Δ), ROY4048 (SUM1-1 sir2Δ), and ROY4053 (SUM1 SIR2), the HML-E and HMR-I silencers flanked the MATα genes. Patch-mating assays using a MATα tester lawn were performed to monitor silencing of the MATα reporter gene.
SUM1-1-mediated repression was not stably inherited:
Thus far, we found that only the mating-type genes were efficiently repressed by Sum1-1p in the absence of Sir2p. This may be due to the inherent difference in the assays used to measure repression of the MAT genes vs. the ADE2 and URA3 reporters (van Leeuwen and Gottschling 2002). The mating assay used to measure repression of the MAT genes was akin to taking a snapshot since it measured repression only during the G1 phase of the cell cycle, and not repression through the cell cycle or inheritance of repression through multiple cell cycles. On the other hand, repression of ADE2, URA3, and TRP1 measured by colony formation on medium lacking these nutrients or containing 5-FOA was akin to a movie in that repression was measured throughout the cell cycle and over multiple generations. We do not believe that the differences in repression between the MAT genes and URA3 were due to different turnover rates in their mRNAs since transcripts of these genes have a similar half-life of ∼3 min (Herrick et al. 1990). This raised the possibility that Sum1-1p was capable of repressing URA3 and the other reporters but this repression was transient and not sustained.
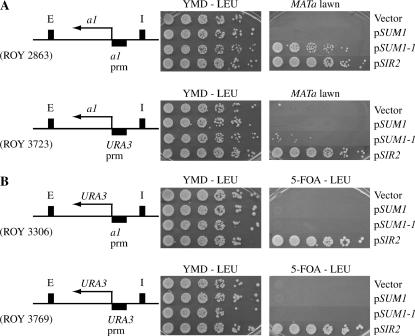
To address these issues, we initially generated two HMR alleles in sir2Δ sum1Δ strains: one allele contained the MATa1 coding region under control of the URA3 promoter while the second allele was a control that contained the MATa1 coding region under control of its own promoter (Figure 4A). If the URA3 promoter was sensitive to Sum1-1p-mediated repression, at least during the G1 phase of the cell cycle, then these cells would be able to mate. This assay also allows us to monitor solely the two promoters without having to worry about differences in the stability of the RNA or protein. Strains were transformed with plasmids expressing Sum1-1p, Sum1p, or Sir2p and cells were grown in selective medium. Mating assays were performed in minimal medium (selecting for the plasmids) to monitor repression of the promoters and our results showed that both the MATa1 and URA3 promoters were repressed by Sum1-1p, although the MATa1 promoter was repressed to a much greater extent than the URA3 promoter. While the repression of the URA3 promoter is slight, it is reproducible, and an integrated version of SUM1-1 at its chromosomal location was able to better repress the URA3 promoter (data not shown). These results are consistent with our earlier results that Sum1-1p was better at repressing the MATa1 promoter compared to the URA3 promoter (see Figure 1D). Wild-type Sum1p did not repress these hybrid genes, while Sir2p completely restored silencing.
Figure 4.
SUM1-1 repression was promoter specific and unstable. (A) MATα sir2Δ sum1Δ strains in which transcription of the MATa1 coding region was driven by the MATa1 promoter (ROY2863) or by the URA3 promoter (ROY3723) at the HMR locus were transformed with vector alone (pRS425), pSUM1 (pRO709), pSUM1-1 (pRO711), or pSIR2 (pRO46). Transformants were grown selectively and fivefold serial dilutions were spotted onto YMD plates lacking leucine to monitor growth or onto YMD plates with a MATa tester lawn (MATa his4) to monitor for expression of the MATa1 gene using a mating assay. (B) MATα sir2Δ sum1Δ strains with the URA3 coding region under the control of the MATa1 promoter (ROY3306) or the URA3 promoter (ROY3769) were transformed with vector alone (pRS425), pSUM1 (pRO709), pSUM1-1 (pRO711), or pSIR2 (pRO46). The transformants were grown selectively and fivefold serial dilutions were spotted onto YMD plates without leucine as controls for growth or onto YMD plates containing 5-FOA (5-FOA–LEU) to monitor for stable repression of URA3.
We next addressed the question of whether Sum1-1p-mediated repression was stably inherited over many generations. We generated two HMR alleles in sir2Δ sum1Δ strains: in one allele, the URA3 coding region was placed under the control of the URA3 promoter, and in the second allele, the URA3 coding region was placed under the control of the MATa1 promoter (Figure 4B). Once again this allows us to monitor solely the two promoters without having to worry about differences in the stability of the RNA or protein. These strains were transformed with plasmids that expressed Sum1-1p, Sum1p, or Sir2p and repression was monitored by growth on 5-FOA-containing plates. Neither strain could grow on 5-FOA media, indicating that the repression mediated by Sum1-1p was transient. Cells expressing wild-type Sum1p were also not able to repress the reporter genes, whereas Sir2p stably silenced both promoters. These results clearly demonstrated that Sum1-1p-mediated repression of URA3 and even the MATa1 promoter was not stably inherited for many generations and may reflect a general characteristic of the repressive state mediated by Sum1-1p.
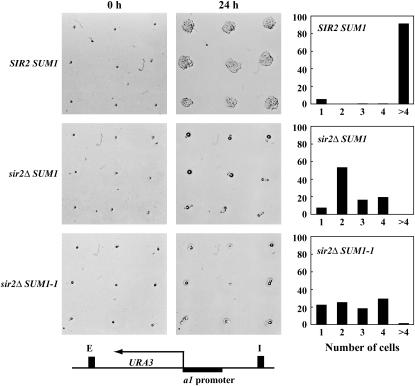
While Sum1-1p-mediated repression was not inherited for many generations, it was possible that Sum1-1p-mediated repression was stably inherited for a few generations but was not sufficient to form a visible colony on 5-FOA. To determine the number of generations in which the repressed state was inherited, we monitored the division of single cells over a period of 24 hr. We used three different strains: SIR2 SUM1, sir2Δ SUM1, and sir2Δ SUM1-1. The HMR locus in these strains was modified such that the URA3 coding region was under the control of the MATa1 promoter. These strains were initially grown in YPD and >200 individual cells were manipulated for each strain and placed on plates lacking or containing 5-FOA. In the absence of FOA, the vast majority of cells divided and formed colonies (data not shown). Also as expected, the vast majority of wild-type SIR2 SUM1 cells divided and formed colonies within 24 hr on plates containing 5-FOA (Figure 5). While most of the sir2Δ SUM1 cells did not divide more than once and nearly 60% of the microcolonies had only two cells, 15–20% had three or four cells each. It should also be pointed out that the microcolonies scored with three and four cells could actually be two cells with large buds.
Figure 5.
Sum1-1p-mediated repression was not stably inherited. Three different MATα strains with the URA3 coding region under the control of the MATa1 promoter were generated. ROY4043 was SIR2 SUM1 while ROY4042 was sir2Δ SUM1 and ROY3364 was sir2Δ SUM1-1. The strains were grown in liquid YPD medium, and then placed onto YMD plates with 5-FOA. Single cells were micromanipulated on the plates and the plates were photographed to monitor growth and division of the cells. At 24 hr postmicromanipulation, the number of cells in each microcolony were counted. Approximately 200 microcolonies were counted for each strain and the data were plotted as a percentage of the total.
The sir2Δ SUM1-1 cells also did not divide to form colonies on 5-FOA. The manipulated cells divided once or twice since there was an even distribution from one to four cells in the microcolonies. Since SUM1-1-mediated repression results in only 40% of cells in a population being repressed (Chi and Shore 1996), and since we were unable to determine which micromanipulated cells were repressed and which were derepressed at the start of this experiment, our data suggest that repression mediated by SUM1-1 was at the most stably inherited for two generations and more likely no more than one generation.
SUM1-1-mediated repression was not stably maintained:
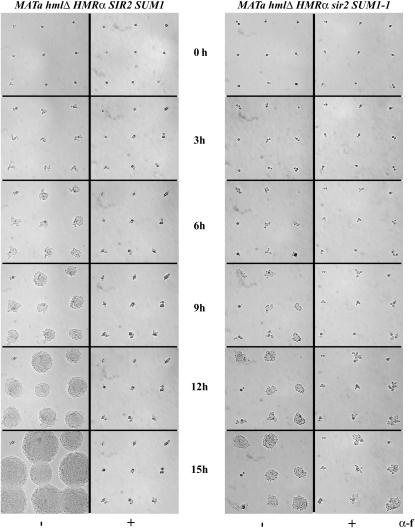
Our data showed that SUM1-1-mediated repression was not stably inherited. We next asked if SUM1-1-mediated repression was stably maintained in the G1 phase of the cell cycle using a modified shmoo-bud assay (Enomoto and Berman 1998). To perform this analysis, we used three MATa hmlΔ HMRα strains. One of these was SIR2 SUM1, one was sir2Δ SUM1, and the third was sir2Δ SUM1-1. As shown in Figure 2, the MATα genes at HMR were repressed by SUM1-1.
Cells were grown in YPAD and then transferred to YPAD liquid medium containing α-factor for 3 hr to arrest the cells in the G1 phase of the cell cycle. The sir2Δ SUM1 strains never arrested in α-factor. For the other two strains, single cells that had formed shmoo projections were then micromanipulated on regions of YPAD plates containing or lacking α-factor (Figure 6). Cells were monitored for the maintenance of their shmoo projections or the appearance of buds. When shmooed cells were placed on medium lacking α-factor, wild-type and sir2Δ SUM1-1 strains were able to exit from the arrest, bud, and form microcolonies within a few hours.
Figure 6.
Sum1-1p-mediated repression was not stably maintained. MATa hmlΔ HMRα strains with SIR2 SUM1 or sir2Δ SUM1-1 alleles were initially grown in liquid YPAD medium. The cells were arrested with α-factor for 3 hr in liquid medium and then streaked onto YPAD plates. Single cells were micromanipulated on the regions of the plate containing or lacking α-factor and the micromanipulated cells were photographed every 3 hr for growth and cell division.
On α-factor-containing plates, wild-type cells maintained their shmoo projections and remained arrested for close to 15 hr, often forming multiple shmoo projections. While a considerable number of SUM1-1 sir2Δ cells arrested in α-factor in liquid medium, when these arrested cells were manipulated onto plates containing α-factor and monitored over a period of time, a significant number of the arrested, shmooed cells escaped the arrest and began forming buds and microcolonies. Analyses of the data indicate that cells remained arrested for 6–9 hr before they escaped the arrest and began forming microcolonies, suggesting that Sum1-1p-mediated repression was not stably maintained (Figure 6 and Table 2). We do not believe that Sum1-1p-containing cells were exiting the arrest due to overexpression of Bar1p since it has been shown that SUM1-1-containing cells have significantly lower levels of BAR1 RNA (Lynch et al. 2005).
TABLE 2.
Single cell analyses for repression of HMRα
|
MATahmlΔ HMRα
|
||||
|---|---|---|---|---|
|
SIR2SUM1
|
sir2Δ SUM1-1
|
|||
| α-Factor | + | − | + | − |
| Budding | 0 | 88 | 55 | 78 |
| Shmoo | 100 | 12 | 45 | 22 |
At 1 hr after micromanipulation, the cells in each microcolony were monitored for the presence of shmooed undivided single cells vs. cells that had divided multiple times to form microcolonies with greater than two cells per colony. The strains monitored were MATa hmlΔ HMRα with SIR2 SUM1 or sir2Δ SUM1-1 alleles. Numbers are percentages.
The SUM1-1-repressed state spreads:
One of the hallmarks of SIR-mediated silencing is that the Sir proteins spread between and beyond the HMR-E and HMR-I silencers up to the flanking barrier elements, and the genes present in this region are repressed independently of their orientation or location (Donze et al. 1999). While SUM1-1 is present at different locations across the HMR domain (Rusche and Rine 2001), it is not known whether these regions are transcriptionally repressed.
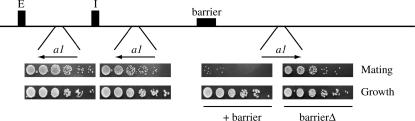
To test whether genes located at any position or orientation within the HMR domain were repressed by SUM1-1, the native MATa1 gene was mutated and rendered nonfunctional, and MATa1 reporter genes were placed at three different locations along the HMR domain. MATa1 was placed either between the two silencers, to the right of the HMR-I silencer but between the silencer and the barrier element, or outside the barrier element as shown schematically in Figure 7. MATa1 was chosen as the reporter gene because it was most efficiently repressed by SUM1-1. As expected, the MATa1 gene located between the two silencers was repressed. It was also repressed when present to the right of the HMR-I silencer between the silencer and the barrier element, but not when it was located beyond the barrier element. Furthermore, overexpression of Sum1-1p alone or with Hst1p did not extend the repressed domain beyond the barrier element (data not shown). These results suggest that Sum1-1p-mediated repression encompassed the entire HMR domain and spread beyond the silencer and up to the barrier element.
Figure 7.
SUM1-1-repressed genes within a large domain. MATα SUM1-1 sir2Δ strains (ROY 3322, ROY 3324, ROY 3259, and ROY4029) containing the MATa1 reporter gene integrated at different locations were grown in YPD liquid medium and 3 μl of fivefold serial dilutions were spotted onto mating lawns to assay for the expression of the MATa1 genes. A schematic shows the sites of insertion and orientation of the MATa1 reporter gene integrated within the HMR locus. “BarrierΔ” refers to a replacement of the HMR barrier sequences with pUC DNA.
The repressive domain generated by the Sir proteins blocks transcription of genes independently of their orientation. Similarly, the repressive domain generated by Sum1-1p also repressed genes independently of their orientation since transcription of the MATa1 gene present between the two silencers was repressed in either orientation (data not shown).
The spread of Sir-protein-mediated silencing is blocked by barrier elements (Donze and Kamakaka 2001; Oki et al. 2004). We therefore inquired if the spread of Sum1-1p-mediated repression was also blocked by the barrier element. We replaced the entire HMR right barrier with pUC DNA and monitored expression of the MATa1 gene that was present outside the barrier. While Sum1-1p was unable to repress MATa1 when the barrier was intact, in the absence of the barrier, Sum1-1p was able to spread and partially repress the reporter gene. The analysis of the semiquantitative spot-mating assay suggests that in ∼5–10% of the cells, the MATa1 gene located beyond the barrier became repressed when the barrier was deleted. While we were able to map Sum1-1p by chromatin immunoprecipitation at the HMR silencers, we were not able to see an increase in the spread of this protein when the barrier was deleted (data not shown). We believe that this is due to the fact that repression by Sum1-1p, beyond the barrier, was present in only a small percentage of cells (Figure 7).
Tethered Sum1-1p repressed HMRΔI:
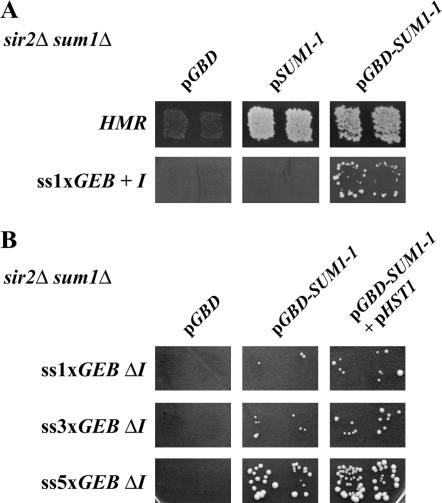
Sum1-1p is recruited to HMR via interactions with ORC (Rusche and Rine 2001; Sutton et al. 2001; Lynch et al. 2005). This model implies that if Sum1-1p were recruited to the silencer, it would be able to silence in the absence of the ORC-binding site. To test this model, we generated sir2Δ sum1Δ strains with a synthetic HMR-E silencer containing Gal4-binding sites in place of the ORC-binding site (Chien et al. 1993; Fox et al. 1997). We also generated fusion proteins with the Gal4 DNA-binding domain fused in frame to SUM1-1. The Gbd-Sum1-1p chimera was active since it was able to repress wild-type HMR in the absence of Sir2p almost to the same extent as untagged Sum1-1p (Figure 8A). We then determined whether the fusion protein could silence MATa1 at the synthetic silencer containing a single Gal4p-binding site in place of the ORC-binding site at the HMR-E silencer. As Figure 8A shows, direct recruitment of Gbd-Sum1-1p was able to repress the reporter gene in the absence of the ORC-binding sites at HMR-E, while Sum1-1p was unable to silence this allele of HMR, presumably because it was not recruited to this locus.
Figure 8.
SUM1-1-repressed genes in the absence of the HMR-I silencer. (A) MATα sir2Δ sum1Δ strains bearing a wild-type or synthetic HMR-E silencer containing one Gal4p-binding site (GEB) were generated. Strains were transformed with vector or plasmids expressing SUM1-1 (pRO711) or GBD-SUM1-1 (pRO707) protein chimeras. Patch-mating assays using a MATa tester lawn were performed to monitor silencing of the MATa1 reporter gene. (B) MATα sir2Δ sum1Δ strains bearing a synthetic HMR-E silencer without HMR-I were generated. One, three, or five GAL4-binding sites replaced the ARS element at HMR-E (ROY4039 HMRss1xGEBΔI, ROY4040 HMRss3xGEBΔI, and ROY4041 HMRss5xGEBΔI). Strains were transformed with vector or plasmids expressing GBD-SUM1-1 (pRO707) and HST1 (pRO713) and patch-mating assays using a MATa tester lawn were performed to monitor silencing of the MATa1 reporter gene.
The HMR-I silencer is necessary for Sum1-1p-mediated repression (Sutton et al. 2001) although its role in repression is not clear. We tested the requirement for HMR-I by using strains with a synthetic silencer that contained one, three, or five binding sites for Gal4p in place of the ORC-binding sites at the HMR-E silencer but lacked the HMR-I silencer (Figure 8B). Under these conditions, Gbd-Sum1-1p was able to repress the MATa1 gene but only when there were multiple binding sites for Gal4p. These results demonstrated that the requirement for the HMR-I silencer could be bypassed by increased or more efficient recruitment of Sum1-1p to the silenced domain.
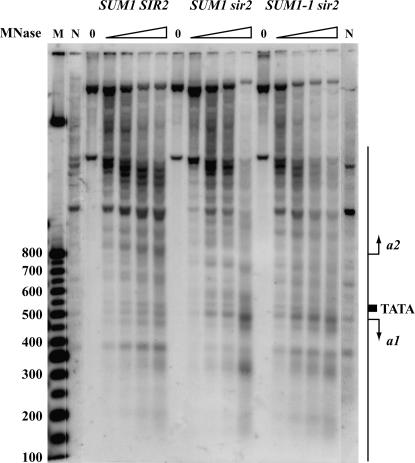
Sum1-1p-generated changes in the nucleosomal organization at HMR:
The presence of the Sir proteins at HMR generates a characteristic organization of the nucleosomes (Ravindra et al. 1999), and it has been suggested that this special organization might be involved in the mechanism of Sir-mediated silencing. We asked if this characteristic pattern of nucleosome organization is recreated in the presence of Sum1-1p. We mapped the locations of the nucleosomes at the HMR locus by micrococcal nuclease digestion and indirect end labeling and the analysis of the pattern in a sir2Δ SUM1-1 revealed characteristics of both wild-type and sir2Δ SUM1 strains (Figure 9). Since SUM1-1-mediated repression occurs in only a fraction of cells in the population, one possibility is that the nucleosomal pattern observed was a composite of the patterns present in wild-type and sir2Δ SUM1 cells. We are currently unable to sort silenced cells from nonsilenced cells to distinguish between this and other possible scenarios.
Figure 9.
Indirect end-labeling analyses of the HMR domain. Permeabilized spheroplasts from wild type (JRY4013), sir2Δ SUM1 (JRY4563), and sir2Δ SUM1-1 (ROY1924) strains were digested with micrococcal nuclease. After deproteinization and restriction enzyme digestion, the DNA was resolved on an agarose gel, blotted, and hybridized with a probe specific to HMR.
DISCUSSION
Transcriptional repression is either gene specific or general (reviewed in Gray and Levine 1996; Courey and Jia 2001): gene-specific repressors are usually short-range local repressors that affect transcription by blocking the function of specific activators in the immediate vicinity of the operator without affecting distal activators. General repression is usually long range, where the repressor encompasses an entire chromatin domain and is often referred to as silencing. Silencing is not gene specific and most genes packaged in the silenced chromatin are rendered inactive. Furthermore, and most importantly, the repressed state is stably inherited over many generations.
A key difference between local and long-range repressors is that the latter encompass large domains of chromatin into a repressed state that can repress most genes placed anywhere within this domain. Sir-mediated silencing at the HMR and HML loci are classic examples of the latter and most genes placed in these silenced domains are repressed (reviewed in Moazed 2001; Rusche et al. 2003). Sum1p is an example of a sequence-specific local repressor (Xie et al. 1999) while SUM1-1 is a neomorphic allele of SUM1 that transforms the wild-type protein from a promoter-specific repressor to a long-range repressor capable of repressing genes at HMR (Rusche and Rine 2001; Sutton et al. 2001). We have investigated Sum1-1p-mediated repression at HMR and our data suggest that this repressor is not as pleiotropic in repressing different promoters as the Sir proteins. We did not observe robust repression of the TRP1, URA3, or ADE2 reporter genes (Figure 1), but as previously reported we did find that the MATa genes were repressed at HMR and, interestingly, even at HML (Figure 2).
It is interesting that while the MATα genes were not repressed at HML, these genes were repressed by Sum1-1p when placed at the HMR locus. We showed that this specificity of repression was due to the presence of specific silencers, with the HMR-E silencer being more efficient for repression compared to the HML silencers. These data are consistent with observations that the HMR silencers are more proficient than the HML silencers in Sir-mediated repression (Shei and Broach 1995). However, a recent report has shown that wild-type Sum1p was bound to the D-element of the HML-E silencer and was necessary for Sir-mediated silencing at HML (Irlbacher et al. 2005). It is therefore paradoxical that HMR-E, rather than HML-E, is a better silencer for Sum1-1p-mediated repression. Since Sum1-1p is recruited via interactions with ORC (Rusche and Rine 2001; Sutton et al. 2001; Lynch et al. 2005), one possibility is that the HMR-E silencer is better at recruiting the mutant protein, given the large number of ORC-binding sites present around this silencer (Palacios DeBeer and Fox 1999). An alternative possibility is that in the absence of wild-type Sum1p, the HML-E silencer is weakened and is therefore unable to efficiently recruit Sum1-1p.
In addition to silencer specificity, promoter specificity may also be involved in influencing Sum1-1p-mediated repression since it is believed that MATα genes are more active than MATa genes, which have no known transcriptional activators (Siliciano and Tatchell 1984, 1986). Our data also suggest that the MATα promoter might be stronger than the MATa promoter since the former could overcome SUM1-1 repression at HML. Thus, the observed differences in Sum1-1p-mediated repression of different genes may be a composite of promoter specificity or strength and silencer specificity or strength. The ability of Sum1-1p to silence some promoters and not others and the role of promoter strength is reminiscent of silencing at telomeres where Sir-mediated telomeric silencing is also promoter specific and depends on promoter strength (reviewed in van Leeuwen and Gottschling 2002).
Repression mediated by Sum1-1p is partial and only a fraction of the cells in a population are repressed at any one time (Laurenson and Rine 1991). In this aspect, too, Sum1-1p repression is more akin to telomeric silencing where repression is observed only in a fraction of the cells in a population (Gottschling et al. 1990). However, the partial repression mediated by Sum1-1p is different from the partial repression mediated by the Sir proteins at telomeres. Silencing at telomeres is epigenetically stable in that, in the fraction where the promoter is silenced, that state of the promoter is propagated for many generations while the cells where the promoter is active remain active for many generations (Gottschling et al. 1990; Iida and Araki 2004). Like telomeric silencing, SUM1-1-mediated repression is partial but our data with the URA3 and MATa1 promoters (using the same transcript reporters) showed that neither promoter was stably repressed and that the repressed state was not stably inherited over many generations. We showed that Sum1-1p-directed repression either could be disrupted in the same mitotic cycle (i.e., a maintenance defect) as the silencing defects observed in cac1Δ mutants (Enomoto and Berman 1998) or could be lost following cell division. Thus the partial repression observed in SUM1-1 cells was different from the partial repression observed for Sir-mediated silencing (Pillus and Rine 1989; Sussel et al. 1993; Gottschling et al. 1990; Mahoney et al. 1991).
One key difference between local gene-specific repressors and long-range repressors is that the latter usually spread along the chromatin to encompass a large chromatin domain into a repressed state. Silencing mediated by the Sir proteins initiates at specific elements (silencers) and then spreads along the chromatin (Luo et al. 2002; Rusche et al. 2002). This encroaching silenced chromatin is actively restricted from spreading by barrier elements (Donze et al. 1999). Localization studies with Sum1-1p have shown that, as with Sir proteins, this protein is also present across the entire HMR domain and it is believed that Sum1-1p recruited to the silencers by ORC spreads along the HMR locus with the aid of Hst1p (Rusche and Rine 2001; Sutton et al. 2001). While Sum1-1p is present across the entire HMR domain, the functional consequences of the physical presence across the domain were not known. We have now shown that while URA3 was not robustly and stably repressed when placed at different sites within this domain (Figure 1 and data not shown), the MATa1 gene was repressed in an orientation- and location-independent manner across the entire domain (Figure 4), suggesting that, as with the Sir proteins, Sum1-1p could also spread and form a repressive chromatin domain. These results are consistent with recent data showing that Sum1-1p-mediated recruitment at ORC-binding sites and the resultant spreading from these sites does lead to repression of some neighboring genes (Lynch et al. 2005).
Our data also showed that the repressed domain was not constrained between the silencers but extended beyond them and that genes located in this flanking region were also repressed by Sum1-1p. Furthermore, our result also showed that genes repressed by Sum1-1p did not necessarily have to be flanked by silencers. Previous data have suggested a requirement for both HMR-E and HMR-I silencers to be present, flanking a gene for efficient repression by this protein (Sutton et al. 2001). We have now shown that the requirement for HMR-I can be partially bypassed by increasing the recruitment of Sum1-1p to the synthetic HMR-E silencer. While the exact function of HMR-I in Sum1-1p-mediated repression is not clear, it is likely that the role of HMR-I is to recruit Sum1-1p with the aid of ORC present at this silencer. However, we cannot exclude the possibility that HMR-I functions to stabilize the Sum1-1p complex recruited at HMR-E in a manner ascribed to proto-silencers (Boscheron et al. 1996).
Also, similar to Sir-mediated silencing (Oki et al. 2004), the spread of the SUM1-1 repressed state could be disrupted by barrier elements, suggesting commonalities in the mechanisms that underlie the spread of these different repressor proteins and that spreading is important for this repression. Current models for Sum1-1p-mediated repression at HMR suggest that the interaction between ORC and Sum1-1p enables its own recruitment as well as that of Hst1p (through its interaction with Sum1-1p), leading to deacetylation of the histones and the propagation of the repressed state along the chromatin fiber (Lynch et al. 2005).
In conclusion, we have shown that Sum1-1p is a long-range repressor since it encompasses an entire chromatin domain to repress genes independently of their position or orientation within the domain. However, unlike Sir-mediated silencing, which is promoter nonspecific and stably inherited, repression mediated by Sum1-1p is more robust for certain promoters and is not stably inherited over many generations.
Acknowledgments
We thank members of the Kamakaka lab for suggestions and criticisms. We also thank J. Rine, D. Shore, and J. Haber for strains and plasmids and A. Gonzalez and C. Aranda for providing facilities and assistance in the analysis of chromatin structure at HMR. We also thank L. Rusche, C. Fox, and N. Dhillon for helpful suggestions during the writing of this manuscript. This work was supported by a National Institutes of Health intramural grant.
References
- Avendano, A., L. Riego, A. DeLuna, C. Aranda, G. Romero et al., 2005. Swi/SNF-GCN5-dependent chromatin remodelling determines induced expression of GDH3, one of the paralogous genes responsible for ammonium assimilation and glutamate biosynthesis in Saccharomyces cerevisiae. Mol. Microbiol. 57: 291–305. [DOI] [PubMed] [Google Scholar]
- Bedalov, A., M. Hirao, J. Posakony, M. Nelson and J. A. Simon, 2003. NAD+-dependent deacetylase Hst1p controls biosynthesis and cellular NAD+ levels in Saccharomyces cerevisiae. Mol. Cell. Biol. 23: 7044–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscheron, C., L. Maillet, S. Marcand, M. Tsai-Pflugfelder, S. M. Gasser et al., 1996. Cooperation at a distance between silencers and proto-silencers at the yeast HML locus. EMBO J. 15: 2184–2195. [PMC free article] [PubMed] [Google Scholar]
- Buck, S. W., and D. Shore, 1995. Action of a RAP1 carboxy-terminal silencing domain reveals an underlying competition between HMR and telomeres in yeast. Genes Dev. 9: 370–384. [DOI] [PubMed] [Google Scholar]
- Chi, M. H., and D. Shore, 1996. SUM1–1, a dominant suppressor of SIR mutations in Saccharomyces cerevisiae, increases transcriptional silencing at telomeres and HM mating-type loci and decreases chromosome stability. Mol. Cell. Biol. 16: 4281–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien, C. T., S. Buck, R. Sternglanz and D. Shore, 1993. Targeting of SIR1 protein establishes transcriptional silencing at HM loci and telomeres in yeast. Cell 75: 531–541. [DOI] [PubMed] [Google Scholar]
- Courey, A. J., and S. Jia, 2001. Transcriptional repression: the long and the short of it. Genes Dev. 15: 2786–2796. [DOI] [PubMed] [Google Scholar]
- Cvrckova, F., and K. Nasmyth, 1993. Yeast G1 cyclins CLN1 and CLN2 and a GAP-like protein have a role in bud formation. EMBO J. 12: 5277–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze, D., and R. T. Kamakaka, 2001. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. EMBO J. 20: 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze, D., C. R. Adams, J. Rine and R. T. Kamakaka, 1999. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev. 13: 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenhofer-Murray, A. E., R. T. Kamakaka and J. Rine, 1999. A role for the replication proteins PCNA, RF-C, polymerase epsilon and Cdc45 in transcriptional silencing in Saccharomyces cerevisiae. Genetics 153: 1171–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto, S., and J. Berman, 1998. Chromatin assembly factor I contributes to the maintenance, but not the re-establishment, of silencing at the yeast silent mating loci. Genes Dev. 12: 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, C. A., A. E. Ehrenhofer-Murray, S. Loo and J. Rine, 1997. The origin recognition complex, SIR1, and the S phase requirement for silencing. Science 276: 1547–1551. [DOI] [PubMed] [Google Scholar]
- Gottschling, D. E., O. M. Aparicio, B. L. Billington and V. A. Zakian, 1990. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63: 751–762. [DOI] [PubMed] [Google Scholar]
- Gray, S., and M. Levine, 1996. Transcriptional repression in development. Curr. Opin. Cell Biol. 8: 358–364. [DOI] [PubMed] [Google Scholar]
- Herrick, D., R. Parker and A. Jacobson, 1990. Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol. Cell. Biol. 10: 2269–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida, T., and H. Araki, 2004. Noncompetitive counteractions of DNA polymerase epsilon and ISW2/yCHRAC for epigenetic inheritance of telomere position effect in Saccharomyces cerevisiae. Mol. Cell. Biol. 24: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irlbacher, H., J. Franke, T. Manke, M. Vingron and A. E. Ehrenhofer-Murray, 2005. Control of replication initiation and heterochromatin formation in Saccharomyces cerevisiae by a regulator of meiotic gene expression. Genes Dev. 19: 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, H., Y. Fukuda, K. Murata and A. Kimura, 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T., T. Chiba, R. Ozawa, M. Yoshida, M. Hattori et al., 2001. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98: 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar, A. J., S. N. Kakar, J. M. Ivy, J. B. Hicks, G. P. Livi et al., 1985. SUM1, an apparent positive regulator of the cryptic mating-type loci in Saccharomyces cerevisiae. Genetics 111: 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenson, P., and J. Rine, 1991. SUM1-1: a suppressor of silencing defects in Saccharomyces cerevisiae. Genetics 129: 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenson, P., and J. Rine, 1992. Silencers, silencing, and heritable transcriptional states. Microbiol. Rev. 56: 543–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livi, G. P., J. B. Hicks and A. J. Klar, 1990. The sum1–1 mutation affects silent mating-type gene transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 10: 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo, S., and J. Rine, 1995. Silencing and heritable domains of gene expression. Annu. Rev. Cell Dev. Biol. 11: 519–548. [DOI] [PubMed] [Google Scholar]
- Luo, K., M. A. Vega-Palas and M. Grunstein, 2002. Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 16: 1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, P. J., H. B. Fraser, E. Sevastopoulos, J. Rine and L. N. Rusche, 2005. Sum1p, the origin recognition complex, and the spreading of a promoter-specific repressor in Saccharomyces cerevisiae. Mol. Cell. Biol. 25: 5920–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney, D. J., R. Marquardt, G. J. Shei, A. B. Rose and J. R. Broach, 1991. Mutations in the HML E silencer of Saccharomyces cerevisiae yield metastable inheritance of transcriptional repression. Genes Dev. 5: 605–615. [DOI] [PubMed] [Google Scholar]
- McCord, R., M. Pierce, J. Xie, S. Wonkatal, C. Mickel et al., 2003. Rfm1, a novel tethering factor required to recruit the Hst1 histone deacetylase for repression of middle sporulation genes. Mol. Cell. Biol. 23: 2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed, D., 2001. Common themes in mechanisms of gene silencing. Mol. Cell 8: 489–498. [DOI] [PubMed] [Google Scholar]
- Oki, M., L. Valenzuela, T. Chiba, T. Ito and R. T. Kamakaka, 2004. Barrier proteins remodel and modify chromatin to restrict silenced domains. Mol. Cell. Biol. 24: 1956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios DeBeer, M. A., and C. A. Fox, 1999. A role for a replicator dominance mechanism in silencing. EMBO J. 18: 3808–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, M., K. R. Benjamin, S. P. Montano, M. M. Georgiadis, E. Winter et al., 2003. Sum1 and Ndt80 proteins compete for binding to middle sporulation element sequences that control meiotic gene expression. Mol. Cell. Biol. 23: 4814–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillus, L., and J. Rine, 1989. Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell 59: 637–647. [DOI] [PubMed] [Google Scholar]
- Ravindra, A., K. Weiss and R. T. Simpson, 1999. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating-type locus HMRa. Mol. Cell. Biol. 19: 7944–7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche, L. N., and J. Rine, 2001. Conversion of a gene-specific repressor to a regional silencer. Genes Dev. 15: 955–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche, L. N., A. L. Kirchmaier and J. Rine, 2002. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol. Biol. Cell 13: 2207–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche, L. N., A. L. Kirchmaier and J. Rine, 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72: 481–516. [DOI] [PubMed] [Google Scholar]
- Schmitt, M. E., T. A. Brown and B. L. Trumpower, 1990. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 18: 3091–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shei, G. J., and J. R. Broach, 1995. Yeast silencers can act as orientation-dependent gene inactivation centers that respond to environmental signals. Mol. Cell. Biol. 15: 3496–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F., 1991. Getting started with yeast. Methods Enzymol. 194: 3–21. [DOI] [PubMed] [Google Scholar]
- Sikorski, R. S., and P. Hieter, 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano, P. G., and K. Tatchell, 1984. Transcription and regulatory signals at the mating type locus in yeast. Cell 37: 969–978. [DOI] [PubMed] [Google Scholar]
- Siliciano, P. G., and K. Tatchell, 1986. Identification of the DNA sequences controlling the expression of the MAT alpha locus of yeast. Proc. Natl. Acad. Sci. USA 83: 2320–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel, L., D. Vannier and D. Shore, 1993. Epigenetic switching of transcriptional states: cis- and trans-acting factors affecting establishment of silencing at the HMR locus in Saccharomyces cerevisiae. Mol. Cell. Biol. 13: 3919–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, A., R. C. Heller, J. Landry, J. S. Choy, A. Sirko et al., 2001. A novel form of transcriptional silencing by Sum1–1 requires Hst1 and the origin recognition complex. Mol. Cell. Biol. 21: 3514–3522.11313477 [Google Scholar]
- van Leeuwen, F., and D. E. Gottschling, 2002. Assays for gene silencing in yeast. Methods Enzymol. 350: 165–186. [DOI] [PubMed] [Google Scholar]
- Wu, X., and J. E. Haber, 1996. A 700 bp cis-acting region controls mating-type dependent recombination along the entire left arm of yeast chromosome III. Cell 87: 277–285. [DOI] [PubMed] [Google Scholar]
- Xie, J., M. Pierce, V. Gailus-Durner, M. Wagner, E. Winter et al., 1999. Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J. 18: 6448–6454. [DOI] [PMC free article] [PubMed] [Google Scholar]